Abstract
It is now well accepted that many forms of experimental hypertension and human essential hypertension are caused by increased activity of the sympathetic nervous system. However, the role of region-specific changes in sympathetic nerve activity (SNA) in the pathogenesis of hypertension has been difficult to determine because methods for chronic measurement of SNA in conscious animals have not been available. We have recently combined indirect, and continuous and chronic direct, assessment of region-specific SNA to characterize hypertension produced by administration of angiotensin II (Ang II) to rats consuming a high-salt diet (Ang II–salt hypertension). Angiotensin II increases whole-body noradrenaline (NA) spillover and depressor responses to ganglionic blockade in rats consuming a high-salt diet, but not in rats on a normal-salt diet. Despite this evidence for increased ‘whole-body SNA’ in Ang II–salt hypertensive rats, renal SNA is decreased in this model and renal denervation does not attenuate the steady-state level of arterial pressure. In addition, neither lumbar SNA, which largely targets skeletal muscle, nor hindlimb NA spillover is changed from control levels in Ang II–salt hypertensive rats. However, surgical denervation of the splanchnic vascular bed attenuates/abolishes the increase in arterial pressure and total peripheral resistance, as well as the decrease in vascular capacitance, observed in Ang II–salt hypertensive rats. We hypothesize that the ‘sympathetic signature’ of Ang II–salt hypertension is characterized by increased splanchnic SNA, no change in skeletal muscle SNA and decreased renal SNA, and this sympathetic signature creates unique haemodynamic changes capable of producing sustained hypertension.
Hypertension is a major risk factor for deaths due to cardiovascular disease and, alarmingly, statistics for 2004 indicate that more than a quarter of the US population has hypertension. The World Health Organization predicts that by the year 2020, hypertension will be the greatest single leading cause of death and disability globally (WHO, 2002). Thus, there can be little debate that insights leading to improved therapeutic treatment of hypertension will have a substantial impact on the health and wellbeing of society. Advances in the development of new antihypertensive therapies are critically dependent on an improved understanding of the long-term regulation of arterial pressure in health and disease. One area of increasing attention in this regard is the sympathetic nervous system.
Elevated sympathetic nerve activity (SNA) as an aetiological factor in human essential hypertension was once controversial, but is now widely accepted (Esler, 1997; Guyenet, 2006). However, at the present time our collective understanding of the mechanisms linking changes in SNA to the pathogenesis and maintenance of hypertension is fairly limited. Much of the work in this field has focused on one of three questions. (1) Is sympathetic activity increased or decreased in hypertension? (2) What is the role of the arterial baroreceptor reflex in hypertension? (3) To what extent is neurogenic hypertension due to increased SNA to the kidney? Although these questions are important, they reflect a fairly limited view of all of the possible avenues for investigation (Osborn et al. 2005). The idea that the sympathetic nervous system acts as a single entity that ‘goes up or down’ is a vast oversimplification which was, in part, necessitated by the limited technology available for the direct continuous measurement of SNA to individual vascular beds in conscious animals. While we acknowledge the importance of arterial baroreceptors and neural control of the kidney in long-term control of arterial pressure, we strongly believe that non-baroreflex control of non-renal vascular beds is also important in some forms of hypertension.
