Abstract
Background:
To evaluate the antitumour activity and safety of metronomic cyclophosphamide vs megestrol acetate in progressive and advanced cancer patients having exhausted all effective therapies under standard care.
Methods:
Patients were randomly assigned to receive orally metronomic cyclophosphamide (50 mg b.i.d) or megestrol acetate (160 mg only daily) until intolerance or progression (RECIST 1.0). The primary efficacy end point was a 2-month progression-free rate (PFR2m). According to Optimal Simon's design and the following assumptions, namely, P0=5%, P1=20%, α=β=10%, the treatment is considered as effective if atleast 5 out of 44 patients achieved PFR2m.
Results:
Between September 2006 and January 2009, 88 patients were enrolled. Two patients experienced grade 3–4 toxicities in each arm (4%). One toxic death occurred in the megestrol acetate arm as a consequence of thrombosis. The metronomic cyclophosphamide arm reached the predefined level of efficacy with a PFR2m rate of 9 out of 44 and a PFR4m rate of 5 out of 44. The MA arm failed to achieve the level of efficacy with a PFR2m of 4 out of 44 and a PFR4m of 1 out of 44. The median overall survival was 195 and 144 days in the metronomic cyclophosphamide arm and megestrol acetate arm, respectively.
Conclusion:
Metronomic cyclophosphamide is well tolerated and provides stable disease in such vulnerable and poor-prognosis cancer patients. This regimen warrants further evaluations.
Keywords: palliative chemotherapy, megestrol acetate, metronomic cyclophosphamide, randomised phase II trial, sarcoma
The care of cancer patients with a good performance status (PS) but having exhausted all available effective therapies under standard care represents a daily challenging situation for medical oncologists. Three possibilities may be discussed: exclusive palliative care, inclusion in phase I trials or treatment with a non-validated regimen, sometimes without a strong scientific basis. We know very well that a large proportion of such patients are not eligible for contemporary phase I trials because of an increasing number of eligibility criteria (Penel et al, 2008). Many patients with a good PS are reluctant to accept palliative care exclusively, and many medical oncologists are reluctant to propose exclusive palliative care in patients having a good PS. Nevertheless, the administration of an off-labeled chemotherapy regimen risks exposing patients to unnecessary toxicity without reasonable hope of clinical benefit, and unduly raises the cost of care. Considering these facts and the common nature of this situation, we explored different possibilities of treatment and retained metronomic cyclophosphamide and megestrol acetate as valuable options warranting further evaluation.
Metronomic chemotherapy refers to the frequent administration of chemotherapy, often daily, with no prolonged drug-free breaks, at doses significantly lower than the maximum tolerated dose (Kerbel and Kamen, 2004). One of the most frequently explored drugs for such uses is cyclophosphamide. In preclinical models, the metronomic administration of this bifunctional alkylating agent showed its ability to inhibit angiogenesis by inducing pericytes and endothelial cell dysfunction and apoptosis (Browder et al, 2000; Pietras and Hanahan, 2005; Yap et al, 2005). In retrospective series or clinical studies, metronomic cyclophosphamide alone or in combination provided some evidence of efficacy in several types of cancers, such hormone-refractory prostate cancer (Glode et al, 2003; Lord et al, 2007), heavily pretreated sarcoma (Casanova et al, 2004; De Pas et al, 2004; Stempak et al, 2006), ovarian cancer (Garcia et al, 2008) or breast cancer (Colleoni et al, 2006; Dellapasqua et al, 2008).
Megestrol acetate is currently used to improve appetite and increase weight in cancer-associated anorexia–cachexia syndrome (Mantovani et al, 1998; Desport et al, 2000; Berenstein and Ortiz, 2005; Yavuzsen et al, 2005). In 1993, the US Federal Drug Administration approved megestrol acetate for the treatment of anorexia, cachexia or unexplained weight loss in patients with AIDS. The mechanisms by which megestrol acetate increases appetite is largely unknown, but some data suggest an action on the pro-and anti-inflammatory interleukin network, especially a reduction in circulating tumour necrosis factor-α (Mantovani et al, 1998). A large meta-analysis had recently shown its ability to improve appetite and weight gain in cancer patients (Berenstein and Ortiz, 2005), and one randomised clinical trial showed its ability to improve the quality of life (Westman et al, 1999). Nevertheless, some studies had also pointed out the risk of phlebitis and pulmonary embolism related to MA (Rowland et al, 1996; Loprinzi et al, 1999). Furthermore, megestrol acetate had provided anecdotal objective responses for some hormone-independent solid tumours (Ravaud et al, 2008).
