Abstract
Preadipocyte factor 1 (Pref-1) is an EGF-repeat-containing transmembrane protein that inhibits adipogenesis. The extracellular domain of Pref-1 is cleaved by TNF-α converting enzyme to generate the biologically active soluble form of Pref-1. The role of Pref-1 in adipogenesis has been firmly established by in vitro and in vivo studies. Pref-1 activates ERK/MAPK and upregulates Sox9 expression to inhibit adipocyte differentiation. Sox9 directly binds to the promoter regions of CCAAT/enhancer-binding protein-β and CCAAT/enhancer-binding protein-δ in order to suppress their promoter activities in preventing adipocyte differentiation. Here, we describe the function of Pref-1 in adipocyte differentiation and the recent findings on the mechanisms by which Pref-1 inhibits adipocyte differentiation.
Keywords: adipocyte differentiation, CCAAT/enhancer-binding protein-β, CCAAT/enhancer-binding protein-δ, EGF-like domain, extracellular signaling-regulated kinase/mitogen-activated protein kinase, preadipocyte factor 1, Sox9, TNF-α converting enzyme
Adipose tissue functions for energy storage and partitioning and also exerts a dynamic role as an endocrine organ. Accumulation of adipose tissue is the result of increased fat cell size with increased triacylglycerol storage, as well as a result of increased number of fat cells arising from differentiation of preadipocytes into mature adipocytes. Paradoxically, both obesity resulting from excess adipose tissue, as well as lipodystrophy, which causes dysfunction in the secretory function of adipose tissues, are commonly associated with diverse pathologies including diabetes, cardiovascular diseases and immunosuppression. Therefore, it is important to elucidate and characterize the mechanisms involved in adipogenesis, as well as the factors that regulate adipocyte differentiation and function.
Adipose tissue contains adipocytes and stromal vascular cells represented by preadipocytes, monocytes/macrophages, endothelial cells and other cells types. The adipocyte lineage develops from mesenchymal cells that undergo commitment and differentiation processes to become adipocytes. Mesenchymal cells can also become myocytes, chondrocytes and osteoblasts that generate muscle, cartilage and bone tissues. Preadipocyte cell lines, such as 3T3-L1 and 3T3-F442A, are widely used for studying adipocyte differentiation. The mesenchymal cell line C3H10T1/2 and mouse embryonic fibroblasts (MEFs) are also used for studying mesenchymal cell commitment and differentiation into adipocytes. During the last two decades, adipocyte differentiation has been extensively studied at the transcriptional level. PPAR-γ and CCAAT/enhancer-binding proteins (C/EBPs) are transcription factors that have been shown to play critical roles in adipocyte differentiation. C/EBP-δ and C/EBP-β are increased early during differentiation to induce PPAR-γ and C/EBP-α for adipocyte differentiation. These transcription factors in turn induce many genes involved in adipocyte function, such as adipocyte fatty acid-binding protein (aFABP; aP2), stearoyl CoA desaturase 1 (SCD-1) and fatty acid synthase (FAS). Various hormones, growth factors and extracellular matrix proteins (ECM) play regulatory roles in adipocyte proliferation and/or differentiation. Some of these molecules are secreted by adipose tissue itself – either by adipocytes or preadipocytes.
Preadipocyte factor 1 (Pref-1) was originally cloned from a 3T3-L1 preadipocyte library [1]. Pref-1, and its soluble form, are also referred to as Delta-like 1 (Dlk1), pG2, Fetal antigen 1 (FA1) or Zona glomerulosa-specific factor (ZOG), depending on the context in which they were originally identified [1–4]. Pref-1 is highly expressed in preadipocytes and absent after adipocyte differentiation. In adults, Pref-1 expression is significantly detected only in certain cell types such as preadipocytes, pancreatic islet β cells, thymic stromal cells, adrenal gland cells and the pituitary [1,5–7]. However, Pref-1 is widely expressed in developing embryonic tissues, such as the tongue, lungs, liver, vertebra, skeletal myotubes, pancreas, placenta and ovarian cells, but the expression is rapidly reduced after birth [1,8]. Pref-1 is also detected in some tumors and tumor cell lines of neuroendocrine origin [3]. Increased levels of circulating Pref-1 are found in maternal serum in concentrations that correlate with the number of fetuses in utero in rodents. Pref-1 is a paternally expressed imprinted gene located in an imprinted syntenic region in mouse chromosome 12, human chromosome 14 and sheep chromosome 18, along with other imprinted genes, meg8, dat, gtl2, peg11 and antipeg11 [9–12]. The majority of imprinted genes code for proteins that regulate fetal growth and organogenesis. In addition, Pref-1 has been shown to affect multiple differentiation processes, including adipogenesis [1,13,14], chondrogenesis [15], osteoblas-togenesis [15,16], hematopoiesis [17], adrenal and neuroendocrine cell differentiation, as well as B-cell differentiation [18]. Pref-1 has also been reported to be involved in tumorigenesis [19,20]. Overall, Pref-1 may function as a soluble factor, maintaining proliferating cells in an undifferentiated state during development. In this review, we focus on describing the function of Pref-1 in adipocyte differentiation and the underlying molecular mechanisms.
