Abstract
AIMS
The present study aimed to evaluate the associations between medication use and falls and to identify high risk medications that acted as a trigger for the onset of falls in an acute care hospital setting.
METHODS
We applied a case-crossover design wherein cases served as their own controls and comparisons were made within each participant. The 3-day period (days 0 to −2) and the 3-day periods (days −6 to −8, days −9 to −11 and days −12 to −14) before the fall event were defined as the case period and the control periods, respectively. Exposures to medications were compared between the case and control periods. Odds ratios (OR) and 95% confidence intervals (CI) for the onset of falls with respect to medication use were computed using conditional logistic regression analyses.
RESULTS
A total of 349 inpatients who fell during their hospitalization were recorded on incident report forms between March 2003 and August 2005. The initial use of antihypertensive, antiparkinsonian, anti-anxiety and hypnotic agents as medication classes was significantly associated with an increased risk of falls, and these ORs (95% CI) were 8.42 (3.12, 22.72), 4.18 (1.75, 10.02), 3.25 (1.62, 6.50) and 2.44 (1.32, 4.51), respectively. The initial use of candesartan, etizolam, biperiden and zopiclone was also identified as a potential risk factor for falls.
CONCLUSIONS
Medical professionals should be aware of the possibility that starting a new medication such as an antihypertensive agent, including candesartan, and antiparkinsonian, anti-anxiety and hypnotic agents, may act as a trigger for the onset of a fall.
Keywords: acute care hospital, case-crossover study, fall, medication use, risk factor
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Benzodiazepines, antidepressants, antipsychotic agents, anti-arrhythmic agents, opioid analgesics and antihypertensive agents including α-receptor antagonists and β-receptor antagonists, but not angiotensin II receptor antagonists, have been implicated as risk factors for falls among community dwelling elderly people, and those in aged care hospitals and nursing homes.
WHAT THIS STUDY ADDS
Using a case-crossover design, the study's findings provide the first evidence suggesting that newly initiating treatment using an angiotensin II receptor antagonist, candesartan, or etizolam, biperiden and zopiclone may be potential risk factors for falls in acute hospitals.
Introduction
Falls are the most common type of inpatient hospital accident, reportedly accounting for up to 70% of all inpatient accidents [1]. Fall rates per 1000 patient-days vary from 2.2 in large tertiary university hospitals to 9.1 in geriatric hospitals [1–8]. Approximately 30% of falls in hospitals lead to physical injury, with 4–6% being serious [6, 9]. For inpatients, falls are associated with serious physical and emotional injury, poor quality of life, increased length of hospital stay, admission to a long-term care facility and increased cost [10–14]. Therefore, it is important to identify and characterize triggers for risk factors for falls in hospitals to assure the quality of hospital care and patient safety.
While many studies on falls in elderly populations have been conducted in community and long-term care facilities, less is known about falls in acute care hospitals. The frequency of falls is higher in institutions such as nursing homes and hospitals than in the community setting [15]. Contributing factors for falls can be divided into two main categories: those specific to the individual (intrinsic) and those related to the environment (extrinsic) [16–31]. Intrinsic factors include age-related changes such as a decline in vision, hearing, musculoskeletal functioning, mobility, and physical activity, and health status-related factors such as the presence of a variety of chronic and acute illnesses, e.g. diabetes, cognitive deficits and Parkinson's disease. Extrinsic factors include poor lighting, loose carpets, slippery flooring and a lack of handrails. Although previous studies have identified risk factors for falls, comparative studies on falls in hospitals have had limitations including variations in study design, setting, patient population, definition of risk factors and care-related factors such as patient-to-nurse ratio. These problems significantly decrease the quality, consistency, and comparability of observational studies on falls in hospital.
Medications related to an increased risk of falls include benzodiazepines, antidepressants, antipsychotic agents, antihypertensive agents, anti-arrhythmic agents and opioid analgesics. In elderly people receiving multiple medications, polypharmacy itself is a risk factor for falls [18, 19]. Yet, many observational studies on falls in hospitals fail to focus on medication use as a risk factor for falls because the majority of falls in hospital settings have a multi-factorial aetiology. For example, in acute care hospitals a variety of medications are often prescribed to patients and new medications are frequently added as required by additional symptoms. It is quite possible that the initial use of a newly initiated medication rather than the long-term use of the same medication presents a higher risk for falls. Thus we hypothesized that newly initiated medications are associated with a greater risk for falls than ongoing medications.
