Abstract
Adaptive radiation therapy for liver cancer has the potential to reduce normal tissue complications and enable dose escalation, allowing the potential for tumor control in this challenging site. Using adaptive techniques to tailor treatment margins to reflect patient specific breathing motions and image-guidance techniques can reduce the high dose delivered to surrounding normal tissues while ensuring the prescription dose is delivered to the tumor. Several treatment planning and delivery techniques have been developed for use in the liver, including a margin to encompass the full breathing motion, mean position techniques, which evaluate the probability of tumor location during breathing, breath hold, gating, and tracking. Patient selection, clinical workflow, and quality assurance must be considered and developed prior to integrating these techniques into clinical practice.
1. Rationale & potentials
Primary liver cancer and liver metastases are leading causes of worldwide cancer morbidity and mortality1,2. Surgery results in five-year survival rates of 30–60% in selected patients with hepatocellular carcinoma, intrahepatic cholangiocarcinoma and colorectal liver metastases. However, less than 20% of patients are surgical candidates3,4. Ablative therapies can control tumors less than 4 cm in diameter5,6, but larger tumors, those adjacent to large vessels and cancers with vascular involvement are generally not eligible for standard local therapies. Advances in chemotherapy and targeted biologic therapies have led to improved outcomes, providing rationale for the increased use of local therapies, such as radiation therapy, to treat sites of isolated or ‘oligo’ metastases, e.g. in the liver. As long-term survival is only possible with the addition of local therapies to systemic therapies, there is strong rationale for improved and increased use of local therapies in primary and metastatic liver cancer.
Radiation therapy has historically had a limited role in the treatment of liver cancer, due partially to the low whole liver tolerance to radiation dose (5% risk of toxicity following 28 in 2 Gy fractions for primary liver cancer). It has now been established that very high doses of radiation can be delivered to portions of the liver safely, as long as enough dose is spared from the remaining liver7. Other challenges in delivering radiation therapy to focal liver cancers are that the cancers are often multi-focal, locally advanced and/or adjacent to extrahepatic normal tissues.
With technical advantages in liver cancer imaging and radiation therapy planning and delivery, tumorcidal doses have been delivered to liver tumors using standard fractionation, hyperfractionation and stereotactic body radiation therapy (SBRT), with sustained local control seen in 70–90% of tumors. For tumors requiring a large volume of liver to be irradiated or those close to extrahepatic normal tissues, lower doses need to be delivered with a reduced chance of local control. The dose-response for tumor control has been observed in many series8. By reducing the volume of normal tissues irradiated, individualized motion management, image guidance and adaptive radiotherapy can facilitate dose escalation to liver cancers.
2. Patient variations: Intra-treatment motion, inter-treatment variation and dose response induced variation
Substantial variations in breathing motion are seen between patients, with a wide range of amplitudes of liver breathing motion (5 to 35 mm), with most of the change in the superior-inferior (SI) direction, followed by the anterior-posterior (AP) direction.9 A ‘one size fits all’ approach to PTV construction will either treat excessive normal tissue or under dose the clinical target volume (CTV) in some patients. In addition to variations in breathing amplitude, the position of the liver (mean position or any phase of breathing, e.g. exhale) relative to bones, can vary, and this can be of similar magnitude to the breathing amplitude. This change has been referred to as a baseline shift in liver. Baseline shifts can occur from the time of planning to the time of treatment, between fractions and possibly within fractions. These shifts are largest between fractions rather than within the time of a fraction. In one series of 29 patients treated with treatment times per fraction of 25 minutes or less (mean 12 minutes), intra-fraction shifts were only seen in one patient with substantial pain.10 For longer treatment times, intra-fraction shifts may be more substantial. Baseline shifts in liver position are one of the most important sources of geometric uncertainty that should be included in PTV margins and/or planning if they are not accounted for with image guidance.
Image Guidance
Image guidance has many roles in the treatment of liver cancer. It allows the evaluation and correction for baseline shifts, which may cause a systematic error in treatment delivery. The inter-fraction variation in patient-specific breathing motion amplitude can also be verified at each fraction using image guidance. Although when analyzed over a population, the average variation is small, there is a potential for substantial reductions or increases in breathing amplitude, especially in patients with pain and those treated with a long fractionation schedule.10 In addition, the potential exists for changes in anatomy to occur over the course of treatment. Thus image guidance can monitor breathing amplitude, can quantify and correct for baseline shifts in liver position, as well as provide information about changing organ shape, which may trigger replanning if beyond a certain threshold. Image guidance techniques, including orthogonal kV or MV imaging, kV or MV cone-beam CT, MVCT, and ultrasound have been previously described11–25.