Pref-1 structure & cleavage
Preadipocyte factor 1 is made as a transmembrane protein and four major alternative splicing forms, Pref-1A–D, are present at varying levels, depending on the tissues [21]. Spliced variants have also been found in other species [22]. Pref-1 contains six EGF-like repeats in the extracellular domain, which maintain the conserved spacing of six cysteines for three disulfide bonds, as well as the other amino acids characteristic of EGF-like repeat motifs [1,3,4]. The largest full-length Pref-1 form, Pref-1A, encodes 385 amino acids with six EGF-like repeats. Alternative splicing generates three major shorter forms of Pref-1; Pref-1B–D, each containing in-frame deletions in the extracellular juxtamembrane region or part of the sixth EGF-like repeat domain (Figure 1). In preadipocytes, multiple transmembrane forms of Pref-1, ranging from 50 to 60 kDa, are found in the cell membrane due to, in part, post-translational modifications of N-linked oligosaccharides and sialic acids in the extracellular domain [13,23]. Pref-1 is proteolytically cleaved at the extracellular domain at two sites to generate soluble forms of Pref-1. Pref-1A and Pref-1B, are cleaved at a juxtamembrane site and at a site closer to the N-terminus to generate a larger 50 kDa and a smaller 25 kDa soluble form, respectively. The Pref-1C and Pref-1D, owing to the larger deletions at the juxtamembrane region that includes the aforementioned cleavage site, are cleaved only at the N-terminal site to generate the smaller 25 kDa soluble form. Using various inhibitors and in vitro approaches, one of the ADAM family members, TNF-α converting enzyme (TACE; ADAM 17), has been shown to be responsible for the cleavage of Pref-1 at the juxtamembrane region to generate the large 50 kDa soluble form [24]. Overexpression of TACE increases this Pref-1 cleavage. Conversely, siRNA-mediated knockdown of TACE decreases the release of the large soluble form from the membrane form. Moreover, this cleavage is undetectable or markedly decreased in cells bearing mutated TACE.
Figure 1. Pref-1 structure and its mechanism for inhibition of adipogenesis.
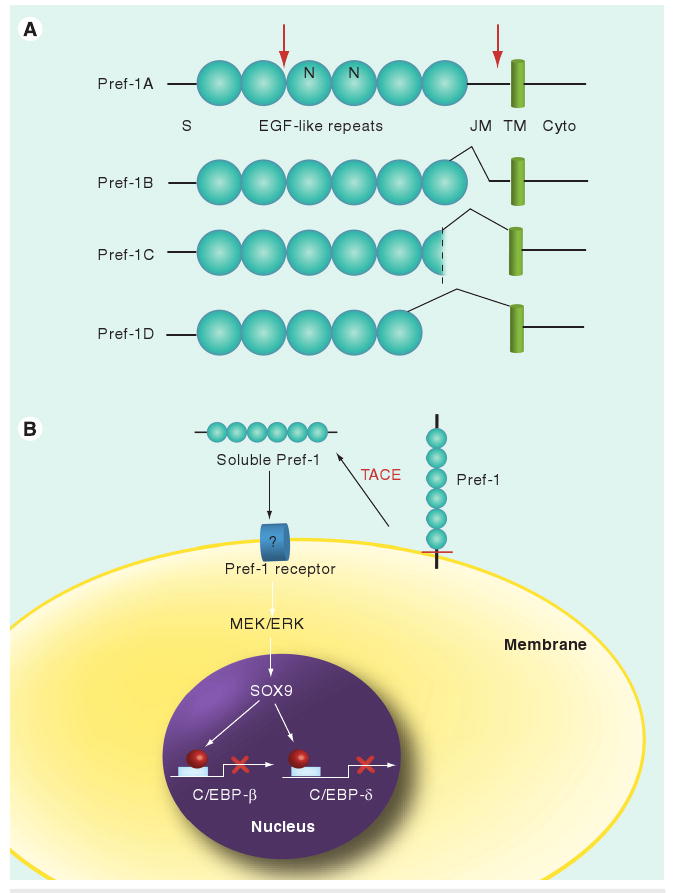
(A) Domain structure of Pref-1 isoforms. Cleavage sites are marked red. (B) Pref-1 inhibition of adipogenesis. Pref-1 is cleaved by TACE at the juxtamembrane region generating 50 kDa soluble Pref-1. Soluble Pref-1 binds to the putative Pref-1 receptor at the preadipocyte membrane and activates MEK/ERK/MAPK that, in turn, increases Sox9 expression. Sox9 binds to C/EBP-β and C/EBP-δ promoter regions to suppress their transcription, resulting in inhibition of adipocyte differentiation.
