Abstract
Traditional antibody-mediated neutralization of HIV-1 infection is thought to result from the binding of antibodies to virions, thus preventing virus entry. However, antibodies that broadly neutralize HIV-1 are rare and are not induced by current vaccines. We report that four human anti-phospholipid monoclonal antibodies (mAbs) (PGN632, P1, IS4, and CL1) inhibit HIV-1 CCR5-tropic (R5) primary isolate infection of peripheral blood mononuclear cells (PBMCs) with 80% inhibitory concentrations of <0.02 to ∼10 µg/ml. Anti-phospholipid mAbs inhibited PBMC HIV-1 infection in vitro by mechanisms involving binding to monocytes and triggering the release of MIP-1α and MIP-1β. The release of these β-chemokines explains both the specificity for R5 HIV-1 and the activity of these mAbs in PBMC cultures containing both primary lymphocytes and monocytes.
The development of strategies to induce antibodies that potently inhibit HIV-1 infection is the highest priority for HIV-1 vaccine development (Mascola et al., 2005a). Rare human mAbs (2F5, 4E10, and Z13 against the membrane-proximal region of gp41 [Muster et al., 1993; Stiegler et al., 2001; Zwick et al., 2001], IgG1b12 against the CD4 binding site of gp120 [Roben et al., 1994], and mAb 2G12 against gp120 high mannose residues [Sanders et al., 2002]) have been developed that can broadly neutralize HIV-1 in vitro. Passive infusion of 2F5 and 2G12, along with hyperimmune HIV immunoglobulin (HIVIG), has protected nonhuman primates from SIV-HIV chimeric virus (SHIV) infections in vivo (Baba et al., 2000; Mascola et al., 2000). To date, no immunogen has induced neutralizing antibodies that mimic these rare broadly neutralizing antibodies. Despite intense investigation, it remains unclear why, in both animal models and humans, these kinds of broadly neutralizing antibodies are not routinely induced (Binley et al., 2008; Li et al., 2009; Sather et al., 2009; Shen et al., 2009). Several hypotheses have been advanced, including lack of immunogens that reflect the native trimer (Burton et al., 2005) and immunoregulatory constraints on production of rare species of broadly neutralizing antibodies (Haynes et al., 2005; Dunlop et al., 2008).
The methods used to test antibodies for their ability to inhibit HIV-1 infectivity can yield strikingly different results. Screening assays for anti–HIV-1 activity have used transfected epithelial cell targets that express CD4 and CCR5, T cell lines which express CXCR4 and which can be used to measure virus induced syncytia, and assays using primary human PBMC and primary isolates of HIV-1 (Mascola et al., 2005b). Panels of HIV-1 isolates that have differing sensitivity to inhibition in these assays have been suggested as a way to determine the potency of responses generated by vaccine candidates (Mascola et al., 2005b). Other assays, including assays of antibody-dependent cell-mediated cytotoxicity (Tyler et al., 1989) and antibody-dependent cell-mediated virus inhibition (ADCVI; Forthal et al., 2001), measure the ability of different components of the immune system to act in concert to inhibit HIV-1 infection by destroying infected targets and inhibiting virus replication. It is likely that any successful vaccine candidate will need to harness multiple arms of the immune system to be successful. This point has been emphasized by the failures of strategies aimed at inducing primarily B cell (Pitisuttithum et al., 2006) or T cell (Sekaly, 2008) immunity, as well as the recent promising results from the RV144 trial (Dolgin, 2009).
One possible strategy to circumvent the problem of the induction of anti–HIV-1 antibodies is to search for antibodies with novel specificities that can inhibit HIV-1 infectivity and that could be potentially elicited by a vaccine strategy. Recently, Brown et al. (2007) have described a mouse mAb against phosphatidylinositol phosphate that neutralizes HIV-1 only in PBMC cultures and not in pseudovirus infection assays using epithelial cell targets. We now report that four human anti-phospholipid mAbs can inhibit HIV-1 infection, and we demonstrate that the mechanism of inhibition is stimulation of an innate anti–HIV-1 response, including the release of soluble chemokines which block HIV-1 entry.
RESULTS
Analysis of anti-phospholipid antibody inhibition of HIV-1 infectivity in epithelial cell–based pseudovirus infection and PBMC infection inhibition assays
First, the ability of anti-phospholipid mAbs (Table I) to inhibit R5 HIV-1 envelope (Env) pseudoviruses B.PVO, B.6535, and C.DU123 in the TZM-bl epithelial cell pseudovirus infectivity assay was determined (Montefiori, 2005). None of these anti-phospholipid mAbs could inhibit pseudovirus infectivity, whereas broadly neutralizing mAb 4E10 potently neutralized all three viruses in this assay (Table S1). Similarly, none of these anti-phospholipid mAbs inhibited fusion-competent CXCR4-tropic (X4) B.MN or CCR5-tropic (R5) B.ADA and B. AD8 HIV-1 mediated fusion in Sup-T1 T cells, whereas the broadly neutralizing mAbs 2F5 and 4E10 did inhibit syncytia formation (Table S2).
Table I.
Anti-phospholipid monoclonal antibodies in this study
| mAb | Heavy chain | Light chain | Binds cardiolipin/PS | Binds HIV-1 Env | Binds directly to β2GP1 | Binding to CL/PS dependent on β2GP1 | mAb origin |
| IS4 | γ3 VH1 | λ Vλ2 | ++ | − | + | Yes | APS subject |
| CL1 | γ3 VH1 | λ Vλ3 | ++ | − | ++ | No | SLE subject with APS |
| P1 | γ1 | λ | ++ | − | +++ | No | SLE subject with APS |
| PGN632 | γ1 | λ | ++ | − | + | No | uninfected and healthy subjecta |
| PGN401 | γ1 | κ | + | − | + | Yes | humanized mouse mAb |
| B1 | γ1 | κ | + | − | +++ | Yes | APS subject |
| B2 | γ3 | κ | ++ | − | + | Yes | APS subject |
| PGN635 | γ1 | λ | + | − | + | Yes | uninfected and healthy subjecta |
| PGN634 | γ1 | λ | + | − | + | Yes | uninfected and healthy subjecta |
| 2F5 | γ3 | κ | + | + | + | No | HIV-1 + subject |
| 4E10 | γ3 | κ | + | + | + | No | HIV-1 + subject |
Derived from an antibody library from normal subjects. PGN632 was further engineered to optimize binding to PS.
In contrast, four of the nine anti-phospholipid mAbs (IS4, CL1, P1, and PGN632) inhibited HIV-1 virion infectivity in PBMCs when tested against primary virus isolates corresponding to the aforementioned Env pseudoviruses (Table II). A mixture of broadly neutralizing mAbs (IgG1b12, 2F5, and 2G12, termed TriMab) was used as a positive control. Interestingly, the mAbs in Table I that bound phospholipids in the presence and absence of β2GP1 (Fig. S1, A and B) were able to inhibit HIV-1 infectivity, whereas mAbs that did not bind phospholipids in the absence of β2GP1 had much more variable inhibitory effects on HIV-1 infection, with only IS4 showing consistent strong HIV-1 inhibitory activity (Table II). These data suggested that the mode of action of P1, PGN632, CL1, and IS4 might be mediated through binding to the lipids of the virion, the target cell, or both in a specific orientation or packing.
Table II.
Assay for anti-phospholipid mAb inhibition of HIV-1 infectivity in a PBMC-based virus infection inhibition assay
| Primary HIV-1 isolates | mAbsa | |||||||||
| IS4 | CL1 | P1 | PGN632 | PGN401 | B1 | B2 | PGN635 | PGN634 | TriMabb | |
| µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | |
| B.PVO | 0.34 | 0.21 | 4.5 | 0.03 | >50 | >50 | >50 | >50 | >50 | 0.64 |
| B.6535 | 0.07 | 0.38 | 4.0 | <0.02 | >50 | >50 | >50 | >50 | >50 | 2.4 |
| C.DU123 | 0.06 | 0.19 | <0.02 | <0.02 | 4.5 | >50 | >50 | 8.2 | >50 | >25 |
mAbs are shown as IC80 values (concentration of antibody resulting in 80% reduction of infection).
TriMab = equal concentration mixture of IgG1b12, 2F5, and 2G12.
Anti-phospholipid mAbs selectively inhibit infection of R5 HIV-1
Next, the breadth of neutralization of PGN632, P1, IS4, and CL1 mAbs was determined. Of eight R5 primary isolates tested, HIV-1 infectivity was inhibited by each of the four mAbs (Table III). When X4 viruses were tested, however, none of the four HIV-1 strains were inhibited. Recently, several env genes from transmitted/founder HIV-1 have been identified and isolated from plasmas of subjects soon after transmission (Keele et al., 2008). Thus, the ability of the anti-phospholipid mAbs to inhibit the infectivity of five such transmitted/founder Envs was tested using replication-competent Renilla luciferase reporter HIV-1 encoding the respective env gene sequences. Four of the Envs were R5 tropic and one (B.WEAU410) was dual R5/X4 tropic (Keele et al., 2008). Two reporter viruses expressing reference/chronic Envs were included as controls (B.BaL [AY426110] and B.SF162LS [EU1123924]). Anti-phospholipid mAbs were able to inhibit the infectivity of four R5 transmitted/founder viruses but not the dual-tropic R5/X4 B.WEAU3 strain (Table IV). In addition, mAb PGN632 inhibited the infectivity of R5 SIVmac251 in rhesus PBMC (unpublished data). Thus, anti-phospholipid antibodies could inhibit the infectivity of R5 primary and transmitted/founded HIV-1 isolates but could not inhibit the infectivity of any virus strains that used CXCR4 as a coreceptor.
Table III.