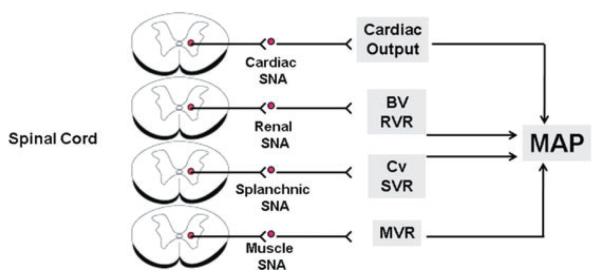
Thus, the question of how the sympathetic nervous system is linked to hypertension is much more complex than ‘is SNA increased?’ As illustrated in Fig. 1, SNA to the heart, kidneys, gastrointestinal tract and skeletal muscle all could impact long-term control of arterial pressure through a variety of mechanisms. It is unlikely that SNA is uniformly increased or decreased to these target organs but rather, the intensity of SNA to different regions over time will differ depending on a variety of inputs to hypothalamic and brainstem circuits that regulate sympathetic nerve discharge. While some forms of hypertension may be associated with one ‘sympathetic signature’, for example increased cardiac and renal SNA, other forms may be characterized by an entirely different signature. Indeed, studies using noradrenaline (NA) spillover methodology reveal substantial regional SNA heterogeneity in different subgroups of human hypertensives, with renal and muscle SNA consistently increased but SNA to the heart, skin and other regions more variable (Grassi et al. 1998; Esler, 2000; Grassi & Esler, 2002). Such heterogeneity in regional NA spillover has also been reported in animal models of hypertension (Patel et al. 1981).
Figure 1. Theoretical pathways linking organ-specific changes in SNA to long-term control of mean arterial pressure.
Theoretical mechanisms whereby changes in cardiac, renal, splanchnic and skeletal muscle sympathetic nerve activity (SNA) can impact long-term control of mean arterial pressure (MAP), resulting in neurogenic hypertension. Changes in SNA result from supraspinal inputs, which ultimately affect inputs to sympathetic preganglionic neurons in the spinal cord. Abbreviations: BV, blood volume; RVR, renal vascular resistance; SVR, splanchnic vascular resistance; Cv, venous capacitance; and MVR, muscle vascular resistance.
The concept of a sympathetic signature refers to more than just a regional pattern of activation; it also incorporates timing, intensity and impact of SNA on arterial pressure. Thus, precise definition of the sympathetic signature associated with any form of hypertension requires direct long-term recording of SNA to individual cardiovascular target organs, as well as assessment of the impact of that SNA on target organ function. Until very recently, direct long-term recording of SNA in conscious animals has been nearly impossible. Most studies have therefore been limited to the use of indirect methods for quantification of SNA regulation of arterial pressure. Such methods, including surgical sympathetic denervation of target organs and acute pharmacological blockade of sympathetic neurotransmission, while useful, do not provide the true measure of time-dependent changes in SNA necessary to define a sympathetic signature.
In this paper, we review studies from our own laboratories and others which support the hypothesis that hypertension caused by increased plasma angiotensin II (Ang II), in combination with a high-salt diet, is associated with a specific sympathetic signature. Support for this hypothesis is provided by studies using indirect and, more recently, direct assessment of SNA in conscious animals and humans. The hallmark of this signature is increased SNA to the splanchnic circulation in contrast to decreased SNA to the kidneys. We will conclude with a discussion of the clinical relevance of understanding the consequences of any specific sympathetic signature in the pathogenesis of cardiovascular diseases.
The Ang II–salt model of hypertension in the rat
Recently, we established a national consortium to investigate systematically, at the molecular, cellular and whole-animal levels, the pathogenesis of neurogenic hypertension (Osborn et al. 2007a). The strategy of the Neurogenic Cardiovascular Diseases Consortium (NCDC) is to study a single animal model intensely at all levels of biological control and then integrate our findings to achieve a detailed understanding of the chosen model with the goal of developing novel therapies to treat neurogenic hypertension.
Our choice of the model to study was based on several considerations. First, in addition to being neurogenic, the model had to have close links to human essential hypertension. Second, the species studied needed to be one in which the latest state-of-the-art technologies used in all participating laboratories could be implemented. Third, the model needed to have a ‘time zero’, thus permitting longitudinal measurements of cardiovascular function before the induction of hypertension and during the critical transition phase between normotension and hypertension. Fourth, the model needed to be easily and reliably produced in all laboratories. Finally, it was preferable to use a model on which there was a large database in the literature from investigators who use a variety of experimental techniques and approaches not presently available among our collaborating laboratories. After careful consideration of all these factors, we chose the model in which Ang II is administered peripherally to the rat in combination with a high-salt diet (i.e. the ‘Ang II–salt model’).