Surrogates
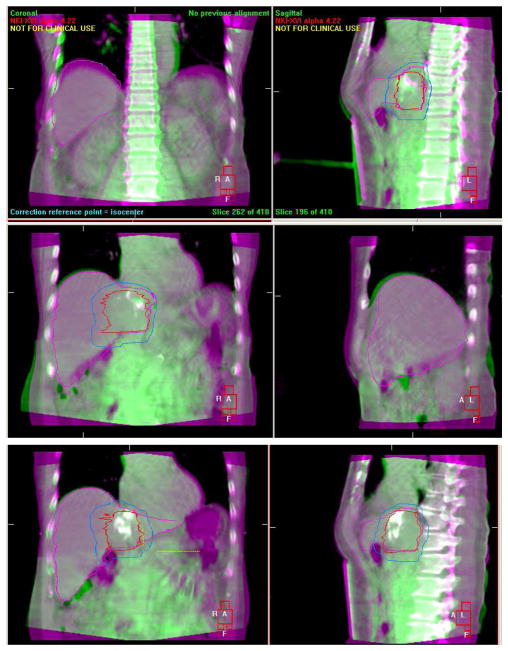
Liver tumors are generally only visible on imaging obtained with intravenous (IV) contrast, which is not routinely used at the treatment unit. Thus, a surrogate for the tumor must be used for image guidance. Surrogates that have been used clinically include the diaphragm-liver interface, the liver volume, natural, iatrogenic and implanted fiducials. Figure 1 shows the improvement in image guidance, based on the alignment of Lipidiol, which is visible on CT and CBCT, as the registration progresses from laser alignment to the external skin marks, to bony alignment, to liver alignment. Understanding the relationship between each surrogate and the tumor and normal tissue is important to successfully deliver the intended dose while adequately sparing the normal tissues. Surrogates, however, may not move in the same manner as the liver tumors. The liver is a complex and deformable tissue, which can be affected by the mechanics of breathing, which may differ from day to day, and also stomach filling and positioning of the bowel. These effects may result in local liver deformation. In a study of 12 patients treated with volumetric image guidance under a 6 fraction SBRT protocol, the average difference in tumor positioning between rigid and deformable registration was small, with an absolute mean of less than 1 mm in each direction.14 In 15% of the fractions evaluated, the difference in the tumor position exceeded 3 mm when using deformable registration. This highlights the importance of evaluating the fit of the surrogates and neighboring normal tissues for image guidance.
Figure 1.
Improvements in image guidance with surrogates closer to the tumor: initial alignment, based on lasers (top), based on bony alignment (center), and based on the liver (bottom). Lipidiol in the tumor, visible on CT and CBCT, enables evaluation of the tumor registration.
3. Planning and Delivery Strategies
Several strategies aim to account for breathing motion in the planning and delivery of the conformal radiation dose. There are several techniques available to perform 4D radiotherapy for the liver, including methods that incorporate the breathing motion into the treatment plan, inhibit breathing motion while delivering the radiation, inhibit the delivery of radiation except when the patient is in a specific breathing phase, and tracking the breathing motion with the delivery of the radiation.
Patient Specific Margins - Internal Target Volume (ITV) Approach
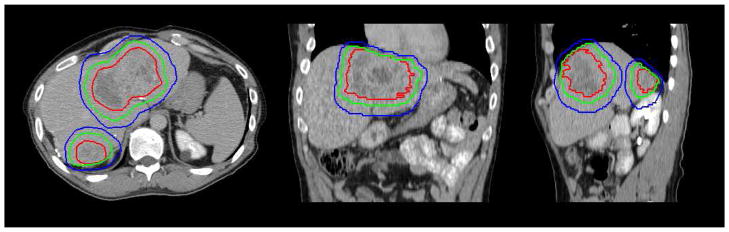
The simplest type of ‘adaption’ is to develop patient specific treatment plans based on individualized breathing motion.26 For example, with no specific breathing motion management strategy, the patient’s specific breathing motion is included within the PTV. Implementation of this technique requires the selection of a reference breathing phase and the measurement of the patient specific breathing motion. Historically, a recommended reference phase has been the exhale position, as this position has been shown to be more stable between breathing cycles as well as between treatment sessions. The gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV) based on an exhale reference position are shown in Figure 2.
Figure 2.
Axial (left), coronal (center), and sagittal (right) view of the GTV (red), CTV (green), and PTV (blue) shown on the exhale breath hold, contrast enhanced image, with an asymmetric margin based on the individual breathing motion
Once the reference phase has been selected, the normal breathing motion, relative to this phase must be measured. Ideally, this measurement is done in 3D as out of plane motion is possible with 2D, or planar imaging, for motion assessment. Several imaging techniques may be used to measure motion including CT, fluoroscopy, and MR. With CT, either 4D CT or inhale and exhale breath hold CT scans may be used.27,28 4D CT scans gives the benefit of measuring normal relaxed breathing and additional geometric information beyond the inhale and exhale states. However, often, a patients’ breathing is not regular and artifacts may exist. These artifacts may be small and not limit the use of the scans or they may be large and require rescanning the patient prior to treatment planning. Or, if artifacts are due to very irregular breathing, one 4DCT is not sufficient to represent all the variability in breathing that may occur for that patient. Obtaining imaging in inhale and exhale breath hold reduces the possibility of artifacts, as long as the patient can comfortably hold their breath for the duration of the scan. With this approach, care must be used to ensure that the patient is not taking a deep instead of normal breathing, which may result in an overestimation of the breathing amplitude. In addition, typically, the tumor cannot be seen on CT without the use of IV contrast. The optimization of contrast with 4D CT is under investigation. Tumor or a shadow where the tumor is may be visualized on a 4D CT if it is acquired immediately following IV injection, or in the very delayed phase.29 Another approach is to obtain an exhale breath hold image with IV contrast with a 4D CT immediately following it, while enough contrast remains for the tumor to be identified for motion measurement purposes.