We used a case-crossover design to overcome many of the difficulties involved selecting an appropriate control series for cases. The case-crossover design is an observational design characterized by self-matching, such that case and control data are obtained from the same subject [32]. In this design, cases serve as their own controls, and a participant's exposure at the time of the event of interest (case period) is compared with another period when the participant was not a case (control period). Therefore the sampling of additional control participants is not required. In the analysis, each participant is considered as a stratum in a case-control study and comparisons are made within each participant. Therefore, possible confounding effects including age, sex, personality and other stable patient-specific covariates from differences between individuals are eliminated, and the focus of the analysis is on the variable of interest. Thus, the case-crossover method is an optimal study design to assess high risk medications that act as triggers for the onset of falls among hospitalized patients.
The present case-crossover study aimed to evaluate associations between medication use and falls among hospitalized patients and to identify high risk medications that could act as triggers for the onset of falls.
Methods
Setting, participants and data collection
This retrospective, case-crossover study was conducted at Fukuoka Tokushukai Medical Centre, a 600-bed acute care hospital in Japan. Fifteen clinical departments were included in the study: Internal Medicine, General Surgery, Cranial Nerve Surgery, Plastic Surgery, Orthopaedic Surgery, Urology, Psychosomatic Medicine, Rehabilitation, Cardiovascular Medicine, Dialysis, Nephrology, Otorhinolaryngology, Anesthesiology, Cardiovascular Surgery and Ophthalmology. An inpatient fall was defined as an incident in which a patient suddenly and involuntarily came to rest upon the ground or another surface and was registered with an incident report form submitted by a nurse or another hospital employee who discovered the fall. Data were collected from incident report forms and medical records during the 30 months between March 1 2003 and August 31 2005. Incident report forms included clinical department, patient demographics, date, time, location, circumstances surrounding falls and the severity of injuries. Medical records included patient demographics such as sex, age, weight, height, admission date, concurrent disease, medication history and clinical laboratory test results such as blood urea nitrogen (BUN), serum creatinine and liver enzymes (AST and ALT). Specifically, medication history during the 2 weeks prior to any fall event was carefully investigated. The present study was reviewed and approved by the Fukuoka Tokushukai Medical Centre Institutional Review Board. The need for written informed consent from patients who had fallen was waived because the present study was part of a hospital-based quality improvement project and posed no risk to patients or to their privacy.
Study design
A case-crossover design was used to assess the association between exposure (medication use) and transient outcome (fall). In the present study, we defined the case period (risk period) as the 3-day period (days 0 to −2) just before the fall event based on the elimination half-life of the prescribed medications. To increase the consistency of comparable controls and to enhance the robustness of the analyses, we estimated three control periods for each case and defined control periods as days −6 to −8, days −9 to −11 and days −12 to −14 before the fall event. The 3-day period (days −3 to −6) before the fall event between the case period and the control period was estimated as the wash-out period (Figure 1). The living status of all patients was hospitalization during the period investigated, including both control and case periods.
Figure 1.
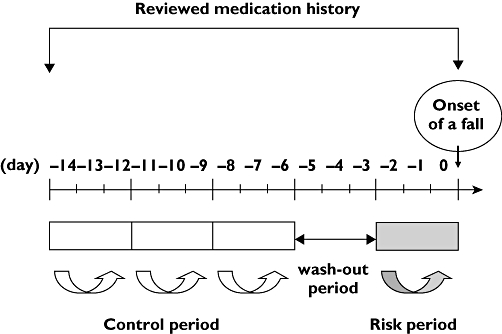
Definition of risk, control and wash-out periods in the present case-crossover study. The 3-day period (days 0 to −2) just before any fall event was defined as the case period based on the elimination half-life of the prescribed medications. The three control periods for each case were estimated to increase the consistency of comparable controls and to enhance the robustness of the analyses. The 3-day periods (days −6 to −8, days −9 to −11 and days −12 to −14) before the fall event were defined as the control period. The 3-day period (days −3 to −6) before the fall event between the case period and the control period was assigned as the wash-out period
Statistical analysis
We first performed univariate analysis to select covariates, adjusting for multivariable analysis. All variables with a significant association with falls, P value < 0.05, in univariate analysis, were entered into a staged multivariable analysis. The strength of association between medication use and falls was evaluated using odds ratios (OR) and 95% confidence intervals (CI). To estimate the OR of falls with respect to medication use, we used conditional logistic regression analyses for 1–3 matching. The 98 medications prescribed were classified into nine groups: hypnotic, anti-anxiety, antipsychotic, antihistamine, antidiabetic, antihypertensive, antiparkinsonian, anti-ulcer agents and diuretics (Table 1). Results were categorized by medication class or by specific medication. In the first analysis, we evaluated the associations between medication classes and falls. In the second analysis, we identified the specific medications that act as triggers for the onset of falls. The analysis was done separately for patients under and over 75 years of age. All statistical analyses were performed using SAS version 8.2 (SAS Institute, Inc., Cary, NC).
