Abstract
Rationale
Previous studies indicated that ethanol could be self-infused into the posterior ventral tegmental area (p-VTA) and that activation of local serotonin-3 (5-HT3) receptors was involved. 5-HT1B and 5-HT2A receptors are involved in the effects of 5-HT and ethanol on VTA dopamine neurons.
Objective
The current study used the intracranial self-administration (ICSA) procedure to determine the involvement of local 5-HT1B and 5-HT2A receptors in the self-infusion of ethanol into the p-VTA.
Materials and methods
Female Wistar rats were implanted unilaterally with a guide cannula aimed at the p-VTA. Seven days after surgery, rats were placed into the two-lever operant conditioning chambers for ICSA tests. The tests consisted of four acquisition sessions with self-infusion of 200 mg% ethanol alone, two or three sessions with co-infusion of the 5-HT1B antagonist GR 55562 (10, 100, or 200 μM) or the 5-HT2A antagonist R-96544 (10, 100, or 200 μM) with 200 mg% ethanol, and one final session with 200 mg% ethanol alone.
Results
During the acquisition sessions, all rats readily self-infused ethanol and discriminated the active from inactive lever. Co-infusion of GR 55562, at all three doses, had no effect on the self-infusion of ethanol. In contrast, co-infusion of R-96544, at the two higher doses, attenuated responding on the active lever for ethanol infusion (p<0.05).
Conclusion
The results suggest that the reinforcing effects of ethanol within the p-VTA are modulated, at least in part, by activation of local 5-HT2A, but not 5-HT1B, receptors.
Keywords: Intracranial self-administration, Ventral tegmental area, Ethanol, Serotonin-1B receptor, Serotonin-2A receptor, GR 55562, R-96544
Introduction
The mesolimbic dopamine (DA) system, originating from the mesencephalic ventral tegmental area (VTA), mediates, at least in part, the reinforcing and rewarding effects of ethanol (Koob 1992; Koob et al. 1998; McBride and Li 1998; Wise 1996). A series of studies, using the intracranial self-administration (ICSA) technique, demonstrated that ethanol can be directly self-infused into the posterior VTA (p-VTA) of both Wistar and alcohol-preferring rats, identifying the p-VTA as a neurosubstrate for the reinforcing effects of ethanol (Gatto et al. 1994; Rodd-Henricks et al. 2000;Rodd et al. 2004, 2005b). The self-infusion of ethanol, which was dependent on activation of local DA neurons (Rodd et al. 2004, 2005b), was prevented by co-infusion of serotonin-3 (5-HT3) receptor antagonists, suggesting that the reinforcing effects of ethanol within the p-VTA are modulated partially by activation of local 5-HT3 receptors (Rodd-Henricks et al. 2003; Rodd et al. 2005b).
Evidence indicates that 5-HT transmission within the VTA can regulate local DA neuronal activity and the effects of ethanol. Projections from 5-HT neurons in raphe nuclei terminate in the VTA and form synapses on VTA DA neurons (Azmitia and Segal 1978; Herve et al. 1987; Parent et al. 1981; Van Bockstaele et al. 1994). Electrophysiological and pharmacological studies demonstrated that 5-HT activated a large proportion of VTA DA neurons (Pessia et al. 1994), facilitated DA release from the VTA slice (Beart and McDonald 1982), and enhanced DA release in the nucleus accumbens when infused into the VTA in vivo (Guan and McBride 1989). In addition, 5-HT has been shown to potentiate the excitation of ethanol on VTA DA neurons (Brodie et al. 1995). The effects of 5-HT can be mediated by 5-HT3 receptors within the VTA. Local administration of a 5-HT3 receptor agonist stimulated VTA DA neuronal activity (Campbell et al. 1996; Liu et al. 2006), whereas 5-HT3 receptor antagonists decreased spontaneous firing rates of VTA DA neurons (Minabe et al. 1991; Rasmussen et al. 1991) and prevented ethanol-induced DA release (Campbell et al. 1996).
In addition to 5-HT3 receptors, evidence also indicated that the effects of 5-HT within the VTA could be modulated by 5-HT1B and 5-HT2A receptors (Brodie et al. 1995; Pessia et al. 1994; Yan and Yan 2001; Yan et al. 2005). 5-HT1B receptors are located within the VTA (Bruinvels et al. 1993; Pazos and Palacios 1985) and these receptors were suggested to modulate the DA-releasing effects of intra-VTA infusion of 5-HT (Guan and McBride 1989). Systemic or intra-VTA administration of 5-HT1B agonists increased DA release in the nucleus accumbens (Boulenguez et al. 1996; Yan and Yan 2001), which could be blocked by 5-HT1B antagonists (Hallbus et al. 1997). Furthermore, intra-VTA administration of a 5-HT1B agonist prolonged, whereas an antagonist attenuated, ethanol-induced DA release in the nucleus accumbens (Yan et al. 2005). 5-HT2A receptors are also found in the VTA (Doherty and Pickel 2000; Nocjar et al. 2002). The stimulating effect of 5-HT on VTA DA neurons was mimicked by 5-HT2A agonists in vitro, whereas 5-HT2A antagonists blocked the effects (Pessia et al. 1994). Moreover, 5-HT2A agonists were shown to potentiate ethanol-induced excitation on VTA DA neurons (Brodie et al. 1995). Given the effects of 5-HT1B and 5-HT2A receptors in the VTA, the objective of the current study was to determine the effects of antagonism of these receptors on the self-infusion of ethanol into the p-VTA.
