Abstract
AIMS
Low-molecular-weight heparins (LMWHs) are used globally to treat thromboembolic diseases; however, there is much debate on how to prescribe effectively for patients who have renal impairment and/or obesity. We aimed to investigate the strategies used to dose-individualize LMWH therapy.
METHODS
We conducted an online survey of selected hospitals in Australia, New Zealand (NZ), United Kingdom (UK) and the United States (US). Outcome measures included: the percentage of hospitals which recommended that LMWHs were prescribed according to the product label (PL), the percentage of hospitals that dose-individualized LMWHs outside the PL based on renal function, body weight and anti-Xa activity and a summary of methods used to dose-individualize therapy.
RESULTS
A total of 257 surveys were suitable for analysis: 84 (33%) from Australia, 79 (31%) from the UK, 73 (28%) from the US and 21 (8%) from NZ. Formal dosing protocols were used in 207 (81%) hospitals, of which 198 (96%) did not adhere to the PL. Of these 198 hospitals, 175 (87%) preferred to dose-individualize based on renal function, 128 (62%) on body weight and 48 (23%) by monitoring anti-Xa activity. All three of these variables were used in 29 (14%) hospitals, 98 (47%) used two variables and 71 (34%) used only one variable.
CONCLUSIONS
Dose-individualization strategies for LMWHs, which contravene the PL, were present in 96% of surveyed hospitals. Common individualization methods included dose-capping, use of lean body size descriptors to calculate renal function and the starting dose, followed by post dose anti-Xa monitoring.
Keywords: anti-coagulants, low-molecular-weight heparins, obesity, renal disease, survey
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Low-molecular-weight heparins (LMWHs) are effective anti-coagulants for the treatment of thromboembolic diseases.
LMWHs are hydrophilic drugs with clearance related to lean body weight and renal function.
Poor subject outcomes are linked to the inadequate dosing of LMWHs.
WHAT THIS STUDY ADDS
Minimal adherence to the product label occured when dosing LMWHs.
Hospitals preferred to dose-individualize using a variety of methods that included dose-capping, post-dose monitoring of anti-Xa activity and the use of lean body size descriptors to calculate a starting dose and renal function.
Introduction
The low-molecular-weight heparins (LMWHs) are anti-coagulants used in the treatment and prevention of thromboembolic diseases [1, 2]. At therapeutic doses, the LMWHs are appealing alternatives to other anti-coagulants such as unfractionated heparin (UFH) due to their linear pharmacokinetics and more predictable dose–response relationship. Linear pharmacokinetics help facilitate simple fixed or weight based dosing, without the need for plasma anti-Xa (aXa) monitoring, which is reflected in the current product labels (PLs) [3–7].
LMWHs are hydrophilic compounds with clearance (CL) described by a composite of renal elimination and metabolism [8], processes that are proportional to lean body weight (LBW) [9]. Subjects who are dosed using total body weight (TBW) are therefore at risk of supra-therapeutic drug exposure, which can result in excessive inhibition of factor-Xa and adverse events such as bleeding. This is particularly problematic in subjects with renal impairment and/or obesity and has resulted in much uncertainty and debate on how to dose LMWHs in these populations. To add to this dilemma, little effectiveness or adverse outcome data exist for these populations as they were generally excluded from confirmatory clinical trials [10–12]. As specific dosing regimens in these subjects are unknown, clinicians often elect to choose empirical dose strategies in an effort to normalize drug exposure to that of a subject who was typically recruited during the drug development process.
Selecting optimal dosing strategies for subjects with renal impairment and/or obesity is an important issue for clinicians. The number of people worldwide who are overweight or obese has now reached epidemic proportions with approximately 1.6 billion adults now classified as overweight (body mass index ≥ 25 kg m−2) and 400 million obese (body mass index ≥ 30 kg m−2) [13]. In Australia, 62.7% of females and 72.1% of males are now overweight or obese and these figures are matched in the United Kingdom (UK) and the United States (US) [13]. The worldwide prevalence of renal disease is also escalating and mirrors the increase in diabetes, obesity and hypertension [14].
As there appears to be much debate on the most suitable dosing and monitoring strategies for LMWHs, in particular for populations with obesity and/or renal impairment, we aimed to investigate how these subjects were being dosed in contemporary clinical practice. Firstly we aimed to quantify the proportion of hospitals that dose LMWHs according to the PL. Second, we sought to determine the proportion of hospitals that individualize the dose of LMWHs using one or more of either: renal function, body weight, or the post-dose monitoring of aXa-activity. Finally, we explored the specific methods that hospitals use to dose-individualize therapy thus enabling hospitals to compare and critique their own dose strategies against their global counterparts.
Methods
Survey
The survey was designed using a formal, iterative and collaborative process. A draft survey was pre-tested by senior clinical pharmacists at five separate hospitals throughout Australia and after modification, the final survey was uploaded onto a web-based survey tool, Checkbox®[15]. The questions were designed to allow participants to describe their institution's dosing recommendations and monitoring strategies as accurately as possible. Non-judgmental questions were used that avoided any reference to current literature or the PL and participants were instructed to consult with other experts in the hospital to obtain accurate answers to the questions. Participants were asked to choose which of the following LMWHs, enoxaparin, dalteparin or tinzaparin, were most widely used at their hospital and if dosing was guided by a formal protocol. A ‘protocol’ was defined as any written protocol, policy or guideline approved for use within the surveyed hospital. Details about the therapeutic indication and the origin of the protocol, for example local, state or county, were requested. Additional demographics included hospital location and bed capacity. The remainder of the survey was divided into sections that allowed participants to describe dose-individualization strategies, the use of post-dose monitoring of aXa-activity and any contraindications to the LMWHs (not listed in the PL).