C/EBP: CCAAT/enhancer-binding protein; Cyto: Cytoplasmic region; EGF: Epidermal growth factor-like repeat; ERK: Extracellular signaling-regulated kinase; JM: Juxtamembrane; MEK: MAPK/ERK kinase; N: N-linked glycosylation sites; Pref-1: Preadipocyte factor 1; S: Signal sequence; TACE: TNF-α converting enzyme; TM: Transmembrane domain.
Inhibition of adipocyte differentiation by Pref-1
Upon treatment with adipogenic agents comprised of synthetic glucocorticoid, dexamethasone (DEX), isobutylmethylxanthine (IBMX) and insulin, preadipocytes, such as 3T3-L1 cells and 3T3-F442A cells, undergo adipocyte differentiation, displaying lipid droplets in the cytoplasm visualized by Oil red O staining and marked induction in expression of adipocyte markers, such as FAS, SCD-1 and aP2, as well as adipogenic transcription factors, C/EBP-α and PPAR-γ. By contrast, Pref-1 is highly expressed in preadipocytes, but is decreased during differentiation and is absent in mature adipocytes [1,25]. Downregulation of Pref-1 reflects the degree of adipocyte differentiation and therefore Pref-1 is widely used as a preadipocyte marker. Pref-1 is downregulated specifically by one of the adipogenic agents, DEX. The inhibitory role of Pref-1 in adipocyte differentiation has been well established in cultured cells. In 3T3-L1 cells, constitutive expression of Pref-1 by stable transfection of Pref-1A markedly lowers the degree of adipocyte differentiation. Conversely, decreasing Pref-1 levels by transfection of the Pref-1 antisense sequence greatly enhances adipocyte differentiation. Using alternative splicing forms of Pref-1A–D and a mutant form of Pref-1 (Pref-1Δ21) with deletion of the cleavage site at the juxtamembrane domain, it has been demonstrated that only the 50 kDa large soluble form of Pref-1 (released from Pref-1A and B) inhibits adipocyte differentiation, and the other smaller alternative splicing products of Pref-1, including Pref-1C, Pref-1D, the membrane form (Pref-1Δ21) or smaller 25 kDa soluble form, do not inhibit differentiation [21]. Overexpression of TACE lowers the degree of differentiation by increasing the release of the 50 kDa soluble form from the cell membrane. Conversely, knockdown of TACE or treatment with inhibitors of TACE enhances differentiation confirming the biological role of this cleavage. The 50 kDa soluble form generated by TACE-mediated cleavage is the only biologically functional form of Pref-1 that can inhibit adipocyte differentiation [24]. This fact further demonstrates the importance of cleavage for bioactivity, similar to many other signal proteins such as EGF and TNF-α. Pref-1 null MEFs have also been used to examine the effect of Pref-1 in adipocyte differentiation. As judged by lipid accumulation and adipocyte marker expression, 90% of Pref-1 null MEFs differentiate into adipocytes. Overexpression of Pref-1A by lentivirus infection or treatment with soluble Pref-1 decreases the degree of differentiation of Pref-1 null MEFs by 60%, further confirming the inhibitory role of Pref-1 in adipocyte differentiation [15,26].
