Abstract
Setting
A public tuberculosis (TB) referral hospital in KwaZulu-Natal, South Africa.
Objective
To present treatment outcomes of patients with extensively drug resistant tuberculosis (XDR-TB) patients and HIV co-infection with and without HAART.
Methods
Retrospective cohort study. Eligible patients had drug susceptibility testing that met a consensus definition for XDR-TB, and agreed to treatment. Therapy was based on drug susceptibilities, available medications, and patient tolerance.
Results
60 XDR-TB patients initiated therapy with a median number of 5.5 drugs. Of these 43 (72%) were HIV+, and 21 (49%) were on anti-retroviral therapy. 29 HIV infected patients (67%) had available CD4 counts; median CD4 count was 200.5 (S.D. 127.4). 31/60 patients (52%) had adverse events (AEs), and 17/60 patients (28%) had severe AEs. During follow-up, 12/60 (20%) experienced sputum culture conversion, while 25/60 (42%) patients died. None of the following was significantly associated with mortality: HIV status, previous MDR diagnosis or severe AEs.
Discussion
In this study it was possible to treat HIV/XDR-TB co-infected patients, and prolong survival in a resource limited setting. We highlight the challenges in treatment, including high frequencies of AEs and death. Expanded identification of cases, prompt referral for treatment, and attention to management of co-morbidities may facilitate successful treatment of in XDR-TB in HIV infected patients.
Keywords: Extensively drug resistant tuberculosis, HIV/AIDS, treatment, South Africa
Introduction
Mycobacterium tuberculosis (M. tuberculosis) is a leading cause of mortality world-wide with 1.7 million deaths from tuberculosis disease (TB) in 2006. [1,2] Although the global incidence of tuberculosis has stabilized over the past five years, TB causes disproportionate disease and mortality in Africa, largely attributable to TB/HIV co-infection (TB/HIV).[3] Multi-drug resistant TB (MDR-TB) and extensively drug resistant TB (XDR-TB) largely attributed to a failure of public health systems, are increasingly identified as one of the greatest challenges to TB control efforts in Africa and world-wide.[4,5] The phenomena of XDR-TB with HIV co-infection, first described in an outbreak in South Africa,[6] threaten to overwhelm fragile health systems.[7]
Drug resistant tuberculosis is not a new phenomenon. Resistance to streptomycin emerged in the very first TB treatment trial, and as each new therapeutic agent has been introduced drug-resistant TB organisms have emerged.[8] The most resistant form of TB, XDR-TB is defined as resistance to isoniazid, rifamycin, fluoroquinolones and one of three injectable second-line anti-TB agents: capreomycin, kanamycin, and amikacin.[9] XDR-TB cases are being increasingly reported world-wide and comprise a rising percentage of all drug resistant TB cases.[1,10]
In particular, XDR-TB cases are causing increasing alarm in South Africa where endemic HIV makes for a deadly and fast moving epidemic.[11] A report published in 2006 from Tugela Ferry, a rural sub-district in KwaZulu-Natal (KZN), South Africa, showed that MDR-TB was present in 221 (41%) of 542 patients with positive cultures for MTB and 53 (9.7%) of these patients had XDR-TB.[5] Among the XDR-TB patients with a known HIV status all were HIV seropositive. In that setting, XDR-TB was rapidly fatal with a median survival of 16 days after specimen collection. However, because the diagnosis of XDR-TB by drug susceptibility testing takes several weeks, these patients died before appropriate therapy could be administered.
Before 2005, only one or two XDR-TB cases were referred annually to King George V Hospital (KGV), the regional TB referral hospital in KZN, South Africa. Since 2005 the number of XDR-TB admissions at KGV has doubled annually: 33 cases in 2005, 74 XDR cases in 2006, and 148 cases in 2007. (personal communication, Dr. Iqubal Master, KGV). Since admission depends on having the results of extensive drug susceptibility testing, and survival to referral, these numbers likely represent only a portion of XDR-TB cases in the province. However, it is unclear whether the increase in XDR-TB cases represents a true increased incidence or rather reflects increased testing of isolates for resistance to second line anti-tuberculosis drugs.
