Abstract
The delivery of spatially modulated radiation fields has been shown to impact on in vitro cell survival responses. To study the effect of modulated fields on cell survival, dose response curves were determined for human DU-145 prostate, T98G glioma tumour cells and normal primary AGO-1552 fibroblast cells exposed to modulated and non-modulated field configurations delivered using a 6 MV Linac with multi-leaf collimator. When exposed to uniform fields delivered as a non-modulated or modulated configuration, no significant differences in survival were observed with the exception of DU-145 cells at a dose of 8 Gy (p = 0.024). Survival responses were determined for exposure to non-uniform modulated beams in DU-145 and T98G and showed no deviation from the survival response observed following uniform non-modulated exposures. The results of these experiments indicate no major deviation in response to modulated fields compared to uniform exposures.
Keywords: IMRT, modulated fields, cell survival, radiobiology
1. Introduction
The aim of radiotherapy is to deliver a dose of radiation to the tumour volume that is sufficient to kill all the tumour cells whilst at the same time minimising damage to the surrounding normal tissues. Significant advances have been made in the technical delivery of radiotherapy using complex beam delivery approaches coupled with sophisticated imaging. These include Intensity Modulated Radiation Therapy (IMRT) which has the ability to improve dose distributions in a way that results in higher conformity around the tumour target whilst reducing dose to normal tissues. The clinical evidence for the effectiveness of IMRT is encouraging (Veldeman et al 2008). Our understanding of the effects of radiation exposure at the cellular level have been driven by an assumption that direct DNA damage triggers response in proportion to dose (Hall & Giaccia 2006). This also assumes that for variable doses delivered across a tumour the response is directly proportional to the dose received by each individual tumour cell. Other factors related to tumour heterogeneity, such as the individual radiosensitivity, proliferation status and the level of localised hypoxia, for example will contribute to the survival response. Recently it has become clear that cell to cell communication plays a major role and that in response to radiation exposure bystander responses may occur where cells respond to their neighbours being irradiated (Prise & O’Sullivan 2009). Advanced delivery approaches for therapy have two potential differences compared to conventional techniques: prolonged dose delivery and spatial modulation. Prolonged fraction times are often inherent in the delivery of IMRT plans due to the increased number and complexity of fields and the increased time required to deliver radiation due to MLC movement either during or between radiation exposures (Kuperman et al 2008). A number of theoretical studies have predicted reduced cell kill when delivering similar doses over a protracted period (Mu et al 2003, Fowler et al 2004, Wang et al 2003, Paganetti 2005). In vitro studies have also found reduced cell kill due to protracted delivery times (Ogino et al 2005, Shibamoto et al 2004, Bewes et al 2008).
IMRT beams are by definition spatially modulated. It has been reported following modulated delivery that communicating cells exhibited different survival characteristics to cells which had inhibited communication under the same delivery conditions (Suchowerska et al 2005). Claridge Mackonis et al 2007 assessed cell survival in vitro through comparison of delivery of a uniform field (to 100% of the cell population) to delivery to 25 % of the cell population as either a single region or as three parallel stripes within the tissue culture flask. Differences in cell survival were observed both in irradiated areas communicating with non irradiated areas and in non irradiated areas communicating with irradiated areas when compared to uniformly irradiate cells at the same dose level (Claridge Mackonis et al 2007). This has generated some discussion in the literature with respect to these observations (Ross and Klassen 2009; Claridge Mackonis et al 2009).
Moiseenko et al 2007 compared acute treatment plans to IMRT plans in vitro for head and neck patients and found decreased cell kill in IMRT plans. This work assessed cell survival outcome following IMRT compared to acute radiotherapy delivery. Further studies revealing outcomes comparing modulated to non-modulated field delivery are important as differences are consistently being revealed (Moiseenko et al 2007, Bewes et al 2008) although this is not seen clinically (Veldeman et al 2008). The aim of this work is to identify the effects of different modulated radiation fields on cell survival. Unlike other studies, a number of different dose levels have been studied in order to generate dose response curves for both modulated and non-modulated fields. Experiments were performed using human normal and tumour cell lines.