Table 1.
List of nine classes of medication and 98 specific medications prescribed to cases in this study
| Medication classes | Specific medication (n) |
|---|---|
| Hypnotic agents | brotizolam (25), flunitrazepam (25), lormetazepam (3), midazolam (8), nitrazepam (12), quazepam (3), rilmazafone (1), triazolam (16), zolpidem (40), zopiclone (41) |
| Anti-anxiety agents | alprazolam (2), bromazepam (12), clotiazepam (2), cloxazolam (4), diazepam(21), ethyl loflazepate (3), etizolam (42), lorazepam (5), tandospirone (2) |
| Antipsychotic agents | chlorpromazine (8), haloperidol (31), prochlorperazine (1), promethazine (21), quetiapine (2), risperidone (7), sulpiride (3), thioridazine (2), timiperone (1) |
| Antihistamines | cetirizine (1), d-chlorpheniramine (6), clemastine (1), cyproheptadine (0), epinastine (4), fexofenadine (1), homochlorcyclizine (0), hydroxyzine (10), ketotifen (1), mequitazine (3), olopatadine (4), oxatomide (2), promethazine (1) |
| Antidiabetic agents | glibenclamide (8), gliclazide (4), glimepiride (10), insulin (8), metformin (8), nateglinide (3), pioglitazone (1), voglibose (14) |
| Antihypertensive agents | alprenolol (0), amlodipine (48), atenolol (8), benidipine (6), betaxolol (1), bisoprolol (2), candesartan (41), captopril (0), carvedilol (10), clonidine (1), delapril (0), diltiazem (26), doxazosin (8), efonidipine (1), enalapril (19), imidapril (11), losartan (22), metoprolol (1), nicardipine (6), nifedipine (35), nilvadipine (6), nisoldipine (16), nitrendipine (0), perindopril (4), prazosin (7), propranolol (1), temocapril (1), valsartan (25), verapamil (3) |
| Diuretics | azosemide (6), canrenoate (0), furosemide (37), spironolactone (23), trichlormethiazide (5) |
| Antiparkinsonian agents | amantadine (11), biperiden (25), cabergoline (7), droxidopa (3), levodopa (13), pergolide (5), pramipexole (1), selegiline (2), tiapride (15), trihexyphenidyl (4) |
| Anti-ulcer agents | cimetidine (4), famotidine (14), lafutidine (20), ranitidine (41), roxatidine (1) |
Results
A total of 349 inpatient falls were recorded on incident report forms during the 30 months between March 1 2003 and August 31 2005. Tables 2 and 3 show the characteristics and the admitting diagnoses of fallers (cases) in the present case-crossover study, respectively. The mean ± SD age of cases was 71.5 ± 14.8 years. Table 4 shows the classes and numbers of medications prescribed in the present case-crossover study. The median numbers of medications prescribed during the case and the control period were 7 and 6, respectively.
Table 2.
Characteristics of cases in this study (n= 349)
| Characteristic | n (%) | n (%) | |
|---|---|---|---|
| Gender | Clinical departments | ||
| Male | 194 (55.6) | Internal Medicine | 120 (34.4) |
| Female | 155 (44.4) | Cardiovascular Medicine | 48 (13.8) |
| Age (years) | Cranial Nerve Surgery | 36 (10.3) | |
| 20–29 | 5 (1.4) | General Surgery | 33 (9.5) |
| 30–39 | 6 (1.7) | Orthopaedic Surgery | 33 (9.5) |
| 40–49 | 9 (2.6) | Rehabilitation | 24 (6.9) |
| 50–59 | 34 (9.7) | Psychosomatic Medicine | 18 (5.2) |
| 60–69 | 69 (19.8) | Plastic Surgery | 14 (4.0) |
| 70–79 | 109 (31.2) | Urology | 12 (3.4) |
| 80–89 | 96 (27.5) | Nephrology | 3 (0.9) |
| 90–99 | 21 (6.0) | Anesthesiology | 2 (0.6) |
| Ophthalmology | 2 (0.6) | ||
| Cardiovascular Surgery | 1 (0.3) | ||
| Dialysis | 1 (0.3) | ||
| Otorhinolaryngology | 1 (0.3) | ||
| Unknown* | 4 (1.2) |
A lack of description in an incident report.
Table 3.
