Abstract
Angiotensin II (AngII) – induced hypertension in experimental animals has been proposed to be due in part to activation of the sympathetic nervous system. This sympathetic activation appears to be accentuated in animals consuming a high salt diet (AngII-salt hypertension). However, accurate quantification of sympathetic activity is difficult and controversy remains. A particularly important question is: What are the critical vascular beds targeted by increased sympathetic nerve activity (SNA) in AngII-salt hypertension? To address this issue, mean arterial pressure (MAP) and renal (RSNA) or lumbar SNA (LSNA) were continuously recorded during a 5 day control period, 11 days of AngII (150 ng/kg/min, sc) and a 5 day recovery period in conscious rats on a high salt (2% NaCl) diet. Whereas MAP reached a new steady-state level of 30-35 mmHg above control levels by the end of the AngII period, RSNA decreased by 40% during the first 7 days of AngII and then returned towards control levels by day 10 of AngII. In contrast, LSNA remained at control levels throughout the AngII period. In another experiment we measured hindlimb norepinephrine (NE) spillover in conscious rats on normal (0.4%) or high (2.0%) salt diets before and during 14 days of AngII administration. AngII had no significant affect on hindlimb NE spillover in either group. We conclude that chronic AngII modulates renal and lumbar SNA differentially in rats consuming a high salt diet and that AngII-salt hypertension in the rat is not caused by increased SNA to the renal or hindlimb vascular beds.
Keywords: hypertension, renal nerve activity, lumbar nerve activity, norepinephrine spillover, sympathetic
INTRODUCTION
Hypertension caused by chronic infusion of angiotensin II (AngII) in experimental animals is likely mediated, at least in part, by activation of the sympathetic nervous system. Sympathoexcitation by circulating AngII in experimental animals was first demonstrated over three decades ago 1, however, the degree to which the sympathetic nervous system contributes to AngII-induced hypertension, relative to non-neural mechanisms, remains an area of active investigation with conflicting results. Chemical sympathectomy has been reported both to have no effect 2 and to totally block 3 hypertension during chronic AngII infusion in rats. Plasma norepinephrine concentration has been reported to be unchanged by chronic AngII infusion in one study 4 and increased in another 5. Other methods of assessing sympathetic control of arterial pressure consistently indicate increased net neurogenic pressor activity during chronic AngII infusion. Ganglion-blockade, adrenergic receptor blockade, and centrally acting sympatholytic drugs all cause a much larger fall in arterial pressure in AngII-infused animals than in normotensive controls 6-10.
One explanation for the apparently conflicting results obtained in studies attempting to relate AngII and sympathetic nerve activity (SNA) is that the magnitude of the sympathoexcitatory actions of AngII seems to be strongly affected by the prevailing sodium chloride (salt) intake. For example, in one study chronic infusion of AngII in rats did not increase plasma norepinephrine in rats on normal salt intake, but did so in rats on high salt intake 5. We have also reported that AngII administration increases overall sympathetic activity in rats on a high salt diet, but not in rats on a normal salt diet. This was established using two methods to assess “whole body” sympathetic activity, i.e. whole body norepinephrine spillover 11 and ganglionic blockade 12.
In contrast to whole body indicators of sympathetic outflow, indirect measures of organ specific sympathetic activity in conscious animals have not generally supported the hypothesis that chronic AngII is sympathoexcitatory. Kline et al 6 found no change in norepinephrine turnover in the heart, kidney, gastrointestinal tract or skeletal muscle of the rat in response to AngII. Two indirect measures of renal sympathetic activity, renal norepinephrine spillover 4 and the split bladder technique 13, suggests that AngII infusion actually decreases SNA to the kidney in conscious dogs. However, all of these studies were conducted in animals on a normal salt intake.
Assessing sympathetic activity by direct measurement of sympathetic nerve discharge is difficult due to the challenge of maintaining neural recordings for long periods of time in conscious animals. Although SNA recordings generally can be maintained for a few days following electrode implantation, stress from surgery may affect basal nerve activity and responses to interventions. Moreover, comparison of SNA between animals is not possible since the absolute level of multiunit nerve activity is determined by the quality of electrode contact with the nerve and the degree of tissue reaction at the implantation site. Furthermore, a universally accepted method for quantifying SNA has not been established 14, 15. With this caveat in mind, there is one report that splanchnic SNA is elevated in conscious rats after 14 days of AngII administration compared to vehicle treated animals 16. Interpretive limitations of between-animal comparisons of SNA can be circumvented by measuring SNA before, during and after chronic AngII administration in the same animal; however this is technically very difficult. One group has successfully made such measurements using continuous radiotelemetric recording of renal SNA in rabbits 17, 18. The results led to the same conclusion as earlier findings using indirect methods 4, 13 that chronic AngII treatment decreases sympathetic activity to the renal vascular bed. It is important to note that all of the studies discussed above were conducted in animals on a normal salt diet. Therefore the sympathoexcitatory actions of AngII may not have been fully expressed.