2. Methods
Cell culture
The human prostate cancer cell line (DU-145) was grown in RPMI-1640 with L-glutamine (Lonza, UK) supplemented with 10% fetal bovine serum, 1% penicillin / streptomycin (Gibco, UK). The human glioma cell line (T98G) was grown in Eagle’s minimum essential medium (Lonza, UK) supplemented with 10% fetal bovine serum and 1% penicillin / streptomycin. The human fibroblast cell line (AGO-1552) was grown in Eagle’s minimum essential medium with deoxyribonucleosides and deoxyribonucleotides supplemented (Lonza, UK) with 20% fetal bovine serum and 1% penicillin / streptomycin. All cell lines were obtained from Cancer Research UK and maintained at 37°C in a humidified atmosphere of 5% CO2.
Clonogenic Assay
Cell survival was determined by clonogenic assay. Sub confluent cells were removed from flasks by incubating in a 1:1 solution of 0.25 % trypsin and 1 mM EDTA (Gibco, UK). Following detachment, cells were centrifuged and re-suspended in fresh culture media before counting using a Coulter counter set at a threshold calibrated for the cell line. Appropriate cell numbers were plated for survival analysis using the clonogenic assay technique of Puck and Marcus (1956). Cells were allowed to adhere overnight. Immediately prior to irradiation, cell culture flasks were filled with serum free medium and sealed. Cells were irradiated at room temperature (25 ± 2 °C). Following irradiation, serum free medium was removed and replaced with complete culture medium. Cell cultures were incubated for 10-14 days at 37°C in 5% CO2 in air and 95% humidity before staining with crystal violet. Colonies exceeding 50 cells were scored as representing surviving cells. All exposures were performed in triplicate on at least three independent occasions. On each occasion unexposed controls were prepared and treated as sham exposures.
Irradiation Setup
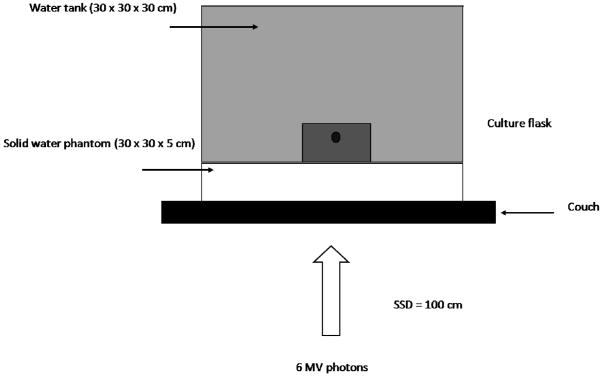
Cells were irradiated in T75 culture flasks (Nunc, UK) with a 6 MV photon beam produced by a Varian 600CD medical linear accelerator with 120 leaf millennium MLC (Varian Medical Systems, Palo Alto, CA) calibrated according to the UK Code of Practice (IPSM, 1990). Full scatter conditions were achieved by filling the flask with culture medium prior to irradiation and submersing in a water phantom on top of a 30 × 30 cm, 5 cm deep block of solid water which was placed on the treatment couch. The gantry was placed at 180° and the couch placed so that the source to surface distance (SSD) to the couch top was 100 cm. All calculations for set monitor units (MU) included a factor to account for the attenuation of the couch. The set-up, as shown in figure 1, was CT scanned using a Siemens Emotion 6 (Siemens AG, Erlangen, Germany) and a plan using Nucletron Oncentra MasterPlan (OMP) (Nucletron BV, Veenendal, The Netherlands) created to ensure uniform irradiation of the T75 flask using a 20 × 20 cm field and to deliver the number of monitor units required to give the prescribed dose to the flask. Cell survival at 9 dose points was measured for AGO-1522 cells (0 – 6.4 Gy) and 6 dose points for DU-145 and T98G cells (0 – 8 Gy).
Figure 1.
Schematic representation of irradiation setup for delivery of dose to cell culture flasks. Cell monolayers were irradiated under full scatter conditions in a Perspex water phantom. A Varian 600CD linear accelerator was used to deliver 6 MV photons in a variety of different field configurations.