Orthogonal imaging can also be used, such as fluoroscopy or cine MR. Fluoroscopy is limited to measuring the motion of the diaphragm or a fiducial, and this motion may differ from the motion of the tumor, depending on the location of the tumor within the liver.28 Cine MR imaging can be useful as the tumor can be seen on MR without contrast enabling direct measurement of the tumor motion, with increased temporal resolution. The limitation of cine MR is that if the tumor motion is in the out of plane direction, it may limit the accuracy of the measurement within that plane. In addition, for the same patient, the measurement on MR and fluoroscopy may not be the same. In a study of 35 patients imaged on both fluoroscopy and cine MR, 8 patients had a difference in SI motion of more than 10 mm (diaphragm motion measured on fluoroscopy and tumor boundary measured on cine MR); in 5 patients, fluoroscopy measurements were less than cine MR, and in 3 patients, fluoroscopy measurements were greater than cine MR.28
Once the individual patient motion measurements are obtained, one must apply them for a PTV margin. Traditionally, the PTV margin is established to capture the entire range of the breathing motion for the patient, that is, if the patient reference position is at exhale and the breathing motion is 10 mm SI, the PTV margin is 10 mm in the inferior direction (plus a component for setup uncertainty and reproducibility of exhale in all directions, e.g. an additional 5 mm with IGRT or more if IGRT is not to account for baseline shifts).
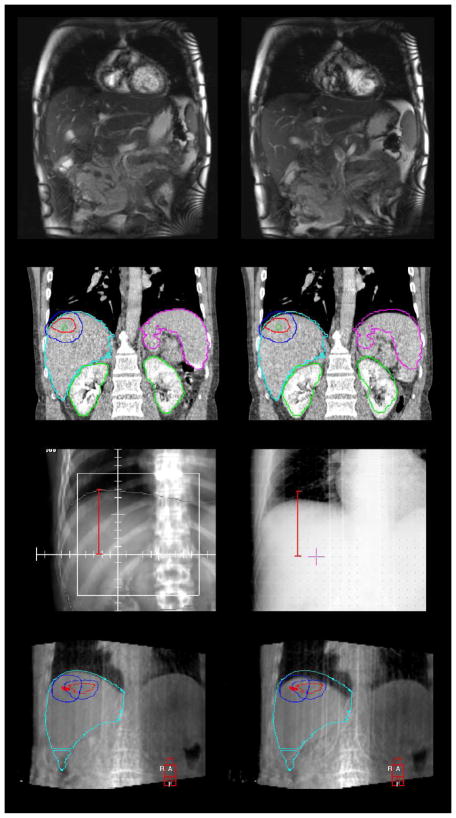
In this scenario, image guidance must be used to align the patient, at the reference state at the time of treatment to the reference state at the time of planning. In addition, imaging must be used to measure the breathing motion. Depending on the in-room imaging available, different strategies can be used. For 2D imaging, an AP and lateral image should be obtained for alignment and is often obtained at exhale breath hold if that is the reference position. Alternatively, a fluoroscopy sequence can be obtained in the AP and lateral direction and the exhale position selected from the sequence for alignment. Non-orthogonal image pairs may also be used to position inserted fiducial markers. Once the reference position is matched, the breathing motion can be measured using fluoroscopy and compared to the treatment plan. The process becomes clearer if 4D volumetric imaging is available at treatment and planning. If the exhale position is the reference, the exhale reconstruction of the 4D image can be obtained and aligned to the reference image. The breathing motion can then be verified using the remainder of the 4D images. In addition, the breathing motion can be verified using the remaining images. Figure 3 shows a comparison of different imaging techniques for motion assessment.
Figure 3.
Methods of measuring breathing motion: cine MR frames (top), 4D CT (upper-middle), online fluoroscopy (lower-middle, right) compared to DRR (left), and 4D CBCT (lower)
Adaptive Inverse Planning - Mean Position Approach
Recently, with the advent of 4D CT, which includes intermediate positions, it has been suggested that the mean position be the reference position.30–32 The mean position enables a statistical-based margin for breathing to be generated, apposed to an inclusive margin which has been historically used. This assumes that breathing motion is a random uncertainty, similar to setup uncertainties.
