Abstract
Background
The Population Council studied a pre-coital contraceptive microbicide vaginal product containing levonorgestrel (LNG) as active component and Carraguard® gel as a vehicle (Carra/LNG gel) for couples who engage in occasional unplanned intercourse. The objective of this study was to evaluate the effect of sexual intercourse after vaginal application of Carra/LNG gel on serum levels of LNG in women and to assess LNG absorption by the male partner.
Study Design
This was a randomized, cross-over, pharmacokinetic study including an abstinence arm and an arm in which couples engaged in sexual intercourse between 2 and 4 h after gel application. In each study arm, each woman received a single application of Carra/LNG gel (0.75 mg in 4 mL gel) followed by serial blood samples taken at 0, 1, 2, 4, 8, 24 and 48 h after gel application for LNG measurements. In the intercourse arm, LNG was measured in blood samples taken from the male partner before intercourse and at 4, 8 and 24 h after gel application in the female partner.
Results
Time concentration curves for serum LNG levels showed a mean Cmax of 7.8±5.5 and 8.3±5.7 nmol/L, a mean Tmax of 6.2±5.9 and 7.5±5.7, and comparable area under the curve for the intercourse and abstinence arm, respectively. Pharmacokinetic parameters presented large variability between subjects, but excellent reproducibility within each subject. LNG was undetectable in 10 out of 12 male partners.
Conclusion
Sexual intercourse does not appear to interfere with vaginal absorption of LNG after application of a Carra/LNG gel. A vaginal pre-coital contraceptive gel is feasible.
Keywords: Pharmacokinetics, LNG, Vaginal absorption, Emergency contraception, Pre-coital contraceptive gel
1. Introduction
More than 20 years have passed since the first AIDS case report, and in spite of worldwide intensive efforts to find a way to stop the AIDS pandemic, the grim reality is that today nearly 40 million people are living with HIV [1]. For sexually active women, the only available way for prevention is to be in a mutually monogamous relationship with an HIV-negative partner, or the use of condoms. Neither of these prevention measures is in the control of women, and both depend on her ability to negotiate condom use or on the faithfulness of her partner. Furthermore, many couples engage in unplanned sexual intercourse, which puts them at risk for both pregnancy and sexually transmitted diseases, including HIV. In an effort to respond to the need of dual protection for couples who engage in occasional intercourse, the Population Council initiated the development of a pre-coital contraceptive microbicide which contained the potential microbicide Carraguard® used as the vehicle for the progestin levonorgestrel (LNG) as the contraceptive, which would prevent fertilization.
Carraguard® (carrageenan PDR98-15: CARRA), the lead microbicide of the Population Council, had shown antiviral and antibacterial properties against HIV, herpes simplex virus type 2 and Neisseria gonorrhoeae [2–7] in vitro, and its safety had been confirmed by laboratory and Phase I and II clinical studies [6,8–10].
The synthetic progestin LNG was chosen for this combined microbicide/contraceptive product since it has been widely used as an emergency contraceptive. A large multicenter study reported a pregnancy rate of 1.8% and 1.5%, after administration of two doses of 0.75 mg LNG given 12 h apart or a single dose of 1.5 mg [11].
The feasibility of the Carra/LNG vaginal gel was first tested in a pharmacokinetic study comparing two formulations containing 750 and 1500 mcg of LNG in 4 mL of Carraguard®. Maximum serum levels between 3.5 and 22.01 nmol/L were attained at 4–12 h following application confirming the vaginal absorption of this steroid [12].
Based on those results, a proof of concept study that evaluated whether a single vaginal administration of 0.75 mg Carra/LNG gel applied prior to intercourse would interfere with the ovulatory process demonstrated that the Carra/LNG 0.75 mg gel adversely affected the ovulatory process as effectively as or even better than the oral administration of either 0.75 or 1.5 mg of LNG (as a single or split dose) [13].
In those previous studies, women were abstinent after gel application, but since this product was being developed to be used prior to sexual intercourse with the dual function of contraceptive on demand and microbicide, it was imperative to assess the effect of sexual intercourse on LNG absorption by both female and male partners.
The objective of this trial was therefore to evaluate the effect of sexual intercourse after vaginal application of 0.75 mg LNG dispersed in Carraguard® (Carra/LNG gel) on serum levels of LNG in women. In order to assess whether very low doses of LNG may be absorbed through the epithelium of the penis, LNG serum levels were also measured in the male partner. Secondary objectives were to assess male and female tolerability based on symptoms, genital findings and adverse events reported by each partner. Preliminary acceptability was assessed by questionnaires completed by each partner upon completion of the study.