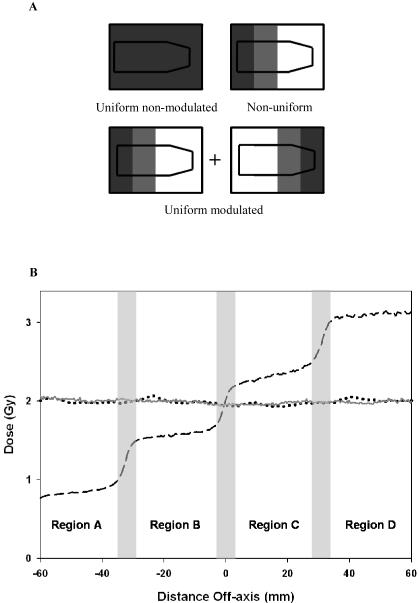
Cells were exposed to three different spatial field configurations; uniform non-modulated, uniform modulated and non-uniform modulated. These are shown schematically in figure 2 (a). The uniform non-modulated field was an ‘open’ field setup defined by the primary and secondary collimators. The uniform-modulated field was delivered through a series of spatially modulated segmented fields to create a uniform dose. This was defined using a the MLC to create a step wedge consisting of five segments of differing size to create a modulated beam (Ezzell et al, 2003) with the X and Y jaws placed at the same dimensions of the largest segment (X1 = 9.5 cm, X2 = 6.5 cm, Y1 = 10 cm and X2 = 10 cm). When delivered dynamically as an equal and opposite step-wedge using the opposing leaf bank from the same gantry angle, a uniform field results from the combination of these two step-wedges to create a uniform modulated field. The non-uniform modulated field was defined using the same step wedge delivered dynamically in one direction with dose prescribed to the centre of the flask. Profiles of doses along the flask are shown in figure 2(b). This simplified set-up is in 2D whereas patient IMRT plans use segmented fields delivered using a variety of gantry angles to create a uniform 3D dose distribution encapsulating the tumour.
Figure 2.
Measured profiles in the direction of MLC’s (GT). Figure 2a shows a schematic representation of dose profile for each of the different field configurations. Figure 2 b shows actual profile using Gafchromic film delivering 2 Gy to the centre of the culture flask. Three different field configurations were delivered; uniform non-modulated (solid line), uniform modulated (dotted line) and non-uniform modulated (dashed line). The non-uniform modulated configuration was separated into four dose regions by excluding the penumbra and defined as regions A – D.
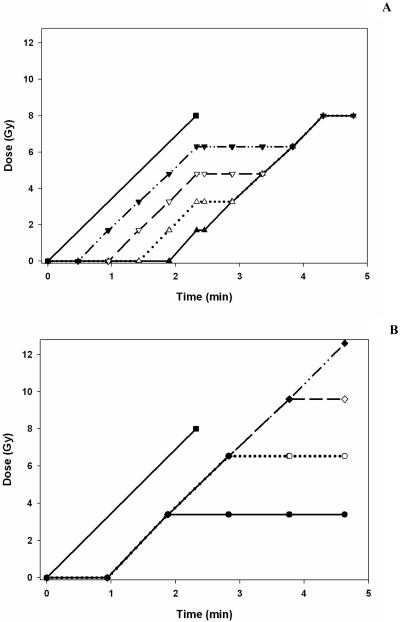
Figure 3 (a) illustrates how dose is accumulated over time at a nominal dose rate of 400 MU / min for the uniform non-modulated set-up, compared to the accumulation of dose with time for each region for a uniform modulated field. At the start and end a ‘redundant’ segment which does not irradiate the flask is shown as a flat gradient. The flat gradient at the centre is the time to load the opposite direction step wedge. A similar representation of dose delivery for the non-uniform modulated field dose acquisition in each region with the uniform non-modulated field is shown in figure 3 (b).
Figure 3.
Representation of dose accumulation with time for different field configurations. Figure 3a shows dose accumulation with time for the uniform non-modulated field (■ solid line) and uniform modulated field delivered to region A (▲ solid line); B (△ dotted line); C (▽ line); D (▼ dash line). Figure 3b shows dose accumulation with time for the uniform non-modulated field (■ solid line) and non-uniform modulated field delivered to region A (● solid line); B (○ dotted line); C (◇ dashed line); D (◆ dot dash line).