There are two methods for calculating the PTV margin for mean target position, the technique presented by Wolthaus, et. al., which focuses on population planning techniques and individualized patient breathing for use in a margin recipe, and a second by Hugo, et. al., which uses patient specific breathing motion and planning information. The use of the mean target position as the reference for planning is treatment has been shown to be a more robust and reliable reference position compared to other positions (i.e. inhale, exhale, or mid-excursion) for the lung.31 Research is needed to confirm these findings in the liver, where the tumor is not easily visible on non-contrast enhanced images (e.g. CBCT). These approaches benefit from using the temporo-spatial information directly in the IMRT optimization.33
Wolthaus et. al. have proposed using a margin recipe to generate breathing PTV margins for breathing34. The mid ventilation position which accounts for hysteresis is constructed as a reference and a margin is generated which aims to ensure that at least 95% of the prescribed dose to the CTV is delivered for 90% of the population. Therefore, if the patient can be setup to the mean breathing position, the margin due to breathing motion, which is a random uncertainty, is smaller than the entire breathing extent (as is typical in the ITV based approach) This reduces the volume of the PTV, however it also changes the goal of the PTV from full prescription dose coverage, in the ITV model, to 95% prescription dose to the CTV for 90% of the patient population. Tailoring a margin recipe, which includes the beam penumbra, has not been evaluated for the liver, to date. Hugo, et. al. has proposed using the mean target position and calculating the target margin using the patient specific probability density function for motion and the individual dose distribution.35
If the mid ventilation position is the reference, the mid ventilation position at treatment delivery must be obtained. This mid ventilation position at treatment can be more challenging to obtain, especially with 2D imaging. One solution is to obtain fluoroscopy during normal breathing and extract the normal inhale and exhale position to derive the mid ventilation position. Similar to the standard ITV approach, the image guidance for the mean approach is easier to implement with 4D imaging at the treatment unit. The mid-ventilation position can be extracted and aligned to the reference mid-ventilation position and the remaining images can be used to verify the treatment margins assigned at planning.
Breath Hold
An alternative approach when the patient has a breathing excursion that exceeds 5 mm, is to image and treat the patient at a pre-defined breath hold position, either voluntarily or with assistance.11,13 The use of this technique enables a large reduction in the PTV margin for patients with large breathing excursions, and therefore a reduction in normal tissue irradiation, either liver or neighboring critical structures. The adaptation of the treatment planning and delivery process for breath hold must be done with caution. First, the reproducibility of the breath hold must be established on a patient-specific basis. Not all patients are suitable candidates for breath hold, for some the variation from breath hold to breath hold within the same treatment session is as large as the breathing motion. Ideally, the patient is examined under fluoroscopy for reproducibility of breath hold position. Once reproducibility of the breath hold is confirmed, the patient should be imaged for treatment planning in the breath hold position. There is more experience in using exhale breath hold, although feasibility of inhale breath hold has also been demonstrated for liver cancer.
Image guidance for treatment delivery, to confirm the breath hold position, is warranted, as the inter-fraction variation of breath hold position has been shown to vary from 3.4 to 4.4 mm.11,13 Image guidance can be performed using 2D coronal and sagittal images at breath hold or using volumetric 3D images obtained at the breath hold position. Typically, patients cannot hold their breath for the full duration of the 3D CBCT acquisition (60 seconds) and therefore the acquisition must be obtained in increments, allowing the patient to breath between segments of image acquisition. In this approach, the soft tissue liver can then be aligned to the reference image.
Gating
An additional adaptation technique is to gate the treatment beam to a certain breathing position, rather than enabling a breath hold for the patient. Similar requirements exist, compared to the breath hold technique, however the burden of breath hold is now removed from the patient and placed onto the treatment machine, to turn off and on the beam at the required times. The reference position of the patient must be determined at planning in addition to the variation from the reference position that is allowed during treatment (e.g. the exhale position, +/− 3 mm from that position). The reference position must be verified at each treatment delivery, in the same manner as describe for the free breathing technique. Typically, a surrogate is used to identify when the patient is in the reference position, therefore, in addition to verifying the reference position, the correlation between the surrogate and the breathing position must be verified at each treatment. Recent research has shown that combining external and internal (e.g. implanted fiducials) surrogates can improve the accurate of respiratory gating. Briere and colleagues have recently shown, through offline evaluation of electronic portal images acquired in cine mode, that for a cohort of 5 patients, the inter-fractional contribution of the external monitoring device to the random error was 2.0 mm in the SI direction.18 The contribution to the systematic error was 0.9 mm and to the intra-fractional random error, 1.0 mm. Shirato and colleagues have reported on the use of a real-time tumor-tracking (RTRT) device which includes fluoroscopic tracking of an implanted fiducial marker near the tumor.25 Kitamura reported on the motion of 20 liver tumors using this device and found the average tumor motion was 4 mm (LR) 5 mm (AP), and 9 mm (SI), but ranged up to 12 mm (LR and AP) and 19 mm (SI).36