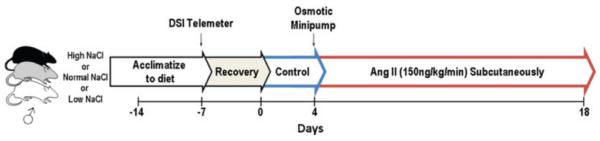
The protocol for producing the model is illustrated in Fig. 2. Seven days prior to instrumentation, male Sprague–Dawley rats (Charles River, Wilmington, MA, USA) are placed on either a low- (0.1%), normal- (0.4%) or high-NaCl diet (2.0%) for the duration of the study. Rats are instrumented with a radio-telemeter (Data Sciences International (DSI), St Paul, MN, USA) for continuous recording of arterial pressure. Following a 7 day control period, Ang II is administered at a dose of 150 ng kg−1 min−1 subcutaneously via an osmotic minipump (Alzet, Cupertino, CA, USA) for 14 days.
Figure 2. Protocol for induction of Ang II–salt hypertension.
Standard protocol for producing the Ang II–salt model of hypertension for interlaboratory study. Rats are acclimated to a low- (0.1%), normal- (0.4%) or high-NaCl diet (2.0%) prior to study. See text for details.
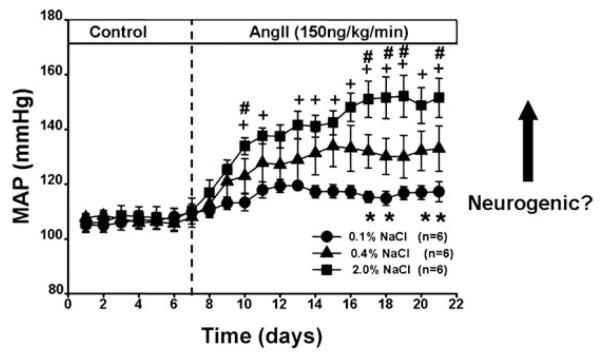
Figure 3 illustrates the response of mean arterial pressure (MAP) to Ang II administration in rats on low-, normal- and high-salt diets. Note that Ang II has minimal effects on MAP in rats on a low-salt diet, and that the hypertensive response is directly related to dietary salt intake. The central hypothesis of the NCDC is that simultaneous increases in plasma Ang II and dietary salt intake produce signals that converge on central autonomic pathways to chronically activate the sympathetic nervous system, which is a major contributor to hypertension in this model (Osborn et al. 2007a). In the studies reviewed below it is important to note that the contribution of dietary salt to the chronic sympathoexcitatory actions of circulating Ang II has not consistently been considered. In other words, ‘Ang II-induced hypertension’ is not necessarily the same as ‘Ang II–salt-induced hypertension’. This distinction will be noted when comparing studies throughout the paper.
Figure 3. Salt sensitivity of Ang II–salt hypertension.
Responses of mean arterial pressure (MAP) to 14 days of Ang II treatment in rats on diets containing 0.1, 0.4 or 2.0% NaCl. Twenty-four hour averages were analysed by two-way analysis of variance for repeated measures followed by the Holm–Sidak method for all post hoc comparisons (SigmaStat version 3.5). Statistically significant differences (P < 0.05) between groups on given days are indicated as: 0.1 versus 0.4% (*), 2.0 versus 0.1% (+) and 2.0 versus 0.4% (#).
‘Whole-body’ sympathetic nervous system activity is increased in Ang II–salt hypertension
The response of ‘whole-body’ SNA to Ang II administration has been assessed indirectly in a number of studies. Two traditional methods of determining the importance of increased SNA in hypertension caused by increased circulating Ang II yielded conflicting results. Chemical sympathectomy was reported either to have no effect (Onishi et al. 1987) or to totally block hypertension (Simon & Csiky, 1998) during chronic Ang II infusion in rats. Plasma NA concentration was unchanged by chronic Ang II infusion in one study (Carroll et al. 1984) and increased in another (Sato et al. 1991). Other methods for assessment of sympathetic control of arterial pressure consistently indicate increased net neurogenic pressor activity during chronic Ang II infusion. Ganglionic blockade, adrenergic receptor blockade and centrally acting sympatholytic drugs all cause a much larger fall in arterial pressure in Ang II-infused animals than in normotensive control rats (Luft, 1989; Kline et al. 1990; Cox & Bishop, 1991; Gorbea-Oppliger & Fink, 1994; Li et al. 1996).
