Abstract
Objectives
This investigational study assessed the suppressive effect of 10 percent povidone iodine (PI) coupled with elimination of active carious lesions on salivary mutans streptococci (MS) populations in children with severe early childhood caries (S-ECC).
Methods
77 children (38 females, 39 males) were treated for S- ECC in one session; a 0.2 ml PI solution was applied to the dentition after dental surgery was completed and immediately wiped off. The subjects aged from 2 to 5 years (mean = 3.78 years) at baseline. Whole nonstimulated saliva samples were obtained at baseline, 30 days, 60 days, and 90 days post dental surgery. Samples were placed on ice and processed within 2 hours. The MS level in each sample was expressed as colony forming units (CFUs) per ml of saliva.
Results
Approximately 50 percent of subjects had a >95 percent reduction in CFU/ml of saliva at each time point after baseline. The percentages of subjects with a >50 percent reduction in MS level were 85 percent at 30 days, 83 percent at 60 days, 84 percent at 90 days. The median (25th, 75th percentiles) CFUs/ml of saliva counts were 8.40 × 105 (1.49 × 105, 5.00 × 106) at baseline (n = 77), 4.12 × 104 (8.40 × 103, 1.89 × 105) at 30 days (n = 74), 4.62 × 104 (7.00 × 103, 1.36 × 105) at 60 days (n = 70), and 5.09 × 104 (1.16 × 104, 1.00 × 105) at 90 days (n = 70). The changes from baseline to 30 days, 60 days, and 90 days were statistically significant (P < 0.0001).
Conclusions
PI coupled with dental surgery has a significant suppressive effect on salivary MS levels in the setting of S-ECC for at least 90 days. These data strongly suggest that treatment with PI may be an important adjunct to dental surgery for S-ECC.
Keywords: dental caries, povidone iodine, mutans streptococci
Introduction
Early childhood caries (ECC), previously known by such terms as nursing caries, baby bottle tooth decay, and bottle mouth, is a major public health problem (1). The American Academy of Pediatric Dentistry (AAPD) has defined ECC as “the presence of 1 or more decayed, missing or filled tooth surfaces in any primary tooth in a child 71 months of age or younger” (2). Severe early childhood caries (S-ECC) is the most damaging form of ECC and has reached near-epidemic proportions among babies and young preschool children worldwide regardless of race or ethnicity; children living in poverty are at very high risk (1). AAPD guidelines differentiate S-ECC from ECC as a consequence of a more extensive disease process based on age and number of carious, filled, or missing tooth surfaces (2). S-ECC is an infectious disease that is most frequently characterized by an overwhelming mutans streptococci (MS) infection (3) and decay-promoting feeding behaviors (4–8). The current community standard of care for treatment of S-ECC is usually restricted to removal and restoration of carious teeth, application of topical fluoride, counseling regarding decay-promoting feeding behaviors, and oral hygiene instructions. Dental surgery has minimal impact on oral MS reservoirs in the setting of S-ECC (9–11), and counseling regarding feeding behaviors by dental clinicians, for the most part, has not been successful (4,7). Not surprisingly, approximately 40 percent of children treated for S-ECC under general anesthesia (GA) relapse with new carious lesions within 6–12 months post treatment (9,12–14). Improved clinical outcomes for S-ECC are likely to be realized through treatment strategies that are not a financial burden on health resources and focus on the infectious aspect of this disease. Suppressing oral MS reservoirs with an antimicrobial agent may be a promising approach. In this regard, the US Food and Drug Administration has approved 10 percent povidone iodine (PI) as a safe presurgical disinfectant to the skin and mucous membranes of children. Results from an earlier placebo-controlled clinical trial showed that PI has suppressive effects on oral populations of MS even in the setting of S-ECC. Intraoperative treatment (under GA) of the subjects included restoration or extraction of carious teeth coupled with application of a 1.23 percent acid phosphate fluoride (APF) gel. The experimental group had PI applied to their dentition and the control subjects, a placebo. Oral MS levels were significantly suppressed in the experimental group relative to baseline in the follow-up period but not in the control group (15). This finding indicates that one topical application of a 1.23 percent APF gel, coupled with dental surgery in the setting of S-ECC, has no prolonged effect on oral MS levels. These findings, coupled with other reports on the utility of topical iodine solutions in suppressing MS (16, 17), strongly indicate that a clinical trial is warranted to determine the utility of PI for prevention of relapse in children treated for S-ECC. One critical question relevant to the design of such a trial is how often PI needs to be applied post dental surgery to suppress oral MS populations to levels that may be associated with a decreased risk for relapse. Accordingly, to extend the work of others, the objective of this study was to estimate how often PI needs to be applied post dental surgery in combination with the aforementioned community standard of care.