Among HIV uninfected patients in developed countries, XDR-TB is difficult to treat and has worse treatment outcomes than MDR-TB.[12,13] Treatment outcomes of XDR-TB among HIV co-infected patients in developing countries have not been reported. This study represents the first published treatment outcomes data among XDR-TB patients in Africa, the majority of whom are HIV/TB co-infected.
Methods
Study Participants
Participants were enrolled at KGV in Sydenham, KZN. Complicated and drug resistant TB cases are referred to KGV at the discretion of the treating physician and are admitted depending on patient acuity and bed availability. Eligible participants were adults patients admitted to KGV between December 1, 2006 and May 31, 2007 with culture proven TB and M. tuberculosis drug susceptibility testing meeting the revised WHO criteria for XDR-TB.[14] In addition, patients had to agree to initiate treatment with appropriate second and third-line anti-tuberculosis therapy. All anti-tuberculosis treatment regimens were determined by KGV physicians based on DST and adverse drug reactions.
Study Design
Patients who met eligibility criteria were identified retrospectively and information was collected by chart review. Data, including demographics, risk factors and adverse drug reactions, and treatment outcomes, were collected retrospectively. Treatment outcomes of enrolled patients were followed through till October 31, 2007. Standard drug-resistant TB treatment outcome definitions were used to define outcome.[15] Treatment outcomes were Death, Culture Conversion (at least 2 negative cultures 30 days apart), Treatment Default (treatment interrupted for 2 or more consecutive months for any reason), or continued therapy without culture conversion. Adverse drug reactions were recorded qualitatively by clinical staff, and were considered severe if they resulted in a significant change in clinical status, or required a change of anti-tuberculosis regimen.[16] In addition, significant electrolyte abnormalities (potassium<2.5mmol/L, or magnesium<1.5mmol/L) if attributed to medication use, were considered to be severe adverse drug reactions.
The cause of death of patients who died during the study period was determined through patient chart review, review of imaging studies, discussion with treating physician at time of death, and laboratory studies by one of the authors. Study protocol was approved by Biomedical Research Ethics Committee of the University of KwaZulu-Natal and the Institutional Review Board of Boston University Medical Center.
Drug Susceptibility Testing
Drug susceptibility testing (DST) for 1st and 2nd line TB medications was performed at the provincial TB referral laboratory in Durban, South Africa. Culture positivity was determined using the BACTEC MGIT 960 fluorometric system (Becton Dickinson, Sparks, MD). Drug susceptibility to isoniazid, rifampin, ethambutol, streptomycin, ethionamide, ofloxacin, and kanamycin was determined using the modified proportional growth method on 7H11 agar according to standard techniques. [17,18] Notably drug susceptibility to capreomycin, paraminosalacylic acid (PAS), and pyrazinamide was not available during this period because of technical and resource availability issues.
Statistical Analysis
All participants in the study were included in an analysis of risk factors for survival versus death. Univariate statistical associations between survival status and categorical variables were tested using the Cox proportional hazards model. Ninety-five percent confidence intervals were calculated by using a normal approximation of the binomial distribution. Kaplan-Meier survival curves were calculated both from time of diagnosis and from time of treatment with appropriate anti-tuberculosis medications. Subjects were censored when lost to follow up or if still event free and in follow up at the end of study period. Statistical analysis was performed with SAS version 9.1 software (SAS Institute, Inc., Cary, North Carolina).