However, recently released disappointing results of a large Phase III effectiveness trial conducted by the Population Council in South Africa failed to demonstrate the effectiveness of Carraguard® in preventing male-to-female HIV transmission during vaginal intercourse [14]. Therefore, the expectation that led to the development of this potential contraceptive microbicide was not realized. Nevertheless, the results merit publication as the need for dual protection remains strong and second-generation microbicides are being developed for evaluation and potential use. Furthermore, the concept of a vaginally delivered pre-coital emergency contraceptive, on its own, warrants further development as the preliminary results indicated superiority of the vaginal application to the oral administration of LNG in suppressing ovulation [13].
2. Methods and materials
The study was conducted at ICMER in Santiago, Chile, and PROFAMILIA in Santo Domingo, Dominican Republic. Approval was granted by the ethics committee of each center and by the institutional review board of the Population Council (New York, NY, USA).
2.1. Study subjects
A total of 12 healthy women and their male partners were enrolled, six couples at each center, after providing their informed consent. The female participants were 29 to 40 years old, with regular menstrual cycles, protected from pregnancy by tubal ligation, at low risk for sexually transmitted infections, with a body mass index (BMI) of ≤30, normal liver function, no clinical evidence of deep epithelial vaginal disruption as judged by naked eye examination and with no contraindications to oral contraceptive use. Subjects were instructed to avoid use of any intravaginal medication or lubrication, as well as all other concomitant medications, unless medically necessary. The male partners were healthy men, aged 33–61 years, with no epithelial lesions in the penis or genital area, at low risk for sexually transmitted infections, with normal sexual function and willing to abstain from condom use on the day of sexual intercourse after gel application.
2.2. Study drug
Carra/LNG formulation is a suspension solution combining Carraguard® and levonorgestrel. It was manufactured at the Population Council’s Center for Biomedical Research in the laboratory of David M. Phillips (New York, NY, USA). Carraguard® contains purified water, 3% PDR98-15 carrageenan and ρ-hydroxybenzoic methyl ester (methyl paraben) as a preservative. Hydrochloric acid and phosphate buffered saline are used to adjust the pH to 7 and raise tonicity. The synthetic progestin levonorgestrel [D(–)-13β-ethyl-17α-ethinyl-17β-hydroxygon-4-en-3-one] was added as micronized crystals yielding a suspension solution. Each subject package consisted of one individually packaged 5-mL syringe containing 0.75 mg LNG in 4 mL of study gel in a self-seal pouch.
2.3. Study design
This was a randomized, cross-over, pharmacokinetic study. There were two arms in the study: an abstinence arm and an intercourse arm. Each woman participated in both arms in a randomly assigned order. After completing one arm, she crossed-over to the other arm, separated by a washout period of 28±2 days between gel applications. The total duration of the study was approximately 6 weeks.
For each arm, the woman received one application of 0.75 mg Carra/LNG gel administered by the investigator and remained recumbent for 10 min thereafter. In order to avoid menses in the 48-h sampling period, gel application took place within the period from cessation of menses up to the seventh day before the expected date of next menses. In the abstinence arm, couples abstained from intercourse for 48 h after the single application. In the intercourse arm, couples engaged in sexual intercourse between 2 and 4 h after application. Female partners were advised to abstain from vaginal (internal) washing following intercourse, though they were allowed to wash the external genitalia.
Blood samples for the measurement of LNG were taken at 0, 1, 2, 4, 8, 24 and 48 h after each gel application in the female partner. In the intercourse arm, LNG was assayed in blood samples taken from the male partner before sexual intercourse and at 4, 8 and 24 h after the time of vaginal application in his female partner.
2.4. Levonorgestrel measurements
LNG in serum was measured at the Center for Biomedical Research of the Population Council, New York, by a conventional radioimmunoassay (RIA) method using a commercial kit (Immunometrics, London, UK). All samples from a subject were analyzed in the same assay run. The method is an extraction RIA and utilizes tritium-labeled LNG and the specific anti-LNG polyclonal antibody. The sensitivity of the assay was found to be 150 pmol/L (47 pg/mL). The assay is controlled through the use of three internal quality control specimens (low, medium and high with the LNG concentrations at 666, 1000 and 7000 pmol/L, respectively) in every assay. The intra- and inter-assay coefficients of variations were less than 10% in the LNG assays for the three controls used.