Methods
Study Design and Specific Aims
An exploratory study design was utilized in this investigation to determine the time course and duration of suppression of oral reservoirs of MS in children with S-ECC following the removal and restoration of carious teeth, application of a 10 percent PI solution followed by an application of 1.23 percent APF foam, and counseling regarding feeding behaviors and oral hygiene instruction. The case definition of S-ECC was according to the guidelines of the AAPD (2).
Study Population
The study population was comprised of 92 children between the ages of 24 and 60 months who consecutively presented to the Ambulatory Surgical Center of Strong Memorial Hospital at the University of Rochester Medical Center for dental surgery because of S-ECC. The study participants were recruited into the study by the study coordinator (MTW) on the day of surgery. All of the subjects were apparently healthy and not taking sweetened oral medications at their time of entry into the study. Health and medication status was determined by reviewing the preoperative physical examination by the pediatrician as well as interviewing the parent or primary care taker. This assessment was carried out by the attending surgeon (RJB) for every subject in the study. All of the subjects met the AAPD criteria for a diagnosis of S-ECC. As the objective of the study was to assess the time course and duration of the suppression of oral reservoirs of MS, all subjects, following restoration or extraction of carious teeth, received an application of 10 percent PI solution followed by an application of 1.23 percent APF foam.
Baseline and Follow-Up Procedures
Caries examinations
Caries examinations were carried out by one examiner (MTW) who was trained and calibrated by another author (RB) who has extensive experience in caries clinical trials. The caries examinations utilized the Dundee Selectable Threshold Method (18, 19). The subjects were examined at their time of entry into the study (baseline) and at follow-up visits at 30, 60, and 90 days post baseline. This approach for caries diagnosis was utilized to ensure that caries diagnosis was uniform and consistent to meet criteria for entry into the study.
Baseline procedures
Preoperative counseling regarding decay-promoting feeding behaviors and oral hygiene instructions was given to the parent(s) or caretaker(s) on the day of dental surgery. Preventive education was carried out by one of the authors (RJB) to make certain that a uniform message was given to all study participants. A saliva sample was obtained prior to administering preoperative medications. The subject was then taken to the operating room where dental surgery was performed under GA. Prior to initiating dental surgery; the baseline caries examination was performed. Dental surgery used an aggressive approach, which has been reported by our group earlier (9). After completion of dental surgery, 0.2 mL of PI was applied to the dentition with a cotton ball and immediately wiped off with a gauze sponge. Finally, 1.23 percent APF foam was applied to the dentition and immediately wiped off after digital application prior to emergence from GA.
Follow-up procedures
Each subject was recalled at 30, 60, and 90 days post dental surgery. At the time of each follow-up visit, a saliva sample was obtained. A $30 volunteer fee per follow-up visit was given to enhance compliance with follow-up.
Sampling and Bacteriologic Procedures
Sampling procedures
Whole, nonstimulated saliva samples were obtained through a disposable saliva ejector attached to a 15 mL sterile centrifuge tube, which in turn was attached to a vacuum pump (20). Approximately 2 mL of saliva was collected for each sample. After collection, the saliva was immediately transported on ice to the laboratory and processed within 2 hours.
Bacteriologic procedures
Each saliva sample was subjected to sonication (on ice to prevent overheating) by a Branson Sonifier 150 (Branson Ultrasonics Corp., Danbury, CT, USA) (three 15-second pulses with 30-second intervals at 7 watts) to disperse cell clumps and initiate dechaining of streptococci. This dispersion technique results in cell survival rates that maximize MS counts (21). The saliva samples were then serially diluted and plated onto mitis salivarius agar (Becton, Dickinson, and Co., Sparks, MD, USA) with bacitracin (Sigma-Aldrich Co., St. Louis, MO, USA) (MSB) as described earlier (22). The MSB plates were then incubated in 5 percent CO2 at 37 °C for 48 hours when the number of colony forming units (CFUs) was determined. The number of MS in each saliva sample was expressed as CFUs/mL of saliva.