Results
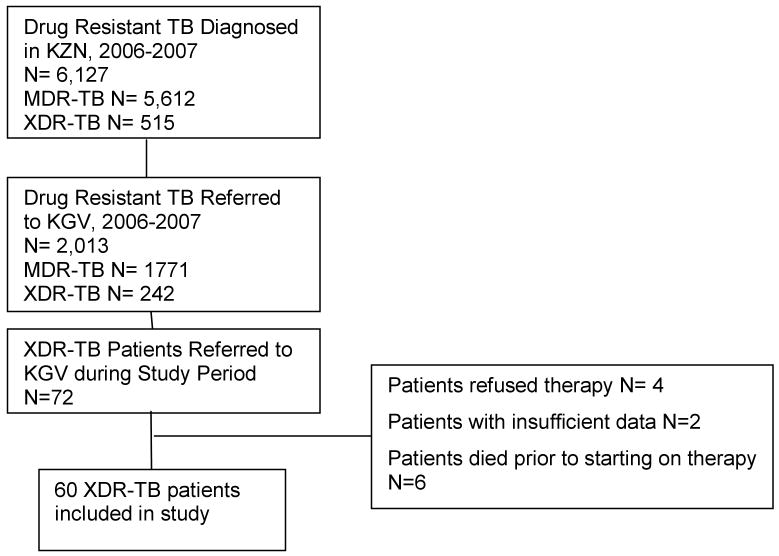
Between 2003 and 2007, 515 patients were diagnosed with XDR-TB throughout KZN province. [19] Seventy-two patients were referred to KGV Hospital during the study period with a diagnosis of XDR-TB. (Figure 1) Ten patients were not started on therapy for XDR-TB either because they refused therapy, absconded from hospital, or died before therapy was initiated. Sixty-two patients were started on therapy for XDR-TB, and information is available on sixty patients who comprise the study cohort (records not available for 2 patients). (Table 1)
Figure 1. XDR-TB patients eligible for analysis in study.
Table 1. Demographic characteristics of extensively drug resistant tuberculosis (XDR-TB) patients initiated on XDR-TB therapy during study period.
| XDR-TB Patients (% total) |
||
|---|---|---|
| Sex | Male | 26 (43) |
| Female | 34 | |
| Age (years) | <20 | 3 (5) |
| 20-35 | 28 (47) | |
| 36-50 | 24 (40) | |
| >50 | 6 (10) | |
| Median Age | 35.0 | |
| (S.D.) | (11.6) | |
| HIV Status | Positive | 43 (72) |
| Negative | 12 | |
| Unknown | 5 | |
| CD4 Count (cells/mm3) | Known | 29 (48) |
| Not Determined | 14 | |
| Median CD4 Count | 200.5 | |
| (S.D.) | (127.4) | |
| On HAART* | Yes | 21 (35) |
| No | 22 | |
| Adverse Events | Yes | 31 (52) |
| Severe† | 17 | |
| No | 20 | |
| Unknown | 9 | |
| Previous TB Treatment | Yes | 42 (70) |
| No | 11 | |
| Unknown | 7 | |
| Previous MDR-TB** Treatment | Yes | 22 (37) |
| No | 28 | |
| Unknown | 10 | |
| Health Care Worker | Yes | 3 (5) |
| No | 57 | |
| Type of Tuberculosis | Pulmonary | 43 (72) |
| Extrapulmonary | 8 | |
| Unknown | 9 | |
HAART – Highly active antiretroviral therapy
MDR-TB – Multi drug resistant tuberculosis
Severe Adverse Drug Reactions- resulted in significant change in clinical status, significant electrolyte abnormalities or required a change of anti-tuberculosis regimen.