We carried out a multicentre randomised phase II trial to evaluate the safety and efficacy of metronomic cyclophosphamide vs megestrol acetate in cancer patients with a good PS and having exhausted all available effective treatments under standard care.
Patients and methods
Inclusion criteria
Patients were eligible if they had biopsy-proven cancer, were at least 18 years of age, had a good PS (⩽2), had exhausted all effective or validated therapies under standard care (chemotherapy, immunotherapy, molecular-targeted therapy and hormonal therapy), had progressive and measurable disease (according to Response Evaluation Criteria in Solid Tumour (RECIST 1.0) (Therasse et al, 2000)) before inclusion and were using effective contraception. They had to be able to swallow.
Exclusion criteria
Patients excluded from the study were those who had had hypercalcaemia, breast cancer or low-grade stromal endometrial sarcoma, previous history of thrombosis or pulmonary embolism, dysphagia or malabsorption, neutrophil count <1500 mm–3, uncontrolled underlying comorbid disease, or any condition or underlying comorbid disease that may alter compliance. Pregnant or breastfeeding women were not eligible.
Randomisation and site coordination
Patients were randomly (1 : 1) assigned to treatment with megestrol acetate or metronomic cyclophosphamide after registration through the Centre Oscar Lambret Clinical Research Unit. We used the method of random permuted block for randomisation.
Treatment plan
Patients received both treatments until progression (RECIST 1.0) or intolerance, as complementary treatment of best supportive care. There was no planned dose modification. In the metronomic cyclophosphamide arm, as previously reported (Suvannasankha et al, 2007), treatment consisted of cyclophosphamide 50 mg b.i.d orally. In the acetate megestrol arm, the treatment consisted of acetate megetrol 320 mg once daily orally, as previously reported (Gebbia et al, 1996; De Conno et al, 1998; Westman et al, 1999; Yavuzsen et al, 2005).
Study end points and data analysis
The primary efficacy end point was the progression-free rate (PFR) at 2 months. Secondary end points were PFRs at 4 and 6 months, toxicity according to the National Cancer Institute Common Toxicity Criteria (Version 3.0), overall survival and median time to progression, quality of life and rate of stable weight.
Consequently, during the study, patients underwent clinical and haematological evaluations at days 1, 15, 30 and 60, and every 2 months thereafter. Disease was assessed by comparing unidimensional tumour measurements (CT scan or MRI) on pre- and per-treatment imaging studies at 2, 4 and 6 months. We assessed response according to the RECIST 1.0. An independent third-party radiologist panel reviewed imaging studies to verify all imaging procedures carried out during the period of treatment with the trial drug, to ensure consistent unbiased application of RECIST 1.0. We defined ‘stable weight’ as weight loss <10% in comparison with baseline weight. Patients were surveyed at baseline, day 30 and day 60 using an auto-questionnaire (EORTC QLQC30 core questionnaire (Aaronson et al, 1993)). For all parameters, we carried out an intent-to-treat analysis.
Statistical considerations
The number of patients was initially calculated using an ‘Optimal Simon's Design’ two-stage design (P0=5%, P1=20%, α=β=10%). Planned inclusion was 44 patients per arm. This design allowed the opening of the second stage if the PFR at 2 months was at least 1 out of 12. At the end of the second stage, if the PFR at 2 months was at least 5 out of 44, the treatment was defined as efficient.
Description of the populations used in the study was based on percentages and their 95% confidence intervals (95% CI) for categorical data, and median and extreme values for continuous data. The Kaplan–Meier method was used to calculate the median progression-free and overall survivals and their 95% CI.
The phase II randomised design did not allow a formal comparison between both arms.
Ethical considerations
Study investigations were conducted after approval by the regional ethics committee (‘Comité de Protection des Patients Nord-Ouest III’, date of approval) and after declaration to the French Health Products Safety Agency (‘Agence Française de Sécurité Sanitaire et des Produits de Santé’, date of approval: June 2006). Informed consent was obtained from each patient. This study was registered in the European Clinical Trials Register (EudraCT No2006-003074-10, June 2006). The study was conducted in agreement with the Declaration of Helsinki and the International Conference on Harmonisation of Good Clinical Practise guidelines.