Statistical analysis
The significance of the mean change from baseline to each follow-up visit (30, 60, and 90 days) as well as the mean changes between the three follow-up visits in log10-transformed MS levels was assessed by using a repeated measures analysis of variance model that used all available data from each subject. Results from this model are expressed as the geometric mean percentage reduction from baseline level and associated 95 percent confidence interval (CI) and P value. The analyses were performed only for subjects who had MS levels obtained at baseline and at least one follow-up visit.
Protection of human subjects
This study was approved by the Research Subjects Review Board of the University of Rochester Medical Center. Written informed consent was obtained from the parent (or legal guardian) of all subjects enrolled.
Results
Characterization and Disposition of Subjects
Ninety-two subjects were enrolled in the study and treated with PI post dental surgery. Fifteen (16 percent) of these subjects were excluded from the analyses because of loss to all follow-up visits (n = 9) or MS baseline levels that were not available (n = 6). Among the 77 subjects that were included in the analyses, there were 39 boys and 38 girls with a mean age of 3.78 years (standard deviation: 0.77 years). A majority of the subjects were African American (53 percent), 32 percent were Caucasian, 8 percent were of mixed race, 3 percent were Asian, and 4 percent were other. A total of 18 percent of the children were Latino. A total of 66 subjects (86 percent) were present at all 4 visits. For the 77 subjects included in the analyses, MS levels were obtained for 291 of the 308 possible subject visits (94 percent): 77 at baseline, 74 at Day 30, 70 at Day 60, and 70 at Day 90.
Baseline Caries Data
Using the Dundee Selectable Threshold Method, it was found that the study sample (n = 77) had an average of 6.9 white spot, 3.0 brown stain, 12.2 dentinal, and 0.8 pulp exposure lesions per subject out of a median of 88 tooth surfaces per subject examined at baseline.
Analyses of MS Levels
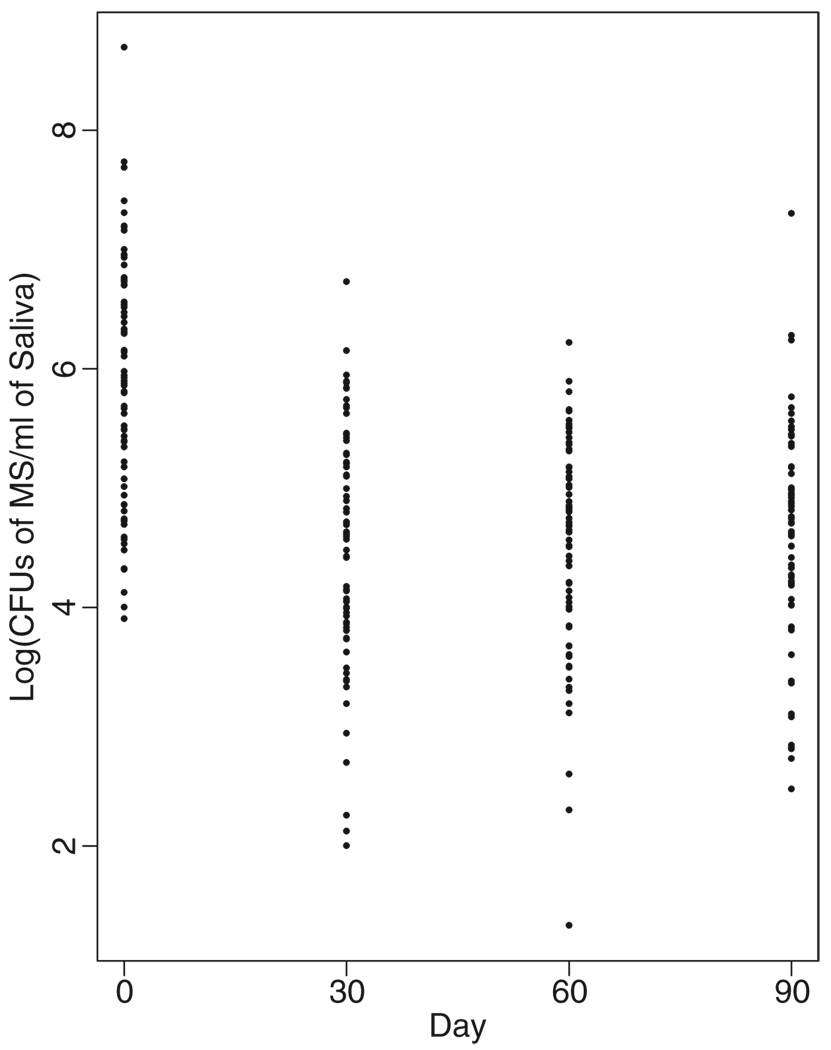
The medians and interquartile ranges of the distributions of MS level (CFU/mL of saliva) at each visit are presented in Table 1. A marked reduction in MS levels was apparent for each post baseline follow-up visit. The geometric mean percentage reductions from baseline in MS levels were 96.0 percent (95 percent CI, 92.9 percent to 97.7 percent, P < 0.0001) at Day 30, 96.0 percent (95 percent CI, 92.5 percent to 97.9 