Abstract
The cockroach, Periplaneta americana represents a basal insect lineage that undergoes the ancestral hemimetabolous mode of development. Here, we examine the embryonic and post-embryonic functions of the hox gene Scr in Periplaneta as a way of better understanding the roles of this gene in the evolution of insect body plans. During embryogenesis, Scr function is strictly limited to the head with no role in the prothorax. This indicates that the ancestral embryonic function of Scr was likely restricted to the head, and that the posterior expansion of expression in the T1 legs may have preceded any apparent gain of function during evolution. In addition, Scr plays a pivotal role in the formation of the dorsal ridge, a structure that separates the head and thorax in all insects. This is evidenced by the presence of a supernumerary segment that occurs between the labial and T1 segments of RNAiScr first nymphs and is attributed to an alteration in engrailed (en) expression. The fact that similar Scr phenotypes are observed in Tribolium but not in Drosophila or Oncopeltus reveals the presence of lineage-specific variation in the genetic architecture that controls the formation of the dorsal ridge. In direct contrast to the embryonic roles, Scr has no function in the head region during post-embryogenesis in Periplaneta, and instead, strictly acts to provide identity to the T1 segment. Furthermore, the strongest Periplaneta RNAiScr phenotypes develop ectopic wing-like tissue that originates from the posterior region of the prothoracic segment. This finding provides a novel insight into the current debate on the morphological origin of insect wings.
Introduction
The tri-partite division of the insect bauplan into a head, thorax and limbless abdomen is a highly conserved, class-defining feature that differentiates this group from other arthropods. Due to this high conservation, it would seem likely that the developmental networks controlling the establishment of these three regions would also be highly conserved. However, what is only now becoming evident is that there may be an extensive amount of lineage-specific variation in these networks. For example, a recent functional analysis of the paired-domain gene nubbin (nub) in the milkweed bug Oncopeltus fasciatus has shown that the limbless abdomen is established differently as compared to Drosophila (Hrycaj et al., 2008). In a similar fashion, functional studies of the homeotic gene Cephalothorax (Cx), an ortholog of Sex combs reduced (Scr), have shown that in the absence of this gene, an extra supernumerary segment forms between the labial and T1 segments in Tribolium (Shippy et al., 2006). Interestingly, this phenotype has never been reported for similar Scr functional analyses conducted in either Oncopeltus or Drosophila (Chesebro et al., 2009; Hughes and Kaufman, 2000; Pattatucci et al., 1991; Struhl, 1982), and suggests that Tribolium has evolved a variation in the developmental program that acts to properly maintain the separation of the head and thorax that differs from the latter two species. However, what still remains unclear is whether this function of Scr is specific to Tribolium or if it is an ancestral function that has been subsequently lost in both Oncopeltus and Drosophila.
While there is an extensive amount of data available on Scr function during insect development, these studies have been primarily performed in holometabolous species such as Drosophila and Tribolium in which the larval and pupal stages are phenotypically different from the eventual adult morphology (Beeman et al., 1993; Beeman et al., 1989; Curtis et al., 2001; DeCamillis et al., 2001; Pattatucci et al., 1991; Shippy et al., 2006; Struhl, 1982). In contrast, the majority of insect lineages undergo a hemimetabolous mode of development in which the embryo hatches into a nymph that is phenotypically similar to the adult. While there is data available on the roles Hox genes play in the embryonic development of such species (Angelini et al., 2005; Chesebro et al., 2009; Hughes and Kaufman, 2000; Hughes and Kaufman, 2002; Mahfooz et al., 2007), we are only now beginning to understand exactly what roles these genes play during post-embryonic development. In fact, Chesebro et al. (2009) represents the only such study and has shown that the embryonic functions of Scr differ from those observed during post-embryonic development in the hemimetabolous species Oncopeltus (milkweed bug).