Results
Patient characteristics
Between September 2006 and January 2009, we enrolled 88 patients. Baseline characteristics were well balanced in both arms (Table 1). All patients experienced progressive disease before inclusion in the study. The median age was 66 years (CI: 57–71) in the megestrol acetate arm and 61 (CI: 50–72) in the metronomic cyclophosphamide arm. The median time between tumour diagnosis and inclusion was 27 months (CI: 18–36) in the megestrol acetate arm and 33 (CI: 24–42) in the metronomic cyclophosphamide arm.
Table 1. Baseline characteristics.
| Megestrol acetate | Metronomic cyclophosphamide | |
|---|---|---|
| n | 44 | 44 |
| Gender | ||
| Men | 29 (66%, (52–80)) | 27 (61%, (47–75)) |
| Women | 15 (34%, (20–48)) | 17 (39%, (24–53)) |
| Tumour types | ||
| Colon and rectum cancers | 11 (25%, (12–38)) | 11 (25%, (12–38)) |
| Lung cancers | 8 (18%, (6–29)) | 7 (16%, (5–26)) |
| Soft tissue sarcoma | 9 (20%, (8–32)) | 8 (18%, (6–29)) |
| Melanomas | 5 (11%, (2–20)) | 4 (9%, (0–17)) |
| Bladder cancers | 4 (9%, (0–17)) | 3 (6, (0–14)) |
| Gastric cancers | 1 (2%, (0–6)) | 5 (11%, (2–20)) |
| Hepatocarcinoma | 2 (4%, (0–10)) | 1 (2%, (0–6)) |
| Unknown primaries | 2 (4%, (0–10)) | 1 (2%, (0–6)) |
| Other tumours | 2 (4%, (0–10)) | 4 (9%, (0–17)) |
| Metastasis | ||
| Liver | 17 (38%, (24–53)) | 16 (36%, (22–50)) |
| Lung | 17 (38%, (24–53)) | 19 (43%, (28–57)) |
| Extra-abdominal lymph nodes | 9 (20%, (8–32)) | 10 (22%, (10–35)) |
| Abdominal lymph nodes | 5 (11%, (2–20)) | 5 (11%, (2–20)) |
| Pleura | 4 (9%, (0–17)) | 1 (2%, (0–6)) |
| Adrenal gland | 3 (6%, (0–14)) | 6 (13%, (3–23)) |
| Peritoneum | 9 (20%, (8–32)) | 2 (4%, (0–10)) |
| Bone | 3 (6, (0–14)) | 3 (6, (0–14)) |
| Brain | 1 (2%, (0–6)) | 1 (2%, (0–6)) |
| Previously treated by | ||
| Surgery | 31 (70%, (57–84)) | 31 (70%, (57–84)) |
| Radiotherapy | 23 (52%, (37–67)) | 22 (50%, (35–64)) |
| Performance status | ||
| 0 | 22 (50% (35–65)) | 24 (54% (39–69)) |
| 1 | 17 (38% (18–45)) | 17 (38% (24–53)) |
| 2 | 5 (12% (1–20)) | 3 (3% (0–14)) |
| Number of previous systemic treatment lines | ||
| 0 | 0 | 1 (2%, (0–6)) |
| 1 | 12 (27%, (14–40)) | 9 (20%, (8–32)) |
| 2 | 20 (45%, 30–60)) | 21 (47%, (36–66)) |
| 3 | 8 (18%, (6–29)) | 3 (6%, (0–14)) |
| 4 | 1 (2%, (0–6)) | 5 (11%, (2–20)) |
| ⩾5 | 3 (6%, (0–14)) | 5 (11%, (2–20)) |
Toxicities
Table 2 describes all drug-related toxicities. Grade 3 and 4 toxicities were observed in the same range in both arms (4%, (CI: 1–6)). We observed a toxic death in the megestreol acetate arm: a case of Budd–Chiari syndrome (hepatic venous outflow obstruction) in a patient with massive liver metastasis from uveal melanoma. In the metronomic cyclophosphamide arm, we observed interstitial pneumonia in a patient with peritoneal metastasis from retroperitoneal liposarcoma, experiencing stable disease after 6 months of treatment. The most frequent side effects were oedema or hormonal and metabolic disorders in the megestrol acetate arm, and nausea, vomiting or anaemia in the metronomic cyclophosphamide arm. There was neither dose reduction nor transient treatment break in both arms. In the metronomic cyclophosphamide arm, one patient discontinued treatment for toxicity (pneumonitis at 6 months). In the megestrol acetate arm, two patients discontinued treatment for toxicity (phlebitis).