Study population
The sampling population was chosen to enable recruitment of experienced practitioners. Directors of Pharmacy or Medical Information (MI) specialists were chosen to help ensure LMWH dosing protocols or practices (if a protocol did not exist), within their hospital, were accurately described. Potential participants were identified using the following methods. In Australia, all Directors of Pharmacy with an e-mail address listed on the Society of Hospital Pharmacists of Australia website [16] were invited to participate via e-mail. In New Zealand (NZ), the NZ HealthCare Pharmacists' Association provided an e-mail list of the Directors of Pharmacy for all hospitals [17]. Due to confidentiality policies in the UK and US, a list of e-mail addresses for Directors of Pharmacy could not be acquired. In the UK, an online directory of MI pharmacists [18] was used to identify suitable participants at hospitals. In the US, two methods were used to identify potential participants. First, MI departments or MI pharmacists with an e-mail address listed in a recent publication [19] were invited to participate. Second, an invitation to participate was placed on the list server CAMIPR (Consortium for the Advancement of Medication Information, Policy and Research) [20], an online discussion forum for MI specialists.
The survey was initially distributed on November 15, 2008, and recruitment and data collection continued until February 28, 2009. A single response was accepted from each hospital. No compensation or remuneration was offered to any survey participants and confidentiality was assured. The study was approved by the Human Research Ethics Committee at the Mater Hospital, Brisbane, Australia.
Data management and analysis
The Checkbox® survey tool [15] stored all responses and data were downloaded into Excel® to facilitate analysis. All graphical analyses were completed using PRISM (GraphPad Prism version 5.00 for Windows, GraphPad software, 2007 San Diego, California, USA). A multi-variable analysis to determine predictors of non-adherence to the PL was planned for this study.
Data were divided for analysis. Firstly, all data were used to determine the proportion of surveyed hospitals that prescribed LMWHs according to the PL and the proportion of hospitals that used a dosing protocol. Second, to gain an objective assessment of dosing strategies, only the data from hospitals with dosing protocols were used to determine the type and number of dose-individualization variables (renal function, body weight or post-dose monitoring of aXa-activity), used at each hospital. The specific methods used by hospitals to dose-individualize were investigated, along with the type of contraindications outside the PL. A contraindication was deemed as an instruction in the protocol that prohibited the use of the LMWH in a patient group or sub-group.
Results
Recruitment and demographics
The number of invitations, exclusions and survey responses from each country are detailed in Table 1. An e-mail address was available for 451 pharmacists and a total of 257 surveys were completed from the following countries: 84 (33%) from Australia, 79 (31%) from the UK, 73 (28%) from the US and 21(8%) from NZ. Five pharmacists refused to participate and six were excluded as the surveys were completed incorrectly.
Table 1.
Response demographics by country*
| Country | Australia | New Zealand | United Kingdom | United States |
|---|---|---|---|---|
| Hospitals identified | 208 | 31 | 205 | 75† |
| E-mail address not available | 41 | 0 | 15 | 12 |
| Hospitals available for survey | 167 | 31 | 190 | 63 |
| Refused to participate | 3 | 0 | 2 | 0 |
| Exclusions | 4 | 0 | 2 | 0 |
| Total responses | 91 (55) | 21 (68) | 83 (44) | 73 (N/A) |
| Total surveys available for analysis | 84 | 21 | 79 | 73 |
Data presented as number or number (%).
Number of departments listed in the publication used to identify participants. N/A = not applicable as a list server was used to recruit participants.
The demographics of the 257 hospitals are shown in Table 2. The greatest response (51%) originated from hospitals with a bed capacity between 100 and 500. A total of 152 (60%) hospitals had a bed capacity less than 500 and 105 (40%) had a bed capacity greater than 500. The most commonly selected LMWH was enoxaparin, which was chosen by 221 (86%) hospitals. Dalteparin was selected by 23 (9%) of hospitals, across all four countries. Tinzaparin was preferred by 13 (5%) hospitals isolated to the UK. The PLs at the time of survey, along with the licensed dosing strategy based on body weight, renal function and post-dose monitoring of aXa-activity for each LMWH are shown in Table 3. The percentage of hospitals, both with and without protocols and the corresponding dose-individualization variables are shown in Table 4. The 248 (96%) hospitals that did not adhere to the PL preferred to dose-individualize, using one or more variables including renal function in 224 (87%), body weight in 154 (60%) and 52 (20%) monitored aXa-activity after dosing.
Table 2.
Hospital and drug demographics by country (n= 257)*
| Country | Australia | New Zealand | United Kingdom | United States | Total |
|---|---|---|---|---|---|
| Total | 84 (33) | 21 (8) | 79 (31) | 73 (28) | 257 |
| Hospital size | |||||
| <100 beds | 11 (13) | 5 (24) | 1 (1) | 5 (7) | 22 (9) |
| 100–500 beds | 54 (64) | 12 (57) | 22 (28) | 42 (57) | 130 (51) |
| 500–1000 beds | 16 (19) | 4 (19) | 42 (53) | 25 (34) | 87 (34) |
| >1000 beds | 3 (4) | 0 | 14 (18) | 1 (1) | 18 (6) |
| Drug type | |||||
| Dalteparin | 2 (2) | 1 (5) | 16 (8) | 4 (5) | 23 (9) |
| Enoxaparin | 82 (98) | 20 (95) | 50 (63) | 69 (95) | 221 (86) |
| Tinzaparin | 0 | 0 | 13 (17) | 0 | 13 (5) |
Data presented as number (%).