Preadipocyte factor 1 is widely expressed in multiple tissues in the embryonic stage and has been reported to be involved in embryonic development in humans and in animal models. Here, we are focusing on the Pref-1 effect on adipogenesis. To define the in vivo role of Pref-1, we generated Pref-1 null mice [27] as well as transgenic mice overexpressing Pref-1 [28]. Approximately 50% of Pref-1 null mice die within 2 days of birth and surviving mice are smaller than wild-type mice at birth and weaning age. However, the weight of white fat depots including inguinal, retroperitoneal and gonadal, are significantly higher than those of wild-type mice. Histological analysis and gene expression examination of adipocyte markers, such as C/EBP-α, PPAR-γ, FAS, SCD-1 and aFABP, have demonstrated that the increased fat pad weights resulted from increased adipogenesis. Moreover, these mice also develop enlarged livers with increased lipid content, elevated circulating triglycerides, free fatty acids and cholesterol – factors normally associated with obesity. Since only the soluble form of Pref-1 inhibits adipocyte differentiation, we generated Pref-1 transgenic mice overexpressing the Pref-1 extracellular domain as a human Fc-fusion protein using the aP2 promoter, which drives transgenic gene expression mainly in adipose tissue. aP2-Pref-1-hFc transgenic mice show a marked decrease in adipose tissue mass and reduced expression of adipocyte markers, including C/EBP-α, SCD-1, FAS and ADSF/resistin [28,29]. Mice overexpressing Pref-1-hFc exclusively in the liver under the control of the albumin promoter also demonstrate a decrease in adipose mass and adipocyte marker expression, suggesting an endocrine mode of action of Pref-1. Pref-1 null and transgenic mice show defects similar to maternal uniparental disomy (UPD) 12 and paternal UPD12 in mice, respectively, and syntenic maternal and paternal UPD14 syndromes in humans. In this regard, in a study analysing polymorphisms in a 109 kb genomic region encompassing Pref-1 on human chromosome 14, among 1025 French and German families comprised of both parents and extremely obese offspring, researchers found a single nucleotide polymorphism (rs1802710) associated with child and adolescent obesity [30]. Furthermore, the callipyge phenotype, characterized by decreased fat mass and muscular hypertrophy in sheep, is evident only when the mutation is paternally inherited in heterozygous animals and is correlated with an abnormally high level of Pref-1 expression during the postnatal period [12]. Overall, these in vivo observations are consistent with in vitro studies, demonstrating that Pref-1 is a negative regulator of adipogenesis.
Mechanism for Pref-1 inhibition of adipocyte differentiation
The Pref-1 effect has been linked to IGF-1 and ERK–MAPK signaling pathways: the inhibitory effect of Pref-1 in adipocyte differentiation has been reported to be via the inhibition of IGF receptor signaling of ERK–MAPK activation [31]. On the other hand, others have reported enhanced IGF-1-dependent ERK activation by downregulation of Pref-1 [32]. In this regard, Pref-1 has also been reported to interact with IGFBP-1 [33]. Recently, by treatment of Pref-1 null MEFs with soluble Pref-1, the ERK1/2–MAPK pathway has been found to be responsible for the Pref-1 effect [26]. Treatment with the 50 kDa soluble Pref-1 can markedly induce phosphorylation or activation of ERK1/2, but not p38 or JNK MAPK in serum-free conditions. This clearly demonstrates that Pref-1 increases ERK1/2 phosphorylation and activation in a time- and dose-dependent manner. During adipocyte differentiation of MEFs, wild-type MEFs show a transient burst of ERK phosphorylation immediately after the addition of adipogenic agents and a second increase in ERK phosphorylation peaking at day 2 of differentiation. However, Pref-1 null MEFs show only the first, but not the second, peak of ERK phosphorylation. The second ERK phosphorylation coincides with the Pref-1 expression pattern during MEF differentiation into adipocytes. Prevention of this second ERK phosphorylation by use of the MAPK/ERK kinase (MEK) inhibitor PD98059 or siRNA-mediated knockdown of ERK enhances adipocyte differentiation, indicating that Pref-1 activation of MEK–ERK is required for Pref-1 inhibition of adipogenesis. Microarray analysis revealed Sox9 as a downstream target gene of Pref-1. Sox9 is a high-mobility-group-box DNA-binding transcription factor, and has been well documented for its critical role in chondrogenesis during skeletal development. Sox9 is expressed in all chondroprogenitor cells, specifically enriched in immature chondroblasts, but its expression is abolished in hypertrophic chondrocytes. Sox9 expression is also detectable in 3T3-L1 cells and in MEFs, and Sox9 expression is downregulated during adipocyte differentiation, matching Pref-1 expression. Also similar to Pref-1, Sox9 is highly detected in the stromal vascular fraction of adipose tissue, but not in the adipocyte fraction. Lentivirus-mediated overexpression of Sox9 in MEFs leads to decreased adipocyte differentiation, indicating the requirement of Sox9 downregulation for adipocyte differentiation. The increase in Sox9 expression by Pref-1 treatment can be blocked by the MEK inhibitors PD98059 or U0126, as well as by knockdown of ERK1/2, indicating that Pref-1 induces Sox9 expression through activation of the ERK1/2 pathway. By chromatin immunoprecipitation and electrophoretic mobility shift assays, Sox9 has been shown to directly bind to the -675–645 promoter region of C/EBP-β and -1770–1740 promoter region of C/EBP-δ. In addition, Sox9 can suppress C/EBP-β and C/EBP-δ promoter activities [15]. Overall, it has been demonstrated that Pref-1 inhibits adipocyte differentiation by preventing Sox9 downregulation.