Table 2. Treatment-related toxicities.
| Megestrol acetate | Metronomic cyclophosphamide | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| Fatigue | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Hot flashes | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| Anorexia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Compulsive eating | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyper-triglyceridemia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epigastralgia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Diarrhoea | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aphtosis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Anaemia | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 |
| Neutropaenia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Phlebitis | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Gynecomastia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galactorrhoea | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Libido alteration | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dysuria | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Interstitial pneumonia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Oedema | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 5 | 5 | 2 | 0 | 1 | 10 | 4 | 2 | 0 | 0 |
Efficacy
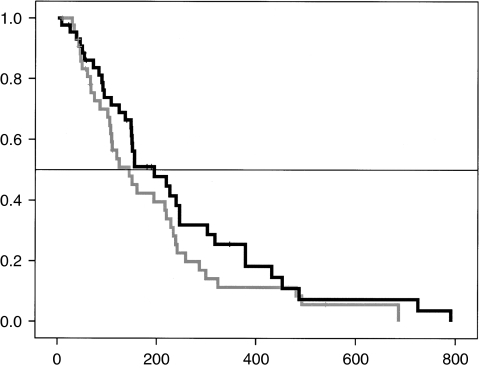
At the end of the first stage, both treatments achieved statistical requirement, allowing the commencement of the second stage (at least one non-progression at 2 months among 12 patients). The megestrol acetate arm failed to reach the desired threshold of efficacy (Table 3). On the other hand, the metronomic cyclophosphamide arm successfully achieved the pre-defined level of efficacy with a PFR at 2 months of 9 out of 44 (20%, (8–32)). In total, six patients experienced stable disease at 4 months: one patient with metastatic limb liposarcoma treated with megestrol acetate, three patients with soft tissue sarcoma treated with metronomic cyclophosphamide, one patient with metastatic squamous cell skin cancer treated with metronomic cyclophosphamide and one patient with renal cell cancer treated with metronomic cyclophosphamide. The median duration of treatment was 57 days (52–61) with megestrol acetate and 58 (54–61) with metronomic cyclophosphamide. The median time to progression was 60 days (59–61) in both arms. Quality of life (Table 4) and weight stabilisation were similar in both arms. The median overall survival was 144 days (82–200) in the megestrol acetate arm and 195 (102–287) in the metronomic cyclophosphamide arm (Figure 1).
Table 3. Efficacy outcomes.
| Megestrol acetate (n=44) | Metronomic cyclophosphamide (n=44) | |
|---|---|---|
| Progression-free rate at 2 months | 4 (9%, (0–17)) | 9 (20%, (8–32)) |
| Progression-free rate at 4 months | 1 (2%, (0–6)) | 5 (11%, (2–20)) |
| Progression-free rate at 6 months | 1 (2%, (0–6)) | 2 (4%, (0–10)) |
| 90-day mortality | 12 (27%, (14–40)) | 9 (20%, (8–32)) |
| Stable weight at day 30 | 11 (25%, (12–38)) | 6 (13%, (3–23)) |
| Stable weight at day 60 | 6 (13%, (3–23)) | 4 (9%, (0–17)) |
| Stable QOL at day 30 | 22 (50%, (35–64)) | 20 (45%, (30–60)) |
| Stable QOL at day 60 | 10 (22%, (10–35)) | 18 (41%, (26–55)) |
Table 4. Quality of live assessments (median and extreme values).