Table 3.
Product label (PL) for the subcutaneous administration of LMWHs in the treatment of a thromboembolic disease (PL as of 1 November 2008)
| LMWH | Dalteparin | Enoxaparin | Tinzaparin* |
|---|---|---|---|
| Acute coronary syndromes | 120 IU kg−1 twice a day to a maximum of 10 000 IU twice a day | 1 mg kg−1 twice a day† | Not indicated |
| Venous thromboembolic disease | 100 IU kg−1 twice a day or 200 IU kg−1 daily‡ | 1 mg kg−1 twice a day or 1.5 mg kg−1 daily | 175 IU kg−1 daily |
| Maximum dose | Refer to each indication | NR | NR |
| Individualization based on renal function | NR | 1 mg kg−1 daily if CLCR <30 ml min−1§ | Contraindicated if age >90 years with a CLCR≤60 ml min−1§ |
| Individualization based on age | NR | NR† | Contraindicated if age >90 years with a CLCR≤ 60 ml min−1§ |
| Individualization based on anti-Xa activity | NR | NR | NR |
Tinzaparin is not licensed for use in Australia.
For the treatment of acute ST-segment elevation myocardial infarction in the US for subjects ≥ 75 years of age, the dose is 0.75 mg kg−1 every 12 h. The PL was updated to incorporate these changes in Australia and NZ during the recruitment period. There is no dose reduction in the UK.
Dose approved in all countries except Australia. The maximum dose is 18 000 IU daily.
CLCR= creatinine clearance, assumed to be calculated using the Cockcroft-Gault equation where total body weight is the body size descriptor used [33]. IU, International units; NR, not recommended in the product label.
Table 4.
Dose-individualization variables used outside the product label (PL)*
| LMWH | Dalteparin | Enoxaparin | Tinzaparin | Total |
|---|---|---|---|---|
| All hospitals | 23 (9) | 221 (86) | 13 (5) | 257 |
| Renal function | 21 (91) | 194 (88) | 9 (69) | 224 (87) |
| Weight | 16 (70) | 134 (61) | 4 (31) | 154 (60) |
| Anti-Xa activity | 6 (26) | 44 (20) | 2 (15) | 52 (20) |
| Dose outside PL† | 23 (100) | 215 (97) | 10 (77) | 248 (97) |
| Hospitals with protocols | 21 (10) | 173 (84) | 13 (6) | 207 |
| Renal function | 19 (91) | 147 (85) | 9 (69) | 175 (85) |
| Weight | 13 (62) | 111 (64)) | 4 (31) | 128 (62) |
| Anti-Xa activity | 5 (24) | 41 (24) | 2 (15) | 48 (23) |
| Dose outside PL† | 21 (100) | 167 (97) | 10 (77) | 198 (96) |
Data presented as number (%).
Indicates one or more dose-individualization variables per hospital.
Protocol based dose-individualization
A formal LMWH dosing protocol was used in 207 (81%) of the 257 surveyed hospitals. The majority of protocols, 133 (65%), were formulated within the participant's hospital with the remaining 35% standardized at a broader level that included an even distribution across cities, trusts and states/counties. A total of 111 (54%) of hospitals with a protocol had a bed capacity less than 500 and 96 (46%) had a bed capacity greater than 500.
A total of 198 (96%) hospitals used a protocol that did not adhere to the PL. As this was an overwhelming result, a multi-variable analysis to determine predictors of non-adherence was not attempted. The number of hospitals that dosed outside the PL for each LMWH is shown in Table 4. The dose was individualized using renal function in 175 (85%) hospitals, body weight in 128 (62%) hospitals and post-dose monitoring of aXa-activity in 48 (23%) hospitals. All three variables were used in 29 (14%) hospitals, while 98 (47%) used two variables and 71 (34%) used only one variable. A plot of the percentage of hospitals that used none, one, two or three variables vs. hospital size is shown in Figure 1. This plot shows that there was a greater percentage of larger hospitals that used three variables to dose-individualize when compared with smaller hospitals (25% vs. 2%) which may, in part, be due to these hospitals having better access to aXa assays. Participants from many smaller hospitals indicated that they would monitor aXa-activity if the assay was available. The plot also shows that if only one variable was used to dose-individualize outside the PL, then renal function was the most preferred option. Finally, if aXa-activity was monitored, then it was usually used in combination with dose-individualization based on renal function.
Figure 1.