Breadth of anti-phospholipid mAb inhibition of HIV-1 infectivity against 12 R5 and X4 HIV-1 and SHIV primary isolates in the PBMC infectivity assay
| Primary HIV-1 isolates | mAbsa | Coreceptor usage | |||||
| IS4 | CL1 | P1 | PGN632 | anti-RSV | TriMabb | ||
| µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | ||
| B.TORNO | 0.58 | 0.73 | 17 | 0.09 | >50 | 0.03 | CCR5 |
| B.PVO | 0.34 | 0.21 | 4.5 | 0.03 | >50 | 0.64 | CCR5 |
| B.6535 | 0.07 | 0.38 | 4.0 | <0.02 | >50 | 2.4 | CCR5 |
| C.DU123 | 0.06 | 0.19 | <0.02 | <0.02 | >50 | >25 | CCR5 |
| C.DU156 | 2.8 | 2.6 | 16 | 0.06 | >50 | >25 | CCR5 |
| C.DU151 | 3.1 | 1.1 | 1.2 | <0.02 | >50 | >25 | CCR5 |
| C.DU172 | 1.1 | 0.62 | 0.55 | <0.02 | >50 | >25 | CCR5 |
| SHIV 162P3 | 5.2 | 1.2 | 1.6 | <0.02 | >50 | 0.46 | CCR5 |
| SHIV 89.6P | >50 | >50 | >50 | >50 | >50 | 1.5 | CXCR4/CCR5 |
| A.92UG029 | >50 | >50 | >50 | >50 | >50 | >50 | CXCR4 |
| B.MN | >50 | >50 | >50 | >50 | >50 | 0.26 | CXCR4 |
| AE_01.NI1052 | >50 | >50 | >50 | >50 | >50 | >50 | CXCR4 |
mAbs are shown as IC80 values.
TriMab = equal concentration mixture of IgG1b12, 2F5, and 2G12.
Table IV.
Breadth of anti-phospholipid mAb inhibition of HIV-1 infectivity against R5 and R5/X4 replicating HIV-1 Renilla reporter viruses engineered to express five transmitted/founder env genes, B.BaL env, and B.SF162LS env in the PBMC infectivity assay
| HIV-1 genes in NL-LucR.T2A-ENV.ecto virusesa | mAbsb | Coreceptor usage | |||||
| IS4 | CL1 | P1 | PGN632 | Anti-RSV | 4E10 | ||
| µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | ||
| B.BaL | 0.58 | 2.2 | 0.11 | <0.02 | >50 | 1.6 | CCR5 |
| B.WITO | 0.06 | <0.02 | <0.02 | <0.02 | >50 | 0.16 | CCR5 |
| B.CH040 | <0.02 | <0.02 | <0.02 | <0.02 | >50 | <0.02 | CCR5 |
| B.CH058 | 0.37 | 0.17 | 0.29 | <0.02 | >50 | 3.6 | CCR5 |
| B.CH077 | 16 | 46 | 5.5 | 0.07 | >50 | 3.0 | CCR5 |
| B.SF162LS | 1.5 | 2.6 | 0.4 | <0.02 | >50 | 2.5 | CCR5 |
| B.WEAU410 | >50 | >50 | >50 | >50 | >50 | 4.5 | CXCR4/CCR5 |
Transmitted env genes were derived from patients WITO4160, 700-01-004-0, 700-01-005-8, 700-01-007-7, and WEAu0575, respectively.
mAbs are shown as IC80 values.
Next the comparative potency and reproducibility of the anti-phospholipid mAb PGN632 was determined in inhibiting HIV-1 B.BaL infectivity by assay of its anti–HIV-1 activity in PBMC from 75 different HIV-1–negative subjects (Fig. S2). HIV-1 infectivity was inhibited by mAb PGN632 in 85% of the PBMCs with a mean 80% inhibitory concentration (IC80) of 0.6 µg/ml and a range of 0.01–24 µg/ml for this 85% of positives. Thus, wide variation was seen in the potency of mAb PGN632 in different donor PBMC, where the mAb exhibited exceptional potency in a majority of cases.
Time course of activity of anti-phospholipid antibodies
To study the kinetics of anti-phospholipid mAb inhibition of HIV-1 infection, mAbs were added at the time of addition of the virus or at 24, 48, and 72 h after adding virus to PBMC. Two of the mAbs were effective when added at later time points (Table V). For both antibodies, the inhibition was attenuated at the later time points. Significantly, both CL1 and PGN632 mAbs inhibited HIV-1 infectivity when added 48 h after the start of the infection with IC80s of 0.22 and 0.07 µg/ml, respectively. These data suggested that anti-phospholipid mAbs were inhibiting virus spread after one or more initial rounds of infection in PBMC cultures.
Table V.
Effect of time of introduction on the inhibitory effect of anti-phospholipid mAbs against B.6535 in the PBMC assay.
| Time after infection | mAbsa | ||||
| IS4 | CL1 | P1 | PGN632 | Anti-RSV | |
| h | µg/ml | µg/ml | µg/ml | µg/ml | µg/ml |
| 0 | 0.91 | 0.19 | 2.4 | <0.02 | >50 |
| 24 | >50 | 0.60 | >50 | 0.18 | >50 |
| 48 | >50 | 0.22 | >50 | 0.07 | >50 |
| 72 | >50 | >50 | >50 | >50 | >50 |
mAbs are shown as IC80 values.
Site of action of anti-phospholipid antibodies
To determine the site of action of anti-phospholipid antibodies, primary isolate B.BG1168 and B.SF162LS pseudovirions produced in PBMC and 293T cells, respectively, were incubated in the presence or absence of soluble CD4 and then captured by immobilized anti-phospholipid, anti-HIV-1, and control mAbs. As expected, the anti-gp120 V3 loop mAb F39F was able to capture HIV-1 virions (Fig. S3 A). In addition, the anti-gp120 CCR5 binding site mAb 17b was able to capture virions in the presence but not in the absence of triggering by soluble CD4. In contrast, none of the anti-phospholipid mAbs were able to capture virions. To verify that the anti-phospholipid antibodies were not reacting with HIV-1 Env, we performed surface plasmon resonance (SPR) analysis of anti-phospholipid antibody reactivity with a series of recombinant Env oligomers. Although 2F5 and 4E10 mAbs bound well to JRFL and CON-S gp140 oligomers, none of the anti-phospholipid antibodies bound to HIV-1 Env (Table I). Similarly, SPR analysis was performed to determine anti-phospholipid mAb binding to intact Aldrithiol-2–inactivated B.ADA virions. P1, IS4, and CL1 mAbs did not bind to HIV-1 virions, whereas PGN632 mAb did bind, although with a fast off rate, likely explaining why this binding was not seen in the virus capture assay shown in Fig. S3 A (Fig. S3, B–D).
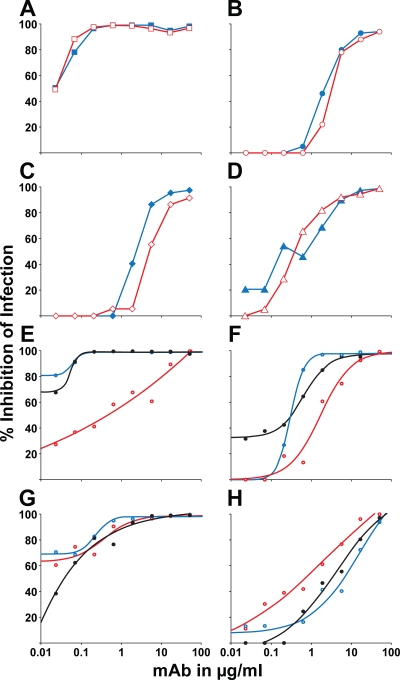
Two variations of the PBMC assay were next performed to further define the site of mAb inhibitory action in PBMC cultures. First, anti-phospholipid mAbs were preincubated with virus for 1 h before addition of the virus-antibody mixture to PHA-activated PBMC. Second, anti-phospholipid mAbs were added first to PHA-activated PBMC for 1 h and then the PBMCs were washed before the virus being added. In both circumstances, the potency of anti-phospholipid mAb inhibition was equal (Fig. 1, A–D). Collectively with the finding that the anti-phospholipid mAbs did not bind stably to virions, these data indicated that the anti-phospholipid antibodies inhibited HIV-1 infectivity by binding only to the PBMC and not to virions.
Figure 1.
Anti-phospholipid mAbs inhibit infection by binding to host cells and inhibition can be blocked by lipids. (A–D) Antibodies were assayed by incubation with the challenge virus stock followed by introduction of the mixture to the target cells (red open points) or by incubation with the PBMC targets and washing away of the excess before virus challenge (blue closed points). For each mAb, similar neutralization curves were seen. (A) PGN632 tested against SHIV SF162P3. (B) CL1 tested against B.QH0692. (C) IS4 tested against B.QH0692. (D) P1 tested against SHIV SF162P3. Data shown are representative of three experiments performed. (E–H) The PBMC infection assay was performed using HIV-1 B.6535 with mAb preincubated with PBS (black curve), 0.5 mM DOPE (blue curve), or 0.5 mM cardiolipin (red curve). (E) PGN632. IC80 = 0.05 µg/ml after preincubation with PBS; IC80 < 0.02 µg/ml after preincubation with DOPE; IC80 = 10 µg/ml after preincubation with cardiolipin (200-fold reduction). (F) CL1. IC80 = 0.53 µg/ml after preincubation with PBS; IC80 = 0.53 µg/ml after preincubation with DOPE; IC80 = 5.8 µg/ml after preincubation with cardiolipin (11-fold reduction). (G) IS4. IC80 = 0.30 µg/ml after preincubation with PBS; IC80 = 0.18 µg/ml after preincubation with DOPE; IC80 = 0.33 µg/ml after preincubation with cardiolipin (no change). (H) P1. IC80 = 16 µg/ml after preincubation with PBS; IC80 = 26 µg/ml after preincubation with DOPE (1.6-fold reduction); IC80 = 7.2 µg/ml after preincubation with cardiolipin. Data shown are representative of three experiments performed.
PBMC target of anti-phospholipid antibodies
To determine the cell type targeted by anti-phospholipid mAbs, different PBMC subsets were infected with HIV-1 Renilla luciferase reporter HIV-1 expressing B.BaL env and tested for inhibition with mAb PGN632 (Table VI). Although HIV-1 NL-LucR.T2A-BaL.ecto infected CD4+ T cells, mAb PGN632 had no effect on CD4+ T cell infection by this R5 HIV-1 isolate. Rather, only subsets of PBMC that contained monocytes (purified monocytes, CD4+ T cell–depleted PBMC) could be protected from infection by the presence of anti-phospholipid mAbs (Table VI). Similar results were obtained using a Renilla luciferase reporter HIV-1 expressing the transmitted HIV-1 env B.WITO (unpublished data). In separate experiments using HIV-1 primary isolates, mAbs PGN632 and CL1 inhibited HIV-1 PVO in PBMC fractions containing monocytes but not in purified CD4+ T cells (Table S3). Thus, although anti-phospholipid mAbs could not directly inhibit CD4+ T cell infectivity with HIV-1, they could mediate abrogation of CD4+ T cell infection by HIV-1 in the presence of peripheral blood monocytes.