Materials and methods
Animals
Experimentally naïve adult female Wistar rats (body weight 270 to 320 g, Harlan Sprague Dawley, Inc., Indianapolis, IN, USA) were used in the present study. Animals were doubly housed in a reverse 12-h light–dark cycle room (light off at 10:00 a.m.), with controlled temperature and humidity, for at least 2 weeks before the beginning of the experiment. Food and water were available ad libitum except during the ICSA test. Although both female and male Wistar rats exhibited similar self-infusion of ethanol into the p-VTA (Rodd-Henricks et al. 2000; Rodd et al. 2004), female rats were used in the present study because they maintain their head size better than male rats for more accurate stereotaxic surgery (Rodd-Henricks et al. 2000). The estrous cycle was not monitored in the present study. Previous studies suggested that estrous cycle did not appear to have a significant effect on ICSA behavior (Ikemoto et al. 1998). Experiments were performed during the dark phase. Protocols used were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine. All experiments were performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Chemical agents
The artificial cerebrospinal fluid (aCSF) consisted of 120 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 2.5 mM CaCl, and 10 mM d-glucose, pH 7.2–7.4. Ethyl alcohol (190 proof) was obtained from McCormick Distilling, Weston, MO, USA. The 5-HT1B receptor antagonist GR 55562 dihydrochloride {3-[3-(dimethylamino)propyl]-4-hydroxy-N-[4-(4-pyridinyl)phenyl]benzamide dihydrochloride} (Walsh et al. 1995) and the 5-HT2A receptor antagonist R-96544 hydrochloride {(2R,4R)-5-[2-[2-[2-(3-methoxyphenyl)ethyl] phenoxy]ethyl]-1-methyl-3-pyrrolidine hydrochloride} (Ogawa et al. 2002; Tanaka et al. 2000) were purchased from Tocris (Ellisville, MO, USA). All chemicals were dissolved in the aCSF solution to the desired concentrations prior to use.
Stereotaxic surgery procedures
Rats were implanted unilaterally with a 22-gauge cannula (Plastics One Inc., Roanoke, VA, USA) aimed at the right p-VTA (AP −5.6 mm, ML +2.1 mm, DV −8.5 mm), as described previously (Rodd-Henricks et al. 2003). Stylets were inserted into the cannulae when no experiments were being conducted. Rats were singly housed after surgery and were allowed to recover from surgery for at least 7 days prior to tests. Rats were habituated and handled on a daily basis during the 7-day period.
General test procedure
The ICSA tests followed procedures previously described (Bozarth and Wise 1981; Gatto et al. 1994). Briefly, during test days, rats were placed into operant conditioning chambers equipped with two levers, one active and one inactive. The active lever was connected to an electrolytic microinfusion transducer system (EMIT) controlled by an operant conditioning control system. The EMIT system is a current generator and is connected to two electrodes that are immersed in a solution-filled cylinder container equipped with a 28-gauge injection cannula. Each response on the active lever (FR1 schedule of reinforcement) produced a 5-s infusion current between the electrodes, resulting in an infusion of 100 nl of solution into the p-VTA. Each infusion was followed by a 5-s timeout period. During both the infusion and timeout periods, responses on the active lever were recorded but did not produce further infusions. The responses on the inactive lever were recorded but did not result in any infusions. The assignment of active and inactive lever was counterbalanced among rats. The duration of each ICSA session was 4 h, and sessions were conducted every other day. The attrition rate was about 10% mostly due to the loss of the cannula during testing.
The effects of co-infusion of GR 55562 on ethanol ICSA
For the ICSA experiments, rats were randomly assigned to one of five groups (n=5–8 per group). Two groups served as controls and self-infused aCSF or 200 μM GR 55562 alone for seven consecutive sessions. Three co-infusion groups self-infused 200 mg% ethanol for the first four sessions (acquisition). During sessions 5 and 6 (co-infusion), rats self-infused GR 55562 (10, 100, or 200 μM) and 200 mg% ethanol. During session 7 (reinstatement), rats self-infused 200 mg% ethanol. Previous findings indicated that 10 μM GR 55562 could attenuate ethanol-induced dopamine increase in the nucleus accumbens (Yan et al. 2005). This procedure of four acquisition sessions, two co-infusion sessions, and one reinstatement session has previously been used to examine ethanol self-infusion into the p-VTA (Rodd-Henricks et al. 2003). In the past, an additional control group (200 mg% ethanol for seven consecutive sessions) was used, and the data indicate that Wistar rats developed stable responding for 200 mg% ethanol for seven sessions (Rodd-Henricks et al. 2003; Rodd et al. 2004). Therefore, this 200 mg% group was not included in the current study. The 200 mg% ethanol has been demonstrated to be an optimal concentration for self-infusion into the p-VTA by Wistar rats (Rodd-Henricks et al. 2000). The 200 mg% ethanol is also physiologically relevant and can be readily obtained in rats under certain drinking conditions (Murphy et al. 1986; Waller et al. 1984).
The effects of co-infusion of R-96544 on ethanol ICSA
For the ICSA experiments, rats were randomly assigned to one of five groups (n=5–8 per group). Two groups served as controls and self-infused aCSF or 200 μM R-96544 alone for eight consecutive sessions. Three co-infusion groups self-infused 200 mg% ethanol for the first four sessions. During sessions 5–7, rats co-infused R-96544 (10, 100, or 200 μM) and 200 mg% ethanol. During session 8, rats self-infused 200 mg% ethanol. The reason for three co-infusion sessions with R-96544 was that three co-infusion sessions were mistakenly performed in the first group of rats; to keep consistency, all following groups were conducted with three co-infusion sessions.
Histology
At the end of experiments, rats were euthanized and bromophenol blue was injected into the p-VTA, as described previously (Gatto et al. 1994; Ikemoto et al. 1998). Brains were quickly removed and frozen at −20°C. Brain sections (40 μm thick) were sliced in a cryostat microtome and stained with cresyl violet for the verification of the placement of injection sites according to the rat brain atlas of Paxinos and Watson (1998).
Statistical analysis
The “group × session” mixed analyses of variance (ANOVAs) with repeated measures on the session were performed on responses on the active lever and the number of the infusions. For each individual group, lever discrimination was determined by “lever (active vs inactive) × session” mixed ANOVA with repeated measures on session. Post hoc Tukey’s b tests were followed when a significant main effect was found (p<0.05). Drug effects were determined by comparing the responses on the active lever during antagonist co-infusion sessions to the average responses on the active lever during acquisition sessions 3 and 4.