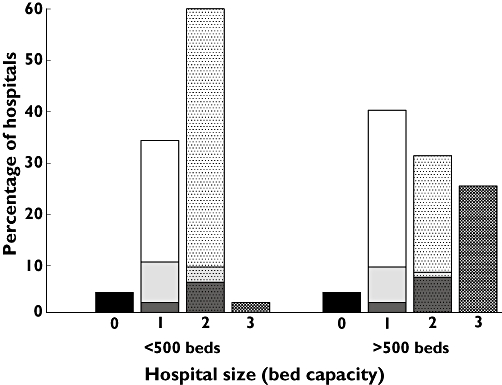
Percentage of hospitals that used none, one, two or three variables to dose-individualize vs. hospital size. Hospital size was dichotomized due to the low number of hospitals with a bed capacity less than 100 and greater that 1000. Column one is sub-divided to indicate which dose-individualization variable is preferred. White box = renal function, light grey box = body weight, dark grey box = anti-Xa activity. Column two is sub-divided to show which ‘combination of variables’ is preferred. White with dot = renal function and body weight, light grey with dot = body weight and anti-Xa activity, dark grey with dot = renal function and anti-Xa activity
Dose-individualization methods
Renal function
A total of 175 (85%) hospitals used a dose-individualization strategy based on renal function that deviated from the PL, including 147 (85%) with enoxaparin, 19 (91%) with dalteparin and 6 (69%) with tinzaparin (Table 4). The common methods to dose-individualize included: a dose reduction at an alternate value of creatinine clearance (CLCR) compared with the PL in 39 (19%) hospitals, the use of UFH instead of a LMWH in 34 (16%) hospitals, the use of a lean body size descriptor (LBSD), such as ideal body weight (IBW) or LBW to calculate CLCR in 133 (64%) hospitals, the use of a linear or graduated dose reduction strategy in 31 (15%) hospitals. Recent literature has demonstrated the benefit of using a LBSD in the Cockcroft-Gault (C-G) equation [21] and this survey showed that using body composition metrics other than TBW was a widespread practice (Figure 2). IBW was the most popular (67% of hospitals), despite its limitations as a body size descriptor in the obese population [22], and it was the most commonly used for all three LMWHs. The equations used to calculate IBW or LBW were not surveyed, although the readers are referred to Green et al.[23] for a summary of commonly used equations. Of interest was that the modification of Diet in Renal Disease (MDRD) equation was used to estimate renal function in 22 (11%) hospitals. However, this equation was not assessed as a dose-individualization method in this survey as its use in dose-individualizing LMWHs is controversial [24–26].
Figure 2.
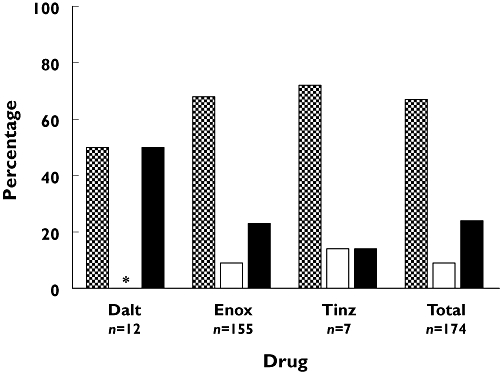
Percentage of body size descriptors used in the Cockcroft-Gault equation [33] for calculating renal function. Dalt, dalteparin; Enox, enoxaparin; Tinz, tinzaparin; IBW, ideal body weight; LBW, lean body weight; TBW, total body weight. n, the number of hospitals that calculate CLCR using the Cockcroft-Gault equation. * Refers to no result. IBW ( ); LBW (
); LBW ( ); TBW (
); TBW ( )
)
Body weight
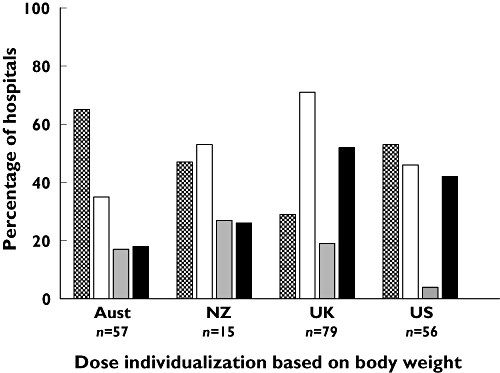
A total of 128 (62%) hospitals dose-individualized outside the PL based on body weight: 111 (64%) that use enoxaparin, 13 (62%) that use dalteparin and 4 (31%) that use tinzaparin. The most common methods of weight based dose-individualization were dose-capping at a pre-determined TBW, which occurred in 96 (46%) hospitals and the use a body size descriptor other than TBW to calculate a weight based dose, which occurred in 32 (16%) hospitals. Figure 3 shows the distribution of this practice across the four surveyed countries. A large percentage of Australian and US hospitals dose capped LMWHs, whereas the UK predominantly followed the PL. Although NZ contributed the least number of hospitals, it had the lowest percentage of compliance with the PL, which was linked to the high use of LBSD for dose-individualization. Only a small percentage of hospitals in the US chose to use this method. Dose capping occurred at a variety of body weights, as represented in Figure 4, which shows the cumulative percentage of hospitals that dose cap vs. TBW. Approximately 15% of hospitals capped the dose before 100 kg and 40% before 150 kg.
Figure 3.
Percentage of hospitals per country that dose-individualize based on body weight. n, the number of hospitals that use protocols per country. Aust, Australia; NZ, New Zealand; UK, United Kingdom and US, United States. Hospitals that cap the dose ( ); Hospitals that do not cap the dose (
); Hospitals that do not cap the dose ( ); Hospitals that do not cap the dose but use a lean body weight descriptor for dose calculation (
); Hospitals that do not cap the dose but use a lean body weight descriptor for dose calculation ( ); Hospitals that use the PL (
); Hospitals that use the PL ( )
)
Figure 4.
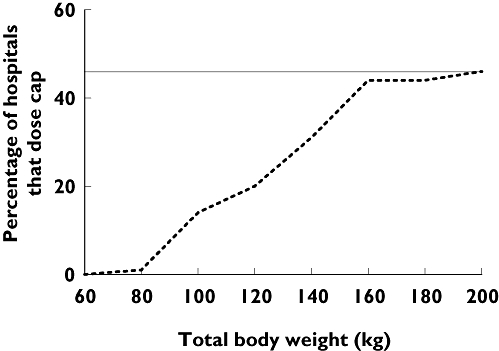
Cumulative percentage of hospitals that dose cap vs. total body weight. The grey line at 46% signifies the total percentage of hospitals that dose cap
Anti-Xa activity monitoring
Forty-eight (23%) hospitals monitored aXa-activity after a dose of a LMWH, despite no dose recommendation based on aXa-activity in the PL. Of these hospitals, 43 (90%) used peak aXa-activity to guide dose-individualization and 41 (85%) used a target range of 500–1000 IU l−1. The predominant method used to dose-individualize was an empirical estimation which occurred in 31 (65%) hospitals, although 10 (21%) hospitals used nomograms or computer assisted methods to predict future doses. The presence of renal impairment was the most popular reason to monitor aXa-activity (Figure 1).