Table VI.
Inhibition of HIV-1 infection with NL-LucR.T2A-BaL.ecto by mAbs in intact PBMC and PBMC cell subsets
| mAb | Target cell populationsa | ||||
| Intact PBMC | CD4+ T cells | CD4+ T cell-depleted PBMC | CD14+ monocytes | CD14+ monocyte-depleted PBMC | |
| µg/ml | µg/ml | µg/ml | µg/ml | µg/ml | |
| PGN632 | 0.29 | >50 | 0.31 | 0.18 | >50 |
| IgG1b12 | 6.2 | 4.6 | 0.57 | 0.65 | 5.1 |
| 2G12 | 0.39 | 4.6 | 0.06 | 0.03 | 5.0 |
| 4E10 | 40 | 41 | 47 | 32 | 42 |
Target cell populations are IC80 values
In addition, we determined if anti-phospholipid antibodies could directly bind to lymphocytes and monocytes. Using flow cytometry to analyze cells treated in the same manner as in the PBMC assay, we found that mAbs PGN632 and CL1 bound to a fraction of CD4+ T cells, whereas the other antibodies tested showed no significant binding to CD4+ T cells (Fig. S4 A). Flow cytometry analysis of monocytes showed binding of all antibodies including control mAb F39F, suggesting Fc receptor-mediated binding of the antibodies. Anti-phospholipid mAbs PGN632 and CL1 showed binding above the background, whereas small shifts were seen for IS4 and P1 (Fig. S4 B).
Determination of the respective roles of the Fab and Fc components of anti-phospholipid antibodies in inhibiting HIV-1 infectivity of PBMC
To determine the role of the phospholipid-binding Fab portion of the anti-phospholipid mAbs in inhibiting HIV-1 infectivity, the PBMC assay was performed with mAbs preincubated with PBS, 2 mM cardiolipin, or 2 mM dioleoylphosphatidylethanolamine (DOPE; Fig. 1, E–H). When tested against B.6535, two of the antibodies, CL1 and PGN632, showed no change in potency when incubated with DOPE but did show a loss of potency after incubation with cardiolipin (reduction in IC80 of 11- and 200-fold, respectively). In contrast, IS4 mAb showed little change upon incubation with either lipid. Thus, these data demonstrated a role of the antigen-specific Fab component of the antibody for mAbs P1, CL1, and PGN632 as preincubation with lipids was able to block the inhibition of HIV-1 infection of PBMC.
To further evaluate the role of the Fab component of recombinant mAb IS4, variants of IS4 that had mutations of the heavy chain CDR3 arginines (which are required for phospholipid binding) were studied (Giles et al., 2006). The panel of recombinant mAbs included IS4 expressed as IgG1 and paired with the native IS4 light chain, IS4 IgG1 heavy chain paired with a light chain from an anti-nucleosome mAb named B3 (which differed solely in its pattern of somatic mutations from IS4VL; Mason et al., 2005) with enhanced cardiolipin and PS binding, and an engineered antibody with the native IS4 light chain paired with IS4 IgG1 containing two arginine-to-serine mutations at positions 96 and 97 in the CDR H3 loop that had been previously shown to abolish phospholipid binding (Giles et al., 2006). The two versions of the antibody that retained cardiolipin and PS binding both inhibited HIV-1 infection of PBMC with Renilla luciferase reporter HIV-1 expressing B.BaL env, whereas the recombinant mutated IS4 mAb that did not bind lipids did not inhibit HIV-1 infectivity (Table VII). Thus, collectively, these data demonstrated that the antigen-specific Fab regions of whole IS4, CL1, P1, and PGN632 mAbs are all required to mediate the anti–HIV-1 effect in vitro.
Table VII.
Effect of mutations of IS4 on inhibition of HIV-1 infection with NL-LucR.T2A-BaL.ecto in the PBMC assay
| Heavy chain | Light chain | Binding to cardiolipin and PS (Giles et al., 2006) | IC50 against B.BaL |
| µg/ml | |||
| IS4 IgG1 VH | IS4 VL | yes | 10.4 |
| IS4 IgG1 VH | B3 VL | yes | 7.4 |
| mutated IS4 IgG1 VHa | IS4 VL | no | >50 |
Mutations in IS4 heavy chain are arginine-to-serine at positions 96 and 97.
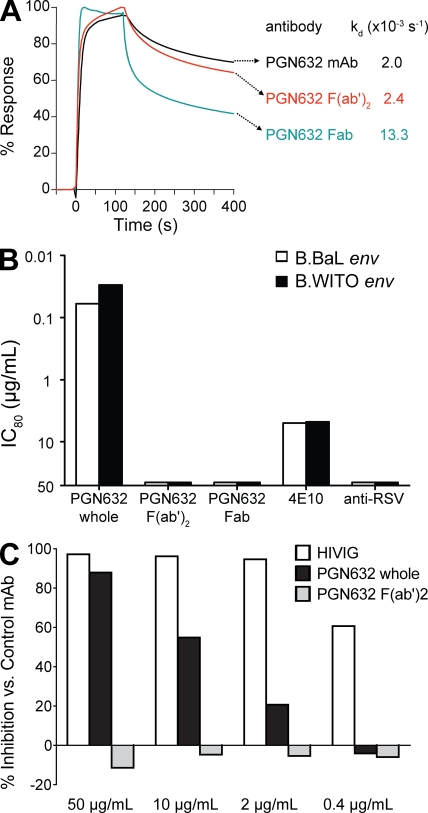
Next, a F(ab′)2 fragment of the most potent mAb, PGN632, was produced and used to determine if the F(ab′)2 bound to phospholipids with kinetics identical to the whole IgG PGN632 and to determine if the PGN632 F(ab′)2 would also inhibit PBMC infection by HIV-1. Although intact PGN632 and the F(ab′)2 fragment had the same kon and koff when assayed for binding to cardiolipin, only the whole IgG1 PGN632 was able to inhibit HIV-1 infection of PBMC (Fig. 2, A and B). Thus, both the Fab and Fc portions of intact whole anti-phospholipid antibodies are required for inhibition of HIV-1 infection of PBMC.
Figure 2.
Intact whole-molecule PGN632 inhibits HIV-1 infection but Fab and F(ab′)2 fragments do not. (A) SPR characterization of the cardiolipin binding of intact PGN632, PGN632 Fab, and PGN632 F(ab′)2. The intact mAb and the bivalent F(ab′)2 have similar kinetics of binding to cardiolipin. In contrast, the monovalent Fab has a greater than fivefold faster koff, indicating the loss of avidity compared with the intact mAb. (B) PBMC assay of PGN632 and fractions. Intact PGN632 inhibited the infection of both NL-LucR.T2A.BaL.ecto and NL-LucR.T2A.WITO.ecto in the PBMC assay (IC80 = 0.06 and 0.03 µg/ml, respectively), as did mAb 4E10 with a less potent IC80 (5.0 and 4.7 µg/ml, respectively). In contrast, both the bivalent F(ab′)2 and the monovalent Fab failed to inhibit, as did the control anti-RSV mAb. Data shown are representative of three experiments performed. (C) ADCVI assay was performed using HIV-1 92US657 as described in Materials and methods. Data shown are representative of three experiments performed. Final antibody concentrations are as shown. Data are normalized to a control mAb. The human antibody preparation HIVIG was used as a positive control. At the highest concentration (50 µg/ml), HIVIG produced 97.2% inhibition and whole intact PGN632 mAb produced 87.9% inhibition, whereas PGN632 F(ab′)2 produced no inhibition (−11.4%). These data demonstrate the requirement for intact mAb containing Fc to mediate inhibition of HIV-1 infection.
ADCVI activity of anti-phospholipid mAb
To determine if the anti–HIV-1 effect of anti-phospholipid antibodies was limited to the PBMC assay alone, mAb PGN632 was tested for ADCVI. CEM.NKr.CCR5 target cells were infected with HIV-1 92US657 and mixed with PBMC effector cells at a 10:1 effector/target cell ratio. PGN632 IgG or PGN632 F(ab′)2 was added, and 7 d later, virus output was measured. ADCVI activity was determined as the percentage of virus inhibition compared with a control mAb (Forthal et al., 2001). Similar to the results for the PBMC assay, whole IgG PGN632 at 50 µg/ml inhibited HIV-1 production by ADCVI (89.6 ± 4.3%), whereas PGN632 F(ab′)2 produced no inhibition (−20.6 ± 11.8%; Fig. 2 C).
Direct effects of anti-phospholipid antibodies on monocytes and CD4+ T cells in vitro
The requirement for the presence of monocytes in PBMC cultures for HIV-1 inhibition suggested that the anti-phospholipid mAbs might have a direct effect on that cell type. Purified monocytes were cultured in the presence of anti-phospholipid mAbs and control mAbs in vitro and assayed for evidence of a proliferative effect of the mAbs by incorporation of 3H-thymidine; however, none of the anti-phospholipid antibodies induced cell proliferation (Fig. S5 A). Similarly, CD4+ T cells cultured under similar conditions showed no evidence of proliferation by incorporation of 3H-thymidine (Fig. S5 A), nor did CD4+ T cells show evidence of increased apoptosis (Fig. S5, B–D). Although control anti–HIV-1 antibodies had little effect on monocyte morphology (Fig. S6 E), culture in the presence of anti-phospholipid mAbs for 4–7 d induced the formation of polykaryons (Fig. S6, A–D). In contrast, culture of monocytes in the presence of 10 µg/ml LPS did not induce polykaryons (Fig. S6 F). Monocytes cultured with anti-phospholipid mAbs were stained for markers of dendritic cells. The majority of polykaryons were monocyte-derived CD11c+, CD68+, and Langerin− (Fig. S6, G, H, and J). There were rare Langerin+ polykaryons (Fig. S6 I), indicating that the anti-phospholipid mAbs induced the formation of polykaryons in dendritic cells as well. Culture of CD4+ T cells showed the development of blast forms (Fig. S6, K–S) but no evidence of polykaryons was seen.