| MA-arm baseline (n=44) | MA-arm at 30 days (n=40) | MA-arm 60 days (n=28) | C-arm baseline (n=44) | C-arm 30 days (n=42) | C-arm 60 days (n=27) | |
|---|---|---|---|---|---|---|
| Physical functioning | 5 (0–10) | 6 (0–10) | 8 (0–10) | 4 (0–10) | 4 (0–10) | 7 (0–10) |
| Role functioning | 2 (0–6) | 3 (0–10) | 5 (0–9) | 3 (1–5) | 4 (0–10) | 5 (0–10) |
| Emotional functioning | 5 (1–9) | 6 (0–9) | 6 (0–9) | 6 (2–10) | 6 (2–10) | 6 (2–10) |
| Cognitive functioning | 3 (0–5) | 4 (0–7) | 4 (1–8) | 3 (1–6) | 4 (1–8) | 4 (1–9) |
| Social functioning | 2 (0–5) | 4 (0–8) | 6 (2–8) | 3 (0–7) | 4 (0–10) | 5 (0–10) |
| Quality of life | 7 (4–7) | 5 (2–7) | 4 (0–7) | 7 (7–5) | 6 (1–7) | 5 (0–7) |
| Fatigue | 5 (0–9) | 7 (0–10) | 8 (2–10) | 5 (0–10) | 6 (0–10) | 8 (0–10) |
| Nausea/vomiting | 6 (0–10) | 6 (2–10) | 6 (0–10) | 7 (0–10) | 7 (0–10) | 6 (0–10) |
| Pain | 3 (0–5) | 4 (0–10) | 6 (3–10) | 3 (1–6) | 4 (2–9) | 5 (0–9) |
| Dyspnoea | 5 (1–8) | 5 (0–9) | 4 (2–9) | 4 (0–8) | 4 (2–10) | 4 (2–9) |
| Sleep disturbance | 8 (2–9) | 7 (2–9) | 8 (3–10) | 7 (5–10) | 8 (2–10) | 7 (0–10) |
| Appetite loss | 3 (0–6) | 4 (0–10) | 4 (1–9) | 4 (1–6) | 4 (0–8) | 5 (0–10) |
| Constipation | 2 (0–6) | 3 (0–8) | 4 (2–10) | 3 (0–5) | 4 (0–9) | 4 (0–7) |
| Diarrhoea | 1 (0–3) | 1 (0–5) | 1 (0–8) | 2 (0–3) | 2 (0–7) | 2 (0–5) |
| Financial difficulty | 4 (0–10) | 6 (1–10) | 6 (1–10) | 5 (0–9) | 5 (0–8) | 6 (0–8) |
Abbreviations: C=cyclophosphamide; MA=megestrol acetate.
There is no statistical significant difference at baseline, at 30 and 60 days in both arms.
Figure 1.
Overall survival (in days). Black line: metronomic cyclophosphamide, grey line: megestrol acetate.
Discussion
We evaluated the efficacy and toxicity of two treatments administered in cancer patients with a good PS and having exhausted all available therapies under standard care. This randomised clinical trial was ethical and conducted taking into account clinical equipoise. Both treatments (metronomic cyclophosphamide and megestrol acetate) gave comparable results in term of secondary end points. Nevertheless, acetate megestrol was responsible for the most severe side effects. Furthermore, metronomic cyclophosphamide was the sole treatment that could reach the predefined primary efficacy end point with a PFR at 2 months of 9 out of 44 and a PFR at 4 months of 4 out of 44.
Megestrol acetate did not reach the primary efficacy end point. Moreover, the rate of patients with stable weight was similar in both arms. Moreover, we had observed that acetate megestrol was the sole treatment responsible for oedemas that could overestimate the weight stabilisation. Acetate megestrol had been associated with compulsive eating and hypertriglyceridemia as a consequence of its orexigen effect. We had observed thrombotic events in two cases in this relatively small sample of patients. One of these thrombotic events led to death. Considering all these facts, we did not recommend administering megestrol acetate in such a population.
This study had some limitations. The choice of acetate megestrol as an internal comparator could be discussed, as endocrine tumours have been excluded (breast cancer, low-grade stromal endometrial sarcoma). Nevertheless, acetate megestrol is commonly administered in this patient population to maintain appetite. Moreover, rare responses have been reported with such hormonotherapy in patients with renal cancer and melanoma (Ravaud et al, 2008). There is no consensual dose for megestrol acetate (from 160 to 480 mg per day) or oral cyclophosphamide (more usually 50 mg per day) is this population. Nevertheless, Gebbia et al have shown that 480 mg of megestrol acetate was not superior to 320 mg (Gebbia et al, 1996). The dose of 50 mg b.i.d of cyclophosphamide has been administered in a previous phase II trial (Suvannasankha et al, 2007) in combination with thalidomide and prednisone without significant toxicity. The study population was a mix of patients with different tumours. The characteristics of the study population were similar to those of patients enrolled in phase I trials, with a 90-day mortality of approximately 20% (Italiano et al, 2008; Penel et al, 2008; Arkenau et al, 2009). In the current area of