The present experiments were conducted with two objectives in mind. The first was to utilize direct continuous recording of SNA in conscious rats to establish whether chronic administration of AngII increases sympathetic outflow in rats consuming a high salt diet. The second objective was to determine whether the response of directly recorded SNA to AngII was uniformly expressed in different vascular beds. Arterial pressure and renal or lumbar SNA were continuously recorded during a 5 day control period, 10 days of AngII and a 5 day recovery period in rats on a 2% NaCl diet. In another experiment, we measured hind limb NE spillover in response to chronic AngII infusion in conscious rats on normal (0.4%) and high (2.0%) salt diets.
METHODS
Animals and diets
Male Sprague Dawley rats (Charles River, Wilmington, MA) ranging in weight from 250 to 350 grams were used in all studies. Upon arrival rats were placed on either a 0.4% (normal) or 2.0% (high) NaCl diet (Research Diets, Inc., New Brunswick, NJ) for at least 1 week prior to instrumentation and remained on these diets for the duration of the protocol. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Minnesota and the Michigan State University Institutional Animal Care and Use Committees.
RSNA and LSNA responses to AngII or vehicle administration in rats on a high salt diet
Surgical instrumentation
Rats were anesthetized (pentobarbital; 65mg/kg, ip) and prepared for placement of a recording electrode on either the renal or lumbar nerve. The construction of the bipolar stainless steel electrode as well as the method for implantation for long-term recording in conscious rats has been described 19-21. Briefly, the left renal nerve was approached retroperitoneally whereas the left lumbar nerve was approached via a ventral midline laparotomy. The quality of the signal was monitored during surgery using a differential amplifier (MEG-1200, Nihon Kohden, Tokyo) output to an oscilloscope (Hitachi, Model VC 6020, Twinsburg, OH) and speaker (Grass Instruments, Model AM8, Quincy, MA). Once the best signal was obtained, as subjectively determined by the oscilloscope trace and auditory signal, the electrode was secured into place using a two-component silicone rubber (604, Wacher-Chemie, Munich, Germany). The electrode cable was then tunneled to the scapular region. Then, a telemeter for continuous recording of arterial pressure and heart rate (model TA11PA-C40, DSI, Intl. St. Paul, MN) was implanted as previously described 22. Incisions were closed and the rats were allowed to recover on a heated pad. At that time the leads of the electrode were attached to an 8 conductor poles electrical swivel (ES8, Biotex, Kyoto). Upon recovery from anesthesia the rats were transferred to our chronic monitoring laboratory and housed individually in custom made Plexiglass® cages inside a Faraday cage to minimize electrical noise. The cages were constructed from 10” diameter Plexiglass® cylinders 17” high. The floor of the cage was a stainless steel grid which was connected to the common ground. The electrical swivel was mounted above the cage and the telemetry receiver was placed behind it to continuously monitor arterial pressure and heart rate.
Data acquisition and analysis
SNA signals, RSNA or LSNA, were amplified by a differential amplifier (MK-2, Bioteck, Kyoto, Japan: gain x 10000 and bandwidth 150-2000 Hz). Part of this output was fed through a voltage integrator (AD-600G, Nihon Kohden, Tokyo, Japan) with a time constant of 0.1 sec. Arterial pressure signals from the telemetry receiver were converted into an analog signal using a calibrated pressure output adapter (model R11-CPA, DSI, Intl. St. Paul, MN). The analog outputs for arterial pressure and differential amplifier, and the voltage integrator were sampled at 1000 Hz using a 14 bit A/D converter (NI-6221, National Instruments, Austin, TX). The sampled data were either saved directly into a computer for offline analysis of the raw waveform, or saved after parameter extraction for 24hr continuous recordings. A custom computer interface for performing these tasks was written in LabView (National Instruments, Austin, TX, USA).
Parameters extracted from the sampled analog signal included the area under the integrated SNA curves, the background noise for the SNA recording, heart rate, and mean arterial pressure. The area under the integrated SNA curves was calculated every 1 s usingLabview (National Instruments, Austin, TX, USA). The background noise level of the SNA signal was set to be the lowest value from a 1s segment of the integrated SNA curve. The validity of this assumption was established by comparing this parameter during free running and after intra-venous infusion of phenylephrine (10 μg) in the same rat. There were no differences in the values obtained during these two conditions, indicating that the lowest value from a 1s segment of the integrated SNA was a good estimate of the background noise (unpublished observation). Heart rate was estimated from the arterial pressure waveform. Ten seconds averages for these values were stored on hard disk through the duration of the study.