Validation of experimental design
The design of the experiments was subject to physical and biological validation. A number of measurements were performed to ensure that the T75 flask was receiving the correct absolute dose using ion chambers and receiving a uniform dose using Gafchromic film (ISP Corp, USA). An NACP-O2 parallel plane ionization chamber was placed at the bottom of the water tank at the centre of the beam and irradiated using the set-up shown in figure 1 using the monitor units generated by OMP. Following calibration of the beam, the dose was confirmed to within 1.5 % of that intended using both setups. Further tests were performed using a Farmer ionisation chamber at the radiological depth in solid water to ensure that the dose from both the uniform non-modulated and uniform modulated were equivalent. They were found to be within 0.2% of each other at 1 Gy.
Gafchromic film was cut into the shape of the T75 flasks and placed at the bottom of the water tank. Each of the fields was delivered to the flask to ensure a dose of 2 Gy on the central axis at the plane of the film (coincident with the plane of the cells). The film was then calibrated and isodoses were observed to ensure correct distribution across the flask. Figure 2(b) shows line profiles along the central axis to help analyse any differences between two set-ups. Table 1 shows the doses attained using the gafchromic film to the non-uniform modulated set-up.
Table 1.
Summary of areas studied within the flask for non-uniform modulated field configuration. Displayed are measured doses to each area depending on the central axis dose. Delivery times for each dose level are also shown
| Central axis dose (Gy) |
Delivery time (mins) |
Region of flask | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
|
| |||||
| 0.5 | 0.33 | 0.21 | 0.40 | 0.59 | 0.78 |
| 1 | 0.62 | 0.42 | 0.80 | 1.18 | 1.56 |
| 2 | 1.18 | 0.84 | 1.58 | 2.34 | 3.11 |
| 4 | 2.33 | 1.68 | 3.16 | 4.68 | 6.23 |
| 8 | 4.66 | 3.36 | 6.32 | 9.36 | 12.46 |
Pilot experiments were conducted comparing cells using phosphate buffered saline (PBS) and culture medium warmed to 37°C prior to irradiation. These studies showed culture medium to provide significant improvement in plating efficiency and reproducibility compared to PBS filled flasks.
Curve fitting and statistical analysis
Surviving fractions (SF) were calculated as the ratio of the number of colonies in the exposed flask to the number of seeded cells corrected for the plating efficiency of sham irradiated control cells. This data was used to plot survival curves fitted to the form SF = exp [− (αD + βD2)] using Origin Pro version 8. Statistical errors on fit values were calculated as the standard error. All experiments were performed in triplicate at least three times with the data presented as ± standard error in all cases. Statistical analysis comparing the survival values for non-modulated and modulated field configurations was carried out using the Mann-Whitney U test. A significance level of 0.05 was used. Calculations were performed using Statistical Package for Social Sciences (SPSS, Chicago, IL).
