Serenoa repens (S. repens), commonly called ‘Saw Palmetto’ (SP), is one of the most commonly used herbal remedies (HRs) for easing symptoms of benign prostatic hyperplasia (BHP). The suggested oral dose is usually 320 mg day−1 of dried extract of SP berries [1]. The biological mechanism of SP in counteracting BHP lies in a pool of phytosterols with anti-steroidal properties able to inhibit the synthesis of dihydrotestosterone [2, 3].
In published clinical trials, adverse events associated with SP are quite rare, generally of mild severity and comparable with placebo and finasteride. However, because of the low number of participants (225 in the largest study) [4], clinical trials are not able to capture rare adverse drug reactions (ADRs). Pharmacovigilance is therefore the most appropriate tool for assessing the safety of both synthetic and herbal drugs in post-marketing clinical practice, through spontaneous reporting.
Concerning the toxicity of S. repens, preparations of this herb have been associated with cholestatic hepatitis [5], as well as liver damage and pancreatitis [6]. Here we report a case of liver injury related to a commercial preparation of S. repens (marketed as a food supplement), including evaluation of the possible presence of contaminants.
In May 2006, a 58 year-old Caucasian man was admitted to the Internal Medicine ward of the ASL5 Hospital (Pisa, Italy) because of severe pain in the right hypochondrium and asthenia. The patient did not have any predisposing factors or chronic disease and denied consumption of any medication, alcohol or substance of abuse. Nevertheless he had taken during the last week a commercially available preparation of S. repens to ease the symptoms of BHP, at the dose suggested by the producer of 3 capsules day−1, equal to 900 mg of dried extract and 660 mg of berry powder. The physical examination revealed slight soreness and tenderness of the right hypochondrium. Blood tests showed hypertransaminasaemia [ALT 1237 IU l−1 (reference values 1–45), AST 550 IU l−1 (1–36), γGT 456 IU l−1 (5–40)], high cholestasis indexes [total bilirubin was 2 mg dl−1 (0.3–1.1); indirect bilirubin was 1.5 mg dl−1 (0.1–0.4)], and LDH 975 UI l−1 (110–220); the patient had previously been diagnosed with Gilbert's syndrome. Other blood tests, renal function, cardiac electrolytes and enzymes were in the normal range. An abdominal ultrasound scan revealed mild liver enlargement suggesting patchy steatosis. Biliary ducts, gallbladder, spleen, portal vein and mesogastrium were normal; BHP was also confirmed.
A diagnosis of acute liver injury was consequently made and further tests were performed in order to establish the aetiology. Virus markers for HBV, HCV, HAV, EBV were negative, while the anti-CMV IgG was positive [11.5 (0.0–15.0)]; a subsequent blood sample confirmed the positivity of anti-CMV IgG, but not IgM. Antigen blood levels and a PCR quantification were negative for CMV.
Meanwhile, a possible association with S. repens extract was hypothesized and the patient was encouraged to discontinue the therapy. All symptoms disappeared within a few weeks and a rapid decrease in all altered markers was also observed; at a follow up conducted 10 days after S. repens discontinuation, all blood tests were found between normal values. Taking into account all these aspects, the attending physician sent an ADR report to the Pharmacovigilance Authority, and causality assessment was graded as ‘probable’ for S. repens[7, 8].
To exclude definitely the presence of contaminants, laboratory analyses on the remaining capsules of SP were performed by means of a high-performance liquid chromatography–mass spectrometry (HPLC-MS; Varian 500-MS LC Ion Trap; Varian, Inc., Palo Alto, CA, USA) and inductively coupled plasma mass spectroscopy (ICP-MS), to rule out the presence of synthetic drugs or chemical contaminants, as well as heavy metals (Figure 1). The HPLC-MS profile of the extract was congruent with that of S. repens berries [9], confirming the correct identification of the herbal drug and the absence of other non-declared herbs. The chemical profiling of the extract also showed that the total amount of fatty acids contained in each capsule accounted for only 22% of total weight (i.e. 345 ± 15 mg out of the recommended dose of 1560 mg day−1).
Figure 1.
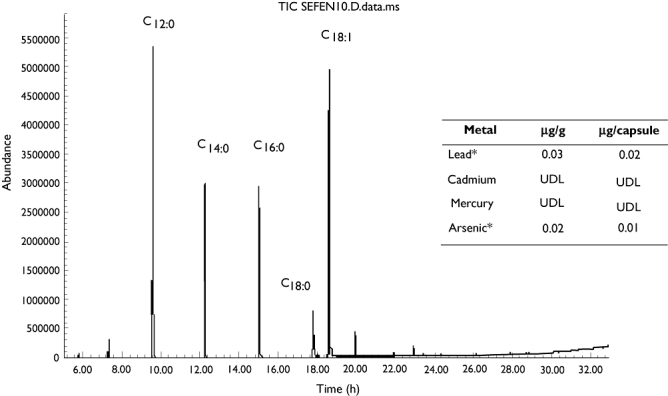
HPLC/MS analysis of the product consumed by the patient. According to Schantz MM et al.[7], peaks compatible only with Serenoa repens were revealed: lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C12:0), oleic acid (C18:0). UDL, under detectable level. *Within accepted levels for alimentary consumption (10 µg l−1)
A recent follow up, conducted more than a year after the episode, confirmed the absence of any clinical and laboratory signs of residual liver injury. This is not the first reported case of liver injury due to S. repens, but no previously published report included a qualitative evaluation of the suspected product. In this case we were also able to exclude both natural, synthetic and chemical contaminants (including heavy metals), as possible alternative causes of acute liver damage, which was definitely attributed to S. repens.