Experimental Protocol
All rats were acclimated to a 2.0% NaCl diet for 1 week prior to instrumentation. Following a 1 week recovery period from surgery, baseline measurements of mean arterial pressure (MAP), heart rate (HR) and either RSNA or LSNA were taken. All variables were recorded continuously 24h/day for the entire protocol. On the 5th day of control measurements, rats were briefly anesthetized with isoflurane, weighed and then an osmotic minipump (Alzet model 2ML2, Durect Corp., Cupertino, CA) filled with either AngII or saline vehicle was implanted. The concentration of AngII was adjusted to deliver an initial dose of 150 ng/kg/min based on body weight at the time of implantation. Rats were returned to their cage and monitored for 10 days of AngII or vehicle infusion. Then rats were again anesthetized with isoflurane, the minipumps were removed and the rats returned to their cage for 5 additional days of monitoring. Thus, beginning 7 days after surgery, the 21 day protocol consisted of 5 control days, 11 days of AngII or vehicle and 5 days of recovery. To quantify the RSNA and LSNA responses to AngII or vehicle the percentage changes in SNA were calculated by taking the mean of these values during the 5 day control period as 100%.
Hindlimb NE spillover and hemodynamic responses to AngII in rats on normal and high salt diets
Surgical instrumentation
Since hind-limb NE spillover predominantly reflects sympathetic activity to skeletal muscle, and should parallel direct measurement of lumbar SNA, we developed a technique to repeatedly assess regional NE spillover to the hind-limbs in rats receiving a chronic infusion of AngII. This required short-term infusion of 3H-NE into the jugular vein to achieve steady-state plasma concentrations, simultaneous sampling of arterial (terminal aorta) and venous (terminal vena cava) blood from the hind-limbs and measurements of hind-limb blood flow (terminal aorta).
Under general anesthesia (2% isoflurane), silicone-tipped catheters were inserted into the terminal aorta, via the left femoral artery and the terminal vena cava via the left femoral vein. Another silicone-tipped catheter was inserted into the right jugular vein and advanced 3 cm’s to the level of the right atrium where it was secured in place. Following a ventral midline laparotomy, the abdominal viscera were reflected and packed with saline soaked gauze. The terminal aorta was isolated from the vena cava and surrounding tissue and a 2mm perivascular flow probe (2SB series, Transonic Systems, Inc) was placed on the isolated section of the aorta just proximal to the iliac bifurcation. The probe cable was passed through the abdominal wall and tunneled subcutaneously to exit the rat between the scapulae. The abdominal viscera were replaced and the incision was closed. The free ends the catheters and the flow probe cable were tunneled subcutaneously to exit the rat between the scapulae into a stainless steel spring attached to the rat by a loosely fitted rubber jacket (Instech Laboratories, Inc, Plymouth Meeting, PA). The rats were then loosely tethered in individual plastic cages to allow continuous access to the catheter and flow probe without handling or disturbing the animal.
Experimental Protocol
In this experiment, rats were acclimatized to a 2% NaCl (n=6) or 0.4% NaCl (n=4) diet and, 10 days after surgery, control measurements were made for 2 days and during 14 days of AngII as described above. Hind-limb NE spillover and hemodynamic variables were measured on control day 2 and AngII infusion days 6, 10 and 14. Hind-limb flow was recorded for approximately 60 minutes each day by connecting the flow probe to a dual channel flowmeter (T206, Transonic Systems, Inc., Ithaca, NY), linked to a computerized data acquisition program (PowerLab). MAP was measured simultaneously by connecting the arterial catheter to a pressure transducer which was linked to the computerized data acquisition program. Hind-limb resistance (HLR) was calculated from hind-limb blood flow (HLF) and mean arterial pressure (MAP) as: HLR = MAP/HLF.
Hind-limb vascular bed NE spillover was measured by applying the radioisotope dilution principle as previously described in our study of whole body NE spillover 11. This requires short-term infusion of 3H-NE to achieve steady-state plasma concentrations, simultaneous sampling of arterial and venous blood from the hind-limbs and hind-limb blood flow measurements. Levo-[ring-2,5,6-3H]-norepinephrine (specific activity = 40-80 Ci/mmol, concentration 1 mCi/ml, PerkinElmer) was infused into the jugular vein as described previously 11. At the end of the 90 minute 3H-NE infusion period a 1 ml arterial blood sample and 1 ml venous blood sample was obtained simultaneously from the aortic catheter and vena cava catheter. Hematocrit (Hct) was measured in duplicate from the arterial blood sample and NE and 3H-NE concentrations were determined as previously described 11. Calculation of hind-limb NE spillover was made using the established methods for radioisotope dilution estimation of regional NE spillover, published by Eisenhofer 23.
Statistical analysis
Data were analyzed by 2-way analysis of variance for repeated measures followed by the Holm-Sidak method for all post-hoc comparisons (SigmaStat version 3.5). The responses of all variables to AngII were compared within groups by comparison to the final day of the control period. A p value less than 0.05 was considered to be statistically significant.