Admitting diagnosis of cases in this study
| Diagnosis | n | Diagnosis | n |
|---|---|---|---|
| Cerebral infarction | 73 | Gastrointestinal bleeding | 3 |
| Cerebral haemorrhage | 33 | Herpes zoster | 3 |
| Chronic renal failure | 30 | Hydrocephalus | 3 |
| Diabetes mellitus | 22 | Intestinal perforation | 3 |
| Parkinson's disease | 21 | Pancreatic cancer | 3 |
| Depression | 17 | Urine tube cancer | 3 |
| Congestive heart failure | 14 | Aortic dissection | 3 |
| Pneumonia | 14 | Asthma | 2 |
| Angina pectoris | 13 | Cholecystitis | 2 |
| Gastric cancer | 10 | Chronic pancreatitis | 2 |
| Burn | 9 | Diabetic retinopathy | 2 |
| Ileus | 9 | Eating disorder | 2 |
| Liver cirrhosis | 9 | Hypoglycaemia | 2 |
| Epilepsy | 8 | Sepsis | 2 |
| Hypertension | 8 | Transient ischaemic attack | 2 |
| Arteriosclerosis obliterans | 7 | Aortic stenosis | 2 |
| Brain contusion | 7 | Aplastic anaemia | 1 |
| Colorectal cancer | 7 | Cataract | 1 |
| Dementia | 7 | Chorea | 1 |
| Lung cancer | 7 | Diabetic nephropathy | 1 |
| Subarachnoid haemorrhage | 7 | Dilated cardiomyopathy | 1 |
| Subdural haematoma | 7 | Duodenal ulcer | 1 |
| Benign prostatic hyperplasia | 6 | Oesophageal varices | 1 |
| Gastric ulcer | 6 | Fever of unknown origin | 1 |
| Alcoholic hepatitis | 5 | Fibroid lung | 1 |
| Head injury | 5 | Hypokalaemia | 1 |
| Hernia | 5 | Infectious endocarditis | 1 |
| Myocardial infarction | 5 | Myelodysplastic syndrome | 1 |
| Alcohol withdrawal syndrome | 4 | Nephrotic syndrome | 1 |
| Alcoholic psychoses | 4 | Osteoarthritis | 1 |
| Cellulitis | 4 | Pancreatitis | 1 |
| Cholelithiasis | 4 | Panic disorder | 1 |
| Hepatocellular carcinoma | 4 | Pyelonephritis | 1 |
| Prostate cancer | 4 | Renal tumour | 1 |
| Traumatic injury | 4 | Respiratory failure | 1 |
| Atrial fibrillation | 3 | Schizophrenia | 1 |
| Bile duct cancer | 3 | Thoracic aortic aneurysm | 1 |
| Bladder cancer | 3 | Thrombocytopenic purpura | 1 |
| Breast cancer | 3 | Thyroid cancer | 1 |
| Chronic obstructive pulmonary disease | 3 | Trigeminal neuralgia | 1 |
| Diabetic acidosis | 3 | Ventricular tachycardia | 1 |
| Drug intoxication | 3 |
Table 4.
Classes and number of medications prescribed to cases (n= 349) in this study
| Case | Control | |||
|---|---|---|---|---|
| n (%) | n (%) | |||
| Day before a fall event | ||||
| Periods | 0 to−2 | −6 to−8 | −9 to−11 | −12 to−14 |
| Classes of medications | ||||
| Hypnotic agents | 134 (38.4) | 122 (35.0) | 113 (32.4) | 108 (31.0) |
| Anti-anxiety agents | 77 (22.1) | 66 (18.9) | 63 (18.1) | 59 (17.0) |
| Antipsychotic agents | 58 (16.6) | 63 (18.1) | 56 (16.1) | 51 (14.6) |
| Antihistamines | 25 (7.2) | 26 (7.5) | 21 (6.0) | 18 (5.2) |
| Antidiabetic agents | 40 (11.5) | 37 (10.6) | 39 (11.2) | 37 (10.6) |
| Antihypertensive agents | 190 (54.4) | 176 (50.4) | 169 (48.4) | 166 (47.6) |
| Diuretics | 52 (14.9) | 53 (15.2) | 51 (14.6) | 49 (14.0) |
| Antiparkinsonian agents | 65 (18.6) | 59 (16.6) | 51 (14.6) | 50 (14.3) |
| Anti-ulcer agents | 76 (21.8) | 72 (20.6) | 67 (19.2) | 67 (19.2) |
| Number of medications | ||||
| 0–5 | 131 (37.5) | 152 (43.6) | 161 (46.1) | 168 (48.1) |
| 6–10 | 141 (40.4) | 140 (40.1) | 138 (39.5) | 129 (37.0) |
| 11–15 | 66 (18.9) | 48 (13.8) | 43 (12.3) | 45 (12.9) |
| 16–20 | 10 (2.9) | 9 (2.6) | 7 (2.0) | 7 (2.0) |
| 21–25 | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Median (min–max) | 7 (0–21) | 6 (0–18) | 6 (0–18) | 6 (0–18) |
Medication classes and falls
In the analysis concerning medication classes, the results using data without patients aged <75 years old were similar to those from all patients. For univariate analysis, antihypertensive, antiparkinsonian, anti-anxiety and hypnotic agents were selected as covariates adjusting for multivariable analysis. For multivariable analysis, the initial use of antihypertensive, antiparkinsonian, anti-anxiety and hypnotic agents was significantly associated with an increased risk of falls, and these ORs (95% CI) were 8.42 (3.12, 22.72), 4.18 (1.75, 10.02), 3.25 (1.62, 6.50) and 2.44 (1.32, 4.51), respectively (Table 5).