Patients included in this study were transferred from 26 different clinics or hospitals representing 7/11 (64%) of health districts in KZN. The most common addresses patients reported were Tugela Ferry (23%), Durban (15%), or Pietermaritzburg (15%). The majority of patients were HIV co-infected (70%), female (57%) and younger than 50 years of age (90%). The majority of patients were treated previously for TB (70%) and a portion had been treated for MDR-TB (37%). Twenty-one of 43 patients with known HIV infection were on highly active antiretroviral therapy (HAART) for HIV using efavirenz-based regimens. Among the 43 patients with HIV infection 9 who were not on HAART had a CD4 count >200 cells/mm3 and were therefore not considered eligible for treatment with ARVs by South African treatment guidelines. Five patients had CD4 counts<200 cells/mm3 but were not on HAART. When stratified by HAART status, patients with HIV infection on HAART were at lower risk for death compared to patients who were HIV infected but not on HAART, however this was not statistically significant (O.R. 0.74 95% CI 0.22-2.49). When further stratified by CD4 count, HIV infected XDR-TB patients with CD4 count below 200 on HAART had substantially lower risk for death compared to patients with CD4 counts less than 200 not on HAART (O.R. 0.094 95% CI 0.007 to 1.22), though this results was not statistically significant.
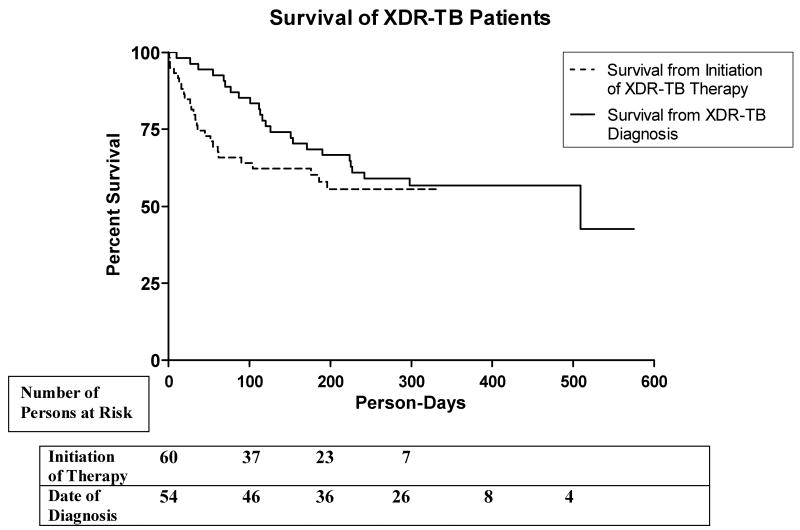
Unadjusted treatment outcomes are presented in Table 2. The longest duration of treatment among patients in this cohort was 11 months; therefore there were no Treatment Failures or Cures according to the standard definitions.[15] ‘Converted Culture’ indicates patients with 2 consecutive cultures at least 1 month apart which were negative at 6 weeks, and no positive smear or culture results within the remaining study period. All treatment outcome categories are mutually exclusive. There were no deaths among patients who converted their TB cultures. Kaplan-Meier survival curves from time of XDR-TB diagnosis and initiation of XDR-TB therapy are shown in Figure 2. Only 54 patients were analyzed from time of diagnosis since time of diagnosis was unknown for 6 patients. Median time between diagnosis and initiation of therapy for patients who died was 88 days. Median time between diagnosis and initiation of therapy for patients who survived was 120 days.
Table 2. Treatment outcomes in a cohort of South African patients with extensively drug resistant tuberculosis (XDR-TB).
| Treatment Outcome | Number (%) |
|---|---|
| Default | 6 (10) |
| Death | 25 (42) |
| Culture Conversion | 12 (20) |
| Continued Treatment | 17 (27) |
| Median Time to Death from Initiation of Therapy, in Days (interquartile range) | 33 (15-61) |
| Median Duration of Follow Up from Initiation of Therapy, in Days (interquartile range) | 183.5 (44-267) |
| Median Duration of Period Between XDR-TB Diagnosis and Initiation of Therapy, in Days (interquartile range) | 83 (61-123) |
| Median Time to Culture Conversion, in Days (interquartile range) | 90 (69-118) |
Figure 2.
Although we looked for associations between risk factors and treatment outcomes there were no statistically significant associations found. (Table 3) In this cohort HIV status did not predict either death or culture conversion. CD4 T-cell count less than 200 did appear to have a substantial association with death, but this association was not statistically significant.