RESULTS
RSNA and LSNA responses to AngII or vehicle administration in rats on a high salt diet
Figure 1 illustrates the responses of mean arterial pressure (MAP) and RSNA to AngII administration in a single rat consuming a high salt diet. Values represent 1 hour averages beginning 7 days after implantation of the electrode and telemeter. AngII increased MAP approximately 40 mmHg by the end of the infusion period and MAP returned promptly to control levels during the recovery period. In this rat, RSNA decreased by approximately 50% from control levels during the entire period of AngII administration with a slight rebound during the recovery period. Five second traces for both variables for Days 3, 14 (AngII day 9) and 19 (Recovery day 3) are also shown.
Figure 1.
Representative data showing hourly values (top panel) for mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) in a single rat before, during and after AngII administration. Bottom shows 5 second traces for arterial pressure (AP) and RSNA on indicated days.
The mean responses of MAP, RSNA and heart rate (HR) of rats on a high salt diet to either AngII or vehicle are shown in Figure 2. AngII resulted in a steady-state increase in MAP of approximately 35 mmHg during the first 5 days of administration. During this same period RSNA decreased by approximately 40% from control levels. Although MAP remained stable for the duration of the AngII infusion period, RSNA tended to return to control levels such that it was not significantly lower compared to the control period or to the vehicle group. HR decreased over the course of the protocol but there was no difference between the AngII and vehicle groups at any time.
Figure 2.
Group data shows the responses of mean arterial pressure (MAP), renal sympathetic nerve activity (RSNA) and heart rate (HR) to AngII or vehicle administration in rats consuming a high salt diet. * = p < 0.05 compared to Control period (within group). # = p < 0.05 between groups.
The responses of mean arterial pressure (MAP) and lumbar sympathetic nerve activity (LSNA) to AngII administration in a single rat consuming a high salt diet are shown in Figure 3 and the group data for responses to AngII or vehicle are shown in Figure 4. Although the MAP and HR responses were similar to that observed in the RNSA protocol, LSNA remained at control levels during the entire period of AngII administration.
Figure 3.
Representative data showing hourly values (top panel) for mean arterial pressure (MAP) and lumbar sympathetic nerve activity (LSNA) in a single rat before, during and after AngII administration. Bottom shows 5 second traces for arterial pressure (AP) and LSNA on indicated days.
Figure 4.
Group data showing the responses of mean arterial pressure (MAP), lumbar sympathetic nerve activity (LSNA) and heart rate (HR) to AngII or vehicle administration in rats consuming a high salt diet. * = p < 0.05 compared to Control period (within group). # = p < 0.05 between groups.
Hind limb NE spillover and hemodynamic responses to AngII administration in rats on normal and high salt diets
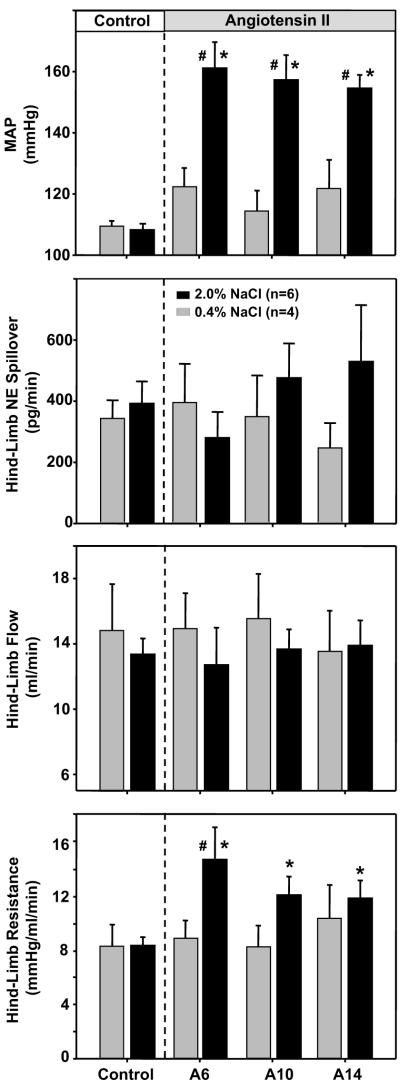
MAP, hind-limb blood flow and hind limb NE spillover were measured on the 2nd day of the control period and on the 6th, 10th and 14th day of AngII administration in rats on a normal or high salt diet (Figure 5). Prior to AngII administration, there were no differences between groups for MAP, hind-limb blood flow, hind-limb vascular resistance or hindlimb NE spillover. On the 6th day of AngII, MAP was significantly increased in rats on a high salt diet but not in rats on a normal salt diet in contrast to hind-limb flow which remained at control levels in both groups. Hind-limb vascular resistance was increased on day 6 of AngII in the high salt group, but did not change in rats on a normal salt diet. By the 10th and 14th days of AngII, MAP remained elevated in high salt rats but hindlimb blood flow and vascular resistance were not different between groups. Finally, although hind limb NE spillover tended to decrease on Day 6 of AngII and appeared to steadily increase above control levels on Day 10 and 14 of AngII, it was not statistically different between groups during the control period or on days 6, 10 and 14 of AngII administration.