Table 5.
Odds ratios and 95% confidence intervals for the onset of a fall with respect to medication classes and specific medication computed by conditional logistic regression
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Medication class | |||
| All age groups | |||
| Antihypertensive agents | 8.42 | 3.12, 22.72 | <0.001 |
| Antiparkinsonian agents | 4.18 | 1.75, 10.02 | 0.004 |
| Anti-anxiety agents | 3.25 | 1.62, 6.50 | 0.001 |
| Hypnotic agents | 2.44 | 1.32, 4.51 | 0.004 |
| Specific medication | |||
| All age groups | |||
| Candesartan cilexetil | 13.92 | 1.71, 113.69 | 0.014 |
| Etizolam | 6.83 | 1.92, 24.26 | 0.003 |
| Bipereiden | 4.34 | 1.57, 11.99 | 0.005 |
| Zopiclone | 4.20 | 1.55, 11.40 | 0.005 |
| Age group <75 years | |||
| Candesartan cilexetil | 11.23 | 1.22, 103.04 | 0.033 |
| Bipereiden | 10.68 | 1.24, 92.24 | 0.031 |
| Age group ≥75 years | |||
| Bipereiden | 3.75 | 1.01, 13.97 | 0.049 |
| Zopiclone | 5.40 | 1.63, 17.93 | 0.006 |
Specific medication and falls
For univariate analysis, candesartan cilexetil, etizolam, biperiden and zopiclone were selected as covariates adjusting for multivariable analysis. For analysis concerning specific medications, the results using data from patients aged <75 years old were somewhat different from those using data from patients aged ≥75 years old. The results of the multivariate analysis were as follows. In all age groups, the initial use of candesartan cilexetil, etizolam, biperiden and zopiclone was significantly associated with an increased risk of falls, and these ORs (95% CI) were 13.92 (1.71, 113.69), 6.83 (1.92, 24.26), 4.34 (1.57, 11.99) and 4.20 (1.55, 11.40), respectively. In the age group <75 years of age, the initial use of candesartan cilexetil and biperiden were significantly associated with an increased risk of falls; these ORs (95% CI) were 11.23 (1.22, 103.04) and 10.68 (1.24, 92.24), respectively. In the age group ≥75 years of age, the initial use of biperiden and zopiclone were significantly associated with an increased risk of falls; these ORs (95% CI) were 3.75 (1.01, 13.97) and 5.40 (1.63, 17.93), respectively (Table 5).
Discussion
Our results suggested that the initial use of the medication classes of antihypertensive, antiparkinsonian, anti-anxiety and hypnotic agents were significantly associated with the onset of falls with eight-, four-, three- and two-fold higher risks, respectively. The initial use of candesartan, etizolam, biperiden and zopiclone were identified as a potential risk factor for falls. To our knowledge, the present study is the first case-crossover study to evaluate the association between medication use and falls and to identify high risk medications that act as triggers for the onset of falls in an acute care hospital setting. Our results are further confirmation that medications that act on the central nervous system are significantly associated with an increased risk of falls. However, we are the first to show that the initial use of candesartan may act as trigger for the onset of falls in acute care hospitals.
Candesartan cilexetil is an inactive ester prodrug that is completely hydrolyzed to the active form, candesartan, during absorption from the gastrointestinal tract. Candesartan is a potent, highly selective antagonist of the angiotensin II receptor subtype 1 [33]. Candesartan cilexetil has been available in Japan since 1999 and is used for the treatment of hypertension and heart failure (NYHA class II-IV). Antihypertensive agents produce orthostatic hypotension, resulting in an increased risk of falls. There are no studies on the association between the use of angiotensin II receptor subtype 1 antagonists and falls. Studies with a large number of participants are needed to determine whether the use of this class of cardiovascular medication is associated with an increased risk of falls. Biperiden, an anticholinergic agent, has been available in Japan since 1964 and is used as adjunctive therapy for all forms of parkinsonism (e.g. idiopathic, postencephalitic and arteriosclerotic) and the control of extrapyramidal disorders secondary to neuroleptic drug therapy (e.g. phenothiazines). The most troublesome side-effects of anticholinergic medications include sedation and mental confusion. Such responses could contribute to adverse events such as falls, delirium, and cognitive impairment in older patients. Etizolam, a thienodiazepine derivative, has been available in Japan since 1983 and is used for anxiety and sleep disorders, as well as for muscle contraction headaches. Based on the pharmacokinetic profiles (half-life = 3.4 h) of the parent compound and its main active metabolite, α-hydroxyetizolam, etizolam can be regarded as a short-acting benzodiazepine [34]. Zopiclone is a cyclopyrrolone that is chemically unrelated to the benzodiazepines and is thought to act on the GABA A receptor complex at a site distinct from, but closely related to, the benzodiazepine binding site. Based on its pharmacokinetic profile (half-life = 5–6 h), zopiclone can also be regarded as a short-acting nonbenzodiazepine [35]. Zopiclone has been available in Japan since 1989 and is used for the treatment of sleep disorders.