Results
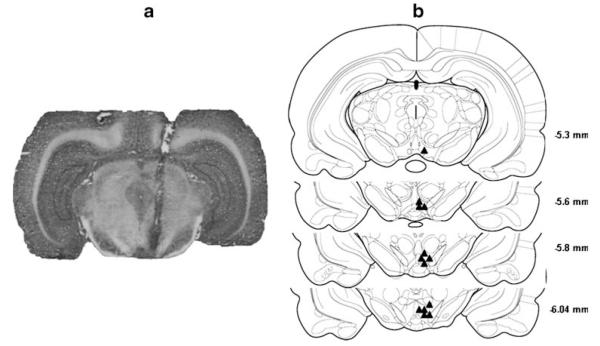
The p-VTA is the VTA region at the level of the interpeduncular nucleus from −5.3 to −6.0 mm relative to bregma (Rodd-Henricks et al. 2000). Figure 1 depicts the representative non-overlapping placements of injection sites within the p-VTA. Only rats with correct placements in the p-VTA were included in analysis. Approximately 80% of animals had injection sites within the p-VTA.
Fig. 1.
a A photomicrograph of a representative brain slice with an injection site within the p-VTA. b Representative non-overlapping placements of the injection sites within the p-VTA. The p-VTA corresponds to coronal sections from 5.3 to 6.0 mm posterior to bregma (Rodd-Henricks et al. 2000). The filled triangles represent the injection sites
The effects of co-infusion of GR 55562 on ethanol ICSA
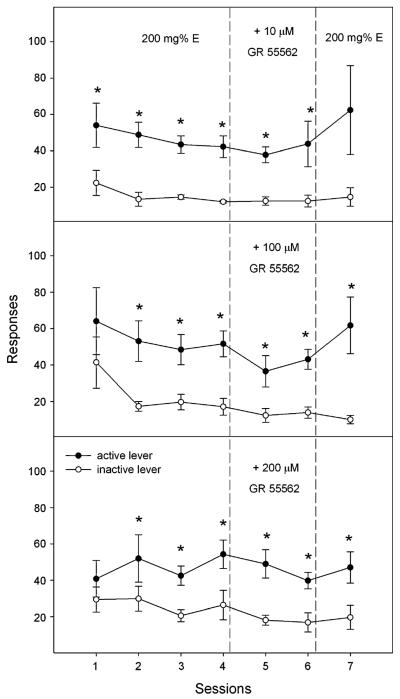
The lever responses for three GR 55562 co-infusion groups are shown in Fig. 2. Lever discrimination was determined by “lever × group” repeated ANOVA conducted in each individual group. The analysis demonstrated that lever discrimination developed for all co-infusion groups (all F values>17.59, all p values<0.005; Fig. 2) but not for the groups that self-infused aCSF or 200 μM GR 55562 (all F values<4.83, all p values>0.09; Table 1). The “group × session” repeated ANOVA performed on the responses on the active lever in the five groups revealed significant effect of group (F 4, 26=6.94, p=0.001) but no significant effect of session or session × group interaction (all F values<1.16, all p values>0.33). The lack of a significant effect of session (p>0.05) suggested that co-infusion of GR-55562, at all three concentrations, did not alter the responses on the active lever for 200 mg% ethanol during co-infusion sessions (Fig. 2).
Fig. 2.
Mean responses (±SEM) on the active and inactive lever by female Wistar rats for self-infusion of 200 mg% ethanol in the first four sessions, 200 mg% ethanol plus 10 μM (top, n=5), 100 μM (middle, n=8), or 200 μM GR 55562 (bottom, n=8) in sessions 5 and 6, and 200 mg% ethanol alone in session 7 into the p-VTA. Asterisks, responses on the active lever were significantly higher than responses on the inactive lever (p<0.05). There was no effect of co-administration of GR 55562 on lever responses for ethanol
Table 1.
Average lever responses and numbers of infusions per session (mean±SEM) for self-infusion of aCSF, GR 55562, and R-96544 alone
| Groups | N | Responses |
Infusions | |
|---|---|---|---|---|
| Active | Inactive | |||
| aCSFa | 10 | 14±2 | 9±2 | 7±1 |
| 200 μM GR 55562 | 5 | 18±9 | 12±6 | 7±3 |
| 200 μM G-96544 | 7 | 20±7 | 12±3 | 8±3 |
Pooled data from aCSF groups since no difference was found between these two groups
The “session × group” repeated ANOVA performed on the number of infusions (data not shown) in all five groups indicated a significant group effect (F 4, 26=8.61, p<0.001) but no significant effect of session or session × group interaction (all F values<1.98, all p values>0.07). Rats had relatively low numbers of infusions for aCSF and 200 μM GR 55562 (Table 1), which was significantly lower than the infusions in the three co-infusion groups (p<0.05). The lack of a significant effect of session suggested that co-infusion of GR 55562 did not alter the number of infusions of 200 mg% ethanol into the p-VTA.
The effects of co-infusion of R-96544 on ethanol ICSA
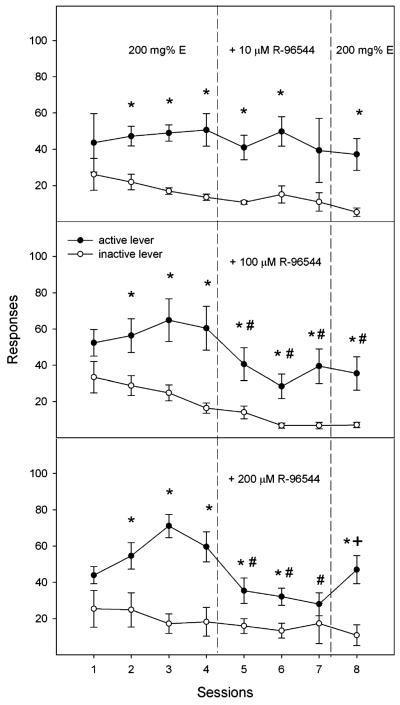
Lever responses for three R-96544 co-infusion groups are shown in Fig. 3. Lever discrimination was determined by “lever × session” repeated ANOVA conducted in each different group. The analysis demonstrated that lever discrimination developed for the three co-infusion groups (all F values>15.85, all p values<0.01; Fig. 3) but not for the control groups (all F values<2.43, all p values>0.17; Table 1).