percent, P < 0.0001) at Day 60, and 95.7 percent (95 percent CI, 92.1 percent to 97.7 percent, P < 0.0001) at Day 90. There were no significant differences in the mean percentage reductions among the follow-up visits (P > 0.79 for all comparisons). The magnitude of the decrease in MS levels was associated with the number of surfaces extracted at baseline. For example, at 30 days, the mean reduction in log10 transformed was 0.91 in subjects with 0–4 surfaces extracted (n = 35), 1.78 in subjects with 5–20 surfaces extracted (n = 30), and 2.03 in subjects with >20 surfaces extracted (n = 9); these values were consistent at 60 and 90 days. All of these reductions, however, remained highly statistically significant (P < 0.0001). Figure 1 provides a graphic display of the MS levels for individual subjects at each visit. The distributions of the percentage reduction from baseline in MS level at each follow-up visit are shown in Table 2. The percentages of subjects with >50 percent reduction in MS level were 85 percent at 30 days, 83 percent at 60 days, and 84 percent at 90 days.
Table 1.
Distributions of MS Level (CFU/mL of Saliva) at Each Visit
| Days post surgery |
CFU/mL of Saliva | |||
|---|---|---|---|---|
| Sample size | Median | 25th percentile | 75th percentile | |
| 0 | 77 | 8.40 × 105 | 1.49 × 105 | 5.00 × 106 |
| 30 | 74 | 4.12 × 104 | 8.40 × 103 | 1.89 × 105 |
| 60 | 70 | 4.62 × 104 | 7.00 × 103 | 1.36 × 105 |
| 90 | 70 | 5.09 × 104 | 1.16 × 104 | 1.00 × 105 |
MS, mutans streptococci; CFU, colony-forming unit.
Figure 1.
Distributions of log10-transformed MS level (CFU/mL of saliva) at each visit. MS, mutans streptococci; CFU, colony-forming unit
Table 2.
Distributions of Percentage Reduction from Baseline in MS Level (CFU/mL of Saliva) at Each Follow-Up Visit
| Days post surgery |
Sample size |
Percentage reduction from baseline in CFU/mL of saliva | ||||
|---|---|---|---|---|---|---|
| <50% | 50–80% | 80–90% | 90–95% | <95% | ||
| 30 | 74 | 14.9 | 14.9 | 10.8 | 10.8 | 48.6 |
| 60 | 70 | 17.1 | 11.4 | 11.4 | 8.6 | 51.4 |
| 90 | 70 | 15.7 | 11.4 | 11.4 | 11.4 | 50.0 |
MS, mutans streptococci; CFU, colony-forming unit.
Adverse Events
Five subjects experienced an adverse event. Of these, 3 subjects required antibiotic therapy for upper-respiratory tract infections during the 90-day follow-up, one subject had his IV infiltrate the soft tissues during GA, and one subject developed ringworm during the 90-day follow-up period. It is also important to report that no adverse events secondary to the PI exposure were observed.