Figure 5.
Response of mean arterial pressure (MA), hind-limb norepinephrine (NE) spillover, hind-limb flow and hind-limb resistance to AngII in rats on a normal (0.4%) salt diet (grey bars) or high salt (2.0%) salt diet (black bars). * = p < 0.05 compared to Control period (within group). # = p < 0.05 between groups.
DISCUSSION
The hypothesis that AngII-induced hypertension is at least partly neurogenically mediated has been tested by numerous investigators over the last 40 years and the topic has been extensively reviewed 1, 24, 25. However, the contribution of dietary salt to the chronic sympathoexcitatory actions of circulating AngII has not consistently been considered and may partially explain the disparate results between some reports in which sympathetic activity is increased by AngII 6-10, 12, 26 and others where it is not 2, 13, 17. In other words, the contribution of the sympathetic nervous system to “AngII-induced hypertension” may not be the same as “AngII-salt induced hypertension”. Another issue is that the sympathoexcitatory actions of AngII may not be uniformly expressed to all organs and therefore measurements of “whole body” SNA may not be consistent with “region specific” SNA.
We recently reported that AngII-induced hypertension increases “whole body” sympathetic activity in rats consuming a high salt (2.0% NaCl) diet, but not rats on a normal salt (0.4% NaCl) diet. This was established by two indirect measures of sympathetic activity, the depressor response to ganglion blockade 12 and whole body norepinephrine spillover 26. We have also reported that the arterial pressure response to AngII is salt sensitive in this model 27. Taken together, these observations led us to the hypothesis that circulating AngII and dietary salt act synergistically to cause neurogenic hypertension 28.
The present study was designed to test this hypothesis by direct continuous measurement of sympathetic nerve activity (SNA) in conscious rats over an approximately 4 week period. Direct 24h/day recording of arterial pressure and either renal or lumbar SNA were begun 1 week after instrumentation and continued during a 5 day control period, 11 days of AngII and a 5 day recovery period in rats consuming a high salt diet. To our knowledge, this is the first reported study in the rat in which long-term continuous recording of SNA has been successfully employed to characterize the role of the sympathetic nervous system in the pathogenesis of any model of hypertension. It is also the first report, in any species, to use direct long-term recording methods to measure the response of two sympathetic nerves, in this case renal and lumbar, in any model of hypertension.
Contrary to our hypothesis, renal SNA decreased by approximately 40% during the first 7 days of AngII-salt hypertension and then returned to control levels by the end of the 11 day AngII period. This response contrasted with lumbar SNA which remained at control levels during the entire protocol. Failure of lumbar SNA to change during AngII administration in rats on a high salt diet was consistent with the observation that hindlimb NE spillover also did not change in rats on either normal or high salt diets. We conclude that AngII-salt hypertension in the rat is not mediated by increased sympathetic activity to the renal or hind limb vascular beds.
The response of renal SNA measured in this study is nearly identical to that reported in the elegant studies of Barrett and colleagues 17. Intravenous infusion of AngII for 7 days in conscious rabbits resulted in an approximately 60% fall in renal SNA as determined by continuous direct measurement by radio telemetry. Similar to the present study, renal SNA tended to return to control levels by the end of the AngII period despite a sustained increase in arterial pressure. A subsequent study from the same group showed that the fall in renal SNA during AngII administration does not occur in sinoaortic denervated rabbits 18 suggesting this response is mediated by the arterial baroreceptor reflex. It is important to note however that the steady-state response of arterial pressure to AngII in that study was not affected by sinoaortic denervation, suggesting that baroreceptor reflex control of renal SNA does not determine the steady state response of arterial pressure to AngII administration. This is consistent with the seminal studies by Cowley et al in which sinoaortic denervation did not affect the steady-state hypertensive response to AngII in conscious dogs 29.
The observation that renal SNA is transiently decreased during AngII administration is consistent with our recent report that bilateral renal denervation does not prevent this model of hypertension 30. Indeed, the initial response of arterial pressure to AngII was greater in renal denervated compared to sham control rats suggesting that the decrease in renal SNA observed in the present study may in fact act to buffer the initial hypertensive actions of AngII. The results of the present study, combined with the effects of renal denervation in this model 30, and the studies of Barrett and colleagues 18 strongly suggest that baroreceptor reflex control of renal SNA buffers the initial pressor response to AngII. However, neither the arterial baroreceptor reflex nor renal SNA determine the steady state level of AngII-induced hypertension.