Preadipocyte factor 1 contains several EGF-like domains that were originally described for EGF and other growth factors. These growth factors function by binding to the EGF receptor, and act as signals for cell proliferation and differentiation. While Pref-1 does not contain the conserved amino acid residues that are required for EGF receptor binding, it shares more similarities with another family of EGF-like repeat containing proteins, the Notch/Delta/Serrate (Jagged) family, which are involved in cell signaling and cell fate determination. Upon binding of Jagged1, Notch was shown to inhibit adipocyte differentiation [34]. On the other hand, others reported Notch to be required for adipocyte differentiation [35]. However, genetic loss-of-function models have demonstrated that the Notch pathway is dispensable for adipocyte differentiation [36]. In relation to this, Pref-1 lacks the Delta Serrate Lin12 (DSL) domain that is conserved in all Notch ligands to mediate receptor-ligand interactions for Notch [37–39]. Nevertheless, Pref-1 has been implicated in Notch signaling [40,41]. Pref-1 has been reported to interact with Notch to inhibit, not activate, Notch signaling [40] to potentiate adipogenesis in a study using a mesenchymal cell line [42]. In this regard, by comparing morphological phenotype, membrane-bound Pref-1, but not the soluble extracellular domain of Pref-1, has been suggested to antagonize the Notch activity in the wing imaginal disk of Drosophila [41]. However, direct interaction of native Pref-1 and Notch has not been demonstrated. In thymocytes, Pref-1 treatment has been shown to induce Hes-1 expression, a target for Notch1 activation [6]. The Notch signaling pathway and its regulation of Pref-1 in the adipocyte differentiation process, if it occurs, requires further investigation.
Conclusion
Preadipocyte factor 1 functions as a soluble factor, maintaining proliferating cells in an undifferentiated state during development. Pref-1 inhibits adipocyte differentiation through preventing downregulation of Sox9 via binding C/EBP-β and δ promoters in order to suppress their activities. Dysregulation of Pref-1 contributes to the development of lipodystrophic disorders, diabetes, diet-induced obesity and related syndromes present in UPD 12 and 14 in mice and humans, respectively.
Executive summary.
Background
Obesity and its associated diseases are a prevalent health problem. In addition to an increase in fat cell size with triacylglycerol storage, an increase in fat cell number can contribute to the development of obesity. The increase in fat cell number arises from adipocyte differentiation of preadipocytes. Elucidating molecules involved in adipocyte differentiation is critical for the development of therapeutic targets for prevention/treatment of obesity.
Preadipocyte factor 1 structure & cleavage
Preadipocyte factor 1 (Pref-1) contains a signal sequence at the N-terminus, a single membrane-spanning domain, EGF-like repeats at the extracellular domain and a short cytoplasmic tail. Pref-1 is cleaved at the juxtamembrane domain by TNF-α converting enzyme generating the bioactive soluble form of Pref-1.
Inhibition of adipocyte differentiation by preadipocyte factor 1
Pref-1 is highly expressed in preadipocytes and is absent in mature adipocytes. Overexpression of Pref-1 markedly lowers the degree of adipocyte differentiation. Conversely, decreasing Pref-1 levels by transfection of the Pref-1 antisense sequence greatly enhances adipogenesis.
A total of 90% of Pref-1 null mouse embryonic fibroblasts (MEFs) differentiate into adipocytes upon treatment with adipogenic agents. Overexpression of Pref-1 by lentivirus infection or treatment with soluble Pref-1 decreases the degree of differentiation of Pref-1 null MEFs by 60%.