Discussion
We found that elimination of active carious lesions, topical application of 1.23 percent APF, and one application of PI significantly suppressed salivary MS populations for at least 90 days relative to baseline. These findings corroborate and extend the findings of an earlier report (15), which showed that MS levels declined initially but then rose toward baseline by 90 days. The magnitude of the suppression observed in this report appeared to be sustained for the entire 90-day study period (Figure 1). One possible explanation for the sustained suppression is that demineralized human enamel is permeable to iodine (23). Results from earlier studies have also demonstrated the utility of topical iodine solutions in suppressing MS. A single 2-minute topical application of 0.2 percent I2-KI solution to the teeth of 9- to 13-year-old children eliminated MS from accessible human tooth sites for up to 13 weeks (16). Another study demonstrated that a single prophylaxis followed by topical application of a 2 percent I2-KI solution immediately after the prophylaxis and again at 3 and 5 days after the prophylaxis significantly reduced MS levels in subjects 18–23 years of age (17). The reduction in MS populations in smooth surface plaque and saliva persisted for 20–24 weeks. However, it must be emphasized that MS level is only a biologic marker for susceptibility to dental caries and that the utility of topical iodine in preventing the disease would be a more accurate indicator of its therapeutic efficacy. In this regard, another investigation assessed the efficacy of bimonthly topical PI in a double-blind, placebo-controlled clinical trial in preventing the development of white spot lesions on the maxillary primary incisors of Puerto Rican babies (24). These babies were at high risk for S-ECC as they were all colonized by MS and had decay-promoting feeding behaviors. A PI solution was applied bimonthly to the dentition of the experimental group for 1 year, and a placebo solution was applied bimonthly to the dentition of the control group for 1 year. Kaplan–Meier estimates (+/− standard error) of the probabilities of disease-free survival at 12 months were 91(+/−5) percent in the experimental group (n = 39) and 54 (+/− 9) percent for the control group (n = 44); P = 0.0013. In a pilot clinical trial (25) on 25 children with S-ECC treated under GA, the children were randomized into experimental and control groups. The experimental group received three topical applications of PI at bimonthly intervals during the first 4 months of the study, and the control group did not receive PI. Examination of the subjects at 6 months post dental surgery demonstrated that 18 percent of the children in the experimental group had dental caries as compared with 63 percent of the children in the control group. Collectively, these studies support the potential of PI in preventing dental caries.
The magnitude of the reduction of salivary MS reservoirs in our study participants is likely associated with a decrease in caries risk (Table 1). Earlier studies (26–29) demonstrated that salivary magnitudes of MS ≤ 104CFU/mL are associated with moderate to low caries risk. As illustrated in Table 1, 50 percent of our study participants had a salivary magnitude of MS ≤ 5.1 × 104CFU/mL at 30, 60, and 90 days post dental surgery. Our study also documented the effect of teeth extracted at baseline on the suppression of MS. Our data showed that the magnitude of the decrease in MS levels was associated with the number of tooth surfaces lost. That is, the greater the number of surfaces lost, the greater the magnitude of MS suppression. Although all of the reductions in MS levels remained highly statistically significant, these data revealed a potential confounder that should be considered when assessing the efficacy of antibacterial agents on the reduction of MS following tooth extraction for S-ECC. This finding has been reported earlier where a reduction in MS levels in the setting of S-ECC was positively correlated to the number of extracted teeth (11). A median of 8 dental surfaces (interquartile range 0–16 surfaces), typically carious primary maxillary incisors, were extracted per subject in our study.
Our exploratory study, coupled with earlier studies, indicate that adjunctive chemotherapy utilizing PI is a treatment strategy that holds promise for the treatment of children with S-ECC. There is reason to expect that recurring administration in a public health venue would also reduce the risk for ECC/S-ECC before its onset in high-risk babies and preschool children. Further, as noted earlier, approximately 40 percent of children treated for S-ECC under GA relapse with new carious lesions post treatment. Frequent, postsurgical application of PI has the potential to reduce substantially the proportion of children who relapse following treatment. Accordingly, randomized double-blind, placebo-controlled trials are needed to determine the utility of PI for prevention of ECC/S-ECC and for relapse in children treated for S-ECC. In such trials, repeated applications of PI over a longer period of time would be required. The results of our study suggest that reapplication of PI treatment would need to be repeated at least every 90 days.
