Indication
Icatibant [1, 2] is indicated in the EU for the symptomatic treatment of acute attacks in patients with hereditary angioedema (HAE).
Mechanism
HAE is a rare disease (occurring in about 1/10 000 to 1/50 000 people) characterized by an absent or dysfunctional C1 esterase inhibitor molecule (C1INH). C1INH is an important regulator in the metabolic pathways of complement, kinin-kallikrein and coagulation factors. The symptoms of the disease are due to local accumulations of bradykinin caused by excessive activation of the complement and kinin pathways as a result of the C1INH deficiency. Bradykinin, a potent inflammatory mediator, causes vasodilatation, stimulates nociceptors and increases vascular permeability, leading to rapid accumulation of fluid in the interstitial tissue with swelling, pain and redness of the affected tissues. These symptoms are mediated by bradykinin B2 receptors. Attacks can be life threatening when laryngeal oedema occurs.
Icatibant, an orphan drug, is a selective, competitive B2 receptor antagonist.
Icatibant has only been tested as treatment during acute attacks, not as prophylaxis, since it is administered intravenously.
Adverse effects
Almost all subjects in the clinical trials developed self-limiting mild reactions (erythema, swelling) at the injection site. There does not appear to be hypersusceptibility in groups of subjects but the study populations are inevitably small. Other common adverse effects include rash, redness of the skin, dizziness, nasal congestion, GI problems and increased blood creatinine phosphokinase concentrations.
Since bradykinin has potential cardioprotective properties, icatibant could in theory impair cardiac function and decrease coronary blood flow. Patients with heart disease should be carefully monitored.
Co-treatment with ACE inhibitors is a contra-indication for icatibant therapy. Icatibant could decrease the antihypertensive effect of ACE inhibitors, since they exert their vasodilating effect partially via increased bradykinin concentrations.
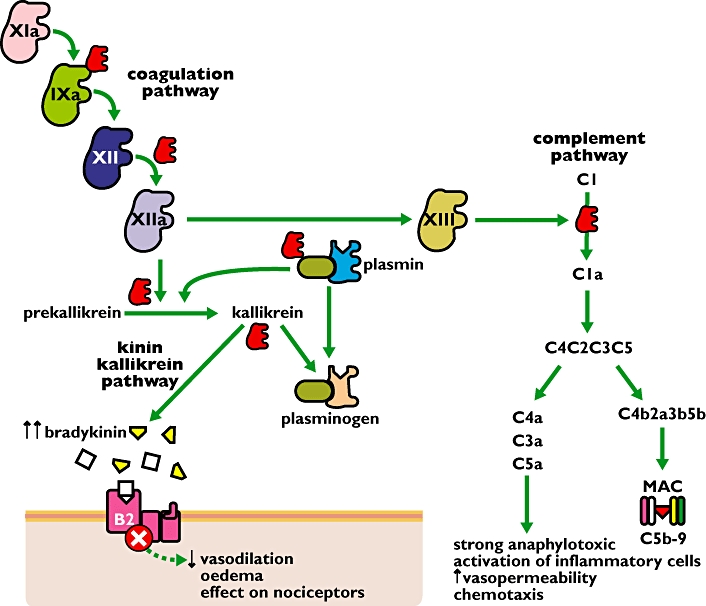
Site of action of icatibant at the bradykinin-2 receptor. In hereditary angioedema (HAE) the C1 esterase inhibitor (C1INH) is deficient. This has consequences for the three metabolic pathways shown. Increased concentrations of bradykinin contribute to the major symptoms of HAE. Icatibant blocks the action of bradykinin and thus relieves the symptoms of vasodilatation, oedema, and pain. Abbreviations: B2: bradykinin-2 receptor; MAC: membrane attack complex; the roman numbers represents the different clotting factors. C1INH ( ); icatibant (□)
); icatibant (□)
Literature
- 1. http://www.emea.europa.eu/humandocs/Humans/EPAR/firazyr/firazyr.htm.
- 2.Bork K, Yasothan U, Kirkpatrick P. Fresh from the pipeline: icatibant. Nat Rev Drug Discov. 2008;7:801–2. [Google Scholar]