In this study, we chose to perform a detailed expression and functional analysis of Scr at both the embryonic and post-embryonic level in the cockroach Periplaneta americana due to the fact that this species represents a phylogenetically older insect lineage as compared to Oncopeltus. Results from our embryonic analysis indicate that, similar to Oncopeltus, the primary functions of Scr are restricted to the head region during embryogenesis with little to no effect on the prothoracic segment. However, in contrast to Oncopeltus, the T1 legs of Periplaneta RNAi-Scr first nymphs are wild type despite distinct embryonic expression of Scr at both the mRNA and protein level in these appendages (Passalacqua et al., 2009). In addition, Periplaneta RNAi-Scr first nymphs also develop an ectopic supernumerary segment between the labial and prothoracic segments reminiscent of the one previously described in Tribolium Cx mutants (Shippy et al., 2006). Similar to what was previously reported in Oncopeltus (Chesebro et al., 2009), the primary effect of Scr during post-embryonic development is to direct the proper growth and development of the prothoracic segment in Periplaneta. In addition, the abolition of Scr during later nymphal stages results in the growth and development of ectopic T1 wings that originate from the paranotal region of the T1 segment.
Materials and Methods
Periplaneta cultures
Original colonies of Periplaneta adults and nymphs were purchased from Carolina Biological Supplies Company and were used to establish laboratory cultures. Both adults and nymphs were reared at 25°C in plastic terrariums with a thick layer of petroleum jelly around the top perimeter to prevent them from escaping and were fed a diet of apples, cat food, and tap water.
Similar to Oncopeltus, Periplaneta also exhibits the hemimetabolous mode of development in which nymphs that hatch from eggs resemble miniature adults except that they lack wings and are sexually immature. Unlike Oncopeltus however, the number of molts between the first instar and the final adult varies quite considerably, ranging anywhere between 6 to 14 times (Bell and Adiyodi, 1982). This variation exists even in controlled environments in which temperature, size of container, and the numbers of individuals reared together are closely monitored (Bell and Adiyodi, 1982). In our rearing conditions, the average number of nymphal molts was approximately 7, with at least one month of time between each nymphal stage. Overall, it takes approximately one year for Periplaneta to develop from egg to adult under our laboratory conditions.
Cloning of Periplaneta americana Scr (Pa-Scr) fragment
Mixed stages of Periplaneta embryos were used for total RNA extraction using Trizol (GibcoBRL/Life Technologies) following the manufacturers protocol. cDNA synthesis, RT-PCR, and cloning were performed as described in Li and Popadic, (2004). Two degenerate primers targeted to the conserved amino acid motifs PQIYPWM (5’ CCR CAR ATH TAY CCR TGG ATG 3’) and WFQNRR (5’ GCT CTA GAC GIC GRT TTT GRA ACC A 3’) were used to generate a 225 bp fragment of Scr that contains the highly conserved homeodomain region. Ten clones were isolated and sequenced. The resulting nucleotide sequences were then compared to each other and previously published Scr data in GenBank and were determined to be a Periplaneta Scr ortholog. In order to obtain a larger fragment of Periplaneta Scr, we used the above sequence as a template to design unique primers for 3’ RACE amplification using the FirstChoice RLM-RACE Kit (Ambion). By using this approach we were able to obtain an additional 420bp of sequence including the stop codon and the 3’ untranslated region (GenBank sequence accession number XXXXXX). Our analysis showed that whereas both fragments yielded comparable results, the larger 3’ RACE fragment produced less phenotypic variation in our RNAi experiments and slightly stronger and more specific signal in our in situ analyses. To address the possibility of non-specific effects, we injected a previously cloned 710 bp fragment of the jellyfish Green Fluorescent Protein (GFP) (Chesebro et al., 2009) into the abdomens of either fertilized Periplaneta adult females or later staged nymphs. All resulting first nymph progeny or emerged adults were indistinguishable from wild type. In addition, Scr in situ analyses performed on random embryos collected from Scr dsRNA injected females from clutch 3 and on showed no staining (Fig. 1E). Together, these results suggest that the phenotypes observed from dsScr injections can be attributed to the specific loss of Scr function.