Induction of PBMC CCR5 chemokines by anti-phospholipid antibodies
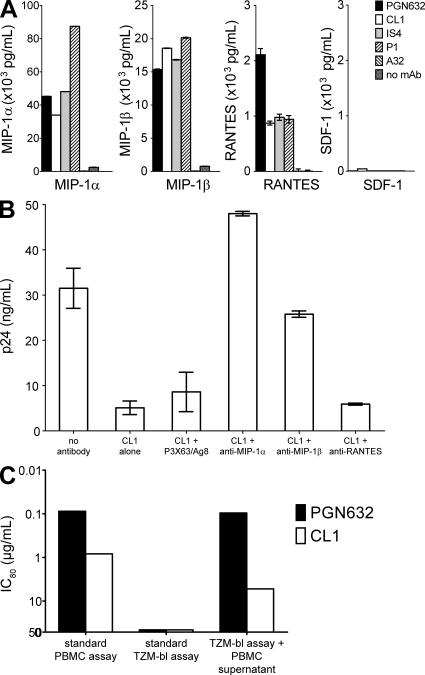
The combination of the specificity of anti-phospholipid mAbs for R5 HIV-1 isolates and the requirement for the presence of monocytes in the target cultures for HIV-1 inhibition suggested that induction of R5 chemokines is important in the mechanism of action of anti-phospholipid mAbs. All four anti-phospholipid mAbs were indeed potent inducers of, primarily, MIP-1α and MIP-1β and, to a lesser extent, RANTES from PBMC but not of the X4 chemokine SDF-1 (Fig. 3 A). When neutralizing antibodies against the R5 chemokines were added to cultures of PBMC, the anti–MIP-1α and anti–MIP-1β antibodies, but not the anti-RANTES antibody, blocked the ability of the anti-phospholipid mAb CL1 to inhibit HIV-1 infection (Fig. 3 B). Thus, the anti-phospholipid antibodies that selectively inhibited the in vitro infectivity of R5 HIV-1 strains also triggered production of MIP-1α and MIP-1β anti–HIV-1 chemokines from peripheral blood monocytes. Prior experiments demonstrated that other mAbs directed against HIV-1 (F39F and 17b), as well as the non–HIV-1 mAb Synagis, did not stimulate the production of chemokines (Fig. S7 A). To determine if the mAbs could have this effect on CD4+ T cells alone, we cultured purified CD4+ T cells in the presence of the anti-phospholipid antibodies and control mAbs and found that none of the antibodies directly stimulated the production of chemokines from CD4+ T cells (Fig. S7 B).
Figure 3.
Chemokines mediate the anti–HIV-1 activity of anti-phospholipid antibodies. (A) Monocytes were cultured in the presence or absence of antibodies, and culture supernatants at 24 h were assayed for the presence of chemokines. The four anti-phospholipid mAbs PGN632, CL1, IS4, and P1 potently induced MIP-1α and MIP-1β, along with lower levels of RANTES. The anti–HIV-1 mAb A32 did not induce any chemokine production. None of the antibodies induced SDF-1. Data shown are means of three wells per point, error bars are SEM, and all are representative of three experiments performed. (B) The activity of CL1 against HIV-1 B.6535 in PBMC was tested in the presence of anti-chemokine blocking antibodies. Productive infection was observed in the absence of any antibody, and that infection was inhibited by the presence of CL1 mAb. Control blocking antibody P3X63/Ag8 did not alter the ability of CL1 to inhibit HIV-1 infection, nor did a blocking anti-RANTES antibody. In contrast, blocking antibodies against MIP-1α and MIP-1β restored infection, abrogating the inhibitory effect of CL1 mAb. Data shown are representative of three experiments performed. (C) The activity of PGN632 and CL1 mAbs were tested in the standard PBMC infectivity assay against NL-LucR.T2A.WITO.ecto (IC80 = 0.08 and 0.80 µg/ml, respectively) and in the TZM-bl pseudovirus neutralization assay using a pseudovirus construct expressing the same env (IC80 >50 for both). Additionally, PBMCs were triggered by incubation with mAb for 24 h and cell-free supernatants transferred to TZM-bl cultures followed by addition of pseudovirus. Supernatants triggered with control antibodies did not inhibit (not depicted), whereas those triggered with PGN632 and CL1 inhibited with IC80 = 0.09 and 5.1 µg/ml, respectively, demonstrating that soluble factors produced in the PBMC cultures mediated inhibition of HIV-1 infectivity. Data shown are representative of three experiments performed.
Endotoxin stimulation of monocytes does not account for the activity of anti-phospholipid antibodies in the PBMC assay
As noted in Materials and methods, all antibody stocks were tested for the presence of endotoxin and were found to either have no detectable endotoxin or to have no more than 1 pg of endotoxin per 1 mg/ml of antibody stock. To directly test the effect of endotoxin in the PBMC assay, the PBMC assay was performed in the presence of LPS. At least 4 µg/ml of endotoxin was required for 80% inhibition of HIV-1 infection, an amount that far exceeded the amount of endotoxin detected in mAb preparations (unpublished data). These data, in concert with those demonstrating the requirement of both the Fab and Fc components of IgG anti-phospholipid mAbs for HIV-1 inhibition, demonstrated that endotoxin contamination was not responsible for the observed anti–HIV-1 activity.
Culture supernatants from anti-phospholipid antibody-stimulated PBMC inhibit HIV-1 pseudovirus infection of TZM-bl cells
The lack of monocytes in the TZM-bl pseudovirus neutralization assay could explain the absence of activity of the anti-phospholipid antibodies in that assay. Therefore, supernatants from PBMC that had been incubated with anti-phospholipid antibodies were transferred into TZM-bl cell cultures to determine if chemokine secretion by monocytes could block pseudovirus infection. As before, anti-phospholipid antibodies alone did not neutralize, nor did supernatants from cells not incubated with anti-phospholipid antibodies. However, supernatants from PBMC incubated with anti-phospholipid antibodies were able to block pseudovirus infection of TZM-bl cells (Fig. 3 C). Thus, transfer of supernatants produced by anti-phospholipid antibody-treated PBMC recapitulated in the TZM-bl assay the HIV-1 inhibitory activity of anti-phospholipid antibodies seen in the PBMC assay.
DISCUSSION
In this paper, we define a mechanism of inhibition of HIV-1 infection of human PBMCs by human anti-phospholipid antibodies. Anti-phospholipid mAbs derived from human subjects can be classified into two types: those that are dependent on the presence of β2GP1 for binding and those that are not (Zhu et al., 1999; Iverson et al., 2002; Ioannou et al., 2007; Lin et al., 2007). Although not an absolute correlation, those mAbs that require β2GP1 for binding to phospholipids have been associated with autoimmune phenomena, including an increase in thrombogenesis and with adverse clinical outcomes (Alving, 2006; Lin et al., 2007), whereas those mAbs that bind directly to phospholipids and that do not require β2GP1 for binding are not (Iverson et al., 2002; Ioannou et al., 2006). In addition, naturally occurring antibodies against phospholipids can be found in healthy individuals and are not associated with negative clinical consequences (Alving, 2006). Thus, antibodies that bind to phospholipids are not always pathological, and those that possess additional beneficial properties, such as virus inhibition (Brown et al., 2007; Soares et al., 2008) or antitumor effects (Ran et al., 2005), could be of interest as candidates for therapeutic monoclonal antibodies or for induction by vaccines.
The mAbs studied in this paper were generated from peripheral blood antibody libraries of healthy subjects or from blood B cells of primary and secondary anti-phospholipid antibody syndrome (APS) subjects. HIV-1–inhibiting anti-phospholipid antibodies were effective up to 48 h after HIV-1 contact with PBMC, broadly neutralized only R5 but not X4 HIV-1, and acted on peripheral blood monocytes to induce R5 chemokines. Collectively, these data explain why anti-phospholipid antibodies inhibit HIV-1 infectivity in PBMC but not in CD4+ CCR5+ transfected epithelial cell assays such as the TZM-bl assay (Montefiori, 2005), and they demonstrate a novel mechanism whereby components of the adaptive immune system can activate antiviral innate immunity.
The target of the anti-phospholipid antibodies in the PBMC HIV-1 infection assay was not HIV-1 virions. Of the four mAbs that inhibited HIV-1 infectivity in PBMCs, only one, PGN632, weakly bound HIV-1 virions in SPR with a fast off rate (Fig. S3, B–D). These mAbs induced chemokines and cytokines to the point of inducing monocyte polykaryon formation in vitro (Fig. S6, A–F). R5 but not X4 chemokines were induced, with the production of MIP-1α and MIP-1β exceeding that of RANTES (Fig. 3 A), and antisera against MIP-1α and MIP-1β abrogated the ability of lipid antibodies to inhibit HIV-1 infectivity of PBMC (Fig. 3 B). Thus, virus inhibition by the anti-phospholipid antibodies is not the result of viral neutralization but rather occurs through in vitro modulation of PBMC by adaptive antibodies, resulting in inhibition of HIV-1 infectivity.
Three of the antibodies (PGN632, P1, and CL1) that inhibit HIV-1 infection bind directly to phospholipids, whereas one (IS4) requires β2GP1 for binding. The importance of the Fab region in binding is shown by the ability of the anionic phospholipid cardiolipin to block the HIV-1 inhibition activity of CL1 and PGN632 mAbs (Fig. 1, E–H). For IS4, the requirement for Fab binding was shown by use of the recombinant IS4 variants with those variants that retain lipid binding showing activity in the PBMC assay. These data are consistent with direct signaling for chemokine induction through lipids or lipid-dependent signaling complexes. PS is a tenable target, as it is recognized by all four HIV-1 inhibitory mAbs, appears to be present constitutively on macrophage lineage cells (unpublished data), and is up-regulated on activated (Bevers et al., 1983) and virally infected cells (Soares et al., 2008). PS is enriched in lipid rafts, membrane microdomains which are known to be associated with cell signaling events. Although cardiolipin is also recognized by the four mAbs, cardiolipin is itself unlikely to be a target as it is predominantly a component of mitochondrial lipid membranes (Schlame and Hostetler, 1997). The differing potencies of the four mAbs also suggested that the particular lipid motif bound by PGN632 and CL1 antibody Fab regions may be similar but that mAbs P1 and IS4 Fab region lipid binding motifs may differ. Thus, it is possible that the activity of certain antibodies involves binding to an unidentified lipid target in addition to PS.