2.5. Other parameters
Each subject kept a record of occurrence and severity of adverse events and medications taken during the study using a specially designed diary. This diary was checked at each visit. External genitalia and vaginal inspection by means of visual examination assessed vaginal tolerability from the perspective of the investigator at baseline and at 48 h post gel application. Gel acceptability was also assessed by structured questionnaires administered to both male and female participants at the end of the study.
2.6. Analysis
Serial serum LNG levels and area under the curve (AUC) for three time periods were compared between the same women in the two different arms of the study (abstinent or with sexual intercourse) using three complementary paired techniques: the Wilcoxon nonparametric paired test, the paired t test and a repeated measure analysis of variance to take into account the variation ascribable to clinic as well as that related to sexual activity. Descriptive statistics were used to define Cmax, Tmax and AUC for LNG levels in each arm of the study. Levonorgestrel levels were also measured in the male partner at baseline, 4, 8 and 24 h post gel application (in the female partner).
3. Results
Subjects at the two centers were comparable regarding age, weight and BMI (Table 1), and therefore the data were pooled. Time–concentration curves for mean serum LNG levels following administration of Carra/LNG with or without sexual intercourse were very similar (Fig. 1 and Table 2). Controlling for clinic differences, the repeated measures ANOVA indicated no significant difference at any time point and none over the entire AUC nor over the two component subsets of the AUC which we calculated. The two other paired tests employed did not control for clinic differences. Both found marginally significant differences (p=.04) at 8 h. The Wilcoxon paired test produced a value of p=.050 at 48 h as well. Neither the paired t nor the paired Wilcoxon test had significant findings concerning the AUC. Pharmacokinetic parameters presented large variability between subjects; however, the reproducibility within each subject was excellent with correlation coefficients of 0.96, 0.80 and 0.89, respectively, for Cmax, AUC and Tmax (all p values <.002) (Table 2).
Table 1.
Characteristics of female and male participants by center
| Participant characteristics |
Female |
Male |
||
|---|---|---|---|---|
| Clinic 03 (n=6) |
Clinic 02 (n=6) |
Clinic 03 (n=6) |
Clinic 02 (n=6) |
|
| Age (years) | 36.0±2.4 | 33.2±3.8 | 40.2±5.6 | 35.8±1.7 |
| Weight (kg) | 59.6±9.1 | 59.6±9.2 | 76.0±12.2 | 72.6±7.2 |
| BMI | 24.5±3.3 | 24.4±3.4 | 27.1±3.9 | 24.8±3.4 |
Values are shown as mean±SD.
Fig. 1.
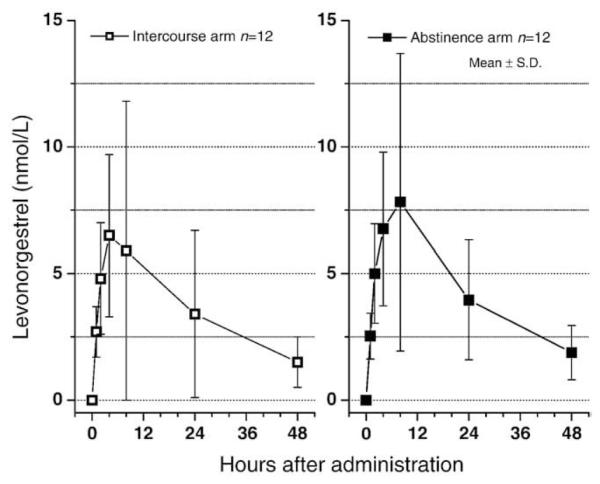
Mean LNG levels following application of Carra/LNG.
Table 2.
Comparison of pharmacokinetic parameters
| Subject no. |
Cmax (nmol/L) |
AUC (nmol/L per hour) |
Tmax (h) |
|||
|---|---|---|---|---|---|---|
| With sex |
Abstinent | With sex |
Abstinent | With sex |
Abstinent | |
| 3001 | 2.9 | 2.9 | 54 | 54 | 4 | 2 |
| 3002 | 21.9 | 24.4 | 466 | 525 | 8 | 8 |
| 3003 | 4.7 | 5.6 | 86 | 137 | 4 | 8 |
| 3004 | 12.8 | 10.8 | 391 | 222 | 8 | 4 |
| 3005 | 9.4 | 11.6 | 119 | 284 | 2 | 4 |
| 3006 | 6.3 | 8.1 | 129 | 193 | 4 | 4 |
| 2001 | 3.0 | 3.6 | 53 | 107 | 4 | 8 |
| 2002 | 9.0 | 7.0 | 334 | 270 | 24 | 24 |
| 2003 | 3.9 | 5.7 | 103 | 168 | 4 | 8 |
| 2004 | 2.9 | 4.8 | 66 | 103 | 4 | 4 |
| 2005 | 7.36 | 6.6 | 134 | 185 | 4 | 8 |
| 2006 | 9.8 | 9.0 | 161 | 276 | 4 | 8 |
| Mean±SD | 7.8±5.5 | 8.3±5.7 | 175±141 | 210±123 | 6.2±5.9 | 7.5±5.7 |
| Range | 2.9–21.9 | 2.9–24.4 | 54–466 | 54–525 | 2–24 | 2–24 |
Nonsignificant differences between two arms.