In contrast to renal SNA, we observed that lumbar SNA remained at control levels, and hind limb NE spillover did not change during AngII administration. This finding is consistent with reports that acute infusion of AngII has no effect on lumbar SNA (which largely targets the skeletal muscle vascular bed) in rats under normal conditions but, when the buffering capacity of the baroreceptor reflex is prevented, a sympathoexcitatory response is observed 31. Similarly, in humans, acute intravenous infusion of AngII increases muscle SNA as long as baroreflex-mediated sympathoinhibition is prevented 32. These observations suggest that the direct effect of circulating AngII to acutely increase SNA to skeletal muscle is effectively buffered by the arterial baroreceptor reflex.
Although we did not observe an increase in lumbar SNA in AngII-salt rats, we cannot rule out skeletal muscle vasculature as a neural target in the pathogenesis of hypertension in this model. Maintenance of a normal level of SNA to this large vascular bed, in the face of elevated arterial pressure, may represent an “inappropriate” level of sympathetic nerve discharge under such conditions. In addition, it was recently reported that the pattern, but not the mean level, of lumbar SNA, was altered in a manner that affected vascular resistance in the spontaneously hypertensive rat 33. Additional studies (e.g. lumbar sympathectomy) are required to more directly address this issue in AngII-salt hypertension.
What is the explanation for the combined results of the present study, in which AngII did not increase SNA to two vascular beds, and those of previous reports from our group 12, 26 which suggest that whole body sympathetic activity is increased by AngII administration in rats on a high salt diet? Based on our previous studies utilizing indirect indicators of SNA 12, 30, 34, we hypothesize that the neurogenic component of AngII-salt hypertension is caused by increased sympathetic activity to the splanchnic vascular bed. This idea is consistent with an early study which reported that intravertebral artery infusion of AngII increased splanchnic, but decreased renal SNA, in the anesthetized dog 35. This hypothesis is also supported by a study in which splanchnic SNA was directly recorded and found to be increased in conscious rats following chronic AngII 7. However, the level of dietary salt intake was not reported and these recordings were conducted within hours of electrode implantation and were essentially a “snapshot” taken at the steady-state phase of AngII-induced hypertension. The hypothesis that splanchnic SNA is increased in AngII-salt hypertension remains to be confirmed by continuous, direct SNA recordings in unstressed rats.
Finally, although the underlying mechanisms remain to be established, the concept that sympathetic outflow is controlled in a differential manner to maintain homeostasis in response to acute stimuli is now well established 36. The present study is the first demonstration, using long-term continuous recording of SNA in conscious animals, that differential control of SNA also occurs in response to a chronic hypertensive stimulus. The mechanisms responsible for this differential pattern of SNA remain to be established. One recent study showed that an acute increase in circulating AngII has disparate effects on firing rates of barosensitive sympathetic premotor neurons in the brainstem 37. Whether such a mechanism can account for differential changes in peripheral SNA during long-term elevations of plasma AngII remains to be established.
Limitations of the Study
Although direct recording of SNA in conscious animals provides, in some ways, a more detailed insight than indirect assessment of neural control of cardiovascular function, the ideal method for quantification of multiunit nerve activity over time has not been agreed upon 14, 15 and therefore, interpretation of such data is not straightforward. In the present study, measurements of SNA were not started until 7 days following electrode placement to insure that the stress of surgery was not a confounding factor in our interpretation. SNA was normalized to the 5 day control period a time-control groupswere included in the study. Based on the time control groups, and the inclusion of a recovery period following 11 days of continuous AngII infusion , we are confident that SNA was stable over the duration of the protocol. In addition, we have interpreted the results of our SNA measurements in combination with indirect assessment of SNA in the AngII-salt model. For example, our observations that renal SNA only transiently decreases in AngII-salt rats are consistent with our observation that renal denervation does not alter the steady-state response of arterial pressure in this model 30. In the present study, the observation that lumbar SNA did not change is consistent with measurements of hindlimb NE spillover and hemodynamics in AngII-salt rats. We conclude that this consistency of the results using direct and indirect measures lessens the limitations of these approaches when used individually.
PERSPECTIVES
Elevated SNA as an etiologic factor in human essential hypertension was once controversial, but is now widely accepted 38, 39. However, at the present time our collective understanding of the mechanisms linking changes in SNA to the pathogenesis and maintenance of hypertension is fairly limited. The question of how the sympathetic nervous system is linked to hypertension is much more complex than “is SNA increased?” since it is unlikely that SNA is uniformly increased to all target organs. Whereas some forms of hypertension may be associated with one “sympathetic signature”, for example increased cardiac and renal SNA, other forms may be characterized by an entirely different signature. The results of the present study suggest that, although the AngII-salt model in the rat is neurogenically driven 26, 30, increased SNA to the renal and skeletal muscle vascular beds are not critical for the pathogenesis of this form of hypertension. Our previous work on this model suggests that increased SNA to the splanchnic vascular bed is central to the development of hypertension in AngII-salt rats 12, 30. We propose that a clear understanding of the “sympathetic signature” associated with different forms of neurogenic hypertension is critical to the development of novel antihypertensive therapies designed to manipulate regional specific sympathetic nerve activity 27.