Numerous studies have indicated the importance of benzodiazepines as risk factors for falls in the elderly. However, the findings are potentially contradictory. Some studies demonstrated an association between the use of long-acting benzodiazepines and the risk of falls among community-dwelling elderly [36–38]. Others have shown that the use of short half-life benzodiazepines is also associated with an increased risk of falls [39, 40]. Most such studies have focused on either nursing home residents or on those dwelling in the community [36–38, 41]. Few studies have been conducted in a hospital setting, where other factors such as the presence of acute illnesses and the frequent addition of new medications could increase the risk of iatrogenic complication and falls [4, 6, 8, 29, 42]. Our results showed that the use of short-acting benzodiazepines was significantly associated with falls, compared with the use of long-acting benzodiazepines. On the other hand, it has been reported that the risk of falls was significantly high only among patients exposed to high doses of benzodiazepines, suggesting that dosage rather than half-life is more important in the evaluation of the association between the use of benzodiazepines and falls [40]. It is well known that benzodiazepines produce dose-related impairment of reaction time and psychomotor function, sedation and muscle relaxation, and these responses may lead to falls. To confirm the association between the use of benzodiazepines and falls, dose–response as well as half-life needs to be considered in analysis.
There are several limitations in the present study. First, the case-cross over design did not allow for alterations of behavioural patterns in patients between the exposed and unexposed periods. Because there were no convincing data providing evidence that no change in diagnoses was observed during the 2-week period in hospital, we could not completely rule out the possibility that the same individuals did not behave in the same manner between the case and control periods. Second, since the information on medication use has been obtained from prescription data, not intake data, we could not confirm patients' compliance with the medication regime prescribed by a physician. Third, we collected information on the dose of prescribed medications, but analyses could not be performed because of the small sample size and low statistical power. Fourth, the ability to generalize our results may be limited because of the small sample size in a single centre setting, although hospital-related confounding effects such as poor lighting, loose carpets and slippery flooring were limited.
In summary, our findings suggest that the initial use of angiotensin II receptor antagonists as antihypertensive agents, antiparkinsonian agents, anti-anxiety agents and hypnotic agents are increased risk factors for falls. Physicians and other medical professionals should be aware of the possibility that medications such as candesartan, etizolam, biperiden and zopiclone, when newly administered to patients, may act as triggers for the onset of falls in acute care hospitals. When these medications are introduced to older patients, a low but effective dose should be used taking into consideration the changes possible in pharmacodynamics and pharmacokinetics. Medical professionals including physicians, nurses and pharmacists should carefully monitor patients for an initial 3 days after starting treatment with any of these medications. Appropriate preventive strategies for falls in acute care hospitals should be considered to assure the quality of hospital care and patient safety.
Competing interests
None declared.
This study was supported, in part, by a Grant-in-Aid for Clinical Epidemiological Research from St Luke's Life Science Institute (Tokyo, Japan).