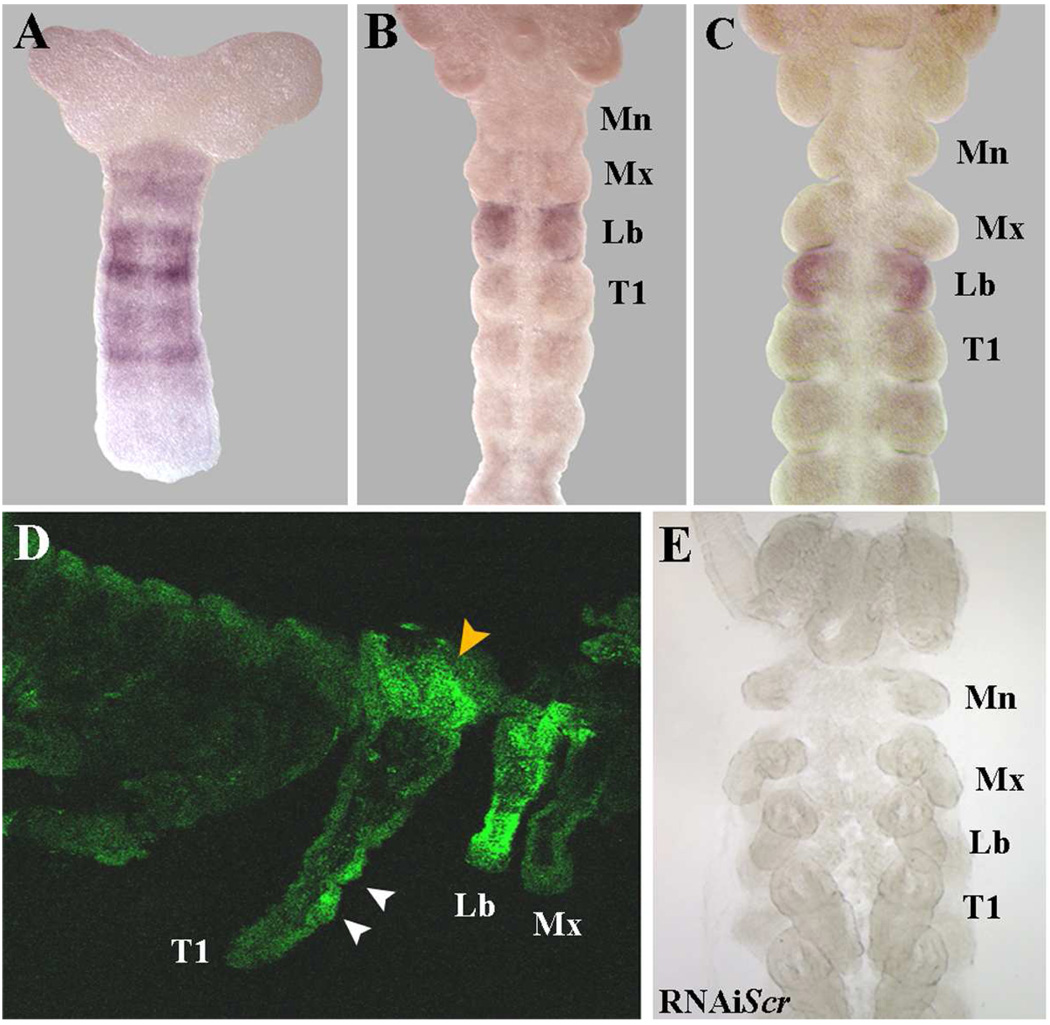
Fig. 1.