In Pref-1 null mice, weights of white fat pads are significantly higher than those of wild-type mice. Adipose tissue from these mice shows high adipocyte differentiation with elevated levels of adipocyte markers such as fatty acid synthase and stearoyl CoA desaturase 1.
aP2-Pref-1, as well as albumin-Pref-1, transgenic mice overexpressing soluble Pref-1 in adipose tissue and liver, respectively, show a marked decrease in adipose tissue mass and reduced expression of adipocyte markers.
Pref-1 null and transgenic mice show distinct defects similar to maternal uniparental disomy (UPD) 12 and paternal UPD12 in mice, respectively, and syntenic maternal and paternal UPD14 syndromes in humans.
A single-nucleotide polymorphism (rs1802710) has been found in a 109 kb genomic region encompassing Pref-1 on human chromosome 14 associated with childhood and adolescent obesity.
The callipyge phenotype, characterized by decreased fat mass and muscular hypertrophy in callipyge sheep is correlated with abnormally high Pref-1 expression during the postnatal period.
Mechanism underlying preadipocyte factor 1 inhibition of adipocyte differentiation
Soluble Pref-1 directly increases phosphorylation of extracellular signaling-regulated kinase (ERK) 1/2 rapidly but not p38 nor c-Jun amino-terminal kinase.
Activation of MAPK/ERK kinase/ERK signaling is required for Pref-1 inhibition of adipogenesis.
Pref-1 increases Sox9 mRNA and protein levels. The increase in Sox9 expression by Pref-1 is through ERK activation.
Sox9 downregulation is required for adipocyte differentiation. Sox9 binds to CCAAT/enhancer-binding protein (C/EBP)-β and C/EBP-δ promoters to suppress their transcription. Pref-1 inhibits adipocyte differentiation by preventing downregulation of Sox9.
Pref-1 has been implicated in Notch signaling, in either a positive or negative fashion. Physiologically relevant Pref-1 receptor and/or interaction partner(s) need to be identified.
Future perspective
The inhibitory role of Pref-1 in adipocyte differentiation has been well studied. However, much remains to be elucidated regarding the mechanism of Pref-1 function. Identification of the Pref-1 receptor would be critical in understanding Pref-1 function. Investigation of membrane and/or extracellular components, receptors or soluble signals, that interact with Pref-1 to mediate Pref-1 signaling will aid in understanding Pref-1 function during adipocyte differentiation. Identification of Pref-1 target genes, which are induced or suppressed by Pref-1 in preadipocytes or MEFs will also be required to clarify the cellular pathways for Pref-1. Pref-1 is expressed mainly in preadipocytes in adults, but widely expressed in embryonic tissues as well as mesenchymal cells. It will be important to investigate the commitment of mesenchymal cells into the adipocyte lineage and the role of Pref-1 in mesenchymal cell commitment and differentiation during adipogenesis.
Acknowledgments
The authors' research was supported in part by NIH DK50828.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact: reprints@futuremedicine.com
Bibliography
- 1.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]; •• First report that demonstrates Pref-1 inhibition of adipocyte differentiation.
- 2.Lee YL, Helman L, Hoffman T, Laborda J. dlk, pG2 and Pref-1 mRNAs encode similar proteins belonging to the EGF-like superfamily. Identification of polymorphic variants of this RNA. Biochim Biophys Acta. 1995;1261:223–232. doi: 10.1016/0167-4781(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 3.Laborda J, Sausville EA, Hoffman T, Notario V. dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J Biol Chem. 1993;268:3817–3820. [PubMed] [Google Scholar]
- 4.Jensen CH, Teisner B, Hojrup P, et al. Studies on the isolation, structural analysis and tissue localization of fetal antigen 1 and its relation to a human adrenal-specific cDNA, pG2. Hum Reprod. 1993;8:635–641. doi: 10.1093/oxfordjournals.humrep.a138110. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson C, Tornehave D, Lindberg K, et al. Growth hormone and prolactin stimulate the expression of rat preadipocyte factor-1/Delta-like protein in pancreatic islets: molecular cloning and expression pattern during development and growth of the endocrine pancreas. Endocrinology. 1997;138:3940–3948. doi: 10.1210/endo.138.9.5408. [DOI] [PubMed] [Google Scholar]
- 6.Kaneta M, Osawa M, Sudo K, Nakauchi H, Farr AG, Takahama Y. A role for pref-1 and HES-1 in thymocyte development. J Immunol. 2000;164:256–264. doi: 10.4049/jimmunol.164.1.256. [DOI] [PubMed] [Google Scholar]
- 7.Halder SK, Takemori H, Hatano O, Nonaka Y, Wada A, Okamoto M. Cloning of a membrane-spanning protein with epidermal growth factor-like repeat motifs from adrenal glomerulosa cells. Endocrinology. 1998;139:3316–3328. doi: 10.1210/endo.139.7.6081. [DOI] [PubMed] [Google Scholar]