3. Results
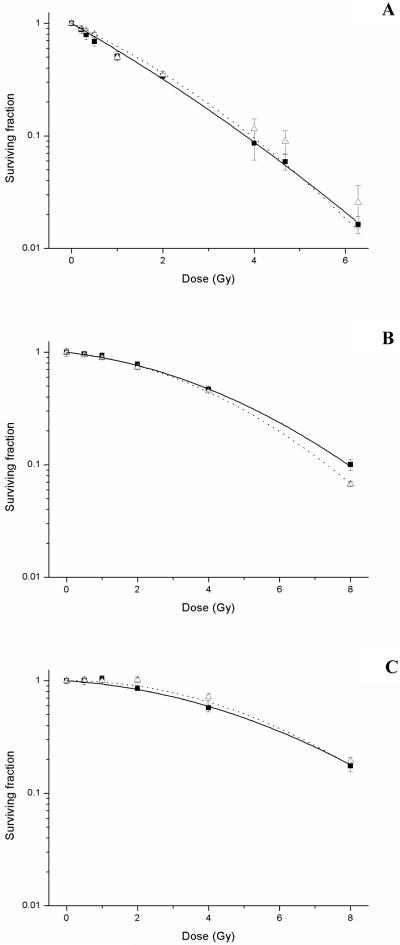
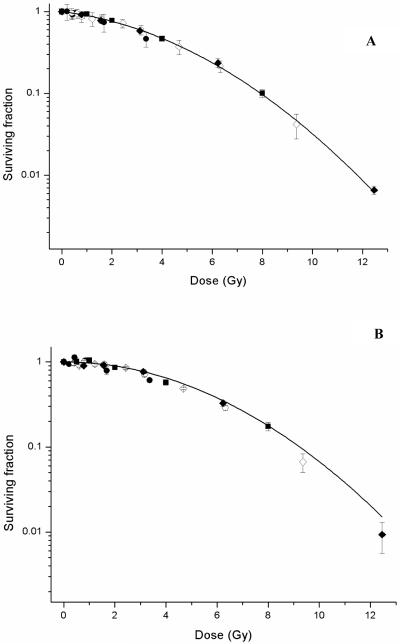
Prior to obtaining cell survival data, dosimetric verification was performed to validate the experimental setup. Survival data for uniform radiation exposures delivered as non-modulated and modulated fields in each of the three cell lines is shown in figure 4 (a-c). These data were fitted to the form SF = exp [− (αD + βD2)]. The parameters of α and β derived from the LQ fit of the survival data for each of the curves are summarised in table 2. Statistical analysis shown in table 3 indicated no significant differences in the response of each of the cell lines between the non-modulated and modulated exposures with the exceptions of DU-145 cell line at a dose of 8 Gy (p = 0.024). Additional measurements were performed at 9.6 Gy and 12.4 Gy in DU-145 cells to further confirm statistically significant differences observed at 8 Gy. Decreased cell survival was observed at 9.6 Gy for modulated exposures (SF = 0.017 ± 0.01) compared to non-modulated (SF = 0.036 ± 0.01) (p = 0.016). This trend was also observed at 12.4 Gy with survival fractions decreasing from 0.003 (± 0.001) to 0.001 (± 0.001) following modulated beam delivery (p = 0.039).
Figure 4.
Radiation dose response curves for AG0-1552B (A), DU-145 (B) and T98G (C) cells following uniform exposures delivered as a non-modulated (■ solid line) and modulated field (△ dotted line). Error bars indicate ± standard error of the mean.
Table 2.
Summary of radiobiological parameters (± standard error of the mean) for cells exposed to uniform doses delivered as modulated or non-modulated fields
| Uniform dose delivery | ||||
|---|---|---|---|---|
| Non-modulated | Modulated | |||
|
| ||||
| Cell line | α (Gy−1) | β (Gy−2) | α (Gy−1) | β (Gy−2) |
|
| ||||
| DU-145 | 0.08 (±0.03) | 0.03 (±0.01) | 0.07 (±0.02) | 0.03 (±0.01) |
| T98G | 0.05 (±0.03) | 0.02 (±0.01) | 0.00 (±0.02) | 0.03 (±0.01) |
| AGO-1522B | 0.53 (±0.05) | 0.02 (±0.01) | 0.43 (±0.08) | 0.04 (±0.02) |
Table 3.
Summary of statistical analysis for cells exposed to uniform doses delivered as modulated or non-modulated fields (± standard error of the mean)
| Uniform field surviving fraction (SF) | ||||
|---|---|---|---|---|
|
|
|
|||
| Cell line | Dose (Gy) | Non-modulated | Modulated | p value |
|
| ||||
| DU-145 | 2 | 0.79 (±0.04) | 0.74 (±0.03) | 0.566 |
| 4 | 0.49 (±0.03) | 0.45 (±0.01) | 0.200 | |
| 8 | 0.11 (±0.01) | 0.07 (±0.01) | 0.024 | |
|
| ||||
| T98G | 2 | 0.86 (±0.03) | 0.98 (±0.05) | 0.085 |
| 4 | 0.58 (±0.03) | 0.69 (±0.04) | 0.058 | |
| 8 | 0.18 (±0.01) | 0.19 (±0.01) | 0.691 | |
|
| ||||
| AGO-1522B | 2 | 0.36 (±0.02) | 0.36 (±0.03) | 0.453 |
| 4 | 0.10 (±0.01) | 0.12 (±0.02) | 0.965 | |
The non-uniform modulated fields were delivered using the MLC to create a step wedge of 4 regions of different dose across the flask. The nominal central axis doses were identical to the doses for the uniform field exposures. The delivery times for the modulated field configuration were approximately double that for the uniform non-modulated configuration. The timings along with the areas and measured doses for the modulated field set up are shown in table 1. Survival analysis for the modulated fields was performed by separating the flask into discrete areas according to dose (regions A-D). Penumbra regions were excluded and plating efficiencies were corrected for area. The survival curves for each of the different region of the modulated fields are shown in figures 5 (a) and (b).