Although the Fab regions of the four anti-phospholipid antibodies were clearly required for HIV-1 inhibition activity, the Fc region was also clearly required. The F(ab′)2 of mAb PGN632, the most potent anti-phospholipid mAb, did not inhibit HIV-1 infectivity in the PBMC assay and did not support ADCVI (Fig. 2, B and C, respectively). This finding was not a result of altered binding of the F(ab′)2 to lipids, as the F(ab′)2 koff for binding to lipids was identical to that of the whole IgG by SPR (Fig. 2 A). Thus, both the Fab and the Fc regions of the anti-phospholipid antibodies, together as an intact molecule, were required for mediating the HIV-1 inhibition effect of the anti-phospholipid antibodies. Triggering via the Fc receptor of IgG has been reported to stimulate chemokine release from natural killer cells (Forthal et al., 2001, 2005), monocytes (Fernández et al., 2002), and dendritic cells (Radstake et al., 2005), and these data raise the possibility of synergy between two modes of triggering (e.g., lipid binding and FcR ligation; polyspecific triggering by antibody Fab plus FcR binding). Although the data in Table VI and Table S3 suggest that monocytes alone can mediate these effects, the role of other cells, such as NK cells, has not been rigorously excluded. It was notable that the inhibitory effect of anti-phospholipid antibodies was seen in only 85% of the PBMC tested. The lack of activity in PBMC that did not support the phenomenon could be the result of an inability to produce the correct chemokines, alteration in a cellular receptor, or differences in signaling pathways. Thus, it will be important to determine the mechanism of induction of chemokines and cytokines by anti-phospholipid antibodies and to determine the roles of each of the cell surface targets of this activity on multiple FcR+ cell types.
It was of considerable interest that anti-phospholipid antibodies did not induce X4 chemokines but only R5 chemokines. In vitro protective effects of R5 chemokines secreted by neonatal natural killer cells have been previously described (Bernstein et al., 2004), and antigen-specific production of R5 chemokines in vitro has been reported to correlate with decreased viral loads in HIV-1–infected subjects (Ferbas et al., 2000). Kinter et al. (1998) reported that R5 virus inhibition through the use of β-chemokines can be correlated with the enhancement of X4 isolate replication. This effect was most pronounced for RANTES and less substantial for MIP-1α. In contrast, in our study RANTES was minimally induced, whereas MIP-1α was increased to the greatest extent (Fig. 3 A) and the use of anti-RANTES antibodies showed little effect on the antiviral activity of mAb CL1 (Fig. 3 B). In addition, Keele et al. (2008) have recently shown that the transmitted/founder virus almost always utilizes CCR5 and not CXCR4. The anti-phospholipid antibodies studied were as potent at inhibiting the infectivity of R5 but not X4 transmitted/founder viruses as they were against chronic HIV-1 strains (Tables III and IV). It will be of interest to dissect the triggering mechanisms of CCR5 versus CXCR4 inhibition chemokines to understand how to differentially trigger both types of anti–HIV-1 innate molecules. Before the use of an intervention based on the findings of this study in human subjects, it will also be important to determine the potential for a shift from R5 to X4 infection in the presence of anti-phospholipid antibody-mediated anti–HIV-1 activity.
That the anti-phospholipid mAbs induced monocyte polykaryon formation in vitro was an indication of the stimulatory capacity of the mAbs for peripheral blood monocytes. In addition to induction of R5 chemokines, lower levels of TNF, IL-6, IL-4, and IFN-γ are induced in vitro. IFN-γ and IL-4 have been reported to induce polykaryon formation (McNally and Anderson, 1995), and it is likely that these cytokines may be the mediators of this in vitro phenomenon. The observation of Langerin+ polykaryons in these cultures suggested that lipid antibodies may also act on dendritic cells as well as peripheral blood monocytes in culture (Fig. S6 I), although we have not studied this exhaustively.
There are prior studies of antibodies from autoimmune disease patients that have shown cross-reactivity with HIV-1 antigens (Douvas and Takehana, 1994; Scherl et al., 2006) and even descriptions of in vitro neutralization by such antibodies (Douvas et al., 1996). Those antibodies have reacted with HIV-1 Env proteins and have not been targeted at host cell or virion lipids. Thus, the antibodies described in this paper represent a novel group of polyspecific antibodies worthy of further investigation to determine their potential role in protecting subjects with these antibodies from R5 HIV-1 infection. The ability of such antibodies to stimulate similar effects in vivo has yet to be determined. In separate studies, plasma was obtained from patients with autoimmune diseases and tested for anti-phospholipid antibody activity and for the presence of β-chemokines with no observed correlation between their levels (unpublished data). In another study, patients infected with HIV-1 and coinfected with syphilis were studied, and it was found that the presence of RPR-positive syphilis, and therefore the presence of anti-cardiolipin antibodies, was associated with greater HIV-1 inhibition in the PBMC assay compared with HIV-1–negative patients with syphilis infection and compared with HIV-1 patients without syphilis (unpublished data). Whether this anti–HIV-1 activity is a result of the same mechanism as that of the mAbs in this study has yet to be determined.
The development of new therapies for the prevention of HIV-1 has suffered major setbacks in efficacy trials of vaccines (Sekaly, 2008) and microbicides (van de Wijgert and Shattock, 2007). In HIV-1 vaccine development, recent studies have demonstrated the extremely early destruction of the immune system and early induction of immunosuppression and apoptosis (Gasper-Smith et al., 2008; Tomaras et al., 2008; Levesque et al., 2009; Stacey et al., 2009), signaling the need for a vaccine that can prime for a very early salutary immune response within days of infection. The novel mechanism outlined in this paper of anti-phospholipid antibody modulation of anti–HIV-1 chemokines accounts for the selective activity of lipid antibodies in PBMC but not epithelial-based cultures. These results suggest that a vaccine could potentially harness the adaptive immune system to trigger innate immunity for an antiviral response, in effect reversing the traditional path of protection of innate to adaptive immunity.
MATERIALS AND METHODS
Antibodies.
MAbs used in this study and their characteristics are shown in Table I. IS4, B1, and B2 are human mAbs originally derived from patients with primary APS (Zhu et al., 1999; Lin et al., 2007). Recombinant native IS4 and variant forms were produced as described previously (Giles et al., 2006). CL1 and P1 are human mAbs derived from patients with systemic lupus erythematosus (SLE) and secondary APS (Zhu et al., 1999; Lin et al., 2007), and PGN632, PGN634, and PGN635 are recombinant mAbs derived from a peripheral blood antibody library from a normal subject. PGN632 was engineered to optimize binding to phosphatidylserine (PS). The cardiolipin binding motifs present in the CDR H3 sequences of IS4, CL1, PGN632, PGN634, and PGN635 are shown in Table S4. Except for the panel of recombinant IS4 mAbs, each cell line was grown in serum-free media and whole immunoglobulin purified using protein A/G preparative columns. The control antibody Synagis (palivizumab) is a humanized mAb against the F-protein of respiratory syncytial virus (MedImmune). Anti-gp41 membrane proximal external region mAbs 2F5 and 4E10 were purchased from Polymun Scientific Immunbiologische Forschung GmbH. All antibodies used for HIV-1 inhibition assays were tested for the presence of endotoxin using a limulus amoebocyte lysate assay and were found to either have no detectable levels of endotoxin or to have <1 pg endotoxin per 1 mg/ml of antibody stock. Data showing the requirement of the mAbs for the presence of β2GP1 for lipid binding are in Fig. S1 (A and B) or have been previously reported (Lin et al., 2007). MAbs 7B2, F39F, 17b, and A32 were gifts of J. Robinson (Tulane University, New Orleans, LA). Goat anti–human IgG (H+L) was purchased from KPL, Inc. All mAbs were used at saturating concentrations.
Flow cytometry.
Flow cytometry staining was performed as described previously (Levesque et al., 2009). Antibodies were titered to optimal concentrations before use. For staining of PBMC using anti-phospholipid mAbs, the antibodies were labeled using a Zenon anti–human Alexa Fluor 488 kit (Invitrogen) according to the manufacturer’s instructions. Staining of CD4+ T cells was performed using two-step immunofluorescence with an FITC-labeled goat anti–human IgG (H+L) reagent (Jackson ImmunoResearch Laboratories). Samples were acquired using an LSR II (BD) and analyzed using FlowJo software (Tree Star, Inc.).
Recombinant Envs and other reagents.
RPMI-1640, PBS, PBS with 1% BSA, and gentamicin were purchased from Invitrogen. Methanol-free formaldehyde 10% was purchased from Polysciences, Inc. Recombinant gp140CF or gp140CFI group M consensus CON-S, JRFL Env oligomers were produced in recombinant vaccinia viruses as secreted proteins as previously described (Liao et al., 2006). LPS was purchased from Sigma-Aldrich.
Lipid binding by ELISA.
Binding of mAbs to aminophospholipids such as PS and cardiolipin was evaluated by ELISA. Lipids were dissolved in hexane at 5 µg/ml, and 100 µl was added to coat wells of 96-well plates. The hexane solution was allowed to evaporate, leaving lipids bound to the plates. Plates were then blocked for 2 h at 37°C with either 2% (wt/vol) bovine serum albumin (lacking β2GP1) in PBS or 10% (vol/vol) fetal bovine serum in PBS to provide a source of β2GP1. Parallel experiments were performed using recombinant β2GP1 in place of fetal bovine serum. The plates were washed three times with PBS. Antibodies (Table I) made in the same diluent used in blocking of the plates were added to the wells and incubated (2 h at 37°C). The plates were washed three times with PBS. Binding mAbs to the immobilized lipids were detected using a goat anti–human IgG (H&L) conjugated to horseradish peroxidase (incubated 45 min at 37°C). The plates were washed three times with PBS. Tetramethyl benzidine substrate was added for color development, followed by 2 M H2SO4 to stop the substrate reaction, and the plates were read at 450 nm.