- 8.Floridon C, Jensen CH, Thorsen P, et al. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt JV, Matteson PG, Jones BK, Guan XJ, Tilghman SM. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Genes Dev. 2000;14:1997–2002. [PMC free article] [PubMed] [Google Scholar]
- 10.Takada S, Tevendale M, Baker J, et al. Delta-like and gtl2 are reciprocally expressed, differentially methylated linked imprinted genes on mouse chromosome 12. Curr Biol. 2000;10:1135–1138. doi: 10.1016/s0960-9822(00)00704-1. [DOI] [PubMed] [Google Scholar]
- 11.Wylie AA, Murphy SK, Orton TC, Jirtle RL. Novel imprinted DLK1/GTL2 domain on human chromosome 14 contains motifs that mimic those implicated in IGF2/H19 regulation. Genome Res. 2000;10:1711–1718. doi: 10.1101/gr.161600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy SK, Freking BA, Smith TP, et al. Abnormal postnatal maintenance of elevated DLK1 transcript levels in callipyge sheep. Mamm Genome. 2005;16:171–183. doi: 10.1007/s00335-004-2421-1. [DOI] [PubMed] [Google Scholar]
- 13.Smas CM, Green D, Sul HS. Structural characterization and alternate splicing of the gene encoding the preadipocyte EGF-like protein pref-1. Biochemistry. 1994;33:9257–9265. doi: 10.1021/bi00197a029. [DOI] [PubMed] [Google Scholar]
- 14.Smas CM, Chen L, Sul HS. Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol Cell Biol. 1997;17:977–988. doi: 10.1128/mcb.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Transmembrane form of Pref-1 is cleaved to generate a soluble Pref-1 to inhibit adipocyte differentiation.
- 15.Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Downregulation of Sox9 is required for adipocyte differentiation and Pref-1 inhibits adipocyte differentiation by preventing downregulation of Sox9. Sox9 directly binds to promoter regions of C/EBP-β and C/EBP-δ to suppress their transcription.
- 16.Abdallah BM, Jensen CH, Gutierrez G, Leslie RG, Jensen TG, Kassem M. Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1. J Bone Miner Res. 2004;19:841–852. doi: 10.1359/JBMR.040118. [DOI] [PubMed] [Google Scholar]
- 17.Ohno N, Izawa A, Hattori M, Kageyama R, Sudo T. dlk inhibits stem cell factor-induced colony formation of murine hematopoietic progenitors: Hes-1-independent effect. Stem Cells. 2001;19:71–79. doi: 10.1634/stemcells.19-1-71. [DOI] [PubMed] [Google Scholar]
- 18.Raghunandan R, Ruiz-Hidalgo M, Jia Y, et al. Dlk1 influences differentiation and function of B lymphocytes. Stem Cells Dev. 2008;17:495–507. doi: 10.1089/scd.2007.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin D, Xie D, Sakajiri S, et al. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006;25:1852–1861. doi: 10.1038/sj.onc.1209219. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Zhang X, Zhang M, et al. Up-regulation of DLK1 as an imprinted gene could contribute to human hepatocellular carcinoma. Carcinogenesis. 2007;28:1094–1103. doi: 10.1093/carcin/bgl215. [DOI] [PubMed] [Google Scholar]
- 21.Mei B, Zhao L, Chen L, Sul HS. Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochem J. 2002;364:137–144. doi: 10.1042/bj3640137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deiuliis JA, Li B, Lyvers-Peffer PA, Moeller SJ, Lee K. Alternative splicing of Delta-like 1 homolog (DLK1) in the pig and human. Comp Biochem Physiol B Biochem Mol Biol. 2006;145:50–59. doi: 10.1016/j.cbpb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Jensen CH, Krogh TN, Hojrup P, et al. Protein structure of fetal antigen 1 (FA1). A novel circulating human epidermal-growth-factor-like protein expressed in neuroendocrine tumors and its relation to the gene products of dlk and pG2. Eur J Biochem. 1994;225:83–92. doi: 10.1111/j.1432-1033.1994.00083.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Sul HS. Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor-α converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol Cell Biol. 2006;26:5421–5435. doi: 10.1128/MCB.02437-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smas CM, Kachinskas D, Liu CM, Xie X, Dircks LK, Sul HS. Transcriptional control of the pref-1 gene in 3T3-L1 adipocyte differentiation. Sequence requirement for differentiation-dependent suppression. J Biol Chem. 1998;273:31751–31758. doi: 10.1074/jbc.273.48.31751. [DOI] [PubMed] [Google Scholar]
- 26.Kim KA, Kim JH, Wang Y, Sul HS. Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol Cell Biol. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon YS, Smas CM, Lee K, et al. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Pref-1 null mice show enhanced adipogenesis and accelerated adiposity.