Fig. 3.
Mean responses (±SEM) on the active and inactive lever by female Wistar rats for self-infusion of 200 mg% ethanol in the first four sessions, 200 mg% ethanol plus 10 μM (top, n=5), 100 μM (middle, n=8), or 200 μM R-96544 (bottom, n=8) in sessions 5, 6, and 7, and 200 mg% ethanol alone in session 8 into the p-VTA. Asterisks, responses on the active lever were significantly higher than responses on the inactive lever (p<0.05); number signs, responses on the active lever are significantly different from the average responses during sessions 3 and 4 (p<0.05); plus signs, responses on the active lever in session 8 are significantly different from the responses during session 7 (p<0.05)
The “session × group” repeated ANOVA performed on the responses on the active lever in all five groups revealed significant effects of session and group and a significant session × group interaction (all F values>1.96, all p values< 0.01). Post hoc comparisons of the responses on the active lever during the first four acquisition sessions among five different groups indicated that the responses for the three co-infusion groups during sessions 2–4 (Fig. 3) were significantly higher than those in the two control groups (all p values<0.05). Furthermore, the responses in the three co-infusion groups during each acquisition session were within the range reported previously (Rodd-Henricks et al. 2000, 2003) and were not statistically different from one another (all p values>0.05).
The significant session × group interaction term was decomposed by holding the “group” constant and examining the “session” effect for the last two acquisition sessions and the three co-infusion sessions (sessions 3–7) in each co-infusion group separately, which allowed an assessment of the effects of co-infusion of R-96544 on ethanol self-infusion. Co-infusion of 10 μM R-96544 did not alter the responses on the active lever (F 4, 16=0.64, p>0.05; Fig. 3, top panel), whereas co-infusion of 100 or 200 μM R-96544 significantly altered the responses on the active lever (all F 4, 28>5.59, all p values<0.002; Fig. 3, middle and bottom panels). Post hoc analysis indicated significantly lower responses on the active lever during co-infusion sessions 5–7 compared to the average responses during session 3 and 4 (all p values<0.05) in the 100 and 200 μM R-96544 co-infusion groups (Fig. 3, middle and bottom panels).
The “session × group” repeated ANOVA performed on the number of infusions in the five groups (data not shown) indicated significant effects of session (F 7, 196=2.75, p=0.01) and group (F 4, 28=4.96, p=0.004) but no significant effect of session × group interaction (F 28, 196=1.23, p=0.21). Rats had relatively low numbers of infusions for aCSF and 200 μM R-96544 (Table 1). Co-infusion of 10 or 100 μM R-96544 did not significantly alter the number of infusions of 200 mg% ethanol into the p-VTA (all F values<2.20, all p values>0.05). However, 200 μM R-96544 significantly reduced the number of infusions of 200 mg% ethanol into the p-VTA (p<0.05) during all three co-infusion sessions (baseline 26±3, fifth session 19±3, sixth session 17±3, and seventh session 16±3).
Discussion
The major finding of the current study is that activation of local 5-HT2A receptors, but not 5-HT1B receptors, may modulate the reinforcing effects of ethanol within the p-VTA. This conclusion is supported by results showing that (a) co-infusion of the 5-HT2A receptor antagonist R-96544 attenuated operant responses for ethanol (Fig. 3) and reduced the number of infusions of ethanol into the p-VTA and (b) co-infusion of the 5-HT1B receptor antagonist GR 55562 had no effect on the self-infusion of ethanol into the p-VTA (Fig. 2).
R-96544 is a potent and selective 5-HT2A receptor antagonist (Ki=1.6 nM; Ogawa et al. 2002). The behavioral effects of R-96544 in reducing ethanol self-infusion into the p-VTA are consistent with the localization of 5-HT2A receptors within the VTA (Cornea-Hebert et al. 1999; Doherty and Pickel 2000; Nocjar et al. 2002; Pazos et al. 1985). The antagonist effect on ethanol self-infusion could be a result of R-96544 blocking the excitation of VTA DA neurons modulated by 5-HT2A receptors directly on DA neurons and/or indirectly through excitatory inputs to the VTA.
The 5-HT2A receptor is one subtype of the G-protein-coupled 5-HT2 receptor family and mediates major excitatory effects of 5-HT (Hoyer et al. 2002). Some in vitro studies suggest that activation of 5-HT2A receptors in the brain depolarizes neurons. Perfusion of VTA slices with 5-HT2A agonists directly depolarized a large portion of VTA DA neurons, which was prevented by administration of 5-HT2A antagonists (Pessia et al. 1994). Activation of 5-HT2A receptors mediated 5-HT-induced depolarization of neurons in the nucleus accumbens, possibly by reducing potassium conductance (North and Uchimura 1989). Moreover, activation of 5-HT2A receptors within the VTA potentiated the excitatory effects of ethanol on DA neurons (Brodie et al. 1995). These studies suggest that activation of 5-HT2A receptors within the VTA may increase DA neuronal activity and blockade of 5-HT2A receptors may decrease DA neuronal activity. Because activation of local DA neurons is involved in the reinforcing effects of ethanol within the p-VTA (Rodd et al. 2004, 2005b), co-infusion of the 5-HT2A antagonist R-96544 could result in reduced DA neuronal activity and decreased self-infusion of ethanol (Fig. 3). In support of the idea that activation of 5-HT2A receptors increases DA neuronal activity, single intravenous injection of the 5-HT2A antagonist M100907 significantly decreased burst firing of VTA DA neurons; repeated intravenous injections of M100907 decreased both burst firing of DA neurons and the number of spontaneously active VTA DA neurons (Minabe et al. 2001). However, some earlier studies reported the opposite effect with a different 5-HT2A antagonist, i.e., ritanserin (Shi et al. 1995; Ugedo et al. 1989).