Acknowledgments
SOURCES OF FUNDING
This study was funded by a NHLBI grant (R01 HL076312) to the Neurogenic Cardiovascular Diseases Consortium.
Footnotes
DISCLOSURES
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ferrario CM. Neurogenic actions of angiotensin II. Hypertension. 1983;5(supplV):V73–V79. doi: 10.1161/01.hyp.5.6_pt_3.v73. [DOI] [PubMed] [Google Scholar]
- 2.Onishi A, Branch RA, Holycross B, Jackson EK. Caffeine enhances the slow pressor response to angiotensin II in rats. Evidence for a caffeine-angiotensin II interaction with the sympathetic nervous system. J Clin Invest. 1987;80:13–16. doi: 10.1172/JCI113038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon G, Csiky B. Effect of neonatal sympathectomy on the development of structural vascular changes in angiotensin II-treated rats. Journal of Hypertension. 1998;16:77–84. doi: 10.1097/00004872-199816010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Carroll RG, Lohmeier TE, Brown AJ. Chronic angiotensin infusion decreases renal norepinephrine overflow in conscious dogs. Hypertension (Dallas) 1984;6:675–681. doi: 10.1161/01.hyp.6.5.675. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y, Ogata E, Fujita T. Role of chloride in angiotensin II-induced salt-sensitive hypertension. Hypertension. 1991;18:622–629. doi: 10.1161/01.hyp.18.5.622. [DOI] [PubMed] [Google Scholar]
- 6.Kline RL, Chow K-Y, Mercer PF. Does enhanced sympathetic tone contribute to angiotensin II hypertension in rats? Eur J Pharmacol. 1990;184:109–118. doi: 10.1016/0014-2999(90)90671-r. [DOI] [PubMed] [Google Scholar]
- 7.Luft FC. Salt and hypertension: Recent advances and perspectives. J Lab Clin Med. 1989;114:215–221. [PubMed] [Google Scholar]
- 8.Cox BF, Bishop VS. Neural and humoral mechanisms of angiotensin-dependent hypertension. Am J Physiol Heart Circ Physiol. 1991;261:H1284–H1291. doi: 10.1152/ajpheart.1991.261.4.H1284. [DOI] [PubMed] [Google Scholar]
- 9.Gorbea-Oppliger VJ, Fink GD. Clonidine reverses the slowly developing hypertension produced by low doses of angiotensin II. Hypertension. 1994;23:844–847. doi: 10.1161/01.hyp.23.6.844. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Dale WE, Hasser EM, Blaine EW. Acute and chronic angiotensin hypertension: neural and non-neural components, time course, dose dependency. American Journal of Physiology. 1996;271:R200–R207. doi: 10.1152/ajpregu.1996.271.1.R200. [DOI] [PubMed] [Google Scholar]
- 11.King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol.(Reg Int.) 2008;4:R1262–R1267. doi: 10.1152/ajpregu.00819.2007. [DOI] [PubMed] [Google Scholar]
- 12.King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension. 2006;48:927–933. doi: 10.1161/01.HYP.0000243799.84573.f8. [DOI] [PubMed] [Google Scholar]
- 13.Lohmeier TE, Hildbrand DA. Renal Nerves Promote Sodium Excretion in Angiotensin-Induced Hypertension. Hypertension. 1998;31:429–434. doi: 10.1161/01.hyp.31.1.429. [DOI] [PubMed] [Google Scholar]
- 14.Burke SL, Head GA. Method for in vivo calibration of renal sympathetic nerve activity in rabbits. J Neurosci Methods. 2003;127:63–74. doi: 10.1016/s0165-0270(03)00121-3. [DOI] [PubMed] [Google Scholar]
- 15.Guild SJ, Barrett CJ, McBryde FD, Van Vliet BN, Head GA, Burke SL, Malpas SC. Quantifying sympathetic nerve activity; problems and pitfalls, the need for standardization. Exp Physiol. 2010;95:41–50. doi: 10.1113/expphysiol.2008.046300. [DOI] [PubMed] [Google Scholar]
- 16.Luft FC, Wilcox CS, Unger T, Kuhn R, Demmert G, Rohmeiss P, Ganten D, Sterzel RB. Angiotensin-induced hypertension in the rat. Sympathetic nerve activity and prostaglandins. Hypertension. 1989;14:396–403. doi: 10.1161/01.hyp.14.4.396. [DOI] [PubMed] [Google Scholar]
- 17.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What Sets the Long-Term Level of Renal Sympathetic Nerve Activity. A Role for Angiotensin II and Baroreflexes? Circ Res. 2003:92. doi: 10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- 18.Barrett CJ, Guild SJ, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension. 2005;46:168–172. doi: 10.1161/01.HYP.0000168047.09637.d4. [DOI] [PubMed] [Google Scholar]