Figure 5.
Comparison of survival data for DU-145 (A) and T98G (B) cells following uniform open and regions for modulated fields. Survival data for each region within the modulated field is shown (region a ●; b ○; c ◇; d ◆) with the data for the uniform open field (■) fitted to the LQ model. Error bars indicate ± standard error of the mean.
4. Discussion
This study was designed to identify the effects of different modulated radiation fields on cell survival. The two main experimental aspects to be addressed were, firstly, do survival responses vary when a uniform dose is delivered as non-modulated or modulated fields and secondly, how does non-uniform dose delivery impact on survival responses. Experiments were undertaken in three different human cell lines using different field configurations for dose delivery as a uniform ‘open’ field, a uniform modulated field and non-uniform modulated fields. The cell lines included a radiosensitive normal primary fibroblast and a radioresistant glioma tumour line (T98G).
No statistically significant differences in the survival responses were observed in each of the three cell lines when exposed to a uniform dose delivered as a non-modulated or modulated field with the exception of DU-145 cells at doses greater than 8 Gy. Differences in radiosensitivity of the cell lines are apparent from the calculated α and β parameters for the uniform open field exposures. The similarities in response to the different uniform fields are reflected in the values for α and β with the exception of a zero value for the α component of the radiation response in the T98G cells. This indicates a zero initial slope to the curve with the responses being driven by the β component for the modulated uniform field.
For cells exposed to modulated non-uniform fields, the survival data was broken down into discrete regions of different dose within the same flask defined in figure 2 as regions A – D. Penumbras were excluded from analysis and plating efficiencies corrected for area. DU-145 cells showed no difference from the predicted uniform field response.
T98G cells showed similar responses to the open field with no statistical difference. Fitting of the data from regions A – D to a LQ was not performed due to the differences in the ranges of dose between the non-modulated and modulated fields as a dose dependency of the LQ fit has been reported in several cell lines including DU-145 (Garcia et al, 2006).
The results from this study compliment other reports concerning the radiobiological response of cells to modulated fields. Suchowerska et al, (2005) observed differences in the response of cells to non-uniform fields deliver using a wedge filter depending on the presence of intracellular communication. These responses appeared to ‘average out’ across the dose range suggesting a dose dependent effect with non-uniform exposures showing less survival than uniform exposure at low doses but more survival at high dose.
Further investigation by Claridge Mackonis et al (2007) measured cell survival under different spatial geometries; a uniform field exposing 100 % of flask to dose, a ‘quarter’ field exposing 25 % of the flask as a single region or ‘striped’ field exposing 25 % of the flask exposes as three parallel stripes. This study showed a non-local component of the response to radiation which they attributed to different types of bystander responses defined as type II and type III depending on the exposure received by communicating cell populations. However, the validity of these findings was challenged by Ross and Klassen (2009). The issues raised by the authors included inaccurate dosimetry due to incomplete shielding and MLC leakage causing cells within shielded regions to receive 4 – 6 % of the dose received by the unshielded cells, presentation of average cell survival data, statistical analyses and conclusions based on limited data sets.
Subsequent defence by Claridge Makonis et al (2009) provided detailed explanation for the observed type II and type III bystander responses observed along with description of the analyses made. Additional data using NCI-H460 non-small cell lung cancer cells in which similar responses were observed was presented in further justification of previous conclusions concerning different types of bystander responses.