The 5-HT2A receptor has long been associated with ethanol-related behavior. Polymorphisms in the 5-HT2A receptor gene are associated with alcohol abuse and dependence (Hwu and Chen 2000; Nakamura et al. 1999). Systemic administration of 5-HT2A antagonists decreased voluntary ethanol intake and preference and reduced operant responses for ethanol in rats (Myers et al. 1992, 1993; Overstreet et al. 1997; Roberts et al. 1998). The current study suggests that the reinforcing effects of ethanol might be modulated, in part, by activation of 5-HT2A receptors within the p-VTA. In addition to ethanol, recent studies have associated 5-HT2A receptors with the effects of other drugs of abuse. 5-HT2A receptor antagonists attenuated DA increase in the nucleus accumbens induced by amphetamine and morphine (Auclair et al. 2004a, b). Antagonism of 5-HT2A receptors also decreased the discriminative stimulus effects of cocaine (Filip et al. 2006), attenuated locomotor hyperactivity and development of behavioral sensitization induced by psychostimulants and morphine (Fletcher et al. 2002; Auclair et al. 2004a, b; Filip et al. 2004), and reduced cocaine-primed reinstatement of operant responding (Fletcher et al. 2002). Taken together, the 5-HT2A receptor plays an important role in the neurochemical and behavioral effects of drugs of abuse.
Although selective for the 5-HT2A receptor subtype, R-96544 exhibits affinity for 5-HT2 receptors (IC50=2.2 nM; Tanaka et al. 2000), which also includes the 5-HT2C subtype, in addition to the 5-HT2A subtype (Hoyer et al. 2002). Evidence shows that 5-HT2C receptors are located in the VTA and regulate DA neuronal activity (Bubar and Cunningham 2007; Clemett et al. 2000). However, the antagonism of 5-HT2C receptors with R-96544 may not be involved in the behavioral effects of R-96544 on self-infusion of ethanol in the p-VTA (Fig. 3). Previous research suggests that activation of VTA 5-HT2C receptors may exert tonic inhibition of VTA DA neurons. Systemic administration of selective 5-HT2C receptor agonists decreased, whereas antagonists increased, basal and evoked VTA DA neuronal activity (Di Matteo et al. 1999; Pierucci et al. 2004). The intra-VTA administration of a 5-HT2C receptor agonist attenuated cocaine- and stress-induced DA overflow (Navailles et al. 2008; Pozzi et al. 2002), whereas local perfusion of a 5-HT2C receptor antagonist into the VTA enhanced 3,4,-methylenedioxymethamphetamine-induced DA overflow in the nucleus accumbens (Bankson and Yamamoto 2004). If R-96544 was acting at 5-HT2C receptors, then its administration should increase DA neuronal activity and further enhance ethanol excitation of DA neurons, resulting in increased, but not decreased (Fig. 3), self-infusion of ethanol. Therefore, the effects of R-96544 within the p-VTA on ethanol self-infusion may not involve 5-HT2C receptors.
In the current study, the R-96544 achieved its effects at relatively high concentrations (100 and 200 μM, Fig. 3). This range of concentrations has been typically used in the ICSA studies with other agents. For example, the DA D2 receptor antagonist sulpiride (1–3 mM) was tested on the self-infusion of the DA reuptake inhibitor nomifensine and phencyclidine (Carlezon and Wise 1996). 5-HT3 receptor antagonists (10–200 μM) or DA D2 agonist quinpirole (10–100 μM) was used to prevent the self-infusion of ethanol and cocaine (Rodd-Henricks et al. 2003; Rodd et al. 2004, 2005a). Co-administration of DA D1 or D2 receptor antagonists (mM range) was used to decrease the self-infusion of cocaine or a mixture of D1 and D2 receptor agonists (Ikemoto 2003; Ikemoto et al. 1997). Even so, it should be remembered that R-96544, at micromolar levels, may have nonspecific effects, e.g., antagonism of DA D2 receptors (IC50=2.4 μM) or 5-HT3 receptors (IC50>5 μM; Tanaka et al. 2000), which has been shown to be involved in the reinforcing effects of ethanol within the p-VTA (Rodd-Henricks et al. 2003; Rodd et al. 2004, 2005b). Therefore, it is possible that high concentrations of R-96544, although given in a very small volume, may be blocking D2 and/or 5-HT3 receptors in addition to 5-HT2A receptors.
After removing R-96544, the responses on the active lever during session 8 did not recover to the preantagonist levels in the 100 and 200 μM R-96544 co-infusion groups (Fig. 3). Although previous studies (Rodd-Henricks et al. 2003; Rodd et al. 2004) reported a recovery of responses, the current study did not observe such a recovery in the reinstatement session. It should be noted that only two co-infusion sessions were conducted in the previous studies (Rodd-Henricks et al. 2003; Rodd et al. 2004), whereas there were three co-infusion sessions in the current study. Perhaps, with the three co-infusion sessions, it will require more than one reinstatement session for responding to return to baseline levels.
The results with the 5-HT1B antagonist GR 55562 indicated that blockade of local 5-HT1B receptors does not alter the self-infusion of ethanol into the p-VTA (Fig. 2), suggesting that activation of local 5-HT1B receptors may not be involved in the reinforcing effects of ethanol in the VTA. In addition, it is possible that 5-HT1B receptors in the p-VTA may not be tonically activated. The 5-HT1B receptors are primarily localized on axon terminals and function as presynaptic autoreceptors and heteroreceptors to regulate neurotransmitter release (Boschert et al. 1994; Maroteaux et al. 1992; Sari et al. 1999). Although intra-VTA application of 5-HT1B agonists increased DA release in the nucleus accumbens (Yan and Yan 2001), reverse microdialysis of 5-HT1B antagonists into the VTA did not alter extracellular dopamine levels in the nucleus accumbens (O’Dell and Parsons 2004; Yan et al. 2005), suggesting that 5-HT1B receptors in the VTA may not tonically modulate DA activity.