Contraindications to LMWHs
Only tinzaparin has a contraindication in its PL (Table 3). Despite this, 19 (9%) and 78 (38%) hospitals had a contraindication in their protocol based on body weight and renal function, respectively. The most common contraindications were a TBW > 150 kg which occurred in 12 (6%) hospitals and a CLCR < 30 ml min−1 (calculated using TBW, IBW or LBW) in 43 (21%) hospitals. Enoxaparin, dalteparin and tinzaparin were contraindicated if the CLCR was <30 ml min−1 in 31 (18%), 7 (33%) and 6 (46%), respectively, despite no contraindication in the PL.
Discussion
The prevalence of protocols for LMWHs has been previously explored [27, 28]. However, we are not aware of any studies that have examined the diversity of dose-individualization strategies. To do this we conducted an international survey of hospitals to determine non-adherence to the PL based on three variables: renal function, body-weight and post-dose monitoring of aXa-activity. We then investigated specific methods used to dose-individualize therapy, in particular for subjects with renal impairment and obesity who are at a high risk of bleeding events to LMWHs. The survey demonstrated that 96% of surveyed hospitals contravene the PL, preferring to dose-individualize with methods such as dose-capping or the use of a LBSD to calculate renal function and starting doses. The authors had originally intended to conduct a multi-variable analysis of the data to determine predictors of non-adherence to the PL. However, the overpowering result meant that an analysis was not warranted.
LMWHs are effective anti-coagulants in the treatment of thromboembolic diseases [1, 2]. They have a simple fixed or weight-based dose regimen, can be administered once or twice daily by subcutaneous injection and can be used in an ambulatory setting, where they are more effective than UFH [29, 30]. Despite these advantages, there was a clear trend to use methods of monitoring and dose-individualization outside the PL, particularly the hospitals that used dalteparin and enoxaparin, where non-adherence rates were 100% and 97%, respectively.
Dose-individualization methods
Renal function
All LMWHs are extensively eliminated by the kidneys resulting in a decrease in CL in renal impairment [31]. Green et al.[8] demonstrated that CL of enoxaparin was best described as a function of both renal (70%) and metabolic elimination (30%) and that a linear dose reduction of 0.1 mg for every 10 ml min−1 loss in renal function, from 80 ml min−1, would best maintain aXa-exposure within the therapeutic range of 500–1000 IU l−1. No LMWH has a linear dose reduction in their PL. The dose reduction recommended at a CLCR of 30 ml min−1 for enoxaparin is either 33% (1.5 mg kg−1 daily to 1.0 mg kg−1 daily) or 50% (1.0 mg kg−1 twice daily to 1.0 mg kg−1 daily) and no dose reduction exists for dalteparin or tinzaparin. A linear or graduated dose reduction is a scientifically and physiologically superior strategy [8] and as this survey showed, is a strategy that is now being adopted worldwide. Figure 5 shows the mean dose of enoxaparin (mg kg−1) vs. renal function in surveyed hospitals, and indicates that on average, hospitals reduced the dose as renal function decreased. It is of interest that the dose reduction began at a CLCR of approximately 60 ml min−1.
Figure 5.

Mean dose (mg kg−1 of enoxaparin) and 95% confidence intervals vs. creatinine clearance (CLCR) for surveyed hospitals (black solid and broken lines). Grey line is the dose according to the product label
Two recent studies have demonstrated an increased risk of bleeding in subjects with renal impairment who were administered enoxaparin. Fox et al.[32], in a retrospective analysis from the ExTRACT-TIMI 25 trial of enoxaparin, found that as renal function declined, a progressive increase in bleeding was observed. Similarly, a meta-analysis by Lim et al.[31], involving all LMWHs, found that enoxaparin was associated with a two- to three-fold increase in the risk of bleeding in subjects with a CLCR < 30 ml min−1. While there are limited bleeding data for tinzaparin and dalteparin, both are renally eliminated and clinical concerns exist as 9 (91%) and 9 (69%) hospitals dose reduced dalteparin and tinzaparin in renal impairment, respectively, in this study.
The method predominantly used by hospitals to dose-individualize in renal impairment was through the use of a LBSD in the C-G equation when estimating renal function. Of the surveyed hospitals, 133 (64%) have adopted this practice and IBW was the most popular body size descriptor used (Figure 2). The C-G equation [33], like other predictive formulae, can furnish erroneously high estimates of glomerular filtration rate (GFR) in obese subjects. Creatinine is a by-product of muscle metabolism and the excess body mass seen in the obese is mostly adipose tissue as opposed to muscle. Therefore, when no individualization is made for the increase in body mass, such as standardization with LBW, CLCR will be routinely overestimated; this can equate to significant clinical ramifications on LMWH dosing. A small study recently confirmed that the estimation of GFR, when normalized using LBW in the C-G equation, was not different between obese and normal weight subjects [21].