‘Whole-body’ sympathetic activity in the Ang II–salt model of hypertension used in our studies has been measured using two indirect methods, both of which suggest that Ang II administration increases overall sympathetic activity in rats on a high-salt diet, but not rats on a normal-salt diet. First, while whole-body NA spillover is similar in rats on normal- and high-salt diets in control conditions, chronic Ang II administration increases NA spillover only in rats on a high salt intake (King et al. 2008b). The second indirect measure is the magnitude of the depressor response to ganglionic blockade with hexamethonium. Again, we have reported that the depressor response to ganglionic blockade is larger in Ang II-treated rats on a high-salt diet than in those on a normal-salt diet (King & Fink, 2006).
Region-specific sympathetic nervous system activity in Ang II–salt hypertension
There are few reports of region-specific changes in SNA during chronic Ang II infusion, and none that we are aware of in which the combined effects of Ang II and a high-salt diet were studied. Kline et al. (1990) found no change in NA turnover in heart, kidney, gastrointestinal tract or skeletal muscle of the rat in response to Ang II, suggesting that Ang II did not alter SNA in these tissues. However, their studies were conducted in rats on a salt intake equivalent to our normal salt level (0.4%).
Renal sympathetic nerve activity
Indirect assessment of renal SNA in conscious dogs suggests that it is decreased during Ang II infusion (Carroll et al. 1984; Lohmeier et al. 2001). This was later confirmed in rabbits in the first study to record SNA directly over a period of weeks in any animal model of hypertension (Barrett et al. 2003).
We have recently achieved continuous and direct renal SNA recordings in our Ang II–salt model and presented our initial results in abstract form (Yoshimoto et al. 2008a). In rats consuming a high-salt diet, MAP increased by approximately 35 mmHg during Ang II treatment. Renal SNA decreased approximately 40% from control values during the first 8 days of Ang II administration, but recovered to baseline levels by the end of the 14 day Ang II treatment period. In another study, we examined the role of renal nerves in this model by comparing the MAP response to Ang II in intact and renal denervated (RDNX) rats consuming a high-salt diet. The steady-state phase of Ang II–salt hypertension was identical between the two groups, but the initial response to Ang II was accelerated in RDNX rats (King et al. 2007). Taken together, these two studies suggest that the transient decrease in renal SNA during Ang II administration, possibly mediated by the baroreceptor reflex, buffers the hypertensive response to Ang II. However, we conclude that renal SNA plays no role in the steady-state phase of Ang II hypertension. This hypothesis is supported by a recent study in conscious rabbits in which baroreceptor denervation abolished the decrease in renal SNA observed during 7 days of Ang II infusion but did not affect the steady-state response of arterial pressure (Barrett et al. 2005). This conclusion is in contrast to a recent study in which RDNX attenuated Ang II hypertension (Hendel & Collister, 2006). However, in this study rats consumed a much higher-salt diet (4.0% NaCl) and Ang II was administered by a different route (intravenous) at a different dose (12 ng kg−1 min−1).
Lumbar sympathetic nerve activity
Acute infusion of Ang II has no effect on lumbar SNA (which largely targets the skeletal muscle vascular bed) in rats in normal conditions but, when the buffering capacity of the baroreceptor reflex is prevented, a sympathoexcitatory response is observed (Xu & Sved, 2002). Similarly, in humans, acute intravenous infusion of Ang II increases muscle SNA as long as baroreflex-mediated sympathoinhibition is prevented (Matsukawa et al. 1991). These observations suggest that the direct effect of circulating Ang II to acutely increase SNA to skeletal muscle is effectively buffered by the arterial baroreceptor reflex.