The results suggest that 5-HT1B receptors in the p-VTA do not modulate the local reinforcing effects of ethanol. However, the results do not exclude the involvement of 5-HT1B receptors in modulating the rewarding and other effects of ethanol. Systemic administration of 5-HT1B receptor agonists suppressed oral ethanol self-administration (Tomkins and O’Neill 2000; Wilson et al. 1998) and the conditioned reinforcing effects of ethanol (Wilson et al. 2000), as well as ethanol-induced aggressive behavior (Miczek and De Almeida 2001), suggesting that activation of 5-HT1B receptors would reduce ethanol effects. Consistent with this idea, 5-HT1B receptor knockout mice have higher ethanol intakes than wild-type mice (Crabbe et al. 1996). However, contrary to these findings, overexpression of 5-HT1B receptors in the nucleus accumbens increased voluntary ethanol drinking (Hoplight et al. 2006). This inconsistency may suggest that 5-HT1B receptors in different brain regions (e.g., the nucleus accumbens, ventral pallidum, etc.) may be differentially involved in regulating ethanol consumption.
In addition to ethanol, 5-HT1B receptors are also involved in effects of psychostimulants. Activation of 5-HT1B receptors potentiated the cocaine-induced increase in extracellular levels of DA in the nucleus accumbens (Parsons et al. 1999; O’Dell and Parsons 2004) and facilitated locomotor stimulation and sensitization of psychostimulants (Neumaier et al. 2002; Przegalinski et al. 2004). On the other hand, antagonism of 5-HT1B receptors decreased the discriminative stimulus effects and locomotor stimulation of psychostimulants (Papla et al. 2002; Filip et al. 2003), reduced self-administration of cocaine (David et al. 2004), and attenuated cocaine-induced sensitization (Przegalinski et al. 2004). However, 5-HT1B receptor knockout mice exhibited enhanced self-administration of cocaine (Rocha et al. 1998), contrary to the antagonist effects (David et al. 2004). The different findings with knockout mice may be due to the compensatory mechanisms. Overall, the 5-HT1B receptor appears to play an important role in the reinforcing effects of ethanol and the psychostimulants.
One limitation of the current study was that the experimental design only tested one antagonist to each receptor type. In general, it is difficult to prove receptor modulation of specific effects due to relative specificity of the antagonist and relatively large doses applied. Thus, the conclusions would be more convincing if more than one antagonist to each of the two receptors had been tested in the current study. Nonetheless, the current study provided evidence that activation of local 5-HT2A receptors, but not 5-HT1B receptors, may be one of the mechanisms modulating the response-contingent behavior reinforced by ethanol in the p-VTA, further confirming the role of 5-HT transmission in the p-VTA in regulating the reinforcing effects of ethanol.
Acknowledgements
This study was supported in part by grants AA12262 and AA10721.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- DA
dopamine
- EMIT
electrolytic microinfusion transducer
- 5-HT
serotonin
- ICSA
intracranial self-administration
- p-VTA
posterior ventral tegmental area
- VTA
ventral tegmental area
Contributor Information
Zheng-Ming Ding, Graduate Program in Medical Neuroscience, Indiana University School of Medicine, Indianapolis, IN 46202, USA; Institute of Psychiatric Research, Department of Psychiatry, Indiana University School of Medicine, 791 Union Drive, Indianapolis, IN 46202-4887, USA.
Jamie E. Toalston, Institute of Psychiatric Research, Department of Psychiatry, Indiana University School of Medicine, 791 Union Drive, Indianapolis, IN 46202-4887, USA; Department of Psychology, Purdue School of Science, Indiana University-Purdue University at Indianapolis, Indianapolis, IN 46202, USA
Scott M. Oster, Institute of Psychiatric Research, Department of Psychiatry, Indiana University School of Medicine, 791 Union Drive, Indianapolis, IN 46202-4887, USA; Department of Psychology, Purdue School of Science, Indiana University-Purdue University at Indianapolis, Indianapolis, IN 46202, USA
William J. McBride, Institute of Psychiatric Research, Department of Psychiatry, Indiana University School of Medicine, 791 Union Drive, Indianapolis, IN 46202-4887, USA
Zachary A. Rodd, Institute of Psychiatric Research, Department of Psychiatry, Indiana University School of Medicine, 791 Union Drive, Indianapolis, IN 46202-4887, USA
References
- Auclair A, Blanc G, Glowinski J, Tassin JP. Role of serotonin2A receptors in the d-amphetamine-induced release of dopamine: comparison with previous data on α1b-adrenergic receptors. J Neurochem. 2004a;91:318–326. doi: 10.1111/j.1471-4159.2004.02714.x. [DOI] [PubMed] [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and α1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioral sensitization to opiates and psychostimulants. Eur J Neurosci. 2004b;20:3073–3084. doi: 10.1111/j.1460-9568.2004.03805.x. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Yamamoto BK. Serotonin–GABA interactions modulate MDMA-induced mesolimbic dopamine release. J Neurochem. 2004;91:852–859. doi: 10.1111/j.1471-4159.2004.02763.x. [DOI] [PubMed] [Google Scholar]
- Beart PM, McDonald D. 5-Hydroxytryptamine and 5-hydroxytryptaminergic-dopaminergic interactions in the ventral tegmental area of rat brain. J Pharm Pharmacol. 1982;34:591–593. doi: 10.1111/j.2042-7158.1982.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Rawlins JNP, Chauveau J, Joseph MH, Mitchell SN, Gray JA. Modulation of dopamine release in the nucleus accumbens by 5-HT1B agonists: involvement of the hippocampo-accumbens pathway. Neuropharmacology. 1996;35:1521–1529. doi: 10.1016/s0028-3908(96)00099-8. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Trifunovic RD, Shefner SA. Serotonin potentiates ethanol-induced excitation of ventral tegmental area neurons in brain slices from three different rat strains. J Pharmacol Exp Ther. 1995;273:1139–1146. [PubMed] [Google Scholar]