Body weight
Currently, all LMWHs are approved with a TBW based dose (mg kg−1 or IU kg−1). Approval based on this metric fails to appreciate the importance of body composition on the CL of LMWHs. LBW is a more accurate body size descriptor than TBW in representing true drug CL in subjects of varying body compositions [9, 22]. Adipose tissue has little metabolic activity, whereas LBW is correlated with 99% of the body's metabolic processes, which includes drug CL [34]. Dosing regimens adjusted by TBW rather than LBW are more likely to result in supra-therapeutic drug exposure, in particular in the obese population, who have an increase in adipose mass with an accompanying but significantly smaller increase in muscle mass. Therefore, obese subjects prescribed LMWHs are at risk of supra-therapeutic aXa-exposures and adverse bleeding events when dosed using TBW. A total of 32 (16%) hospitals chose to dose obese subjects individually using a LBSD such as IBW or LBW. New equations for LBW [35] have recently been developed that are superior to old methods at normalizing drug exposure across a diverse range of body compositions. The other method used to reduce the risk of toxic exposure is an arbitrary dose reduction or a dose ‘cap’. Despite never being formally evaluated, these methods are common as 128 (62%) hospitals contravened the PL, preferring to either dose cap or use a LBSD to calculate a starting dose (Figure 3).
Age
Although not assessed in this survey, age is also an important variable to consider as it is an independent predictor of bleeding and the risk of bleeding increases with age in both the presence and absence of an anti-coagulant [36]. Age is proportional to renal function (refer C-G equation [33]), and therefore it is an indirect determinant of LMWH CL. Recent studies with enoxaparin have observed an increase in the risk of bleeding in subjects ≥ 75 years of age which has led to the addition of age into the dose calculation for enoxaparin (Table 2). Dalteparin and tinzaparin have yet to adopt age based dose-individualization strategies; however, a recent multi-centre trial [37] in elderly subjects with renal impairment was stopped early due to an increase in mortality in the tinzaparin arm. The aim of this study was to assess the safety of tinzaparin, when compared with UFH, in treating DVT or PE in subjects with renal impairment who were ≥ 70 years of age. Subjects recruited were either ≥ 75 years of age with a CLCR≤ 30 ml min−1 (calculated using TBW) or ≥ 70 years old with a CLCR≤ 60 ml min−1 and randomized to either UFH or tinzaparin [37]. When the study was terminated overall mortality was 6.3% in subjects receiving UFH and 11.2% in subjects receiving tinzaparin.
Anti-Xa activity monitoring
Despite the PLs recommending that aXa-activity monitoring would be of benefit in subjects at high risk of bleeding, no dose-individualization algorithms are provided. An aXa-activity > 1000 IU l−1 is linked to an increase in the risk of bleeding [38] and an aXa-activity < 500 IU l−1 has been linked to an increase in re-infarction and mortality [39]. Therefore, it is not surprising that 48 (23%) surveyed hospitals elected to use aXa-activity to dose-individualize LMWHs. A number of studies have proposed dose-individualization strategies to achieve the therapeutic range [40, 41], but none has been prospectively tested using a robust clinical endpoint.
Contraindications to LMWHs
Hospitals will often choose to stop the LMWH rather than continue with the dose recommended in the PL and this occurs predominantly in subjects with a TBW > 150 kg or subjects with a CLCR < 30 ml min−1. These results probably reflect the lack of dosing data in these populations who were often excluded from confirmatory studies [10, 12]. This approach is supported by a recent study reporting a higher risk of bleeding in subjects with a TBW > 150 kg who were dosed at 1 mg kg−1 twice a day (PL) of enoxaparin, compared with subjects who received a lower dose [42].
What are the optimal dosing regimens for LMWHs?
This survey has demonstrated that hospitals did not prescribe LMWHs according to the PL and that formal dose-individualization methods were commonly used. The PLs do recommend dose-individualization strategies based on subject demographics (TBW and renal function). However, these methods are not robust enough to encompass the physiological diversity seen in today's population. In an effort to resolve the dilemma, two dose strategies for enoxaparin were recently proposed; one based on LBW [9] and the other based on renal function [8]. An individualized dose strategy was developed and compared with the PL based dosing in a prospective, randomized controlled trial [43]. The trial demonstrated that dose-individualization as a function of LBW [9] and renal function [8], reduced the relative risk of bleeding to 0.12 (P= 0.03). Whilst this study was small the benefits of dose-individualization were clearly evident.
What does this mean for international dosing practices for LMWHs?
Hospitals have elected to dose-individualize LMWHs with preponderance for guidance documents when dosing subjects with renal impairment and obesity. Given that the PL is not being used, is it time to revisit its current content? We believe so and recommend the development of an international consensus document for dosing LMWH in subjects with renal impairment and obesity. This document would integrate confirmatory evidence with physiological science and contemporary clinical needs to provide peer agreed dose-individualization strategies. It would also detail the correct use of LBSDs and specify how to monitor aXa-activity, interpret the results and make informed dosing decisions. We also propose that data already available from major confirmatory and post-marketing studies are used to quantify the dose–exposure–response relationship (both effectiveness and adverse events) in these populations. While we have a good understanding about the dose-exposure relationship of LMWHs in subjects with renal impairment [8] and obesity [9], we do not know if simply matching exposure in these subjects to those without renal impairment or obesity results in the same effectiveness and prevalence of adverse events. Currently, LMWH dosing in the clinical setting does not match the PL which questions its value to both health care professionals and patients alike.
In conclusion there is minimal adherence to the PL when dosing LMWHs and dose-individualization strategies were recommended in 96% of surveyed hospitals. Common individualization methods included dose-capping, use of lean body weight descriptors to calculate the starting dose and renal function, followed by post-dose monitoring of aXa-activity.