Given the experimental challenges and debate concerning the presentation and interpretation of survival data when using modulated fields, particular attention was paid to the design, analysis and interpretation in this study. Extensive dosimetry measurements were undertaken and penumbra regions excluded from region analysis for the modulated fields to ensure accurate dose delivery to cells. Cells were irradiated in culture medium to improve reproducibility and data was acquired across a broad range of dose to ensure goodness of fit to the LQ model (Garcia et al, 2006). Finally, we present data acquired in two tumour and one normal human cell lines.
In addition to spatial modulation, the delivery of radiation fields in IMRT can be highly temporally regulated. Delivery time has been demonstrated as an important factor influencing radiobiological outcome with several reports estimating a fraction delivery time of 15 – 30 minutes resulting in clinically significant reduced cell kill (Moiseenko et al, 2007; Mogili et al, 2006; Ogino et al, 2005). Ogino et al (2005) demonstrated that by varying fraction number, fraction dose and dose rate in intermittent exposures cell survival was shown to increase compared to acute irradiation for the same dose in IMRT when delivery times were greater than 15 minutes. Bewes et al (2008) observed increased cell survival with decreased average dose rate and concluded that this reduction was outweighed by therapeutic gain in IMRT.
The combined effect of both spatial modulation and protracted delivery time in IMRT was demonstrated in vitro by Moiseenko et al, (2007). Comparison of acute dose delivered using parallel and opposing fields (75 seconds) with a typical dynamic IMRT plan (5 minutes) and IMRT with break for MLC re-initialisation (10 mins) for head and neck cancer showed decreased cell kill in the three cell lines investigated. Although dose rate effects were not the primary subject of this investigation, it is important to highlight the delivery times for the uniform modulated and non-uniform modulated fields were approximately double the time for the uniform open field. This is inherent in any modulated delivery. Given the role of delivery time in determining radiobiological response, it is likely that delivery times differing only by a factor of 2 for the different configurations may contribute to the similarity in the results and merits further investigation as single variable (Bewes et al 2008) and in combination with spatial modulation. Additional investigation is also required to fully characterise the role of radiation induced bystander signalling in response to modulated fields and elucidate the underlying mechanism responsible.
5. Conclusions
This study reports the survival of cells exposed to uniform doses delivered as non-modulated and modulated fields and also non-uniform doses delivered as modulated fields. No significant difference was found between cells exposed to uniform fields delivered as non-modulated fields or modulated fields in most cases except for DU-145 cells at high dose. In addition, cells exposed to non-uniform fields delivered as a modulated field similarly showed no deviation from uniform open field exposures.Our results indicate no major deviation in response to modulated fields compared to uniform exposures.
Acknowledgements
The authors wish to acknowledge financial support from Cancer Research UK (grant number C1513/A7047 to KMP) and Dr Chris Cardwell of the Department of Epidemiology and Public Health, Queen’s University Belfast for his assistance with statistical analyses. C.K.M. is supported by a Health & Social Care Research & Development of the Public Health Agency Training Fellowship Award.