Tracking
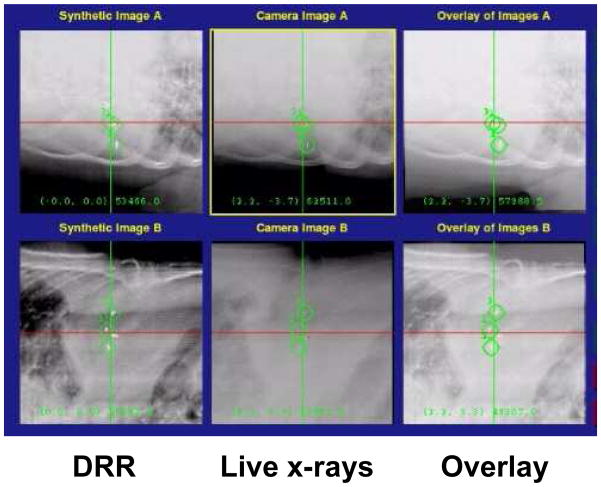
Both the breath hold and gating techniques described above reduce the efficiency of treatment as the beam must be turned off periodically, to allow the patient to breath or to wait until the breathing cycle is in the correct phase. For hypo-fractionated cases, where a larger dose is delivered per treatment, this reduction in efficiency will extend an already lengthy procedure. An alternative approach is to track the tumor as it moves due to respiration. Monitoring the breathing is typically done in the same manner as for gating, either using an external or internal surrogate and monitoring. Additional verification must be performed when treating the patient using a tracking technique compared to the gating technique. With gating, only the gated position and surrogacy must be verified, however, with tracking, the correlation between the surrogate and the entire breathing pattern must be verified. In addition, the relationship of the surrounding anatomy with the tumor must be verified to ensure that the normal tissue dose is not substantially different than was planned. Lieskovsky and colleagues have reported on a phase I dose escalation study of CyberKnife stereotactic radiosurgery for liver malignancies. Early results indicated the safe administration of single fraction 18-22 Gy dose to PTVs which ranged from 11–42 cc.37 An example of tracking based on fiducials is shown in Figure 4.
Figure 4.
Digitally reconstructed radiographs (DRRs) generated from treatment planning images with the fiducials highlighted (left), kV images generated during deliver (center), and the fusion of both images (right). Figure courtesy of Paul J. Keall, Stanford University
4. Eligibility: Advantage, disadvantage and limitations
The eligibility of patients for treatment using the techniques described above varies due many factors including comfort, compatibility with the device, and regularity of breathing. The simple techniques, such as adapting the treatment plan to the patient-specific motion, is possible for all patients. The limitation in this technique is the reproducibility of the patients breathing. The longer the overall treatment duration, i.e. non-SBRT approaches, the more likely the patient will exhibit a different breathing pattern or changes in anatomy during therapy. On the contrary, the larger the number of fractions, the smaller the effect of one treatment fraction being delivered under the conditions of a different breathing cycle. Free-breathing approaches have the advantage of less technological intervention, easier quality assurance, and more efficiency (average treatment with ABC is 21 minutes, average treatment time, excluding images was 13 minutes11). The disadvantage, of course, is the increased irradiation to normal tissues. Depending on the size of the tumor and its location, the increase in irradiation may prohibit the delivery of the prescription dose deemed necessary for sustained tumor control.
Patient compliance and understanding is necessary for treatment protocols using breath hold. It is critical for the patient to understand the importance of maintaining the breath hold during the treatment delivery. The advantages of using the breath hold technique is that the treatment delivery is consistent between each fraction (i.e. the same treatment beam is delivered to the same patient geometry, within the residual uncertainties). The disadvantages include potential discomfort or inconvenience to the patient during breath hold and a reduction in the efficiency of the treatment delivery. The use of this technique is limited to patients who can tolerate holding their breath, ideally for more than 20 seconds at a time, understand and can comply with the instructions, and who can obtain a reproducible breath hold with the device.
For both tracking and gating the eligibility of the patients depends on the ability to accurately correlate a surrogate with the tumor position. Using an internally implanted surrogate improves the likelihood of this correlation, however it involves an invasive procedure. The advantages of these techniques are that there is no inconvenience to the patient, they maintain a normal breathing state, and the dose to the surrounding normal tissue is decreased. The disadvantages of these techniques is a reduction in treatment efficiency, for gating only, and the increased burden of quality assurance, as the treatment delivered each day will vary, likely only subtly, based on the variation of the patients breathing and position, i.e. the ‘beam on’ will vary based on the breathing phase for gating and the position of the beam will depend on the motion of the surrogate in tracking.
5. Patient Selection, Clinical Workflow, QA and Management
The first step in patient selection for different treatment techniques is to evaluate the breathing motion. If the motion is less than 5 mm, than as recommended by the American Association of Physicists in Medicine (AAPM) Task Group Report #76, no adaptation for motion should be performed. It is advisable to verify the lack of substantial motion at the time of first treatment and periodically for standard fraction schedules. The selection of which treatment technique to use depends on the clinical availability and the risk to normal tissues.