Competing interests
B.G. is a paid consultant to various pharmaceutical companies.
We thank Jonathon Hall, Principal Pharmacist Medicines Information, Wessex Drug & Medicines Information Centre, Pharmacy Department, Southampton General Hospital, Southampton, for his assistance with data collection in the UK.
REFERENCES
- 1.Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:188–204. doi: 10.1378/chest.126.3_suppl.188S. [DOI] [PubMed] [Google Scholar]
- 2.Snow V, Qaseem A, Barry P, Hornbake ER, Rodnick JE, Tobolic T, Ireland B, Segal JB, Bass EB. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the Academy of Family Physicians. Ann Intern Med. 2007;146:204–10. doi: 10.7326/0003-4819-146-3-200702060-00149. [DOI] [PubMed] [Google Scholar]
- 3.Pfizer Australia Pty Ltd. Product Information. Fragmin® Injection. Dalteparin sodium. 2006. Available at http://www.pfizer.com.au/ProductInfo.aspx (last accessed 1 November 2008.
- 4.Sanofi-Aventis Australia Pty Ltd. Clexane® Product Information. 2008. Available at http://www.sanofi-aventis.com.au/products/aus_pi_clexane.pdf (last accessed 1 November 2008.
- 5.Datapharm communications Ltd. The electronic Medicines Compendium. 2008. Available at http://emc.medicines.org.uk/ (last accessed 1 November 2008.
- 6.MEDSAFE. New Zealand medicines and medical devices safety authority. 2008. Available at http://www.medsafe.govt.nz/ (last accessed 1 November 2008.
- 7.FDA. U.S. Food and Drug Administration. 2008. Available at http://www.fda.gov/ (last accessed 1 November 2008. [DOI] [PubMed]
- 8.Green B, Greenwood M, Saltissi D, Westhuyzen J, Kluver L, Rowell J, Atherton J. Dosing strategy for enoxaparin in patients with renal impairment presenting with acute coronary syndromes. Br J Clin Pharmacol. 2004;59:281–90. doi: 10.1111/j.1365-2125.2004.02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol. 2003;54:96–103. doi: 10.1046/j.1365-2125.2003.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antman EM, McCabe C, Gurfinkel E, Turpie A, Alexander G, Bernink P, Salein D, de Luna A, Fox K, LaBlanche J-M, Radley D, Premmereur J, Braunwald E. Enoxaparin prevents death and cardiac ischaemic events in unstable angina/non-Q-wave myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) 11B trial. Circulation. 1999;100:1593–601. doi: 10.1161/01.cir.100.15.1593. [DOI] [PubMed] [Google Scholar]
- 11.Cohen M, Demers C, Gurfinkel E, Turpie A, Fromell G, Goodman S, Langer A, Califf R, Fox K, Premmereur J, Bigonzi F, Stephens J, Weatherley B. A comparison of low molecular weight heparin with unfractionated heparin for unstable coronary artery disease. N Engl J Med. 1997;337:447–52. doi: 10.1056/NEJM199708143370702. [DOI] [PubMed] [Google Scholar]
- 12.Klein W, Buchwald A, Hills S, Monrad S, Sanz G, Turpie A, Van der Meer J, Olaisson E, Underland S, Ludwig K. Comparison of low molecular weight heparin with unfractionated heparin acutely and with placebo for 6 weeks in the management of unstable coronary artery disease. Fragmin in unstable coronary artery disease study (FRIC) Circulation. 1997;96:61–8. doi: 10.1161/01.cir.96.1.61. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation. Obesity and overweight. What are overweight and obesity? 2007. Available at http://www.who.int/topics/obesity/en/ (last accessed 3 July 2008.
- 14.Barsoum R. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–99. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- 15.Checkbox survey solutions. Checkbox Survey Software®. 2007. Available at http://www.prezzatech.com (last accessed 7 December 2008.
- 16.The Society of Hospital Pharmacists of Australia. SHPA directory of hospital pharmacy. 2007. Available at http://www.shpa.org.au/ (last accessed 28 August 2008.
- 17.New Zealand HealthCare Pharmacists' Association. New Zealand Hospital Pharmacies 2008. 2008. Available at http://www.nzhpa.org.nz/hospitals.htm (last accessed 28 August 2008.
- 18.United Kingdom Medical Information Pharmacists. Medical information Pharmacist directory. 2008. Available at http://www.ukmi.nhs.uk/ukmi/directory/default.asp (last accessed 28 October 2008.
- 19.Koumis T, Cicero L, Nathan J, Rosenberg J. Directory of pharmacist-operated drug information centers in the United States-2003. Am J Health Syst Pharm. 2003;61:2033–42. doi: 10.1093/ajhp/61.19.2033. [DOI] [PubMed] [Google Scholar]
- 20.CAMIPR. Consortium for the advancement of medication information, policy and research. Listserver. 2007. Available at http://rherman.idis.uiowa.edu/CAMIPR/default.htm (last accessed 15 October 2008.