Embryonic expression patterns of Scr in Periplaneta americana. (A) At ≈10% development, Scr mRNA is broadly expressed in the mid-posterior region with two strong bands that correspond to the future Lb/T1 region. (B) At ≈20% development, strong Scr mRNA localizes to the labial segment. (C) Slightly later at ≈25%, Scr continues to be solely expressed in the labium with no signal in the Mx or T1 segments. (D) SCR protein accumulation in an embryo at ≈75% development. While strong expression remains in the labial palps, SCR has now expanded into the T1 segment with clear signal in the dorsal T1 region (orange arrowhead) and in two discrete clusters of cells in the T1 leg (white arrowheads). (E) RNAiScr embryo that has been stained for Scr mRNA accumulation. The lack of signal indicates the complete depletion of Scr.
Abbreviations: Mn = Mandibles, Mx = Maxillary, Lb =Labial, T1 = First thoracic segment
Immunohistochemistry
Various stages of Periplaneta embryos were hand dissected from their oothecae (egg cases) and fixed for either in situ hybridization (as according to Li and Popadic, 2004) or antibody staining (as according to Mahfooz et al., 2004). Riboprobe synthesis for both Periplaneta Sex combs reduced (Scr) and Engrailed (En) as well as the in situ hybridization procedure were performed as described in Li and Popadic, (2004). The clone used to generate the Periplaneta En riboprobe was generously provided by J.P. Couso (University of Sussex, U.K.). Expression of SCR protein was detected using a rat polyclonal antibody generated against a C-terminal fragment of Drosophila SCR kindly donated by D.J. Andrew and M.P. Scott (unpublished). This antibody has been effectively proven to cross react in several hemimetabolous insect species (Passalacqua et al., 2009), Tribolium (Curtis et al., 2001) and in crustaceans (Abzhanov and Kaufman, 1999). The staining was performed as previously described in (Passalacqua et al., 2009). The antibody was detected by using a secondary anti-rat antibody that was conjugated to FITC (The Jackson Laboratory). Detailed protocols on maintaining Periplaneta cultures, collection/fixation of embryos and immunohistochemistry are available upon request.
Preparation of Pa-Scr dsRNA
The cloned cDNA fragments of Periplaneta Scr were linearized with the Not-I and Pme-I restriction enzymes and were subsequently used as templates to generate sense and anti-sense single stranded RNA transcripts using the T3/T7 MEGAscript kit (Ambion). Following ethanol precipitation, the concentration of each transcript was determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). The two strands were annealed by mixing equimolar amounts of the sense and anti-sense transcripts and running a PCR-type reaction that initiated at 85°C, and then slowly cooled as follows: 55°C for 20 minutes; 40°C for 10 minutes; 30°C for 5 minutes.
RNA-interference (RNAi)
Approximately 4uL of a 3ug/uL concentration of Periplaneta Scr dsRNA was prepared and injected into a single side of the abdomen of adult Periplaneta females using a Hamilton syringe with a 32-gauge needle. Three hours following the original injection, an additional 4µ1 of Scr dsRNA of the same concentration was injected into the other side of the abdomen. Injected females were then placed in cages with one male per female. Typically between five and eight pairs of males and females were kept in a single container. Oothecae, each of which contain 12–18 eggs, were collected several times per week and placed in Petri dishes with a moist paper towel and sealed with parafilm. Embryos were allowed to mature at 30°C until hatching, which usually occurred in approximately 30 days, upon which their phenotypes were analyzed. Typically, the first 2–3 clutches were wild type. Subsequent clutches showed phenotypic alterations that persisted for at least 20 clutches before reverting back to wild type.
Maternal RNAi experiments were repeated three times with a total of 22 adult females injected. In short, a total of 820 first nymphs were examined with 432 displaying a wild type phenotype and 388 displaying an RNAiScr phenotype. In addition, a total of 346 Scr-depleted embryos were fixed for subsequent molecular analysis.