Levonorgestrel levels were undetectable (<0.16 nmol/L) in 10 out of the 12 male partners. In one subject, LNG was barely detectable at the 4-h sample (0.186 nmol/L), which was taken 35 min after intercourse. In the remaining subject, LNG levels were 0.51 and 0.34 nmol/L, at the 4- and 8-h sample, respectively (90 min and 51/2 h after intercourse). Both of these subjects had very low BMI (20.7 and 22.0).
One woman reported nausea in the evening of one gel application. No other product-related adverse event was reported.
In response to the acceptability questionnaire, one of the 12 women found the gel messy, and two women reported too much lubrication. One suggested that the gel volume should be decreased. Two men also reported too much lubrication and two also suggested reduction in gel volume.
4. Discussion
The results of this study indicate that sexual intercourse does not appear to interfere with the absorption of LNG administered in the form of a Carra/LNG gel applied in the vagina. The AUC is not significantly different without and with intercourse 2–4 h after administration and there are no reasons to believe that the achieved serum levels will not be appropriate to interfere with the ovulatory process as previously published [13].
However, when serum levels with and without intercourse are compared at each hour after administration, a slight but significant lower level was observed at the 8-h sample, just over 4 h after intercourse, suggesting a possible interference of coitus over vaginal absorption of the drug. That effect is biologically plausible considering that there is some leakage of the gel during intercourse and some gel will adhere to the penis.
The concern that some of the steroid could be transferred to the male partner during intercourse was not confirmed, as only two of the 12 subjects had LNG detectable in their circulation and at very low levels. Levonorgestrel, at higher doses than what is expected to be absorbed by the male partner, has been safely used and well tolerated by men in studies of male contraception, both by oral route (125–500 mcg/day) as well as by subdermal implants (160 mcg/day)[15–19].
This study, however, has a limitation. Sexual intercourse took place at a time close to Cmax, indicating that a large part of the drug had already been absorbed and reached the circulation before coital activity. Should this gel become available to the population, it is possible that many women would apply it immediately before intercourse, not 2–4 h prior to sexual intercourse as in this study. This schedule was chosen for the study due to logistical reasons, as the blood collection interval was only 60 min between application and the 1- and 2-h samples, while the next interval increased to 2 h, allowing sufficient time for the couples to leave the clinic, have intercourse and return within this time frame.
Therefore, the question remains whether LNG absorption would be affected if sexual intercourse took place shortly after gel application, before significant absorption has occurred and at a time when partial loss of the gel by leakage and adherence to the penis might reduce the delivered dose. Consequently, it is necessary to repeat this experimental design, but mimicking closely the expected practical mode of use, namely, with intercourse soon after the initial application of the gel.
As emergency contraception, this pre-coital method will have the added advantage of 0-h interval between coitus and administration, which will undoubtedly enhance its effectiveness, as the inverse correlation between length of that interval and effectiveness has been repeatedly demonstrated [11,20].
The results of this study tend to confirm the feasibility of a vaginally administered pre-coital contraceptive gel reaching antiovulatory serum levels of LNG with no interaction of sexual intercourse on the absorption of the progestin.
Footnotes
NICHD Contraceptive Development Center Grant U54 #29990 funded this work.
References
- [1].UNAIDS WHO AIDS epidemic update: special report on HIV/AIDS. Dec, 2006.