- 28.Lee K, Villena JA, Moon YS, et al. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J Clin Invest. 2003;111:453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Pref-1 overexpressing mice show impaired adipogenesis with lean phenotype.
- 29.Villena JA, Choi CS, Wang Y, et al. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): a new model of partial lipodystrophy. Diabetes. 2008;57:3258–3266. doi: 10.2337/db07-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wermter AK, Scherag A, Meyre D, et al. Preferential reciprocal transfer of paternal/maternal DLK1 alleles to obese children: first evidence of polar overdominance in humans. Eur J Hum Genet. 2008;16:1126–1134. doi: 10.1038/ejhg.2008.64. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Noohr J, Jensen CH, et al. Insulin-like growth factor-1/insulin bypasses Pref-1/FA1-mediated inhibition of adipocyte differentiation. J Biol Chem. 2003;278:20906–20914. doi: 10.1074/jbc.M300022200. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Hidalgo MJ, Gubina E, Tull L, Baladron V, Laborda J. dlk modulates mitogen-activated protein kinase signaling to allow or prevent differentiation. Exp Cell Res. 2002;274:178–188. doi: 10.1006/excr.2001.5464. [DOI] [PubMed] [Google Scholar]
- 33.Nueda ML, Garcia-Ramirez JJ, Laborda J, Baladron V. dlk1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3-L1 cells. J Mol Biol. 2008;379:428–442. doi: 10.1016/j.jmb.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 34.Ross DA, Rao PK, Kadesch T. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3-L1 preadipocytes. Mol Cell Biol. 2004;24:3505–3513. doi: 10.1128/MCB.24.8.3505-3513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garces C, Ruiz-Hidalgo MJ, Font de Mora J, et al. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J Biol Chem. 1997;272:29729–29734. doi: 10.1074/jbc.272.47.29729. [DOI] [PubMed] [Google Scholar]
- 36.Nichols AM, Pan Y, Herreman A, et al. Notch pathway is dispensable for adipocyte specification. Genesis. 2004;40:40–44. doi: 10.1002/gene.20061. [DOI] [PubMed] [Google Scholar]
- 37.Fleming RJ, Scottgale TN, Diederich RJ, Artavanis-Tsakonas S. The gene Serrate encodes a putative EGF-like transmembrane protein essential for proper ectodermal development in Drosophila melanogaster. Genes Dev. 1990;4:2188–2201. doi: 10.1101/gad.4.12a.2188. [DOI] [PubMed] [Google Scholar]
- 38.Kopczynski CC, Alton AK, Fechtel K, Kooh PJ, Muskavitch MA. Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factor of vertebrates. Genes Dev. 1988;2:1723–1735. doi: 10.1101/gad.2.12b.1723. [DOI] [PubMed] [Google Scholar]
- 39.Tax FE, Yeargers JJ, Thomas JH. Sequence of C. elegans lag-2 reveals a cell-signalling domain shared with Delta and Serrate of Drosophila. Nature. 1994;368:150–154. doi: 10.1038/368150a0. [DOI] [PubMed] [Google Scholar]
- 40.Baladron V, Ruiz-Hidalgo MJ, Nueda ML, et al. dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp Cell Res. 2005;303:343–359. doi: 10.1016/j.yexcr.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Bray SJ, Takada S, Harrison E, Shen SC, Ferguson-Smith AC. The atypical mammalian ligand Delta-like homologue 1 (Dlk1) can regulate Notch signalling in Drosophila. BMC Dev Biol. 2008;8:11. doi: 10.1186/1471-213X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nueda ML, Baladron V, Sanchez-Solana B, Ballesteros MA, Laborda J. The EGF-like protein dlk1 inhibits notch signaling and potentiates adipogenesis of mesenchymal cells. J Mol Biol. 2007;367:1281–1293. doi: 10.1016/j.jmb.2006.10.043. [DOI] [PubMed] [Google Scholar]