REFERENCES
- 1.Sutton JC, Standen PJ, Wallace WA. Patient accidents in hospital: incidence, documentation and significance. Br J Clin Pract. 1994;48:63–6. [PubMed] [Google Scholar]
- 2.Vassallo M, Amersey RA, Sharma JC, Allen SC. Falls on integrated medical wards. Gerontology. 2000;46:158–62. doi: 10.1159/000022152. [DOI] [PubMed] [Google Scholar]
- 3.Halfon P, Eggli Y, Van Melle G, Vagnair A. Risk of falls for hospitalized patients: a predictive model based on routinely available data. J Clin Epidemiol. 2001;54:1258–66. doi: 10.1016/s0895-4356(01)00406-1. [DOI] [PubMed] [Google Scholar]
- 4.Hitcho EB, Krauss MJ, Birge S, Claiborne Dunagan W, Fischer I, Johnson S, Nast PA, Costantinou E, Fraser VJ. Characteristics and circumstances of falls in a hospital setting: a prospective analysis. J Gen Intern Med. 2004;19:732–9. doi: 10.1111/j.1525-1497.2004.30387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172–81. doi: 10.1093/gerona/62.10.1172. [DOI] [PubMed] [Google Scholar]
- 6.Krauss MJ, Nguyen SL, Dunagan WC, Birge S, Costantinou E, Johnson S, Caleca B, Fraser VJ. Circumstances of patient falls and injuries in 9 hospitals in a midwestern healthcare system. Infect Control Hosp Epidemiol. 2007;28:544–50. doi: 10.1086/513725. [DOI] [PubMed] [Google Scholar]
- 7.Healey F, Scobie S, Oliver D, Pryce A, Thomson R, Glampson B. Falls in English and Welsh hospitals: a national observational study based on retrospective analysis of 12 months of patient safety incident reports. Qual Saf Health Care. 2008;17:424–30. doi: 10.1136/qshc.2007.024695. [DOI] [PubMed] [Google Scholar]
- 8.Schwendimann R, Bühler H, De Geest S, Milisen K. Characteristics of hospital inpatient falls across clinical departments. Gerontology. 2008;54:342–8. doi: 10.1159/000129954. [DOI] [PubMed] [Google Scholar]
- 9.Ash KL, MacLeod P, Clark L. A case control study of falls in the hospital setting. J Gerontol Nurs. 1998;24:7–15. doi: 10.3928/0098-9134-19981201-05. [DOI] [PubMed] [Google Scholar]
- 10.Bates DW, Pruess K, Souney P, Platt R. Serious falls in hospitalized patients: correlates and resource utilization. Am J Med. 1995;99:137–43. doi: 10.1016/s0002-9343(99)80133-8. [DOI] [PubMed] [Google Scholar]
- 11.Kong KS, Lee Fk FK, Mackenzie AE, Lee DT. Psychosocial consequences of falling: the perspective of older Hong Kong Chinese who had experienced recent falls. J Adv Nurs. 2002;37:234–42. doi: 10.1046/j.1365-2648.2002.02094.x. [DOI] [PubMed] [Google Scholar]
- 12.Aditya BS, Sharma JC, Allen SC, Vassallo M. Predictors of a nursing home placement from a non-acute geriatric hospital. Clin Rehabil. 2003;17:108–13. doi: 10.1191/0269215503cr567oa. [DOI] [PubMed] [Google Scholar]
- 13.Scaf-Klomp W, Sanderman R, Ormel J, Kempen GI. Depression in older people after fall-related injuries: a prospective study. Age Ageing. 2003;32:88–94. doi: 10.1093/ageing/32.1.88. [DOI] [PubMed] [Google Scholar]
- 14.Scuffham P, Chaplin S, Legood R. Incidence and costs of unintentional falls in older people in the United Kingdom. J Epidemiol Community Health. 2003;57:740–4. doi: 10.1136/jech.57.9.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003;(4) doi: 10.1002/14651858.CD000340. CD000340. [DOI] [PubMed] [Google Scholar]
- 16.Cumming RG, Miller JP, Kelsey JL, Davis P, Arfken CL, Birge SJ, Peck WA. Medications and multiple falls in elderly people: the St Louis OASIS study. Age Ageing. 1991;20:455–61. doi: 10.1093/ageing/20.6.455. [DOI] [PubMed] [Google Scholar]
- 17.Ruthazer R, Lipsitz LA. Antidepressants and falls among elderly people in long-term care. Am J Public Health. 1993;83:746–9. doi: 10.2105/ajph.83.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gales BJ, Menard SM. Relationship between the administration of selected medications and falls in hospitalized elderly patients. Ann Pharmacother. 1995;29:354–8. doi: 10.1177/106002809502900402. [DOI] [PubMed] [Google Scholar]
- 19.Hendrich A, Nyhuis A, Kippenbrock T, Soja ME. Hospital falls: development of a predictive model for clinical practice. Appl Nurs Res. 1995;8:129–39. doi: 10.1016/s0897-1897(95)80592-3. [DOI] [PubMed] [Google Scholar]
- 20.Gluck T, Wientjes HJ, Rai GS. An evaluation of risk factors for in-patient falls in acute and rehabilitation elderly care wards. Gerontology. 1996;42:104–7. doi: 10.1159/000213779. [DOI] [PubMed] [Google Scholar]