- 21.Janmahasatian S, Duffull SB, Chagnac A, Kirkpatrick CMJ, Green B. Lean body mass normalizes the effect of obesity on renal function. Br J Clin Pharmacol. 2008;65:964–65. doi: 10.1111/j.1365-2125.2008.03112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han P, Duffull SB, Kirkpatrick CMJ, Green B. Dosing in obesity: a simple solution to a big problem. Clin Phamacol Ther. 2007;82:505–08. doi: 10.1038/sj.clpt.6100381. [DOI] [PubMed] [Google Scholar]
- 23.Green B, Duffull S. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–33. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouin-Thibault I, Pautas E, Mahe I, Descarpentries C, Nivet-Antoine V, Golmard J-L, Siguret V. Is modification of diet in renal disease formula similar to Cockcroft-Gault formula to assess renal function in elderly hospitalized patients treated with low-molecular-weight heparin? J Gerontol. 2007;62A:1300–05. doi: 10.1093/gerona/62.11.1300. [DOI] [PubMed] [Google Scholar]
- 25.Melloni C, Peterson E, Chen A, Szczech L, Newby L, Harrington R, Gibler W, Ohman E, Spinler S, Roe M, Alexander K. Cockcroft-Gault versus modification of diet in renal disease. J Am Coll Cardiol. 2008;51:991–6. doi: 10.1016/j.jacc.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 26.Moranville M, Jennings H. Implications of using modification of diet in renal disease Cockcroft-Gault equations for renal dosing adjustments. Am J Health Syst Pharm. 2009;66:154–61. doi: 10.2146/ajhp080071. [DOI] [PubMed] [Google Scholar]
- 27.Vats V, Nutescu E, Theobald J, Wojtynek J, Schumock G. Survey of hospital guidelines, policies, and protocols for anticoagulants. Am J Health Syst Pharm. 2007;64:1203–8. doi: 10.2146/ajhp060264. [DOI] [PubMed] [Google Scholar]
- 28.Nutescu E, Lewis R, Finley J, Schumock G. Hospital guidelines for use of low-molecular-weight heparins. Ann Pharmacother. 2003;37:1072–81. doi: 10.1345/aph.1C400. [DOI] [PubMed] [Google Scholar]
- 29.Hull R. Treatment of pulmonary embolism: the use of low-molecular-weight heparin in the inpatient and outpatient settings. Thromb Haemost. 2008;99:502–10. doi: 10.1160/TH07-08-0500. [DOI] [PubMed] [Google Scholar]
- 30.Merli G, Spiro T, Olsson C, Abildgaard U, Davidson B, Eldor A, Elias D, Grigg A, Musset D, Rodgers G, Trowbridge A, Yusen R, Zawilska K. Subcutaneous enoxaparin once or twice daily compared with intravenous heparin for the treatment of venous thromboembolic disease. Ann Intern Med. 2001;134:191–202. doi: 10.7326/0003-4819-134-3-200102060-00009. [DOI] [PubMed] [Google Scholar]
- 31.Lim W, Dentali F, Eikelboom JW, Crowther MA. Meta-analysis: Low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med. 2006;144:673–84. doi: 10.7326/0003-4819-144-9-200605020-00011. [DOI] [PubMed] [Google Scholar]
- 32.Fox K, Antman E, Montalescot G, Agewall S, SomaRaju B, Verheugt F, Lopez-Sendon J, Hod H, Murphy S, Braunwald E. The impact of renal dysfunction on outcomes in the ExTRACT-TIMI 25 trial. J Am Coll Cardiol. 2007;49:2249–55. doi: 10.1016/j.jacc.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 33.Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 34.Cheymol G. Effects of obesity on pharmacokinetics. Implications for drug therapy. Clin Pharmacokinet. 2000;39:215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 35.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 36.Moscucci M, Fox KA, Cannon CO, Klein W. Predictors of major bleeding in acute coronary syndromes: the global registry of acute coronary events (GRACE) Eur Heart J. 2003;24:1815–23. doi: 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration. Communication about ongoing safety review. Innohep (tinzaparin sodium injection) 2008. Available at http://www.fda.gov/cder/drug/early_comm/tinzaparin.htm (last accessed 24 April 2009.
- 38.Thrombolysis in Myocardial Infarction (TIMI)11A Investigators. Dose-ranging trial of enoxaparin for unstable angina: results of TIMI 11A. J Am Coll Cardiol. 1997;29:1474–82. [PubMed] [Google Scholar]
- 39.Montalescot G, Collet J, Tanguy ML, Ankri A, Payot L, Dumaine R, Choussat R, Beygui F, Gallois V, Thomas D. Anti-Xa activity relates to survival and efficacy in unselected acute coronary syndrome patients treated with enoxaparin. Circulation. 2004;110:392–98. doi: 10.1161/01.CIR.0000136830.65073.C7. [DOI] [PubMed] [Google Scholar]
- 40.Collet J, Montalescot G, Fine E, Golmard J-L. Enoxaparin in unstable angina patients who would have been excluded from randomized pivotal trials. J Am Coll Cardiol. 2003;41:8–14. doi: 10.1016/s0735-1097(02)02664-5. [DOI] [PubMed] [Google Scholar]
- 41.Hulot J-S, Montalescot G, Lechat P, Collet J-P, Ankri A, Urien S. Dosing strategy in patients with renal failure receiving enoxaparin for the treatment of non-ST-segment elevation acute coronary syndrome. Clin Pharmacol Ther. 2005;77:542–52. doi: 10.1016/j.clpt.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Spinler S, Ou F-S, Roe MT, Gibler WB, Ohman EM, Pollack CV, Alexander K, Peterson ED. Weight-based dosing of enoxaparin in obese patients with non-ST-elevation acute coronary syndromes: results from the CRUSADE initiative. Pharmacotherapy. 2009;29:631–38. doi: 10.1592/phco.29.6.631. [DOI] [PubMed] [Google Scholar]
- 43.Barras MA, Duffull SB, Atherton JJ, Green B. Individualized compared to conventional dosing of enoxaparin. Clin Pharmacol Ther. 2008;83:882–88. doi: 10.1038/sj.clpt.6100399. [DOI] [PubMed] [Google Scholar]