The general clinical workflow begins with motion assessment and selection of appropriate motion management technique. Imaging at treatment planning should include diagnostic quality imaging for tumor delineation as well as motion assessment in all 3 directions, ensuring that the normal breathing pattern is accurately evaluated. At treatment, the baseline reference position should be used for setup and the motion of a surrogate should be evaluated. Substantial changes in the motion should be acted on as necessitated by the clinical protocol.
Quality assurance is critical for all aspects of the adaptive management of liver cancer. First it is important to verify that the correct phase of breathing is transferred to the treatment planning system for planning and use as the reference image. Verification of the breathing motion should be mandated for each treatment fraction. For patients treated at breath hold, image guidance to align and verify the breath hold position should be performed at each treatment fraction, for both hypo-fractionated and standard fractionation techniques. Patients treated with gating or tracking using a surrogate should have verification of the consistency of the surrogate prior to each treatment fraction, again, regardless of fractionation schedule.
The dosimetric impact of the uncertainties associated with the treatment of liver cancer, and therefore the dosimetric indications for the need to pursue a true adaptive approach, are beginning to be investigated. Romero and colleagues have recently reported on the dosimetric impact of daily setup corrections for 14 SBRT patients.38 CT-based corrections reduced the loss in tumor volume coverage from 6.8% to 1.7%, which translates into a reduction in the equivalent uniform dose from 15.5% (no corrections) to 2.3% (with corrections). Dosimetric changes to the organs at risk varied between positive and negative deviations and were found to be independent from the magnitude of the setup error, indicating the potential for dosimetric improvements with the development of online adaptive treatment techniques.
Similar improvements in target coverage with IGRT have been seen for patients treated under breath hold. In a study of 30 patients treated with 6-fraction image guided (MV portal images and kV CBCT projections) SBRT, 28% of the non-IGRT setups were within 3 mm, which increased to 82% when IGRT was employed for setup. The dosimetric implications of these offsets were a reduction in the planned mean PTV coverage from 82.4% to 80.3% (with IGRT) and 66.3% (without IGRT).15 For 29 free breathing patients treated under the same protocol, CBCT images obtained at the beginning and end of each fraction were analyzed offline to evaluate baseline shifts (i.e. the relative change in position of the liver to the vertebral bodies) and intra-fraction changes. Inter-fraction changes dominated the uncertainties, with a systematic error in the SI direction of 2.9 mm (inter-fraction) versus 1.1 mm (intra-fraction). These inter-fraction differences may provide the opportunity to perform adaptive radiotherapy to take advantage of the new geometrical relationship between the tumor and surrounding normal tissue. For example, for tumors near the stomach a change in stomach filling between treatment planning and treatment delivery may enable re-optimization of the treatment plan to further spare dose to the stomach.
Advancements in deformable registration, dose accumulation and volumetric online imaging are enabling the investigation into the use of adaptive protocols for liver RT. Current research is showing the dosimetric impact of changes in breathing motion, baseline shifts, residual setup errors, and the relative deformation of the tumor and surrounding normal tissues using 4D CBCT at each fraction and deformable registration and dose accumulation. Dosimetric differences in tumor coverage as well as normal tissue doses are observed. Most importantly, dosimetric differences in the dose-limiting normal tissues have been observed, indicating that with deformable dose accumulation and adaptive planning, additional dose escalation could be pursued.39
6. Conclusions
IGRT for radiation therapy of the liver can improve the ability to deliver a high dose of radiation to the tumor while maintaining an appropriately low risk of normal tissue toxicity. These techniques range in complexity, both for treatment planning, delivery, and quality assurance, however the potential impact for the treatment of patients with large amplitudes of breathing motion can be significant. Image guidance is necessary for the safe implementation of these techniques, however, improvements in image guidance systems is enabling a streamlined approach for the integration of these complex techniques into the clinical process. Although, to date, the use of adaptive technology for the treatment of liver cancer has mainly been focused on individualizing planning margins and treatment techniques to improve dose escalation for individual patients, ongoing research and emerging technology is paving the way, and indicating the potential benefit of, true adaptive radiotherapy.
Acknowledgments
Support: Dr. Brock is supported by a research chair from Cancer Care Ontario. Research is supported in part by NIH 1R01CA124714-01A2, National Cancer Institute of Canada – Terry Fox Foundation, National Cancer Institute of Canada (NCIC #18207), the Canadian Cancer Society, and Elekta Oncology Systems.