Table 3. Univariate analysis of predictors of mortality in a treatment cohort of South African XDR-TB patients.
| Deceased Number/total (%) |
Survivors Number/total (%) |
P value | Univariate Hazard Ratio (95% CI) |
||
|---|---|---|---|---|---|
| Sex | Female | 13/25 (52) | 21/35 (60) | 0.36 | 0.69 (0.31-1.52) |
| Age (years) | 50+ | 2/25 (8) | 4/35 (11) | 0.66 | 0.67 (0.11-4.00) |
| Previous TB Treatment | Yes | 17/25 (68) | 26/35 (74) | 0.69 | 0.78 (0.23-2.67) |
| Previous MDR-TB Diagnosis | Yes | 8/25 (32) | 14/35 (40) | 0.59 | 0.89 (0.34-2.32) |
| HIV Infected | Yes | 18/23 (78) | 25/32 (78) | 0.90 | 0.96 (0.52-1.78) |
| CD4 count (cells/mm3) | Less than 200 | 7/9 (78) | 9/20 (45) | 0.16 | 3.07 (0.64-14.79) |
| On HAART | Yes | 8/25 (32) | 13/35 (37) | 0.75 | 0.87 (0.38-2.02) |
| Severe AEs* | Yes | 6/18 (33) | 11/32 (34) | 0.94 | 0.94 (0.35-2.51) |
AEs- Adverse Events
Through medical chart, laboratory data, radiology imaging review and discussion with treating physicians, we attempted to determine cause of death for each patient. (Table 4) Etiologies likely associated with the diagnosis of tuberculosis (respiratory failure, hemoptysis) were major causes of death, as were AIDS-related illnesses (progression of HIV disease, septic shock). Adverse events while on treatment causing death due to electrolyte abnormalities were seen in patients being treated with capreomycin. These events decreased over the study period as physicians aggressively supplemented potassium and magnesium for patients prior to treatment with capreomycin.
Table 4. Probable* causes of death among XDR-TB patients started on anti-tuberculosis therapy from December 2006 through to October 2007.
| Cause of Death | Number of Patients |
|---|---|
| Respiratory Arrest | 8 |
| Unable to Ascertain | 7 |
| Adverse Drug Reactions | 4 |
| Septic Shock | 2 |
| Progression of HIV Disease | 2 |
| Massive Hemoptysis | 1 |
| Acute Renal Failure | 1 |
Based on chart and laboratory review as well as discussion with treating physicians.
Discussion
In this cohort it was possible to treat HIV/XDR-TB co-infected patients, prolong survival, and convert XDR-TB cultures even in a resource-limited public health system.[20] Compared to the mortality rates reported among HIV/XDR-TB patients in the Tugela Ferry outbreak, mortality in our cohort was significantly reduced in association with treatment with a regimen consisting of anti-mycobacterial agents to which the patient's M. tuberculosis isolate was susceptible in vitro. The survival curve in the Tugela Ferry outbreak showed median survival of 16 days from time of XDR-TB sputum collection until death. In our study patients survival was prolonged; Kaplan-Meier survival analysis showed 43% survival from time of XDR-TB diagnosis at 509 days although few patients contributed person-time to the study this far into the follow up period. Kaplan-Meier survival analysis from initiation of XDR-TB therapy showed 56% survival at 196 days. This analysis was more robust as there were more participants contributing person-days to this result.
In part this improved survival rate may represent survival bias; patients had to survive for 3.5 months on average before initiation of therapy and enrollment in the study. Although patients from Tugela Ferry comprised the largest subset of patients in this study (23%) they were not a majority; showing that XDR-TB is present throughout the province. A significant difference between the earlier Tugela Ferry report and the cohort presented in this study is the degree of immunosupression due to HIV infection; patients in the Tugela Ferry outbreak had a mean CD4 count 63 as compared to median CD4 count 200 in our cohort. Regardless, the lower mortality rate observed in our study suggests that XDR-TB/HIV co-infection need not have the high case fatality rate observed in that initial report.