molecular-targeted therapies, one could argue the very low level of evidence to investigate both treatments in non-selected patients. Nevertheless, this study was pragmatic and addressed a very common, daily clinical issue. We believe that metronomic chemotherapy, especially metronomic cyclophosphamide, has been underevaluated. Many preclinical data and some clinical evidence suggest that this treatment inhibits angiogenesis (Bocci et al, 2003; Damber et al, 2005; Pietras and Hanahan, 2005; Yap et al, 2005), and some biological markers (circulating vascular endothelial growth factors, circulating endothelial cells, thrombospondin and so on) warrant further investigations as potential predictive factors (Bocci et al, 2003; Kieran et al, 2005; Munoz et al, 2005; Dellapasqua et al, 2008). Some could argue that this study did not formally establish the superiority of metronomic cyclophospahmide over acetate megestrol. Nevertheless, the purpose of randomised phase II trial is not to test such hypothesis but to show in parallel the results of both treatment arms (Cannistra, 2009). The ‘best’ arm is still chosen on the basis of the predetermined tumour size, as previously carried out in single-arm phase II trials. Randomisation minimises some pitfalls inherent to single-arm phase II trials, especially selection biases. Thus, the phase II randomised design leads to a double go–not go decision. The results of this study are sufficiently consistent to stop the evaluation of acetate megestrol in such a population and to favour new studies with metronomic cyclophosphamide in the same population. This study presents two strengths. All patients included experienced progressive disease before inclusion. Therefore, it is unlikely that the high rate of tumour stabilisation observed with metronomic cyclophosphamide was a spontaneous event. The second strength was the third-part CT-scan review that confirms stable diseases.
Eight heavily pretreated patients with sarcoma received metronomic cyclophosphamide in this study. We observed a 4-month non-progression rate of 3 out of 8. One patient experienced a stable disease for more than 30 months. The observed 4-month non-progression rate is in the range of results defining a second-line treatment as effective according to the EORTC recommendations (⩾40%) (Van Glabbeke et al, 2002). A previous rat model study had shown promising antitumour activity of metronomic cyclophosphamide administration in lymphoma and sarcoma (Rozados et al, 2004). De Pas et al had reported their single-centre experience of combined metronomic chemotherapy (cyclophosphamide plus methotrexate) in 17 heavily pretreated sarcoma patients. The median time of treatment was 3 months (range, 2–13), no grade 3–4 toxicity was noticed. Eight patients experienced stable disease (median time to progression 4 months, range 4–47+). Out of these eight patients, five experienced a progressing disease at the time of study entry. Our findings, together with these previous data, justify a further evaluation of metronomic cyclophosphamide in sarcoma patients.
On the contrary, no stable disease at 2 months was observed in patients with gastrointestinal cancers that represent approximately a third of enrolled patients.
Oral metronomic cyclophosphamide can be safely used on a metronomic basis in such a population. The efficacy, low toxicity, low cost (<£ 0.1 per day) and ease of administration of this treatment justify further studies in patients having exhausted all available therapies under standard care. We suggest that this treatment may be used as an internal comparator in further randomised phase II trials testing new options in some situations without established or shared consensual therapy.
Footnotes
Presented in part at the 45th ASCO Annual Meeting, 29 May–2 June 2009, Orlando, FL, USA.
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, De Haes JCJM, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–376 [DOI] [PubMed] [Google Scholar]
- Arkenau HT, Barriuso J, Olmos D, Ang JE, De Bono I, Judson I, Kaye S (2009) Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol 27: 2692–2696 [DOI] [PubMed] [Google Scholar]