Footnotes
Disclosures: Dr. Brock receives research funding from Elekta Oncology Systems, Philips Medical Systems, and RaySearch Laboratories. She also serves on the IMPAC Physics Advisory Board. Dr. Dawson receives research funding from Elekta Oncology Systems.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007 Feb 15;109(4):718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 4.Liu JH, Chen PW, Asch SM, Busuttil RW, Ko CY. Surgery for hepatocellular carcinoma: does it improve survival? Ann Surg Oncol. 2004;11(3):298–303. doi: 10.1245/aso.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery. 2002;132(4):605–611. doi: 10.1067/msy.2002.127545. [DOI] [PubMed] [Google Scholar]
- 6.Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221(1):159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 7.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15(4):279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Ben Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Ten Haken RK, Knol J, Dawson LA, Pan C, Lawrence TS. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005 Dec 1;23(34):8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 9.Langen KM, Jones DT. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001 May 1;50(1):265–278. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- 10.Case RB, Sonke JJ, Moseley DJ, Kim J, Brock KK, Dawson LA. Inter- and Intra-Fraction Variability in Liver Position in Non-Breath Hold Stereotactic Body Radiotherapy. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Eccles C, Brock KK, Bissonnette JP, Hawkins M, Dawson LA. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006 Mar 1;64(3):751–759. doi: 10.1016/j.ijrobp.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 12.Dawson LA, Eccles C, Bissonnette JP, Brock KK. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005 Jul 15;62(4):1247–1252. doi: 10.1016/j.ijrobp.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 13.Dawson LA, Brock KK, Kazanjian S, Fitch D, McGinn CJ, Lawrence TS, Ten Haken RK, Balter J. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001 Dec 1;51(5):1410–1421. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 14.Brock KK, Hawkins M, Eccles C, Moseley JL, Moseley DJ, Jaffray DA, Dawson LA. Improving image-guided target localization through deformable registration. Acta Oncol. 2008;47(7):1279–1285. doi: 10.1080/02841860802256491. [DOI] [PubMed] [Google Scholar]
- 15.Eccles CL, Craig TD, Dawson LA. Estimated Dosimetric Impact of IGRT in Liver SBRT with ABC. Eur J Cancer. 2007;5(4):125–126. [Google Scholar]
- 16.Hawkins MA, Brock KK, Eccles C, Moseley D, Jaffray D, Dawson LA. Assessment of residual error in liver position using kV cone-beam computed tomography for liver cancer high-precision radiation therapy. Int J Radiat Oncol Biol Phys. 2006 Oct 1;66(2):610–619. doi: 10.1016/j.ijrobp.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Guckenberger M, Sweeney RA, Wilbert J, Krieger T, Richter A, Baier K, Mueller G, Sauer O, Flentje M. Image-guided radiotherapy for liver cancer using respiratory-correlated computed tomography and cone-beam computed tomography. Int J Radiat Oncol Biol Phys. 2008 May 1;71(1):297–304. doi: 10.1016/j.ijrobp.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Briere TM, Beddar S, Balter P, Murthy R, Gupta S, Nelson C, Starkschall G, Gillin MT, Krishnan S. Respiratory gating with EPID-based verification: the MDACC experience. Phys Med Biol. 2009 Jun 7;54(11):3379–3391. doi: 10.1088/0031-9155/54/11/007. [DOI] [PubMed] [Google Scholar]
- 19.Mendez Romero A, Zinkstok RT, Wunderink W, van Os RM, Joosten H, Seppenwoolde Y, Nowak PJ, Brandwijk RP, Verhoef C, Ijzermans JN, Levendag PC, Heijmen BJ. Stereotactic body radiation therapy for liver tumors: impact of daily setup corrections and day-to-day anatomic variations on dose in target and organs at risk. Int J Radiat Oncol Biol Phys. 2009 Apr 20; doi: 10.1016/j.ijrobp.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Wunderink W, Mendez Romero A, Vasquez Osorio EM, de Boer HC, Brandwijk RP, Levendag PC, Heijmen BJ. Target coverage in image-guided stereotactic body radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys. 2007 May 1;68(1):282–290. doi: 10.1016/j.ijrobp.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Teh BS, Paulino AC, Lu HH, Chiu JK, Richardson S, Chiang S, Amato R, Butler EB, Bloch C. Versatility of the Novalis system to deliver image-guided stereotactic body radiation therapy (SBRT) for various anatomical sites. Technol Cancer Res Treat. 2007;6(4):347–354. doi: 10.1177/153303460700600412. [DOI] [PubMed] [Google Scholar]
- 22.Fuss M, Shi C, Papanikolaou N. Tomotherapeutic stereotactic body radiation therapy: Techniques and comparison between modalities. Acta Oncol. 2006;45(7):953–960. doi: 10.1080/02841860600897942. [DOI] [PubMed] [Google Scholar]