Acknowledgment
We thank Stacy Gregoire for technical assistance. This study was funded by NIDCR grant R21 DE016280.
Research Support: NIH/NIDCR grant R21 DE016280
Footnotes
Disclaimers: None
Previous Presentation of this Study: A poster was presented on our preliminary findings at the 2007 IADR meeting in New Orleans.
Contributor Information
Robert J. Berkowitz, Eastman Department of Dentistry University of Rochester Medical Center.
Hyun Koo, Eastman Department of Dentistry University of Rochester Medical Center.
Michael P. McDermott, Department of Biostatistics and Computational Biology, University of Rochester Medical Center.
Mary Therese Whelehan, Eastman Department of Dentistry University of Rochester Medical Center.
Patricia Ragusa, Eastman Department of Dentistry University of Rochester Medical Center.
Dorota T. Kopycka-Kedzierawski, Eastman Department of Dentistry University of Rochester Medical Center.
Jeffrey M. Karp, Eastman Department of Dentistry University of Rochester Medical Center.
Ronald Billings, Eastman Department of Dentistry University of Rochester Medical Center.
References
- 1.Milnes AR. Description and epidemiology of nursing caries. J Public Health Dent. 1996;56:38–50. doi: 10.1111/j.1752-7325.1996.tb02394.x. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatric Dentistry. Definition of early childhood caries (ECC) Pediatr Dent. 2007;29:13. [Google Scholar]
- 3.Berkowitz RJ. Etiology of nursing caries: a microbiologic perspective. J Public Health Dent. 1996;56:51–54. doi: 10.1111/j.1752-7325.1996.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 4.Albert RJ, Cantin RY, Cross HG, Castaldi CR. Nursing caries in the Inuit children of the Keewatin. J Can Dent Assoc. 1988;54:751–758. [PubMed] [Google Scholar]
- 5.Holt RD, Joels D, Winter GB The Camden study. Caries in pre-school children. Br Dent J. 1982;153:107–109. doi: 10.1038/sj.bdj.4804862. [DOI] [PubMed] [Google Scholar]
- 6.Marino RV, Bomze K, Scholl TO, Anhalt H. Nursing bottle caries: characteristics of children at risk. Clin Pediatr (Phila) 1989;28:129–131. doi: 10.1177/000992288902800305. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein P, Domoto P, Wohlers K, Koday M. Mexican-American parents with children at risk for baby bottle tooth decay: pilot study at a migrant farmworkers clinic. ASDC J Dent Child. 1992;59:376–383. [PubMed] [Google Scholar]
- 8.Wendt IK, Hallonstain A, Koch G Part I A longitudinal study. Dental caries in 1- and 2-year-old children living in Sweden. Swed Dent J. 1991;15:1–6. [PubMed] [Google Scholar]
- 9.Graves CE, Berkowitz RJ, Proskin HM, Chase I, Weinstein P, Billings R. Clinical outcomes for early childhood caries: influence of aggressive dental surgery. J Dent Child. 2004;71:114–117. [PubMed] [Google Scholar]
- 10.Peretz B, Sarit F, Eidelman E, Steinberg D. Mutans streptococcus counts following treatment for early childhood caries. J Dent Child (Chic) 2003;70:111–114. [PubMed] [Google Scholar]
- 11.Twetman S, Fritzon B, Jensen B, Hallberg U, Stahl B. Pre- and post-treatment levels of salivary mutans streptococci and lactobacilli in pre-school children. Int J Paediatr Dent. 1999;9:93–98. doi: 10.1046/j.1365-263x.1999.00108.x. [DOI] [PubMed] [Google Scholar]
- 12.Almeida AG, Roseman MM, Sheff M, Huntington N, Hughes CV. Future caries susceptibility in children with early childhood caries following treatment under general anesthesia. Pediatr Dent. 2000;22:302–306. [PubMed] [Google Scholar]
- 13.Berkowitz RJ, Moss M, Billings RJ, Weinstein P. Clinical outcomes for nursing caries treated using general anesthesia. ASDC J Dent Child. 1997;64:210–211. 228. [PubMed] [Google Scholar]