- 19.Miki K, Kosho A, Hayashida Y. Method for continuous measurements of renal sympathetic nerve activity and cardiovascular function during exercise in rats. Exp Physiol. 2002;87:33–39. doi: 10.1113/eph8702281. [DOI] [PubMed] [Google Scholar]
- 20.Miki K, Oda M, Kamijyo N, Kawahara K, Yoshimoto M. Lumbar sympathetic nerve activity and hindquarter blood flow during REM sleep in rats. Journal of Physiology. 2004;557(Pt 1):261–271. doi: 10.1113/jphysiol.2003.055525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miki K, Yoshimoto M, Tanimizu M. Acute shifts of baroreflex control of renal sympathetic nerve activity induced by treadmill exercise in rats. Journal of Physiology. 2003;548(Pt 1):313–322. doi: 10.1113/jphysiol.2002.033050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimoto M, Wehrwein EA, Novotny M, Swain GM, Kreulen DL, Osborn JW. Effect of stellate ganglionectomy on basal cardiovascular function and responses to beta-1 adrenoceptor blockade in the rat. Am J Physiol Heart Circ Physiol. 2008;295:H2447–H2454. doi: 10.1152/ajpheart.00958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhofer G. Sympathetic nerve function:Assessment by radioisotope dilution analysis. Clin Auton Res. 2005;15:264–283. doi: 10.1007/s10286-005-0292-5. [DOI] [PubMed] [Google Scholar]
- 24.Brody MJ, Haywood JR, Touw KB. Neural mechanisms in hypertension. Ann Rev Physiol. 1980;42:441–453. doi: 10.1146/annurev.ph.42.030180.002301. [DOI] [PubMed] [Google Scholar]
- 25.Fink GD. Long-term sympathoexcitatory effect of angiotensin II: A mechanism of spontaneous and renovascular hypertension. Clin Exp Pharm and Phys. 1997;24:91–95. doi: 10.1111/j.1440-1681.1997.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 26.King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1262–1267. doi: 10.1152/ajpregu.00819.2007. [DOI] [PubMed] [Google Scholar]
- 27.Osborn JW, Fink GD. Region specific changes in sympathetic nerve activity in AngII-salt hypertension. Exp Physiol. 2010;95:61–68. doi: 10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 29.Cowley AW, Jr., DeClue JW. Quantification of baroreceptor influence on arterial pressure changes seen in primary angiotensin-induced hypertension in dogs. Circulation Research. 1976;39:779–787. doi: 10.1161/01.res.39.6.779. [DOI] [PubMed] [Google Scholar]
- 30.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Sved AF. Acute sympathoexcitatory action of angiotensin II in conscious baroreceptor-denervated rats. Am J Physiol. 2002;283:R451–R459. doi: 10.1152/ajpregu.00648.2001. [DOI] [PubMed] [Google Scholar]
- 32.Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S-I, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. American Journal of Physiology. 1991;261:R690–R696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- 33.Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol. 2009;587(Pt 3):597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osborn JW, Guzman PP, King A, Fink G. Celiac ganglionectomy abolishes the chronic vasoconstrictor responses to angiotensin II (AngII) in conscious rats consuming a high salt diet. FASEB J. 2007;21 899.893. [Google Scholar]
- 35.Fukiyama K. Central action of angiotensin and hypertension - increased central vasomotor outflow by angiotensin. Japanese Circulation Journal. 1972;36:599–602. doi: 10.1253/jcj.36.599. [DOI] [PubMed] [Google Scholar]
- 36.Morrison SF. Differential control of sympathetic outflow. Am J Physiol . 2001;281:R683–R696. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- 37.McMullan S, Goodchild AK, Pilowsky PM. Circulating angiotensin II attenuates sympathetic baroreflex by reducing barosensitivity of medullary cardiovascular neurones in the rat. J Physiol. 2007;582:711–722. doi: 10.1113/jphysiol.2007.128983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esler M. Sympathetic activity in experimental and human hypertension. In: Bulpitt CJ, editor. Pathophysiology of Hypertension. Vol. 17. Elsevier Science; Amsterdam: 1997. pp. 628–673. [Google Scholar]
- 39.Guyenet PG. The sympathetic control of blood pressure. Nature Reviews Neuroscience. 2006;7:335–346. doi: 10.1038/nrn1902. [Review] [119 refs] [DOI] [PubMed] [Google Scholar]