Isolation of human PBMC, CD4+ T cells, and monocytes.
Peripheral blood and leukophereses were obtained from healthy donors under clinical protocols approved by the Duke University Institutional Review Board. Additional PBMCs were obtained as discarded buffy coats from the American Red Cross. PBMCs were isolated using Hypaque-Ficoll density gradients by standard techniques. Cells were negatively selected for peripheral blood CD4+ T cells or monocytes using an autoMACS Pro Separator (Miltenyi Biotec). Monocytes were also isolated by elutriation of leukopheresis product and repurification using the autoMACS system if needed. Resulting cell preparations were analyzed by staining with CD14, CD20, CD3, CD4, and CD8 antibodies and analyzed on either an LSR II or a Guava EasyCyte Mini-SSC system (Millipore). All CD4+ T cell preparations were ≥95% CD3+CD4+ and monocyte preparations were ≥95% CD14+.
SPR.
Binding of mAbs to lipids, virions, and HIV-1 Env proteins was studied using SPR. SPR studies were performed using standard techniques on a Biacore 3000 (GE Healthcare) as previously described (Alam et al., 2007, 2008).
Virus capture assay.
Virus capture assays were performed by a modification of a published method (Poignard et al., 2003). In brief, 96-well ELISA plates were coated overnight at 4°C with mAbs at 5 µg/ml in 0.1 M sodium bicarbonate, pH 9.6. The plates were washed twice with PBS containing 0.1% Tween-20 and blocked for 1 h at room temperature with 150 µl/well PBS containing 4% whey protein. The plates were washed twice with PBS containing 0.1% Tween-20 and once with plain PBS. Pseudovirus B.BG1168 or B.SF162LS (100 µl at 2–5 × 105 TCID50/ml) was added to the wells alone or after preincubation for 1 h with soluble CD4 at 50 µg/ml. The wells were washed three times with PBS and then harvested using 100 µl/well PBS containing 0.5% Triton X-100. Captured virus was measured using p24 ELISA (Abbott Laboratories).
Neutralization assay in TZM-bl cells.
Neutralizing antibody assays in TZM-bl cells were performed as described previously (Montefiori, 2005). Antibodies were tested starting at a 50 µg/ml final concentration and titered using serial threefold dilutions. Pseudoviruses were added to the antibody dilutions at a predetermined titer to produce measurable infection and incubated for 1 h. TZM-bl cells were added and incubated for 48 h before lysis, after which supernatant was measured for firefly luciferase activity by a luminometer. The data were calculated as a reduction in luminescence compared with control wells and reported as mAb IC50 in µg/ml (Montefiori, 2005). For some experiments, PBMCs or purified monocytes were stimulated by antibodies as described in Neutralization assay in PBMCs, and the conditioned culture supernatants were harvested and then added to TZM-bl cell cultures for detection of the inhibitory activity of triggered supernatants. All Env-pseudotyped viruses were prepared in 293T cells and titrated in TZM-bl cells as previously described (Montefiori, 2005).
Antibody inhibition of HIV-1 induced syncytium formation.
Syncytium inhibition assays were performed using 2,2′-dipyridyl disulfide (Aldrithiol-2) inactivated virions (gift from L. Arthur and J. Lifson, Frederick Research Cancer Facility, Frederick, MD). Antibody prepared in serial dilution was incubated with inactivated virions for 1 h at 37°C. SUP-T1 cells, grown in 10% FBS in RPMI 1640 with 50 µg/ml gentamicin, were added to the antibody-virus mixture and incubated for 16 h at 37°C in 5% CO2. Syncytia were imaged using inverted phase-contrast microscopy and counted. Titers were expressed as the concentration of antibody that inhibited 90% of syncytium formation compared with wells containing no antibody.
Neutralization assay in PBMCs.
PBMC infectivity inhibition assays were performed using primary HIV-1 isolates or SHIV viruses to infect PBMC with infection detected using p24 ELISA as previously described (Pilgrim et al., 1997). In brief, cryopreserved human PBMCs were thawed and activated in culture for 1 d in IL-2 growth medium (RPMI 1640 with 2 mM L-glutamine, 25 mM Hepes, 20% heat-inactivated fetal bovine serum, 5% IL-2, and 50 µg/ml gentamicin) containing PHA-P at 5 µg/ml. Cells were then washed and added to U-bottom wells containing antibody or serum dilutions as appropriate and incubated for 1 h before adding HIV or SHIV isolates at an appropriate dilution. After 24 h, the cells were washed four times with IL-2 growth medium and then incubated for a further 24–96 h. 25 µl of media was removed and incubated with 225 µl 0.5% Triton X-100 and then assayed by p24 ELISA. Data were calculated as a reduction of p24 production compared with control infected wells and expressed as mAb IC80 in micrograms per milliliter. Assay stocks of virus were grown and titrated in PBMC.
In some experiments, PBMC assays were conducted with replication-competent Renilla luciferase reporter viruses engineered to express transmitted and chronic env gene sequences of choice in cis in an isogenic backbone in which all other viral proteins are expressed and in which the reporter gene is genetically stable. The construction and utility of these reagents have been described elsewhere (Ochsenbauer and Kappes, 2009). In brief, the reporter viruses included in this study (collectively named NL-LucR.T2A-ENV.ecto in which ENV is replaced by the designation of the inserted env sequences, respectively) express the respective ectodomains (i.e., all of gp120 and the ectodomain and membrane-spanning domain of gp41) of the Env proteins listed in Table IV. The cytoplasmic domain of gp41 is derived from NL4-3, thereby avoiding chimerisms in tat, rev, and vpu. PBMCs were infected essentially as described in the previous paragraph with viruses produced in PBMC; however, input virus was not washed out after 24 h, and cells were lysed 4 d after infection. The level of infection in each sample was determined by measuring virally encoded Renilla luciferase activity in cell lysates on a luminometer. Data were calculated as a reduction of relative light units compared with control infected wells and expressed as mAb IC80 in micrograms per milliliters. Assay stocks of virus were grown and titrated in PBMC.
Studies of mAbs preabsorbed with lipids were performed with antibody stocks incubated with 2 mM cardiolipin, 2 mM DOPE, or PBS at 37°C for 2 h or overnight, after which the mixture was assayed as described in the previous paragraph. Time course studies were performed by adding mAb at 0, 24, 48, or 72 h. In these experiments, antibody was reintroduced after each wash step so that a constant concentration of antibody was present throughout the assay.
ADCVI assays.
ADCVI antibody activity was measured essentially as described previously (Forthal et al., 2001; Hessell et al., 2007). In brief, target cells were prepared by infecting CEM.NKr.CCR5 cells (National Institutes of Health AIDS Research and Reference Reagent Program) with the R5 HIV-1 clinical isolate 92US657 at an MOI of 0.05 for 24 h. The target cells were washed and incubated with mAbs at indicated final concentrations and with fresh PBMC effector cells from normal healthy donors (effector/target ratio of 10:1). After 7 d, supernatant fluid from duplicate wells was assayed for p24 by ELISA (ZeptoMetrix Corporation). Percentage of virus inhibition was calculated by referencing the p24 from controls wells containing effector cells and isotype-matched mAb at corresponding concentrations.
Induction of polykaryon formation in peripheral blood monocytes and of lymphoblast formation in CD4+ T cells.
Human peripheral blood monocytes (≥95% CD14+) or CD4+ T cells (≥95% CD4+) were brought to a final concentration of 106 cells/ml and placed into 6-well plates or 8-chamber slides and grown at 37°C in 5% CO2 in IL-2 growth media containing 10 µg/ml of antibody or 10 µg/ml of LPS at final concentration. Cells were examined during culture using inverted phase-contrast microscopy. At 4–7 d after the start of culture, chamber slides were cleared of media and Wright-giemsa stained for visual microscopy. Cultures in 6-well plates were harvested by pipetting and cell scraping and cells deposited on slides using Cytospin funnels (Thermo Fisher Scientific) before Wright-giemsa staining or indirect immunohistochemistry. For immunohistochemistry, slides were treated when required for antigen retrieval using standard techniques (Maeda et al., 2002) and stained with saturating amounts of the monoclonal or polyclonal antibodies against Langerin, CD11c (Leica), and CD68 (Dako). Secondary stains were with horseradish peroxidase–labeled polyclonal IgG (H+L) against the species of the primary antibody followed by development with 3,3′-diaminobenzidine in the presence of hydrogen peroxide. Photomicrographs were acquired on a microscope (Vanox AH-3; Olympus) outfitted with a digital camera (D-70; Olympus) and using DP Controller & Manager software (Olympus). Images were acquired at a 60× magnification (Fig. S6, A–F and K–R) using a Dplan Apo 60–0.90 NA objective lens (Olympus) or at a 100× magnification (Fig. S6, G–J) using an SPlan 100–1.25 NA oil lens (Olympus). Images were not manipulated after acquisition other than by cropping and the addition of 10-µm scale bars.
Cellular proliferation by 3H-thymidine incorporation and cell counts.
Intact PBMC, purified monocytes, and purified CD4+ T cells were incubated in the presence of polyclonal stimulation (PHA or pokeweed mitogen) or in the presence of varying concentrations of mAbs as indicated (Fig. S5 A). Cellular proliferation was determined by the incorporation of 3H-thymidine as previously described (Haynes and Fauci, 1978). In parallel experiments, CD4+ T cells were incubated in the presence of 10 µg/ml of mAbs and were assayed by cell counting and viability measurement using an EasyCyte cell counter (Millipore).
Multiplex chemokine assays.