- 14.Eidelman E, Faibis S, Peretz B. A comparison of restorations for children with early childhood caries treated under general anesthesia or conscious sedation. Pediatr Dent. 2000;22:33–37. [PubMed] [Google Scholar]
- 15.Zhan L, Featherstone JD, Gansky SA, Hoover CI, Fujino T, Berkowitz RJ, DenBesten PK. Antibacterial treatment needed for severe early childhood caries. J Public Health Dent. 2006;66:174–179. doi: 10.1111/j.1752-7325.2006.tb02576.x. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons RJ, Depaola PF, Spinell DM, Skobe Z. Interdental localization of Streptococcus mutans as related to dental caries experience. Infect Immun. 1974;9:481–488. doi: 10.1128/iai.9.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caufield PW, Gibbons RJ. Suppression of Streptococcus mutans in the mouths of humans by a dental prophylaxis and topically applied iodine. J Dent Res. 1979;58:1317–1326. doi: 10.1177/00220345790580040301. [DOI] [PubMed] [Google Scholar]
- 18.Fyffe HE, Deery C, Nugent ZJ, Nuttall NM, Pitts NB. In vitro validity of the Dundee Selectable Threshold Method for caries diagnosis (DSTM) Community Dent Oral Epidemiol. 2000;28:52–58. doi: 10.1034/j.1600-0528.2000.280107.x. [DOI] [PubMed] [Google Scholar]
- 19.Fyffe HE, Deery C, Nugent ZJ, Nuttall NM, Pitts NB. Effect of diagnostic threshold on the validity and reliability of epidemiological caries diagnosis using the Dundee Selectable Threshold Method for caries diagnosis (DSTM) Community Dent Oral Epidemiol. 2000;28:42–51. doi: 10.1034/j.1600-0528.2000.280106.x. [DOI] [PubMed] [Google Scholar]
- 20.Leverett DH, Featherstone JD, Proskin HM, Adair SM, Eisenberg AD, Mundorff-Shrestha SA, Shields CP, Shaffer CL, Billings RJ. Caries risk assessment by a cross-sectional discrimination model. J Dent Res. 1993;72:529–537. doi: 10.1177/00220345930720021001. [DOI] [PubMed] [Google Scholar]
- 21.Mundorff SA, Eisenberg AD, Leverett DH, Espeland MA, Proskin HM. Correlations between numbers of microflora in plaque and saliva. Caries Res. 1990;24:312–317. doi: 10.1159/000261289. [DOI] [PubMed] [Google Scholar]
- 22.Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002;46:1302–1309. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashket S, Brudevold F, Yaskell T, Makonnen M. Increased permeability of enamel to iodide ions following the ingestion of cookies varying in sucrose or fat content. Caries Res. 1988;22:193–198. doi: 10.1159/000261105. [DOI] [PubMed] [Google Scholar]
- 24.Lopez L, Berkowitz RJ, Spiekerman C, Weinstein P. Topical antimicrobial therapy in the prevention of early childhood caries: a follow-up report. Pediatr Dent. 2002;24:204–206. [PubMed] [Google Scholar]
- 25.Amin AS, Harrison RL, Benton TS, Roberts M, Weinstein P. Effect of povidone-iodine in children with extensive dental caries. Pediatr Dent. 2004;26:5–10. [PubMed] [Google Scholar]
- 26.Klock B, Krasse B. Microbial and salivary conditions in 9- to 12-year-old children. Scand J Dent Res. 1977;85:56–63. doi: 10.1111/j.1600-0722.1977.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 27.Klock B, Krasse B. Effect of caries-preventive measures in children with high numbers of S. mutans and lactobacilli. Scand J Dent Res. 1978;86:221–230. doi: 10.1111/j.1600-0722.1978.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 28.Klock B, Krasse B. A comparison between different methods for prediction of caries activity. Scand J Dent Res. 1979;87:129–139. doi: 10.1111/j.1600-0722.1979.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 29.Krasse B. Biological factors as indicators of future caries. Int Dent J. 1988;38:219–225. [PubMed] [Google Scholar]