We have measured the chronic responses of lumbar SNA in rats consuming a high-salt diet and subjected to the NCDC Ang II protocol described above. Compared with their own control period, and with vehicle-treated rats, lumbar SNA did not change from control levels during the entire Ang II treatment period (Yoshimoto et al. 2008a). Our observation of no change in lumbar SNA in Ang II–salt hypertension was further confirmed using measurements of hindlimb NA spillover in conscious rats subjected to the same protocol. Measurements were made during the control period and on days 7 and 14 of Ang II infusion, and revealed no evidence of increased SNA to the hindlimb vascular bed (King et al. 2008a).
Failure of lumbar SNA to increase in the Ang II–salt model does not rule out the contribution of this vascular bed as a neural target in the pathogenesis of Ang II–salt hypertension for two reasons. First, as recently reported in the spontaneously hypertensive rat (Simms et al. 2009), it is possible that the pattern of lumbar nerve discharge was altered in a manner that affected vascular resistance, even though the mean level of nerve activity was not increased. Second, maintenance of a normal level of SNA to this large vascular bed, in the face of elevated arterial pressure, may represent an ‘inappropriate’ level of sympathetic nerve discharge in such conditions. The observation that, in the face of the same increase in arterial pressure, lumbar SNA does not change but renal SNA decreases implies that the contribution of the baroreceptor reflex to long-term reflex control of SNA to skeletal muscle is weaker than that for renal SNA. The theoretical role of arterial baroreceptors in ‘shaping’ the sympathetic signature of Ang II–salt hypertension will be discussed in a later section.
Splanchnic sympathetic nerve activity
The explanation for the discrepancy between indirect measurement of ‘whole-body’ sympathetic activity and direct measurement of renal and lumbar SNA appears to be that the neurogenic component of Ang II–salt hypertension is caused by increased SNA to the splanchnic vascular bed. This hypothesis is supported by several studies from our laboratories.
First, coeliac ganglionectomy (CGx), which interrupts sympathetic neurotransmission to the splanchnic vasculature, markedly attenuates Ang II–salt hypertension (King et al. 2007). Second, mean circulatory filling pressure (MCFP) is elevated in Ang II–salt-treated rats (King & Fink, 2006). Since blood volume is not elevated, this increase in MCFP reflects an increase in venomotor tone, resulting in reduced total vascular capacitance (King & Fink, 2006). This Ang II-induced increase in MCFP was not observed in rats on a normal-salt diet, and was eliminated by either CGx or ganglionic blockade (King et al. 2007). These results are consistent with the hypothesis that the combination of Ang II and a high-salt diet activates sympathetic nerves to the capacitance vessels in the splanchnic vascular bed. Coeliac ganglionectomy also markedly alters the systemic haemodynamic profile of Ang II–salt hypertension (Osborn et al. 2007b). In rats consuming a high-salt diet, Ang II-induced hypertension is associated with a decrease in cardiac output and a marked increase in total peripheral resistance. The increase in resistance is nearly abolished by CGx, suggesting that it is neurogenically mediated (Osborn et al. 2007b). Overall, these studies suggest that hypertension in Ang II–salt-treated rats is associated with an increase in SNA to capacitance and resistance vessels of the splanchnic vascular bed.
This hypothesis is supported by a study in which splanchnic SNA was directly recorded and found to be increased in conscious rats following chronic Ang II administration (Luft, 1989). However, the level of dietary salt intake was not reported, and these recordings were conducted within hours of electrode implantation and were essentially a ‘snapshot’ taken at the steady-state phase of Ang II-induced hypertension. The hypothesis that splanchnic SNA is increased in Ang II–salt hypertension remains to be confirmed by continuous, direct SNA recordings in unstressed rats.