References
- Bewes JM, Suchowerska N, Jackson M, Zhang M, McKenzie DR. The radiobiological effect of intra-fraction dose-rate modulation in intensity modulated radiation therapy (IMRT) Phys. Med. Biol. 2008;53:3567–78. doi: 10.1088/0031-9155/53/13/012. [DOI] [PubMed] [Google Scholar]
- Claridge Mackonis E, Suchowerska N, Zhang M, Ebert M, McKenzie DR, Jackson M. Cellular response to modulated radiation fields. Phys. Med. Biol. 2007;52:5469–82. doi: 10.1088/0031-9155/52/18/001. [DOI] [PubMed] [Google Scholar]
- Claridge Mackonis E, Suchowerska N, McKenzie DR, Ebert M, Morrell Jackson M., Bewes J. Reply to ‘Comments on “Cellular response to modulated radiation fields”. Phys. Med. Biol. 2009;54:L15–20. [Google Scholar]
- Ezzell GA, Galvin JM, Low D, Palta JR, Rosen I, Sharpe MB, Xia P, Xiao Y, Xing L, Yu CX, IMRT subcommittee. AAPM Radiation Therapy committee Guidance document on delivery, treatment planning, and clinical implementation of IMRT: report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med. Phys. 2003;30:2089–115. doi: 10.1118/1.1591194. [DOI] [PubMed] [Google Scholar]
- Fowler JF, Welsh JS, Howard SP. Loss of biological effect in prolonged fraction delivery. Int. J. Radiat. Oncol. Biol. Phys. 2004;59:242–9. doi: 10.1016/j.ijrobp.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Garcia LM, Leblanc J, Wilkins D, Raaphorst GP. Fitting the linear-quadratic model to detailed data sets for different dose ranges. Phys. Med. Biol. 2006;51:2813–2823. doi: 10.1088/0031-9155/51/11/009. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Lippincott William & Wilkins; Philadelphia: 2006. [Google Scholar]
- IPSM Code of practice for high-energy photon therapy dosimetry based on the NPL absorbed dose calibration service. Phys. Med. Biol. 1990;35:1355–60. doi: 10.1088/1361-6560/ab99e3. [DOI] [PubMed] [Google Scholar]
- Kuperman VY, Ventura AM, Sommerfeldt M. Effect of radiation protraction in intensity-modulated radiation therapy with direct aperature optimization: a phantom study. Phys. Med. Biol. 2008;53:3279–92. doi: 10.1088/0031-9155/53/12/014. [DOI] [PubMed] [Google Scholar]
- Mogili N, Joiner M, Burmeister J. Effects of IMRT treatment time progression on tumour cell survival. Med. Phys. 2006;33:2294. [Google Scholar]
- Moiseenko V, Duzenli C, Durand RE. In vitro study of cell survival following dynamic MLC intensity-modulated radiation therapy dose delivery. Med. Phys. 2007;34:1514–20. doi: 10.1118/1.2712044. [DOI] [PubMed] [Google Scholar]
- Mu X, Löfroth PO, Karlsson M, Zackrisson B. The effect of fraction time in intensity modulated radiotherapy: theoretical and experimental evaluation of an optimisation problem. Radiother. Oncol. 2003;68:181–7. doi: 10.1016/s0167-8140(03)00165-8. [DOI] [PubMed] [Google Scholar]
- Ogino H, Shibamoto Y, Sugie C, Ito M. Biological effects of intermittent radiation in cultured tumor cells: influence of fraction number and dose per fraction. J Radiat. Res (Tokyo) 2005;46:401–6. doi: 10.1269/jrr.46.401. [DOI] [PubMed] [Google Scholar]
- Paganetti H. Changes in tumor cell response due to prolonged dose delivery times in fractionated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:892–900. doi: 10.1016/j.ijrobp.2005.07.953. [DOI] [PubMed] [Google Scholar]
- Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck TT, Marcus PI. Action of X-rays on mammalian cells. J. Exp. Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CK, Klassen NV. Comments on ‘Cellular response to modulated radiation fields’. Phys. Med. Biol. 2009;54:L11–3. doi: 10.1088/0031-9155/54/5/L02. [DOI] [PubMed] [Google Scholar]
- Shibamoto Y, Ito M, Sugie C, Ogino H, Hara M. Recovery from sublethal damage during intermittent exposures in cultured tumor cells: implications for dose modification in radiosurgery and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2004;59:1484–90. doi: 10.1016/j.ijrobp.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Suchowerska N, Ebert MA, Zhang M, Jackson M. In vitro response of tumour cells to non-uniform irradiation. Phys. Med. Biol. 2005;50:3041–51. doi: 10.1088/0031-9155/50/13/005. [DOI] [PubMed] [Google Scholar]
- Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet. Oncol. 2008;9:367–75. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Li XA, D’Souza WD, Stewart RD. Impact of prolonged fraction delivery times on tumor control: a note of caution for intensity-modulated radiation therapy (IMRT) Int. J. Radiat. Oncol. Biol. Phys. 2003;57:543–52. doi: 10.1016/s0360-3016(03)00499-1. [DOI] [PubMed] [Google Scholar]