Not all the reports of adverse events due to herbal drugs can include a product analysis, making it sometimes difficult to define causality. Indeed it is widely known that the composition of HRs varies, even for commercially distributed products [2].
This case signals relevant alerts from a Public Health perspective: unlike other medications, most HRs are marketed as ‘dietary supplements’, and are used as self-medication, without any clinical control [10]. In the present case the patient had consumed a significant dosage of SP (900 mg day−1 of dried extract plus 660 mg day−1 of berry powder, corresponding to 345 mg day−1 of SP fatty acids) without reporting its use to the physician, until explicitly asked; this further denotes a misconception regarding herbal products which are often erroneously considered devoid of any risk. This is particularly important in the case of S. repens, whose clinical utility for BPH symptoms has been recently questioned, especially when compared with synthetic drugs [11].
In conclusion, spontaneous reporting of ADRs appears to be a pivotal tool in raising ‘signal alarms’ in the field of herbal medicine due to the limited capability of clinical trials to identify rare ADRs, patients' scanty knowledge of the safety of herbal products, as well as the often unclear regulatory aspects.
Competing interests
None to declare.
REFERENCES
- 1.Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, Avins AL. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557–66. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 2.Di Silverio F, Monti S, Sciarra A, Varasano PA, Martini C, Lanzara S, D'Eramo G, Di Nicola S, Toscano V. Effects of long-term treatment with Serenoa repens (Permixon) on the concentrations and regional distribution of androgens and epidermal growth factor in benign prostatic hyperplasia. Prostate. 1998;37:77–83. doi: 10.1002/(sici)1097-0045(19981001)37:2<77::aid-pros3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Singh YN, Devkota AK, Sneeden DC, Singh KK, Halaweish F. Hepatotoxicity potential of saw palmetto (Serenoa repens) in rats. Phytomedicine. 2007;14:204–8. doi: 10.1016/j.phymed.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Avins AL, Bent S, Staccone S, Badua E, Padula A, Goldberg H, Neuhaus J, Hudes E, Shinohara K, Kane C. A detailed safety assessment of a saw palmetto extract. Complement Ther Med. 2008;16:147–54. doi: 10.1016/j.ctim.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid S, Rojter S, Vierling J. Protracted cholestatic hepatitis after the use of prostata. Ann Intern Med. 1997;127:169–70. doi: 10.7326/0003-4819-127-2-199707150-00033. [DOI] [PubMed] [Google Scholar]
- 6.Jibrin I, Erinle A, Saidi A, Aliyu ZY. Saw palmetto-induced pancreatitis. South Med J. 2006;99:611–12. doi: 10.1097/01.smj.0000215642.76198.44. [DOI] [PubMed] [Google Scholar]
- 7.Kelly WN, Arellano FM, Barnes J, Bergman U, Edwards RI, Fernandez AM, Freedman SB, Goldsmith DI, Huang KA, Jones JK, McLeay R, Moore N, Stather RH, Trenque T, Troutman WG, van Puijenbroek E, Williams F, Wise RP. Guidelines for submitting adverse event reports for publication. Drug Saf. 2007;30:367–73. doi: 10.2165/00002018-200730050-00001. [DOI] [PubMed] [Google Scholar]
- 8.Teschke R, Schwarzenboeck A, Hennermann KH. Causality assessment in hepatotoxicity by drugs and dietary supplements. Br J Clin Pharmacol. 2008;66:758–66. doi: 10.1111/j.1365-2125.2008.03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schantz MM, Bedner M, Long SE, Molloy JL, Murphy KE, Porter BJ, Putzbach K, Rimmer CA, Sander LC, Sharpless KE, Thomas JB, Wise SA, Wood LJ, Yen JH, Yarita T, NguyenPho A, Sorenson WR, Betz JM. Development of saw palmetto (Serenoa repens) fruit and extract standard reference materials. Anal Bioanal Chem. 2008;392:427–38. doi: 10.1007/s00216-008-2297-0. [DOI] [PubMed] [Google Scholar]
- 10.Vannacci A, Lapi F, Baronti R, Gallo E, Gori L, Mugelli A, Firenzuoli F. Too much effectiveness from a herbal drug. Br J Clin Pharmacol. 2009;67:473–4. doi: 10.1111/j.1365-2125.2009.03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tacklind J, Macdonald R, Rutks I, Wilt TJ. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2009;2:CD001423. doi: 10.1002/14651858.CD001423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