Cardiac sympathetic nerve activity
The contribution of cardiac SNA to any model of hypertension, using direct or indirect approaches, has received very little attention relative to studies investigating the role of sympathetic nerves to other vascular beds. This is probably due to the difficulty of surgically denervating the heart or directly recording from cardiac sympathetic nerves in conscious animals, particularly in rodents. We have recently reported a method for cardiac denervation in the rat (Yoshimoto et al. 2008b) and found that this procedure has no effect on a different model of hormonally induced salt-dependent hypertension, the deoxycorticosterone acetate–salt model (J.W. Osborn, unpublished observation). However, the contribution of cardiac SNA to Ang II–salt hypertension is currently unknown.
The ‘sympathetic signature’ of Ang II–salt hypertension: a hypothesis
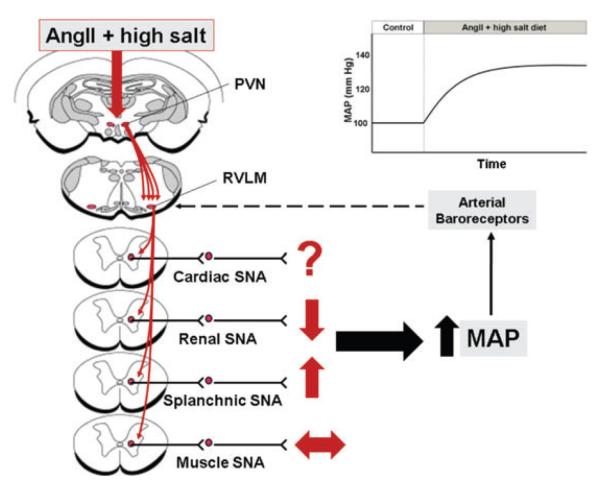
Sympathetic signature incorporates the timing, intensity and impact of SNA on arterial pressure. Studies in our laboratory using both indirect assessment of whole-body SNA, and indirect and direct assessment of organ-specific SNA, have led us to the following hypothesis (Fig. 4). Systemic administration of Ang II to rats consuming a high-salt diet activates the sympathetic nervous system in a highly specialized, organ-specific manner, which ultimately results in hypertension. This pattern of SNA, the sympathetic signature, is defined by increased splanchnic SNA, decreased renal SNA and no change in SNA to the skeletal muscle vascular bed.
Figure 4. Sympathetic signature of Ang II–salt hypertension.
Proposed changes in cardiac, renal, splanchnic and muscle sympathetic nerve activity (SNA), based on studies from the authors’ laboratories and others, which result in long-term increases in mean arterial pressure (MAP). The dashed line from the arterial baroreceptors represents a sympathoinhibitory input to sympathetic premotor neurons in the rostral ventrolateral medulla (RVLM). Excitatory drive to the RVLM by Ang II–salt is proposed to involve the paraventricular nucleus (PVN) of the hypothalamus. See text for details.
At the present time, the mechanisms that ‘shape’ this sympathetic signature are unknown. We propose that circulating Ang II, in combination with a high-salt diet, activates key brain sites, such as the paraventricular nucleus (PVN), that directly drive sympathetic premotor neurons in the rostral ventrolateral medulla (RVLM; Osborn et al. 2007a). What is the explanation for the differential sympathetic response? One possibility is that the central drive is organ specific such that RVLM neurons regulating splanchnic SNA are activated, whereas those controlling muscle and renal SNA are not. This idea is supported by the fact that RVLM sympathetic premotor neurons are organized topographically (McAllen & Dampney, 1990; McAllen & May, 1994). What then explains the transient decrease in renal SNA and lack of change in lumbar SNA in Ang II–salt-treated rats? One hypothesis is that the arterial baroreceptor reflex has a differential impact on the central drive such that it strongly inhibits renal SNA, has less effect on lumbar SNA and has a negligible impact on premotor neurons that regulate splanchnic SNA. Although this is purely speculative and the mechanisms are unknown, Ang II directly applied to the nucleus tractus solitarius (NTS) can evoke selective changes in baroreflex control of different sympathetic outflows (Polson et al. 2007). The presence of salt-sensitive aldosterone-detecting neurons in the NTS (Geerling et al. 2006) provides another possible central site of convergence between Ang II and salt. The extent to which the shape of the sympathetic signature is determined by the balance of organ-specific central excitatory drive and baroreceptor control of organ-specific SNA will be the focus of future studies.