Plasma samples and culture supernatants were analyzed for soluble levels of MIP-1α, MIP-1β, Eotaxin, RANTES, MCP-1, and SDF-1 on the Bio-Plex Xmap system (Bio-Rad Laboratories) using luminex bead-based assays. MIP-1α, MIP-1β, Eotaxin, RANTES, and MCP-1 levels (in picograms per milliliter) were determined with a commercially available kit (Invitrogen) as a custom five-plex assay as per the manufacturer’s instructions. SDF-1 levels (in picograms per milliliter) were determined with a commercially available kit (Bio-Rad Laboratories) as a single analyte assay as per the manufacturer’s instructions. Four-point logarithmic curve fits were performed for both assays on provided standard curves (expected concentration vs. median fluorescence intensity per bead set) using the Bio-Plex system manager software (Bio-Rad Laboratories). The assay’s sensitivities were 5 (Eotaxin), 10 (MIP-1α, MIP-1β, and MCP-1), and 15 pg/ml (RANTES and SDF-1). Reported final results were corrected for any required initial sample dilution in either assay.
Inhibition of anti-phospholipid antibody effect by chemokine blocking antibodies.
Neutralizing anti–MIP-1α, MIP-1β, and RANTES polyclonal antibodies were purchased from R&D Systems. Serial dilutions of the individual anti-chemokine antibodies, as well as a combination of the three antibodies or a negative control mAb P3X63/Ag8, were added to triplicate PBMC cultures (105 cells/well) in 96-well plates. Infectious virus B.6536 was added in the presence of anti-lipid mAb CL1 or negative control mAb 7B2 at subsaturating concentration (3 µg/ml) or in the absence of antibody. At day 5 after infection, 50 µl of culture supernatant from each well was assayed for HIV-1 p24 production as described in Neutralization assay in PBMCs. HIV-1 p24 production from PBMC cultures infected in the absence of anti-phospholipid mAb served as a positive control. Data were calculated as the mean ± SEM of the triplicates for each experimental group.
Online supplemental material.
Fig. S1 shows mAb binding to lipids in the presence and absence of β2GP1. Fig. S2 shows variation in PGN632 inhibition of HIV-1 by donor PBMC. Fig. S3 shows lack of virus capture by anti-phospholipid mAbs and that only PGN632 interacts with HIV-1 virions by SPR. Fig. S4 shows binding of anti-phospholipid mAbs to cells by flow cytometry. Fig. S5 shows no induction of either proliferation or cell death by anti-phospholipid mAbs. Fig. S6 shows monocyte polykaryons and lymphocyte blasts after cell culture in the presence of anti-phospholipid mAbs. Fig. S7 shows additional chemokine assay data from cells cultured in the presence of anti-phospholipid mAbs. Table S1 shows pseudovirus assay data. Table S2 shows syncytium assay data. Table S3 shows PBMC assay data with different cell subsets. Table S4 shows CDR H3 sequence data for anti-phospholipid mAbs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091281/DC1.
Acknowledgments
We thank Joshua A. Eudailey, Marietta M. Gustilo, Damon Ogburn, and Julie E. Hohm for expert technical assistance.
This work was supported by a Collaboration for AIDS Vaccine Discovery grant to B.F. Haynes from the Bill and Melinda Gates Foundation; by the Center For HIV/AIDS Vaccine Immunology (CHAVI; grant U19 AI067854); by a Veterans Affairs Merit Review Award to J.C. Kappes; by research resources of the Genetically Defined Microbe and Expression Core of the UAB mucosal HIV and Immunology Center (R24DK64400); and by core facilities of the Birmingham Center for AIDS Research (P30-AI-27767). Multiplex assays were performed in the Immune Reconstitution and Biomarker Analysis Shared Resource (Duke Human Vaccine Institute, Gregory Sempowski), which is housed in the Regional Biocontainment Laboratory at Duke (UC6 AI58607) and partially supported by the Duke Center for Translational Research (P30 AI51445).
Peregrine Pharmaceuticals, Inc and Affitech AS have a commercial interesting the antibodies named PGN632, PGN634, and PGN635. A. Kavlie is an employee of Affitech AS. Authors on this manuscript have the following financial relationships with Peregrine Pharmaceuticals, Inc.: P.P. Chen received royalty payments from Peregrine’s licensing arrangements with UCLA for mAbs (including CL1, IS4, and P1); the Duke Human Vaccine Institute (B.F. Haynes and T.N. Denny) has a sponsored research agreement and Duke authors (B.F. Haynes, H.X. Liao, and M.A. Moody) have submitted a patent; P.E. Thorpe is a consultant, has a sponsored research agreement, and owns Peregrine stock; M. Soares is a consultant; and S.W. King and C. Chang are Peregrine employees and own Peregrine stock. The authors have no additional conflicting financial interests.
Footnotes
Abbreviations used:
- ADCVI
- antibody-dependent cell-mediated virus inhibition
- APS
- anti-phospholipid antibody syndrome
- DOPE
- dioleoylphosphatidylethanolamine
- Env
- envelope
- HIVIG
- hyperimmune HIV immunoglobulin
- IC80
- 80% inhibitory concentration
- PS
- phosphatidylserine
- R5
- CCR5-tropic
- SHIV
- SIV-HIV chimeric virus
- SLE
- systemic lupus erythematosus
- SPR
- surface plasmon resonance
- X4
- CXCR4-tropic
References
- Alam S.M., McAdams M., Boren D., Rak M., Scearce R.M., Gao F., Camacho Z.T., Gewirth D., Kelsoe G., Chen P., Haynes B.F. 2007. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J. Immunol. 178:4424–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S.M., Scearce R.M., Parks R.J., Plonk K., Plonk S.G., Sutherland L.L., Gorny M.K., Zolla-Pazner S., Vanleeuwen S., Moody M.A., et al. 2008. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 82:115–125 10.1128/JVI.00927-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving C.R. 2006. Antibodies to lipids and liposomes: immunology and safety. J. Liposome Res. 16:157–166 10.1080/08982100600848553 [DOI] [PubMed] [Google Scholar]
- Baba T.W., Liska V., Hofmann-Lehmann R., Vlasak J., Xu W., Ayehunie S., Cavacini L.A., Posner M.R., Katinger H., Stiegler G., et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 10.1038/72309 [DOI] [PubMed] [Google Scholar]
- Bernstein H.B., Kinter A.L., Jackson R., Fauci A.S. 2004. Neonatal natural killer cells produce chemokines and suppress HIV replication in vitro. AIDS Res. Hum. Retroviruses. 20:1189–1195 [DOI] [PubMed] [Google Scholar]
- Bevers E.M., Comfurius P., Zwaal R.F. 1983. Changes in membrane phospholipid distribution during platelet activation. Biochim. Biophys. Acta. 736:57–66 10.1016/0005-2736(83)90169-4 [DOI] [PubMed] [Google Scholar]
- Binley J.M., Lybarger E.A., Crooks E.T., Seaman M.S., Gray E., Davis K.L., Decker J.M., Wycuff D., Harris L., Hawkins N., et al. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651–11668 10.1128/JVI.01762-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B.K., Karasavvas N., Beck Z., Matyas G.R., Birx D.L., Polonis V.R., Alving C.R. 2007. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: role of phosphate-binding subsites. J. Virol. 81:2087–2091 10.1128/JVI.02011-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D.R., Stanfield R.L., Wilson I.A. 2005. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. USA. 102:14943–14948 10.1073/pnas.0505126102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. 2009. Vaccine protects against HIV virus. Nature News.. 10.1038/news.2009.947 [DOI] [Google Scholar]
- Douvas A., Takehana Y. 1994. Cross-reactivity between autoimmune anti-U1 snRNP antibodies and neutralizing epitopes of HIV-1 gp120/41. AIDS Res. Hum. Retroviruses. 10:253–262 10.1089/aid.1994.10.253 [DOI] [PubMed] [Google Scholar]
- Douvas A., Takehana Y., Ehresmann G., Chernyovskiy T., Daar E.S. 1996. Neutralization of HIV type 1 infectivity by serum antibodies from a subset of autoimmune patients with mixed connective tissue disease. AIDS Res. Hum. Retroviruses. 12:1509–1517 10.1089/aid.1996.12.1509 [DOI] [PubMed] [Google Scholar]
- Dunlop D.C., Ulrich A., Appelmelk B.J., Burton D.R., Dwek R.A., Zitzmann N., Scanlan C.N. 2008. Antigenic mimicry of the HIV envelope by AIDS-associated pathogens. AIDS. 22:2214–2217 10.1097/QAD.0b013e328314b5df [DOI] [PubMed] [Google Scholar]
- Ferbas J., Giorgi J.V., Amini S., Grovit-Ferbas K., Wiley D.J., Detels R., Plaeger S. 2000. Antigen-specific production of RANTES, macrophage inflammatory protein (MIP)-1α, and MIP-1β in vitro is a correlate of reduced human immunodeficiency virus burden in vivo. J. Infect. Dis. 182:1247–1250 10.1086/315849 [DOI] [PubMed] [Google Scholar]
- Fernández N., Renedo M., García-Rodríguez C., Sánchez Crespo M. 2002. Activation of monocytic cells through Fc γ receptors induces the expression of macrophage-inflammatory protein (MIP)-1 α, MIP-1 β, and RANTES. J. Immunol. 169:3321–3328 [DOI] [PubMed] [Google Scholar]
- Forthal D.N., Landucci G., Daar E.S. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75:6953–6961 10.1128/JVI.75.15.6953-6961.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal D.N., Landucci G., Phan T.B., Becerra J. 2005. Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1. J. Virol. 79:2042–2049 10.1128/JVI.79.4.2042-2049.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasper-Smith N., Crossman D.M., Whitesides J.F., Mensali N., Ottinger J.S., Plonk S.G., Moody M.A., Ferrari G., Weinhold K.J., Miller S.E., et al. 2008. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J. Virol. 82:7700–7710 10.1128/JVI.00605-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles I., Lambrianides N., Pattni N., Faulkes D., Latchman D., Chen P., Pierangeli S., Isenberg D., Rahman A. 2006. Arginine residues are important in determining the binding of human monoclonal antiphospholipid antibodies to clinically relevant antigens. J. Immunol. 177:1729–1736 [DOI] [PubMed] [Google Scholar]
- Haynes B.F., Fauci A.S. 1978. Activation of human B lymphocytes. VI. Immunoregulation of antibody production by mitogen-induced and naturally occurring cells in normal individuals. Cell. Immunol. 36:294–302 10.1016/0008-8749(78)90273-3 [DOI] [PubMed] [Google Scholar]