- 23.Fuss M, Salter BJ, Cavanaugh SX, Fuss C, Sadeghi A, Fuller CD, Ameduri A, Hevezi JM, Herman TS, Thomas CR., Jr Daily ultrasound-based image-guided targeting for radiotherapy of upper abdominal malignancies. Int J Radiat Oncol Biol Phys. 2004 Jul 15;59(4):1245–1256. doi: 10.1016/j.ijrobp.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Rietzel E, Rosenthal SJ, Gierga DP, Willet CG, Chen GT. Moving targets: detection and tracking of internal organ motion for treatment planning and patient set-up. Radiother Oncol. 2004;73(Suppl 2):S68–S72. doi: 10.1016/s0167-8140(04)80018-5. [DOI] [PubMed] [Google Scholar]
- 25.Shirato H, Shimizu S, Kitamura K, Onimaru R. Organ motion in image-guided radiotherapy: lessons from real-time tumor-tracking radiotherapy. Int J Clin Oncol. 2007;12(1):8–16. doi: 10.1007/s10147-006-0633-y. [DOI] [PubMed] [Google Scholar]
- 26.Ten Haken RK, Balter JM, Marsh LH, Robertson JM, Lawrence TS. Potential benefits of eliminating planning target volume expansions for patient breathing in the treatment of liver tumors. Int J Radiat Oncol Biol Phys. 1997 Jun 1;38(3):613–617. doi: 10.1016/s0360-3016(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 27.Balter JM, Lam KL, McGinn CJ, Lawrence TS, Ten Haken RK. Improvement of CT-based treatment-planning models of abdominal targets using static exhale imaging. Int J Radiat Oncol Biol Phys. 1998 Jul 1;41(4):939–943. doi: 10.1016/s0360-3016(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 28.Kirilova A, Lockwood G, Choi P, Bana N, Haider MA, Brock KK, Eccles C, Dawson LA. Three-dimensional motion of liver tumors using cine-magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2008 Jul 15;71(4):1189–1195. doi: 10.1016/j.ijrobp.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Beddar AS, Briere TM, Balter P, Pan T, Tolani N, Ng C, Szklaruk J, Krishnan S. 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiother Oncol. 2008;87(3):445–448. doi: 10.1016/j.radonc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Wolthaus JW, Schneider C, Sonke JJ, van Herk M, Belderbos JS, Rossi MM, Lebesque JV, Damen EM. Mid-ventilation CT scan construction from four-dimensional respiration-correlated CT scans for radiotherapy planning of lung cancer patients. Int J Radiat Oncol Biol Phys. 2006 Aug 1;65(5):1560–1571. doi: 10.1016/j.ijrobp.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Hugo GD, Liang J, Campbell J, Yan D. On-line target position localization in the presence of respiration: a comparison of two methods. Int J Radiat Oncol Biol Phys. 2007 Dec 1;69(5):1634–1641. doi: 10.1016/j.ijrobp.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hugo G, Vargas C, Liang J, Kestin L, Wong JW, Yan D. Changes in the respiratory pattern during radiotherapy for cancer in the lung. Radiother Oncol. 2006;78(3):326–331. doi: 10.1016/j.radonc.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Trofimov A, Rietzel E, Lu HM, Martin B, Jiang S, Chen GT, Bortfeld T. Temporo-spatial IMRT optimization: concepts, implementation and initial results. Phys Med Biol. 2005 Jun 21;50(12):2779–2798. doi: 10.1088/0031-9155/50/12/004. [DOI] [PubMed] [Google Scholar]
- 34.Wolthaus JW, Sonke JJ, van Herk M, Belderbos JS, Rossi MM, Lebesque JV, Damen EM. Comparison of different strategies to use four-dimensional computed tomography in treatment planning for lung cancer patients. Int J Radiat Oncol Biol Phys. 2008 Mar 15;70(4):1229–1238. doi: 10.1016/j.ijrobp.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 35.Hugo GD, Campbell J, Zhang T, Yan D. Cumulative lung dose for several motion management strategies as a function of pretreatment patient parameters. Int J Radiat Oncol Biol Phys. 2009 Jun 1;74(2):593–601. doi: 10.1016/j.ijrobp.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura K, Shirato H, Seppenwoolde Y, Shimizu T, Kodama Y, Endo H, Onimaru R, Oda M, Fujita K, Shimizu S, Miyasaka K. Tumor location, cirrhosis, and surgical history contribute to tumor movement in the liver, as measured during stereotactic irradiation using a real-time tumor-tracking radiotherapy system. Int J Radiat Oncol Biol Phys. 2003 May 1;56(1):221–228. doi: 10.1016/s0360-3016(03)00082-8. [DOI] [PubMed] [Google Scholar]
- 37.Lieskovsky YC, Koong A, Fisher G, Ho A, Gibbs I, Goodman K. Phase I Dose Escalation Study of CyberKnife Stereotactic Radiosurgery for Liver Malignancies. Int J Radiat Oncol Biol Phys. 2005 Oct 1;63(1):S283. [Google Scholar]
- 38.Romero AM, Zinkstok RT, Wunderink W, van Os RM, Joosten H, Seppenwoolde Y, Nowak PJCM, Brandwijk RP, Verhoef C, Ijzermans JNM, Levendag PC, Heijmen BJM. Stereotactic Body Radiation Therapy for Liver Tumors: Impact of Daily Setup Corrections and Day-to-Day Anatomic Variations on Dose in Target and Organs at Risk. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 39.Velec M, Moseley J, Dawson LA, Brock KK. Accumulated Treatment Dose for Image Guided Liver Radiotherapy using Deformable Registration of 4D Cone Beam CT. Int J Radiat Oncol Biol Phys. 2008 Sep 1;72(1):S149. [Google Scholar]