There have been reported XDR-TB treatment outcome studies from Korea showing XDR-TB cure rate 53.5%, and from Europe showing XDR-TB cure rate 29%. [12,21,22] A recent article describing community-based treatment of XDR-TB in Peru in 48 patients reported 60.4% cured after a median duration of therapy of 24 months.[23] None of these cohorts included HIV co-infected patients. The largest series to date reviewing treatment outcomes among drug-susceptible TB patients with HIV co-infection was the ten year experience of the San Francisco Tuberculosis Control Program. This study showed a similar treatment completion rate among HIV infected and non-infected persons, but a higher rate of death due to tuberculosis, and a strikingly higher overall death rate in the HIV-infected over the course of follow-up.[24] This suggests that HIV infection contributes to the poorer survival outcomes of patients with HIV and TB, and may explain, in part, why our XDR-TB patients had poorer outcomes than patients with XDR-TB but without HIV, such as those reported from Korea and Europe.
One of the findings in our results was the lack of correlation between mortality and HIV status among XDR-TB patients. A possible explanation is that the study was underpowered to detect an association between HIV status and death. However, the best effect estimate indicates no causal association. Increasing the sample size would likely only narrow the confidence intervals around the effect estimate.[25] The unmeasured clinical factors that enabled these particular patients to survive long enough to receive therapy might mitigate against the effect of HIV on clinical outcome.[26] It is important to note that HIV status is associated with other predictors of mortality, that we were not able to entirely control for in this study. Potential factors that might mask a real association between HIV and mortality include the fact that 21/41 HIV-infected patients were on HAART; CD 4 T-cell count was unknown for 14 patients; and HIV status was unknown for 5 patients. Although we have tried to address these issues through stratified analysis, the variables are time varying and interdependent which limits the value of conventional Cox proportional hazards analysis.
Several studies have found that CD4 count is inversely related to clinical outcomes with drug-susceptible and MDR-TB among HIV co-infected patients. [27,28,29] The effect of low CD4 count was seen in patients on and off HAART and is likely independent of HAART. Although no statistically significant association between outcome and CD4 T-cell count was found in our study, there was a clinically significant effect measure (H.R. 3.07 95% C.I. 0.64-14.79). It is a plausible association and likely that a larger study would find this to be statistically significant.
This paper highlights the challenges in providing care for XDR-TB/AIDS co-infected hospitalized patients in the developing world.[5,30] There were not always timely laboratory or information services, full array of second and third line anti-TB medicines, or complete second line drug susceptibility testing. Initially, adverse drug reactions including electrolyte abnormalities were not detected and treated expeditiously. Doctors and nurses are overburdened with significant inpatient and outpatient responsibilities for sick and medically complex patients.[31] Given such limitations high rates of adverse drug reactions, patient default and death are not unexpected. [32,33] Limitations of this study also include the retrospective design, referral bias and selection bias in the cohort.
Data from this study show it is possible to treat patients with XDR-TB/AIDS co-infection in the developing world in the public health care system. Patients with XDR-TB should be diagnosed promptly and treated aggressively despite the fact that they are XDR-TB/HIV co-infected. Treatment of patients with XDR-TB and HIV with HAART according to published guidelines may reduce mortality rates. [34] Prompt referral for treatment and diligent management of co-morbidities and adverse drug reactions may further decrease XDR-TB mortality in HIV co-infected patients.
Acknowledgments
Our appreciation is to the staff at King George V Hospital who made this possible and to the patients in this study.
Dr O'Donnell was supported by the National Institute of Health (NIH) T32 AI52074 (NIAID) and the Centre for AIDS Programme of Research (CAPRISA). CAPRISA is part of the University of KwaZulu-Natal HIV/AIDS Clinical Trials Unit which is funded by the NIH and the US Department of Health and Human Services (DHHS); grant# A1069469). The funding source played no role in the study design or data analysis.