- Berenstein EG, Ortiz Z (2005) Megestrol acetate for the treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev 13(2): CD004316. [DOI] [PubMed] [Google Scholar]
- Bocci G, Francia G, Man S, Lawler J, Kerbel RS (2003) Thrombospondin 1, a mediator of the antiangiogenic effect of metronomic chemotherapy. Proc Natl Acad Sci USA 100: 12917–12922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J (2000) Anti-angiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60: 1878–1886 [PubMed] [Google Scholar]
- Cannistra SA (2009) Phase II trials in journal of clinical oncology. J Clin Oncol 27: 3073–3076 [DOI] [PubMed] [Google Scholar]
- Casanova M, Ferrari A, Bisogno G, Merks JH, De Salvo GL, Meazza C, Tettoni K, Provenzi M, Mazzarrino I, Carli M (2004) Vinorelbine and low-dose cyclophophamide in the treatment of pediatric sarcomas. Pilot study for the Upcoming European Rhabdomyosarcoma Protocol. Cancer 101: 664–671 [DOI] [PubMed] [Google Scholar]
- Colleoni M, Orlando L, Sanna G, Rocca A, Maisonneuve P, Peruzzotti G, Ghisni R, Sandri MT, Zorzino L, Nole F, Viale G, Goldhirsch A (2006) Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: antitumor activity and biological effects. Ann Oncol 17: 232–238 [DOI] [PubMed] [Google Scholar]
- Damber JE, Vallbo C, Albertsson P, Lennernas B, Norrby K (2005) The anti-tumour effect of low-dose continuous chemotherapy may partly be mediated by thrombospondin. Cancer Chemother Pharmacol 2005: 354–360 [DOI] [PubMed] [Google Scholar]
- De Conno F, Martini C, Zecca E, Balzanni A, Venturino P, Groff L, Caraceni A (1998) Megestrol acetate for anorexia in patients with far-advaced cancer: a double-blind controlled clinical trial. Eur J Cancer 34: 1705–1709 [DOI] [PubMed] [Google Scholar]
- De Pas T, Colleoni M, Orlando L, Masci G, Rocca A, Catania C, Curigliano G, Manzoni S, Goldhirsch A, De Braud F (2004) Reply to the article ‘metronomic therapy with cyclophosphamide induces rat lymphoma and sarcoma regression, and is devoid of toxicity’ by V.R. Rozados et al. (Ann Oncol 2004; 15:1543–1550):… and in humans? Ann Oncol 16: 673–677 [DOI] [PubMed] [Google Scholar]
- Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, Pietri E, Colleoni M (2008) Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol 26: 4899–4905 [DOI] [PubMed] [Google Scholar]
- Desport JC, Blanc-Vincent MP, Delabaere G, Bachman P, Beal J, Benamouzig R, Colomb V, Kere D, Melchior JC, Nitenberg G, Raynard B, Schneider S, Senesse P (2000) Standard, options, recommendations (SOR) pour l’utilisation des medicaments orexigènes en cancérologie. Bull Cancer 87: 315–328 [PubMed] [Google Scholar]
- Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, Groshen S, Swenson S, Makland F, Gandara D, Scudder S, Morgan R, Chen H, Lenz HJ, Oza AM (2008) Phase II clinical trial of bevacizumab and low-dose metronomic oral cycclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago and the Princess Margaret Hospital Phase II Consortia. J Clin Oncol 26: 76–82 [DOI] [PubMed] [Google Scholar]
- Gebbia V, Testa A, Gebbia N (1996) Prospective trial of two dose levels of megestrol acetate in the management of anorexia-cachexia syndrome in patients with metastatic cancer. Br J Cancer 73: 1576–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode LM, Barqawi A, Crighton F, Crawford ED, Kerbel RS (2003) Metronomic therapy with cyclophoshamide and dexamethasone for prostate carcinoma. Cancer 98: 1643–1648 [DOI] [PubMed] [Google Scholar]
- Italiano A, Massard C, Bahleda R, Vataire AL, Deutsch E, Magne N, Pignon JP, Vassal G, Armand JP, Soria JC (2008) Treatment outcome and survival in participants of phase I oncology trials carried out from 2003 to 2006 at Institut Gustave Roussy. Ann Oncol 19: 787–792 [DOI] [PubMed] [Google Scholar]
- Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4: 423–436 [DOI] [PubMed] [Google Scholar]
- Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, Klement G, Laforme A, Gordon A, Thomas A, Neuberg D, Browder T, Folkman J (2005) A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol 27: 571–572 [DOI] [PubMed] [Google Scholar]
- Loprinzi CL, Kugler JW, Sloan JA, Maillard JA, Krook JE, Wilwerding MB, Rowland Jr KM, Camoriano JK, Novotny PJ, Christensen BJ (1999) Randomized comparison of acetate megestrol versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol 17: 3299–3306 [DOI] [PubMed] [Google Scholar]