- Bruinvels AT, Palacios JM, Hoyer D. Autoradiographic characterisation and localisation of 5HT1D compared to 5HT1B binding sites in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:569–582. doi: 10.1007/BF00166939. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146:286–297. doi: 10.1016/j.neuroscience.2006.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, Schafer GL. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- David V, Segu L, Buhot MC, Ichaye M, Cazala P. Rewarding effects elicited by cocaine microinjections into the ventral tegmental area of C57BL/6 mice: involvement of dopamine D1 and serotonin1B receptors. Psychopharmacology (Berl) 2004;174:367–375. doi: 10.1007/s00213-003-1767-5. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Pickel VM. Ultrastructural localization of the serotonin 2A receptor in dopaminergic neurons in the ventral tegmental area. Brain Res. 2000;864:176–185. doi: 10.1016/s0006-8993(00)02062-x. [DOI] [PubMed] [Google Scholar]
- Filip M, Papla I, Nowak E, Czepiel K, Przegalinski E. Effects of 5-HT1B receptor ligands microinjected into the ventral tegmental area on cocaine discrimination in rats. Eur J Pharmacol. 2003;459:239–245. doi: 10.1016/s0014-2999(02)02873-x. [DOI] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J Pharmacol Exp Ther. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841. [DOI] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-HT) 5-HT2 receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology (Berl) 2006;183:482–489. doi: 10.1007/s00213-005-0197-y. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100907 and the 5-HT2C receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li T-K. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Guan XM, McBride WJ. Serotonin microinfusion into the ventral tegmental area increases accumbens dopamine release. Brain Res Bull. 1989;23:541–547. doi: 10.1016/0361-9230(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Hallbus M, Magnusson T, Magnusson O. Influence of 5-HT1B/1D receptors on dopamine release in the guinea pig nucleus accumbens: a microdialysis study. Neurosci Lett. 1997;225:57–60. doi: 10.1016/s0304-3940(97)00178-x. [DOI] [PubMed] [Google Scholar]
- Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38:73–79. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hwu HG, Chen CH. Association of 5HT2A receptor gene polymorphism and alcohol abuse with behavior problems. Am J Med Genet. 2000;96:797–800. doi: 10.1002/1096-8628(20001204)96:6<797::aid-ajmg20>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: intracranial self-administration studies. J Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Regional differences within the rat ventral tegmental area for muscimol self-administrations. Pharmacol Biochem Behav. 1998;61:87–92. doi: 10.1016/s0091-3057(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Liu W, Thielen RJ, Rodd ZA, McBride WJ. Activation of serotonin-3 receptors increases dopamine release within the ventral tegmental area of Wistar and alcohol-preferring (P) rats. Alcohol. 2006;40:167–176. doi: 10.1016/j.alcohol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Saudou F, Amlaiky N, Boschert U, Plassat JL, Hen R. Mouse 5-HT1B serotonin receptor: cloning, functional expression, and localization in motor control centers. Proc Natl Acad Sci USA. 1992;89:3020–3024. doi: 10.1073/pnas.89.7.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Li T-K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Miczek KA, De Almeida RM. Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5HT1B agonist anpirtoline. Psychopharmacology. 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- Minabe Y, Ashby CR, Schwartz JE, Wang RY. The 5-HT3 receptor antagonists LY277359 and granisetron potentiate the suppressant action of apomorphine on the basal firing rate of ventral tegmental dopamine cells. Eur J Pharmacol. 1991;209:143–150. doi: 10.1016/0014-2999(91)90162-j. [DOI] [PubMed] [Google Scholar]
- Minabe Y, Hashimoto K, Watanabe K, Ashby CR. Acute and repeated administration of the selective 5-HT2A receptor antagonist M100907 significantly alters the activity of midbrain dopamine neurons: an in vivo electrophysiological study. Synapse. 2001;40:102–112. doi: 10.1002/syn.1031. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li T-K. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Myers RD, Lankford M, Bjork A. Selective reduction by the 5-HT antagonist amperozide of alcohol preference induced in rats by syst44 emic cyanamide. Pharmacol Biochem Behav. 1992;43:661–667. doi: 10.1016/0091-3057(92)90392-s. [DOI] [PubMed] [Google Scholar]
- Myers RD, Landford MF, Bjork A. 5-HT2 receptor blockade by amperozide suppresses ethanol drinking in genetically preferring rats. Pharmacol Biochem Behav. 1993;45:741–747. doi: 10.1016/0091-3057(93)90535-2. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Matsushita S, Nishiguchi N, Kimura M, Yoshino A, Higuchi S. Association of a polymorphism of the 5HT2A receptor gene promoter region with alcohol dependence. Mol Psychiatry. 1999;4:85–88. doi: 10.1038/sj.mp.4000474. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U. Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology. 2008;33:237–246. doi: 10.1038/sj.npp.1301414. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA. Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Roth BL, Pehek EA. Localization of 5HT2A receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience. 2002;111:163–176. doi: 10.1016/s0306-4522(01)00593-0. [DOI] [PubMed] [Google Scholar]