Footnotes
Conflict of Interest Statement: We declare that we have no conflict of interest.
References
- 1.World Health Organization. (WHO) Global tuberculosis control - surveillance, planning, financing. WHO; Geneva: 2008. [Google Scholar]
- 2.Dye C, Bassili A, Bierrenbach AL, Broekmans JF, et al. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008;8:233–43. doi: 10.1016/S1473-3099(07)70291-8. [DOI] [PubMed] [Google Scholar]
- 3.Chaisson R, Martinson N. Tuberculosis in Africa — Combating an HIV-Driven Crisis. N Engl J Med. 2008;358:1089–92. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]
- 4.Horsburgh CR., Jr The global problem of multidrug-resistant tuberculosis: the genie is out of the bottle. JAMA. 2000;283:2575–6. doi: 10.1001/jama.283.19.2575. [DOI] [PubMed] [Google Scholar]
- 5.Raviglione M, Smith I. XDR Tuberculosis — Implications for Global Public Health. 2007. N Engl J Med. 356(7):656–9. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi NR, Moll A, Sturm AW, Pawinski R, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 7.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrugresistant tuberculosis—the perfect storm. J Infect Dis. 2007;196:S86–107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 8.Schluger NW. The impact of drug resistance on the global tuberculosis epidemic. Int J Tuberc L Dis. 2000;4(1):S71–5. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) Extensively drug-resistant tuberculosis (XDR-TB): recommendations for prevention and control. Wkly Epidemiol Rec. 2006;81:430–2. [PubMed] [Google Scholar]
- 10.Andrews JR, Shah NS, Gandhi N, Moll T, Friedland G. Multidrug-resistant and extensively drug-resistant tuberculosis: implications for the HIV epidemic and antiretroviral therapy rollout in South Africa. J Infect Dis. 2007;196:S482–90. doi: 10.1086/521121. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Extensively drug-resistant tuberculosis--United States, 1993-2006. Morb Mortal Wkly Re. 2007;56:250–3. [PubMed] [Google Scholar]
- 12.Kim HR, Hwang SS, Kim HJ, et al. Impact of extensive drug resistance on treatment outcomes in non–HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–5. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 13.Migliori GB, Lange C, Girardi E, Centis R, Besozzi G, Kliiman K, et al. Extensively drug-resistant tuberculosis is worse than multidrug-resistant tuberculosis: different methodology and settings, same results. Clin Infect Dis. 2008;46:958–9. doi: 10.1086/528875. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Revised definition of extensively drug-resistant tuberculosis. MMWR. 2006;55:1176. [Google Scholar]
- 15.Laserson KF, Thorpe LE, Leimane V, Weyer K, Mitnik CD, Riekstina V, et al. Speaking the same language: treatment outcome definitions for multidrug resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–5. [PubMed] [Google Scholar]
- 16.Breen RA, Miller RF, Gorsuch T, Smith CJ, Schwenk A, Holmes W, et al. Adverse events and treatment interruption in tuberculosis patients with and without HIV co-infection. Thorax. 2006;61:791–4. doi: 10.1136/thx.2006.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisner BA, Gatson MA, Wood GL. Evaluation of Mycobacteria Growth Indicator Tubes for susceptibility testing of Mycobacterium tuberculosis to isoniazid and rifampin. Diagnostic Microbiology and Infectious Disease. 1995;22:325–9. doi: 10.1016/0732-8893(95)00147-7. [DOI] [PubMed] [Google Scholar]
- 18.Rusch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol. 2006;44:688–92. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margot B, Wallengren K. Situational Analysis TB drug-resistance in KwaZulu-Natal. Oral presentation. TB Conference; July 4, 2008; Durban, South Africa. [Google Scholar]