Perspectives and significance
Although it is now accepted that increased SNA is associated with every major cardiovascular disease, our understanding of the region-specific and time-dependent nature of changes in SNA is limited. Furthermore, the detailed cardiovascular consequences of any given sympathetic signature, including those that can cause hypertension, are not well described. Thus, there is a critical need to identify the mechanisms responsible for generating specific sympathetic signatures and the associated haemodynamic effects. This information can then be used to develop novel methods for modulating regional sympathetic activity within time frames that will be most efficacious for therapeutic purposes. Indeed, a novel device-based treatment for organ-specific sympathetic denervation to treat hypertensive humans may represent a new direction of therapy (Krum et al. 2009).
Acknowledgements
Studies in the authors’ laboratories were supported by NIH grant RO1 HL076312 to the Neurogenic Cardiovascular Diseases Consortium.
References
- Barrett CJ, Guild SJ, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension. 2005;46:168–172. doi: 10.1161/01.HYP.0000168047.09637.d4. [DOI] [PubMed] [Google Scholar]
- Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res. 2003;92:1330–1336. doi: 10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- Carroll RG, Lohmeier TE, Brown AJ. Chronic angiotensin infusion decreases renal norepinephrine overflow in conscious dogs. Hypertension. 1984;6:675–681. doi: 10.1161/01.hyp.6.5.675. [DOI] [PubMed] [Google Scholar]
- Cox BF, Bishop VS. Neural and humoral mechanisms of angiotensin-dependent hypertension. Am J Physiol Heart Circ Physiol. 1991;261:H1284–H1291. doi: 10.1152/ajpheart.1991.261.4.H1284. [DOI] [PubMed] [Google Scholar]
- Esler M. Sympathetic activity in experimental and human hypertension. In: Bulpitt CJ, editor. Pathophysiology of Hypertension. vol. 17. Elsevier Science; Amsterdam: 1997. pp. 628–673. [Google Scholar]
- Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:995–1055. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Kawata M, Loewy AD. Aldosterone-sensitive neurons in the rat central nervous system. J Comp Neurol. 2006;494:515–527. doi: 10.1002/cne.20808. [DOI] [PubMed] [Google Scholar]
- Gorbea-Oppliger VJ, Fink GD. Clonidine reverses the slowly developing hypertension produced by low doses of angiotensin II. Hypertension. 1994;23:844–847. doi: 10.1161/01.hyp.23.6.844. [DOI] [PubMed] [Google Scholar]
- Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- Grassi G, Esler M. The sympathetic nervous system in renovascular hypertension: lead actor or ‘bit’ player? JHypertens. 2002;20:1071–1073. doi: 10.1097/00004872-200206000-00013. (Editorial comment.) [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Hendel M, Collister JP. Renal denervation attenuates long-term hypertensive effects of angiotensin II in the rat. Clin Exp Pharmacol Physiol. 2006;33:1225–1230. doi: 10.1111/j.1440-1681.2006.04514.x. [DOI] [PubMed] [Google Scholar]
- King A, Yoshimoto M, Osborn JW, Fink GD. Regional hind-limb (HL) hemodynamics and norepinephrine (NE) spillover in chronic angiotensin II (AngII)–salt hypertension in the rat. Hypertension. 2008a;52:e64. [Google Scholar]
- King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension. 2006;48:927–933. doi: 10.1161/01.HYP.0000243799.84573.f8. [DOI] [PubMed] [Google Scholar]
- King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2008b;294:R1262–R1267. doi: 10.1152/ajpregu.00819.2007. [DOI] [PubMed] [Google Scholar]
- King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- Kline RL, Chow K-Y, Mercer PF. Does enhanced sympathetic tone contribute to angiotensin II hypertension in rats? Eur J Pharmacol. 1990;184:109–118. doi: 10.1016/0014-2999(90)90671-r. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- Li Q, Dale WE, Hasser EM, Blaine EW. Acute and chronic angiotensin hypertension: neural and non-neural components, time course, dose dependency. Am J Physiol Regul Integr Comp Physiol. 1996;271:R200–R207. doi: 10.1152/ajpregu.1996.271.1.R200. [DOI] [PubMed] [Google Scholar]