- Haynes B.F., Fleming J., St Clair E.W., Katinger H., Stiegler G., Kunert R., Robinson J., Scearce R.M., Plonk K., Staats H.F., et al. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 308:1906–1908 10.1126/science.1111781 [DOI] [PubMed] [Google Scholar]
- Hessell A.J., Hangartner L., Hunter M., Havenith C.E., Beurskens F.J., Bakker J.M., Lanigan C.M., Landucci G., Forthal D.N., Parren P.W., et al. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 449:101–104 10.1038/nature06106 [DOI] [PubMed] [Google Scholar]
- Ioannou Y., Giles I., Lambrianides A., Richardson C., Pearl L.H., Latchman D.S., Isenberg D.A., Rahman A. 2006. A novel expression system of domain I of human beta2 glycoprotein I in Escherichia coli. BMC Biotechnol. 6:8 10.1186/1472-6750-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou Y., Pericleous C., Giles I., Latchman D.S., Isenberg D.A., Rahman A. 2007. Binding of antiphospholipid antibodies to discontinuous epitopes on domain I of human β(2)-glycoprotein I: mutation studies including residues R39 to R43. Arthritis Rheum. 56:280–290 10.1002/art.22306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson G.M., Reddel S., Victoria E.J., Cockerill K.A., Wang Y.X., Marti-Renom M.A., Sali A., Marquis D.M., Krilis S.A., Linnik M.D. 2002. Use of single point mutations in domain I of β2-glycoprotein I to determine fine antigenic specificity of antiphospholipid autoantibodies. J. Immunol. 169:7097–7103 [DOI] [PubMed] [Google Scholar]
- Keele B.F., Giorgi E.E., Salazar-Gonzalez J.F., Decker J.M., Pham K.T., Salazar M.G., Sun C., Grayson T., Wang S., Li H., et al. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA. 105:7552–7557 10.1073/pnas.0802203105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter A., Catanzaro A., Monaco J., Ruiz M., Justement J., Moir S., Arthos J., Oliva A., Ehler L., Mizell S., et al. 1998. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4(+) T cells: role of signal transduction. Proc. Natl. Acad. Sci. USA. 95:11880–11885 10.1073/pnas.95.20.11880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M.C., Moody M.A., Hwang K.K., Marshall D.J., Whitesides J.F., Amos J.D., Gurley T.C., Allgood S., Haynes B.B., Vandergrift N.A., et al. 2009. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 6:e1000107 10.1371/journal.pmed.1000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Svehla K., Louder M.K., Wycuff D., Phogat S., Tang M., Migueles S.A., Wu X., Phogat A., Shaw G.M., et al. 2009. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J. Virol. 83:1045–1059 10.1128/JVI.01992-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H.X., Sutherland L.L., Xia S.M., Brock M.E., Scearce R.M., Vanleeuwen S., Alam S.M., McAdams M., Weaver E.A., Camacho Z., et al. 2006. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 353:268–282 10.1016/j.virol.2006.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.-S., Chen P.-C., Yang C.-D., Cho E., Hahn B.H., Grossman J., Hwang K.-K., Chen P.P. 2007. Some antiphospholipid antibodies recognize conformational epitopes shared by β2-glycoprotein I and the homologous catalytic domains of several serine proteases. Arthritis Rheum. 56:1638–1647 10.1002/art.22522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Matsuda M., Suzuki H., Saitoh H.A. 2002. Immunohistochemical recognition of human follicular dendritic cells (FDCs) in routinely processed paraffin sections. J. Histochem. Cytochem. 50:1475–1486 [DOI] [PubMed] [Google Scholar]
- Mascola J.R., Stiegler G., VanCott T.C., Katinger H., Carpenter C.B., Hanson C.E., Beary H., Hayes D., Frankel S.S., Birx D.L., Lewis M.G. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 10.1038/72318 [DOI] [PubMed] [Google Scholar]
- Mascola J.R., D’Souza P., Gilbert P., Hahn B.H., Haigwood N.L., Morris L., Petropoulos C.J., Polonis V.R., Sarzotti M., Montefiori D.C. 2005a. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 79:10103–10107 10.1128/JVI.79.16.10103-10107.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J.R., D’Souza P., Gilbert P., Hahn B.H., Haigwood N.L., Morris L., Petropoulos C.J., Polonis V.R., Sarzotti M., Montefiori D.C. 2005b. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 79:10103–10107 10.1128/JVI.79.16.10103-10107.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L.J., Lambrianides A., Haley J.D., Manson J.J., Latchman D.S., Isenberg D.A., Rahman A. 2005. Stable expression of a recombinant human antinucleosome antibody to investigate relationships between antibody sequence, binding properties, and pathogenicity. Arthritis Res. Ther. 7:R971–R983 10.1186/ar1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally A.K., Anderson J.M. 1995. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am. J. Pathol. 147:1487–1499 [PMC free article] [PubMed] [Google Scholar]
- Montefiori D.C. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr. Protoc. Immunol. 12:Unit 12.11. [DOI] [PubMed] [Google Scholar]
- Muster T., Steindl F., Purtscher M., Trkola A., Klima A., Himmler G., Rüker F., Katinger H. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbauer C., Kappes J.C. 2009. New virologic reagents for neutralizing antibody assays. Curr Opin HIV AIDS. 4:418–425 10.1086/508748 [DOI] [PubMed] [Google Scholar]
- Pilgrim A.K., Pantaleo G., Cohen O.J., Fink L.M., Zhou J.Y., Zhou J.T., Bolognesi D.P., Fauci A.S., Montefiori D.C. 1997. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 176:924–932 10.1086/516508 [DOI] [PubMed] [Google Scholar]
- Pitisuttithum P., Gilbert P., Gurwith M., Heyward W., Martin M., van Griensven F., Hu D., Tappero J.W., Choopanya K.; Bangkok Vaccine Evaluation Group 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661–1671 10.1086/508748 [DOI] [PubMed] [Google Scholar]
- Poignard P., Moulard M., Golez E., Vivona V., Franti M., Venturini S., Wang M., Parren P.W., Burton D.R. 2003. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J. Virol. 77:353–365 10.1128/JVI.77.1.353-365.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radstake T.R., van der Voort R., ten Brummelhuis M., de Waal Malefijt M., Looman M., Figdor C.G., van den Berg W.B., Barrera P., Adema G.J. 2005. Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis, and regulation by Fc gamma receptors. Ann. Rheum. Dis. 64:359–367 10.1136/ard.2003.017566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran S., He J., Huang X., Soares M., Scothorn D., Thorpe P.E. 2005. Antitumor effects of a monoclonal antibody that binds anionic phospholipids on the surface of tumor blood vessels in mice. Clin. Cancer Res. 11:1551–1562 10.1158/1078-0432.CCR-04-1645 [DOI] [PubMed] [Google Scholar]
- Roben P., Moore J.P., Thali M., Sodroski J., Barbas C.F., III, Burton D.R. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R.W., Venturi M., Schiffner L., Kalyanaraman R., Katinger H., Lloyd K.O., Kwong P.D., Moore J.P. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293–7305 10.1128/JVI.76.14.7293-7305.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather D.N., Armann J., Ching L.K., Mavrantoni A., Sellhorn G., Caldwell Z., Yu X., Wood B., Self S., Kalams S., Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 10.1128/JVI.02036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherl M., Posch U., Obermoser G., Ammann C., Sepp N., Ulmer H., Dierich M.P., Stoiber H., Falkensammer B. 2006. Targeting human immunodeficiency virus type 1 with antibodies derived from patients with connective tissue disease. Lupus. 15:865–872 10.1177/0961203306071405 [DOI] [PubMed] [Google Scholar]
- Schlame M., Hostetler K.Y. 1997. Cardiolipin synthase from mammalian mitochondria. Biochim. Biophys. Acta. 1348:207–213 [DOI] [PubMed] [Google Scholar]
- Sekaly R.-P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 205:7–12 10.1084/jem.20072681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Parks R.J., Montefiori D.C., Kirchherr J.L., Keele B.F., Decker J.M., Blattner W.A., Gao F., Weinhold K.J., Hicks C.B., et al. 2009. In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. J. Virol. 83:3617–3625 10.1128/JVI.02631-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M.M., King S.W., Thorpe P.E. 2008. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat. Med. 14:1357–1362 10.1038/nm.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A.R., Norris P.J., Qin L., Haygreen E.A., Taylor E., Heitman J., Lebedeva M., DeCamp A., Li D., Grove D., et al. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 83:3719–3733 10.1128/JVI.01844-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler G., Kunert R., Purtscher M., Wolbank S., Voglauer R., Steindl F., Katinger H. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 17:1757–1765 10.1089/08892220152741450 [DOI] [PubMed] [Google Scholar]
- Tomaras G.D., Yates N.L., Liu P., Qin L., Fouda G.G., Chavez L.L., Decamp A.C., Parks R.J., Ashley V.C., Lucas J.T., et al. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449–12463 10.1128/JVI.01708-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D.S., Lyerly H.K., Weinhold K.J. 1989. Anti-HIV-1 ADCC. AIDS Res. Hum. Retroviruses. 5:557–563 10.1089/aid.1989.5.557 [DOI] [PubMed] [Google Scholar]
- van de Wijgert J.H., Shattock R.J. 2007. Vaginal microbicides: moving ahead after an unexpected setback. AIDS. 21:2369–2376 10.1097/QAD.0b013e3282ef83fd [DOI] [PubMed] [Google Scholar]
- Zhu M., Olee T., Le D.T., Roubey R.A., Hahn B.H., Woods V.L., Jr., Chen P.P. 1999. Characterization of IgG monoclonal anti-cardiolipin/anti-β2GP1 antibodies from two patients with antiphospholipid syndrome reveals three species of antibodies. Br. J. Haematol. 105:102–109 10.1111/j.1365-2141.1999.01292.x [DOI] [PubMed] [Google Scholar]
- Zwick M.B., Labrijn A.F., Wang M., Spenlehauer C., Saphire E.O., Binley J.M., Moore J.P., Stiegler G., Katinger H., Burton D.R., Parren P.W. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892–10905 10.1128/JVI.75.22.10892-10905.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]