- 21.Monane M, Avorn J. Medications and falls. Causation, correlation, and prevention. Clin Geriatr Med. 1996;12:847–58. [PubMed] [Google Scholar]
- 22.Ebly EM, Hogan DB, Fung TS. Potential adverse outcomes of psychotropic and narcotic drug use in Canadian seniors. J Clin Epidemiol. 1997;50:857–63. doi: 10.1016/s0895-4356(97)00118-2. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Anderson G, Mittmann N, To T, Axcell T, Shear N. Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet. 1998;351:1303–7. doi: 10.1016/s0140-6736(97)09528-7. [DOI] [PubMed] [Google Scholar]
- 24.Thapa PB, Gideon P, Cost TW, Milam AB, Ray WA. Antidepressants and the risk of falls among nursing home residents. N Engl J Med. 1998;339:875–82. doi: 10.1056/NEJM199809243391303. [DOI] [PubMed] [Google Scholar]
- 25.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47:30–9. doi: 10.1111/j.1532-5415.1999.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 26.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47:40–50. doi: 10.1111/j.1532-5415.1999.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 27.Passaro A, Volpato S, Romagnoni F, Manzoli N, Zuliani G, Fellin R. Benzodiazepines with different half-lives and falling in a hospitalized population: the GIFA study. J Clin Epidemiol. 2000;53:1222–9. doi: 10.1016/s0895-4356(00)00254-7. [DOI] [PubMed] [Google Scholar]
- 28.Frels C, Williams P, Narayanan S, Gariballa SE. Iatrogenic causes of falls in hospitalised elderly patients: a case-control study. Postgrad Med J. 2002;78:487–9. doi: 10.1136/pmj.78.922.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse JM. Enhancing the safety of hospitalization by reducing patient falls. Am J Infect Control. 2002;30:376–80. doi: 10.1067/mic.2002.125808. [DOI] [PubMed] [Google Scholar]
- 30.Hendrich AL, Bender PS, Nyhuis A. Validation of the Hendrich II Fall Risk Model: a large concurrent case/control study of hospitalized patients. Appl Nurs Res. 2003;16:9–21. doi: 10.1053/apnr.2003.YAPNR2. [DOI] [PubMed] [Google Scholar]
- 31.Walker PC, Alrawi A, Mitchell JF, Regal RE, Khanderia U. Medication use as a risk factor for falls among hospitalized elderly patients. Am J Health Syst Pharm. 2005;62:2495–9. doi: 10.2146/ajhp050116. [DOI] [PubMed] [Google Scholar]
- 32.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 33.Sever PS. Clinical profile of the novel angiotensin II type I blocker candesartan cilexetil. J Hypertens Suppl. 1997;15:S9–12. doi: 10.1097/00004872-199715066-00003. [DOI] [PubMed] [Google Scholar]
- 34.Fracasso C, Confalonieri S, Garattini S, Caccia S. Single and multiple dose pharmacokinetics of etizolam in healthy subjects. Eur J Clin Pharmacol. 1991;40:181–5. doi: 10.1007/BF00280074. [DOI] [PubMed] [Google Scholar]
- 35.Goa KL, Heel RC. Zopiclone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy as an hypnotic. Drugs. 1986;32:48–65. doi: 10.2165/00003495-198632010-00003. [DOI] [PubMed] [Google Scholar]
- 36.Ray WA, Griffin MR, Schaffner W, Baugh DK, Melton LJ., III Psychotropic drug use and the risk of hip fracture. N Engl J Med. 1987;316:363–9. doi: 10.1056/NEJM198702123160702. [DOI] [PubMed] [Google Scholar]
- 37.Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA. 1989;262:3303–7. [PubMed] [Google Scholar]
- 38.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 39.Lichtenstein MJ, Griffin MR, Cornell JE, Malcolm E, Ray WA. Risk factors for hip fractures occurring in the hospital. Am J Epidemiol. 1994;140:830–8. doi: 10.1093/oxfordjournals.aje.a117331. [DOI] [PubMed] [Google Scholar]
- 40.Herings RM, Stricker BH, de Boer A, Bakker A, Sturmans F. Benzodiazepines and the risk of falling leading to femur fractures. Dosage more important than elimination half-life. Arch Intern Med. 1995;155:1801–7. [PubMed] [Google Scholar]
- 41.Thapa PB, Gideon P, Fought RL, Ray WA. Psychotropic drugs and risk of recurrent falls in ambulatory nursing home residents. Am J Epidemiol. 1995;142:202–11. doi: 10.1093/oxfordjournals.aje.a117619. [DOI] [PubMed] [Google Scholar]
- 42.Krauss MJ, Evanoff B, Hitcho E, Ngugi KE, Dunagan WC, Fischer I, Birge S, Johnson S, Costantinou E, Fraser VJ. A case-control study of patient, medication, and care-related risk factors for inpatient falls. J Gen Intern Med. 2005;20:116–22. doi: 10.1111/j.1525-1497.2005.40171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