- Lohmeier TE, Lohmeier JR, Reckelhoff JF, Hildebrandt DA. Sustained influence of the renal nerves to attenuate sodium retention in angiotensin hypertension. Am J Physiol Regul Integr Comp Physiol. 2001;281:R434–R443. doi: 10.1152/ajpregu.2001.281.2.R434. [DOI] [PubMed] [Google Scholar]
- Luft FC. Salt and hypertension: recent advances and perspectives. J Lab Clin Med. 1989;114:215–221. [PubMed] [Google Scholar]
- McAllen RM, Dampney RA. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neurosci Lett. 1990;110:91–96. doi: 10.1016/0304-3940(90)90793-9. [DOI] [PubMed] [Google Scholar]
- McAllen RM, May CN. Differential drives from rostral ventrolateral medullary neurons to three identified sympathetic outflows. Am J Physiol Regul Integr Comp Physiol. 1994;267:R935–R944. doi: 10.1152/ajpregu.1994.267.4.R935. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S-I, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol Regul Integr Comp Physiol. 1991;261:R690–R696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- Onishi A, Branch RA, Holycross B, Jackson EK. Caffeine enhances the slow pressor response to angiotensin II in rats. Evidence for a caffeine-angiotensin II interaction with the sympathetic nervous system. J Clin Invest. 1987;80:13–16. doi: 10.1172/JCI113038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007a;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Guzman PP, King A, Fink G. Celiac ganglionectomy abolishes the chronic vasoconstrictor responses to angiotensin II (AngII) in conscious rats consuming a high salt diet. FASEB J. 2007b;21:899.3. (abstract) [Google Scholar]
- Osborn JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2005;288:R846–R855. doi: 10.1152/ajpregu.00474.2004. [DOI] [PubMed] [Google Scholar]
- Patel KP, Kline RL, Mercer PF. Noradrenergic mechanisms in the brain and peripheral organs of normotensive and spontaneously hypertensive rats at various ages. Hypertension. 1981;3:682–690. doi: 10.1161/01.hyp.3.6.682. [DOI] [PubMed] [Google Scholar]
- Polson JW, Dampney RA, Boscan P, Pickering AE, Paton JF. Differential baroreflex control of sympathetic drive by angiotensin II in the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1954–R1960. doi: 10.1152/ajpregu.00041.2007. [DOI] [PubMed] [Google Scholar]
- Sato Y, Ogata E, Fujita T. Role of chloride in angiotensin II-induced salt-sensitive hypertension. Hypertension. 1991;18:622–629. doi: 10.1161/01.hyp.18.5.622. [DOI] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory–sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon G, Csiky B. Effect of neonatal sympathectomy on the development of structural vascular changes in angiotensin II-treated rats. J Hypertens. 1998;16:77–84. doi: 10.1097/00004872-199816010-00012. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Report 2002: Reducing Risks, Promoting Healthy Life. vol. 2002. WHO; Geneva: 2002. [DOI] [PubMed] [Google Scholar]
- Xu L, Sved AF. Acute sympathoexcitatory action of angiotensin II in conscious baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R451–R459. doi: 10.1152/ajpregu.00648.2001. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Miki K, King A, Fink G, Osborn JW. Differential responses of renal and muscle sympathetic nerve activity to chronic angiotensin II administration in rats consuming a high-salt diet. Hypertension. 2008a;52:e64. doi: 10.1161/HYPERTENSIONAHA.109.145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M, Wehrwein EA, Novotny M, Swain GM, Kreulen DL, Osborn JW. Effect of stellate ganglionectomy on basal cardiovascular function and responses to β1-adrenoceptor blockade in the rat. Am J Physiol Heart Circ Physiol. 2008b;295:H2447–H2454. doi: 10.1152/ajpheart.00958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]