- [2].Baba M, Nakajima M, Schols D, Pauwels R, Balzarini J, De Clercq E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antiviral Res. 1988;9:335–43. doi: 10.1016/0166-3542(88)90035-6. [DOI] [PubMed] [Google Scholar]
- [3].Pearce-Pratt R, Phillips DM. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of HIV-1. Biol Reprod. 1996;54:173–82. doi: 10.1095/biolreprod54.1.173. [DOI] [PubMed] [Google Scholar]
- [4].Zacharopoulos VR, Phillips DM. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diag Lab Immunol. 1997;4:465–8. doi: 10.1128/cdli.4.4.465-468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67:5699–708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maguire R, Bergman N, Phillips DM. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytoxicity, antibacterial properties, and sperm immobilization. Sex Transm Dis. 2001;28:259–65. doi: 10.1097/00007435-200105000-00003. [DOI] [PubMed] [Google Scholar]
- [7].Spencer SE, Valentin-Bon IE, Whaley K, Jerse AE. Inhibition of Neisseria gonorrhoeae genital tract infection by leading-candidate topical microbicides in a mouse model. J Infect Dis. 2004;189:410–9. doi: 10.1086/381125. [DOI] [PubMed] [Google Scholar]
- [8].Elias CJ, Coggins C, Alvarez F, et al. Colposcopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception. 1997;56:387–9. doi: 10.1016/s0010-7824(97)00176-5. [DOI] [PubMed] [Google Scholar]
- [9].Coggins C, Blanchard K, Alvarez F, et al. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex Transm Inf. 2000;76:480–3. doi: 10.1136/sti.76.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kilmarx PH, van de Wilgert JH, Chaikummao S, et al. Safety and acceptability of the candidate microbicide Carraguard in Thai women: finding from a Phase II clinical trial. J Acquir Immune Defic Syndr. 2006;43:327–34. doi: 10.1097/01.qai.0000243056.59860.c1. [DOI] [PubMed] [Google Scholar]
- [11].Von Hertzen H, Piaggio G, Ding J, et al. World Health Organization Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803–10. doi: 10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- [12].Sitruk-Ware R, Brache V, Maguire R, et al. Pharmacokinetic study to compare the absorption and tolerability of two doses of levonorgestrel following single vaginal administration of levonorgestrel in Carraguard gel: a new formulation for “dual protection” contraception. Contraception. 2007;75:454–60. doi: 10.1016/j.contraception.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brache V, Croxatto H, Sitruk-Ware R, et al. Effect of a single vaginal administration of levonorgestrel in Carraguard gel on the ovulatory process: a potential candidate for “dual protection” emergency contraception. Contraception. 2007;76:111–6. doi: 10.1016/j.contraception.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Population Council News Release. Johannesburg, South Africa and New York, NY: Feb, 2008. Trial Shows Anti-HIV microbicide is safe, but does not prove it effective. [Google Scholar]
- [15].Bebb RA, Anawalt BD, Khristensen RB, Paulsen CA, Bremner WJ, Matsumoto AM. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab. 1996;81:757–62. doi: 10.1210/jcem.81.2.8636300. [DOI] [PubMed] [Google Scholar]
- [16].Kamischke A, Ploger D, Verherm S, von Eckardstein S, von Eckardstein A, Nieschlag E. Intramuscular testosterone undecanoate with or without oral levonorgestel: a randomized placebo-controlled feasibility study for male contraception. Clin Endocrinol. 2000;53:43–53. doi: 10.1046/j.1365-2265.2000.01024.x. [DOI] [PubMed] [Google Scholar]
- [17].Anawalt BD, Bebb RA, Bremner WJ, Matsumoto AM. A lower dosage levonorgestrel and testoterone combination effectively suppresses spermatogenesis and circulating gonadotropin levels with fewer metabolic effects than higher dosage combinations. J Androl. 1999;20:407–14. [PubMed] [Google Scholar]
- [18].Gonzalo IT, Swerdloff RS, Nelson AL, et al. Levonorgestrel implants (Norplant II) for male contraception clinical trials: combination with transdermal and injectable testosterone. J Clin Endocrinol Metab. 2002;87:3562–72. doi: 10.1210/jcem.87.8.8710. [DOI] [PubMed] [Google Scholar]
- [19].Pollanen P, Nikkanen V, Huhtaniemi I. Combination of subcutaneous levonorgestrel implants and transdermal dihydrotestosterone gel for male hormonal contraception. Int J Androl. 2001;24:369–80. doi: 10.1046/j.1365-2605.2001.00319.x. [DOI] [PubMed] [Google Scholar]
- [20].Piaggio G, von Hertzen G, Grimes DA, VanLook PF. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Task Force on Postovulatory Methods of Fertility Regulation. Lancet. 1999;353:721. doi: 10.1016/s0140-6736(98)05718-3. [DOI] [PubMed] [Google Scholar]