- 20.Farmer P, Kim JY. Community based approaches to the control of multidrug resistant tuberculosis: introducing “DOTS-plus”. BMJ. 1998;317:671–4. doi: 10.1136/bmj.317.7159.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migliori GB, Ortmann J, Girardi E, et al. Extensively drug-resistant tuberculosis, Italy and Germany. Emerg Infect Dis. 2007;13:780–1. doi: 10.3201/eid1305.060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliori GB, Lange C, Centis R, Sotgiu G, Mütterlein R, Hoffmann H, et al. Resistance to second-line injectables and treatment outcomes in multidrug-resistant and extensively drug-resistant tuberculosis cases. Eur Respir J. 2008;31:1155–9. doi: 10.1183/09031936.00028708. [DOI] [PubMed] [Google Scholar]
- 23.Mitnick C, Shin SS, Seung KJ, Rich ML, Atwood SS, Furin JJ, et al. Comprehensive Treatment of Extensively Drug-Resistant Tuberculosis. N Eng J Med. 2008;359:563–74. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahid P, Gonzalez LC, Rudoy I, de Jong BC, Unger A, Kawamura LM, et al. Treatment Outcomes of Patients with HIV and Tuberculosis. Am J Respir Crit Care Med. 2007;175:1199–1206. doi: 10.1164/rccm.200509-1529OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montori VM, Kleinbart J, Newman TB, et al. Tips for learners of evidence-based medicine, 2: measures of precision. CMAJ. 2004;171:611–15. doi: 10.1503/cmaj.1031667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival Bias Associated with Time-to-Treatment Initiation in Drug Effectiveness Evaluation. Am J Epidemiol. 2005;162:1016–23. doi: 10.1093/aje/kwi307. [DOI] [PubMed] [Google Scholar]
- 27.Akksilp S, Karnkawinpong O, Wattanaamornkiat W, Viriyakitja D, Monkongdee P, Sitti W, et al. Antiretroviral therapy during tuberculosis treatment and marked reduction in death rate of HIV-infected patients, Thailand. Emerg Infect Dis. 2007;13:1001–7. doi: 10.3201/eid1307.061506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58:1299–302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 29.Schwander SK, Dietrich M, Mugyenyi P, Kityo C, Okwera A, et al. Clinical course of human immunodeficiency virus type 1 associated pulmonary tuberculosis during short-course antituberculosis therapy. East Afr Med J. 1997;74:543–8. [PubMed] [Google Scholar]
- 30.Sanguanwongse N, Cain KP, Suriya P, Nateniyom S, Yamada N, Wattanaamornkiat W, et al. Antiretroviral therapy for HIV-infected tuberculosis patients saves lives but needs to be used more frequently in Thailand. J Acquir Immune Defic Syndr. 2008;48:181–9. doi: 10.1097/QAI.0b013e318177594e. [DOI] [PubMed] [Google Scholar]
- 31.Zelnick J, O'Donnell M. The impact of the HIV/AIDS epidemicon hospital nurses in KwaZulu Natal, South Africa: nurses' perspectives and implications for health policy. J Public Health Policy. 2005;26:163–85. doi: 10.1057/palgrave.jphp.3200021. [DOI] [PubMed] [Google Scholar]
- 32.Thomas A, Ramachandran R, Rehaman F, Jaggarajamma K, Santha T, Selvakumar N, Krishnan N, Mohan NS, Sundaram V, Wares F, Narayanan PR. Management of multi drug resistance tuberculosis in the field: Tuberculosis Research Centre experience. Indian J Tuberc. 2007;54:117–24. [PubMed] [Google Scholar]
- 33.Basu S, Andrews JR, Poolman EM, Gandhi NR, Shah NS, Moll A, Moodley P, Galvani AP, Friedland GH. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–7. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guidelines for the programmatic managementof drug-resistant tuberculosis. World Health Organization. (WHO); Geneva: 2008. [Google Scholar]