- North RA, Uchimura N. 5-Hydroxytryptamine acts at 5-HT2 receptors to decrease potassium conductance in rat nucleus accumbens neurons. J Physiol. 1989;417:1–12. doi: 10.1113/jphysiol.1989.sp017786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increase in nucleus accumbens dopamine levels. J Pharmacol Exp Ther. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Sugidachi A, Tanaka N, Fujimoto K, Asai F. Pharmacological profiles of R-96544, the active form of a novel 5-HT2A receptor antagonist R-102444. Eur J Pharmacol. 2002;457:107–114. doi: 10.1016/s0014-2999(02)02654-7. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, McArthur RA, Rezvani AH, Post C. Selective inhibition of alcohol intake in diverse alcohol-preferring rat strains by the 5-HT2A antagonists amperozide and FG 5974. Alcohol Clin Exp Res. 1997;21:1448–1454. [PubMed] [Google Scholar]
- Papla I, Filip M, Przegalinski E. Effects of intra-tegmental microinjections of 5-HT1B receptor ligands on the amphetamine-induced locomotor hyperactivity in rats. Pol J Pharmacol. 2002;54:351–357. [PubMed] [Google Scholar]
- Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. RU 24969, a 5-HT1B/1A receptor agonist, potentiates cocaine-induced increases in nucleus accumbens dopamine. Synapse. 1999;32:132–135. doi: 10.1002/(SICI)1098-2396(199905)32:2<132::AID-SYN6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Pessia M, Jiang ZG, North RA, Johnson SW. Actions of 5-hydroxytryptamine on ventral tegmental area neurons of the rat in vitro. Brain Res. 1994;654:324–330. doi: 10.1016/0006-8993(94)90495-2. [DOI] [PubMed] [Google Scholar]
- Pierucci M, Di Matteo V, Esposito E. Stimulation of serotonin2C receptors blocks the hyperactivation of midbrain dopamine neurons induced by nicotine administration. J Pharmacol Exp Ther. 2004;309:109–118. doi: 10.1124/jpet.103.062208. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Acconcia S, Ceglia I, Invernizzi RW, Samanin R. Stimulation of 5-hydroxytryptamine (5-HT2C) receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex. J Neurochem. 2002;82:93–100. doi: 10.1046/j.1471-4159.2002.00947.x. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Papla I, Siwanowicz J, Filip M. Effects of 5-HT1B receptor ligands microinjected into the ventral tegmental area on the locomotor and sensitizating effects of cocaine in rats. Eur Neuropsychopharmacol. 2004;14:217–225. doi: 10.1016/S0924-977X(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Stockton ME, Czachura JF. The 5-HT3 receptor antagonist zatosetron decreases the number of spontaneously active A10 dopamine neurons. Eur J Pharmacol. 1991;205:113–116. doi: 10.1016/0014-2999(91)90781-k. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, McArthur RA, Hull EE, Post C, Koob GF. Effects of amperozide, 8-OH-DPAT, and FG 5974 on operant responding for ethanol. Psychopharmacology (Berl) 1998;137:25–32. doi: 10.1007/s002130050589. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Scearce-levie K, Lucas JJ, Hirois N, Castanon N, Crabbe JC, Nestler EJ, Hen R. Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature. 1998;393:175–178. doi: 10.1038/30259. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharmacol Exp Ther. 2005a;313:134–145. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li T-K, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005b;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology. 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Berge D. Cellular and subcellular localization of 5-hydroxytrptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- Shi WX, Nathaniel P, Bunney BS. Ritanserin, a 5-HT2A/2C antagonist, reverses direct dopamine agonist-induced inhibition of midbrain DA neurons. J Pharmacol Exp Ther. 1995;274:735–740. [PubMed] [Google Scholar]
- Tanaka N, Goto R, Ito R, Hayakawa M, Sugidachi A, Ogawa T, Asai F, Fujimoto K. [2-(ω-Phenylalkyl)phenoxy]alkylamines III: synthesis and selective serotonin-2 receptor binding (2) Chem Pharm Bull. 2000;48:1729–1739. doi: 10.1248/cpb.48.1729. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, O’Neill MF. Effect of 5-HT1B receptor ligands on self-administration of ethanol in an operant procedure in rats. Pharmacol Biochem Behav. 2000;66:129–136. doi: 10.1016/s0091-3057(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Ugedo L, Grenhoff J, Svensson TH. Ritanserin, a 5-HT2 receptor antagonist, activates midbrain dopamine neurons by blocking serotonergic inhibition. Psychopharmacology (Berl) 1989;98:45–50. doi: 10.1007/BF00442004. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Cestari DM, Pickel VM. Synaptic structure and connectivity of serotonin terminals in the ventral tegmental area: potential sites for modulation of mesolimbic dopamine neurons. Brain Res. 1994;647:307–322. doi: 10.1016/0006-8993(94)91330-7. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Gatto GJ, Lumeng L, Li T-K. Intragastric self-infusion of ethanol by ethanol-preferring and - nonpreferring lines of rats. Science. 1984;225:78–80. doi: 10.1126/science.6539502. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Beattie DT, Connor HE. The activity of 5-HT1D receptor ligands at cloned human 5-HT1Da and 5-HT1Db receptors. Eur J Pharmacol. 1995;287:79–84. doi: 10.1016/0014-2999(95)00612-1. [DOI] [PubMed] [Google Scholar]
- Wilson AW, Neill JC, Costall B. An investigation into the effects of 5-HT agonists and receptor antagonists on ethanol self-administration in the rat. Alcohol. 1998;16:249–270. doi: 10.1016/s0741-8329(98)00013-5. [DOI] [PubMed] [Google Scholar]
- Wilson AW, Costall B, Neill JC. Manipulation of operant responding for an ethanol-paired conditioned stimulus in the rat by pharmacological alteration of the serotonergic system. J Psychopharmacol. 2000;14:340–346. doi: 10.1177/026988110001400402. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Yan QS, Yan SE. Activation of 5HT1B/1D receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. Eur J Pharmacol. 2001;418:55–64. doi: 10.1016/s0014-2999(01)00913-x. [DOI] [PubMed] [Google Scholar]
- Yan QS, Zheng SZ, Feng MJ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in ethanol-induced increases in mesolimbic dopaminergic transmission. Brain Res. 2005;1060:126–137. doi: 10.1016/j.brainres.2005.08.051. [DOI] [PubMed] [Google Scholar]