- Lord R, Nair S, Scache A, Spicer J, Somaihan N, Khoo V, Pandha H (2007) Low dose metronomic oral cyclophosphamide for hormone-resistant prostate cancer: a phase II study. J Urol 177: 2136–2140 [DOI] [PubMed] [Google Scholar]
- Mantovani G, Maccio A, Lai P, Massa E, Ghiani M, Santona MC (1998) Cytokine activity in cancer-related anorexia/cachexia: role of the megestrol acetate and medroxyprogesterone acetate. Semin Oncol 25: 45–52 [PubMed] [Google Scholar]
- Munoz R, Shaked Y, Bertolini F, Emmenegger U, Man S, Kerbel RS (2005) Anti-angiogenic treatment of breast cancer using metronomic low-dose chemotherapy. Breast 14: 466–479 [DOI] [PubMed] [Google Scholar]
- Penel N, Vanseymortier M, Bonneterre ME, Clisant S, Dansin E, Vendel Y, Beuscart R, Bonneterre J (2008) Prognostic factors among cancer patients with good performance status screened for phase I trials. Invest New Drugs 26: 53–58 [DOI] [PubMed] [Google Scholar]
- Pietras K, Hanahan D (2005) A multitargeted, metronomic, and maximum-tolerated dose ‘chemo-switch’ regimen is antiangiogenic, producing objective response and survival benefit in a mouse model of cancer. J Clin Oncol 23: 939–952 [DOI] [PubMed] [Google Scholar]
- Ravaud A, Hawkins R, Gardner JP, Von de Maase H, Zantl H, Harper P, Rolland F, Audhuy B, Machiels JP, Petavy F, Gore M, Schoffsky P, El-Hariry I (2008) Lapatinib versus hormone therapy in patients with advanced renal cell carcinoma: a randomized phase III clinical trial. J Clin Oncol 26: 2285–2591 [DOI] [PubMed] [Google Scholar]
- Rowland Jr KM, Loprinzi CL, Shaw EG, Maksymiuk AW, Kurass SA, Jung SH, Kugler JM, Tschetter LK, Ghosh C, Schaefer PL, Owen D, Washburn Jr JH, Webb TA, Maillard JA, Jett JR (1996) Randomized double-blind placebo-controlled trial of cisplatin and etoposide plus megestrol acetate/placebo in extensive-stage small-cell lung cancer: a North Central Group Study. J Clin Oncol 14: 135–141 [DOI] [PubMed] [Google Scholar]
- Rozados VR, Sànchez AM, Gervasoni SI, Berra HH, Matar P, Graciela Scharovsky O (2004) Metronomic therapy with cyclophosphamide induces rat lymphoma and sarcoma regression, and is devoid of toxicity. Ann Oncol 15: 1543–1550 [DOI] [PubMed] [Google Scholar]
- Stempak D, Gammon J, Halton J, Moghrabi A, Koren G, Baruchel S (2006) A pilot pharmacokinetic and antiangiogenic biomarker study of celecoxib and low-dose metronomic vinblastine or cyclophosphamide in pediatric recurrent solid tumors. J Pediatr Hematol Oncol 28: 720–728 [DOI] [PubMed] [Google Scholar]
- Suvannasankha A, Fausel C, Juliar BE, Yiannoutsos CT, Fisher WB, Ansari RH, Wood LL, Smith GG, Cripe LD, Abonour R (2007) Final report of toxicity and efficacy of a phase II study of oral cyclophosphamide, thalidomide, and prednisone for patients with relapsed or refractory multiple myeloma: a Hoosier Oncology Group Trial, HEM01-21. Oncologist 12: 99–106 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Chistian MG, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Van Glabbeke M, Verweij J, Juson I, Nielsen OS, EORTC Soft Tissue and Bone Sarcoma Group (2002) Progression-free rate as the principal end point for phase II trials in soft tissue sarcomas. Eur J Cancer 38: 543–549 [DOI] [PubMed] [Google Scholar]
- Westman G, Bergman B, Albertsson M, Kadar L, Gustavsson G, Thaning L, Andersson M, Straumits A, Jeppson B, Linden CJ, Ewers SB, Andersson H, Mercke C, Hafstrom L, Birck O, Ogum P, Yap R (1999) Megestrol acetate in advanced, progressive, hormone-insensitive cancer. Effects on the quality of life: a placebo-controlled, randomized, multicenter trial. Eur J Cancer 35: 586–595 [DOI] [PubMed] [Google Scholar]
- Yap R, Valiceasa D, Emmenegger U, Kerbel RS, McKay LM, Henkin J, Volpert OV (2005) Metronomic low-dose chemotherapy boosts CD95-dependent antiangiogenic effect of the thrombospondin peptide ABT-510: a complementation antiangiogenic strategy. Clin Cancer Res 15: 6678–6685 [DOI] [PubMed] [Google Scholar]
- Yavuzsen T, Davis MP, Walsh D, Legrand S, Lagman R (2005) Systematic review of the treatment of cancer-associated anorexia and weight loss. J Clin Oncol 23: 8500–8511 [DOI] [PubMed] [Google Scholar]