On average, under our laboratory conditions, there are approximately seven nymphal stages of Periplaneta post-embryonic development. To perform our nymphal RNAi experiments, we injected approximately 4uL of a 3ug/uL Scr dsRNA solution into the abdomens of Periplaneta nymphs at various stages (3rd-4th, 5th-6th, and 7th) using a Hamilton syringe with a pulled glass capillary needle. Briefly, a total of 21 nymphs at stages 3–4, 18 nymphs at stages 5–6 and 19 nymphs at stage 7 were injected. All injections performed at stages 3–6 were boosted with an additional 4uL of Scr dsRNA every three weeks until the nymph either matured to adult or died during post-embryogenesis. In addition, 19 7th staged nymphs were injected only once in order to assess the contributions that Scr solely has during the final nymphal stage. On average, injections at early stages of development (stages 3–4) were generally lethal (80%) with few surviving individuals displaying more moderate phenotypes. These data indicate that there may be a functional requirement for Scr during early stages of Periplaneta of post-embryogenesis and that the complete abolition of Scr transcript during these stages is lethal. A similar situation has been recently reported in Oncopeltus, in which the abolition of Scr during early post-embryonic stages was lethal (Chesebro et al., 2009). In contrast, injections at the 5th–6th nymphal stages generally resulted in moderate to strong phenotypes (61%) with small percentages of weak phenotypes (6%) and lethality (33%). Finally all surviving 7th staged nymphal injections resulted in weak phenotypes only, suggesting that Scr function may be continuously required throughout post-embryogenesis in Periplaneta.
RT-PCR analysis
Periplaneta sixth nymphs were injected with 4µl of a 3µg/µl concentration of Scr dsRNA and allowed to molt into seventh nymphs. At this stage, the T1 plates from three RNAiScr 7th staged nymphs were dissected and the total RNA was extracted in three independent reactions using Trizol (GibcoBRL/Life Technologies). This RNA was subsequently used as a template to generate cDNA utilizing a poly-T primer (Promega). For comparison, total RNA and cDNA was generated from wild type T1 plates in an identical manner. Equal concentrations of cDNA of both wild type and RNAiScr seventh nymphs were subsequently used as templates in individual PCR reactions to assess the amount of Scr transcript that was abolished in injected individuals. Unique Scr primers were designed according to the shorter Periplaneta Scr fragment described in this study. As a positive control, primers were also designed to the Periplaneta 18S ribosomal subunit sequence originally published in Giribet et al., (2001) and were used in both wild type and Scr injected seventh nymphs. The PCR conditions were as follows: 94°C for 3 min; 30 cycles of 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds; one cycle of 72°C for 7 minutes.
Results
I. Embryonic functions of Scr in Periplaneta americana
Embryonic expression patterns of Scr in Periplaneta americana
A 420bp partial cDNA fragment specific to the 3’ end of Periplaneta americana Scr (Pa-Scr) was used to study the patterns of mRNA accumulation throughout embryonic development. At ≈10% development, Scr is broadly expressed in the mid-posterior region of the embryo with two strong bands of expression that correspond to the future Lb/T1 segments (Fig. 1A). At approximately 20% development, strong Scr signal localizes to the labial segment only (Fig 1B). Slightly later in development, when limb buds are fully formed (≈25%), Scr signal remains localized to the labial segment, with no expression in the maxillary or T1 segments (Fig. 1C). As described in Passalacqua et al. (2009), during dorsal closure Scr signal is maintained in the labial appendages and fades away in the mid-ventral region of the segment. At later stages (≈65%), Scr expands into the T1 segment, with clear signal in the dorsal T1 region (Fig. 1D, orange arrowhead) and in two discrete clusters of cells in the T1 legs (Fig. 1D, white arrowheads). Overall, both mRNA and protein expression data (Passalacqua et al., 2009) show that Scr signal is initially confined to the labial segment at early stages of development and does not expand into the T1 segment and its associated appendages until much later. These data suggest that the primary embryonic functions of Scr should be to control the proper development of the labial segment and its appendages, with a secondary role in the T1 segment. In order to test this hypothesis, we employed a parental RNAi (pRNAi) approach to assess the embryonic functions of Scr in Periplaneta. Scr in situ analyses performed on random embryos collected from clutch 3 and on showed no staining, indicating that our pRNAi application completely abolishes its expression (Fig. 1E).
Role of Scr in Periplaneta labial development
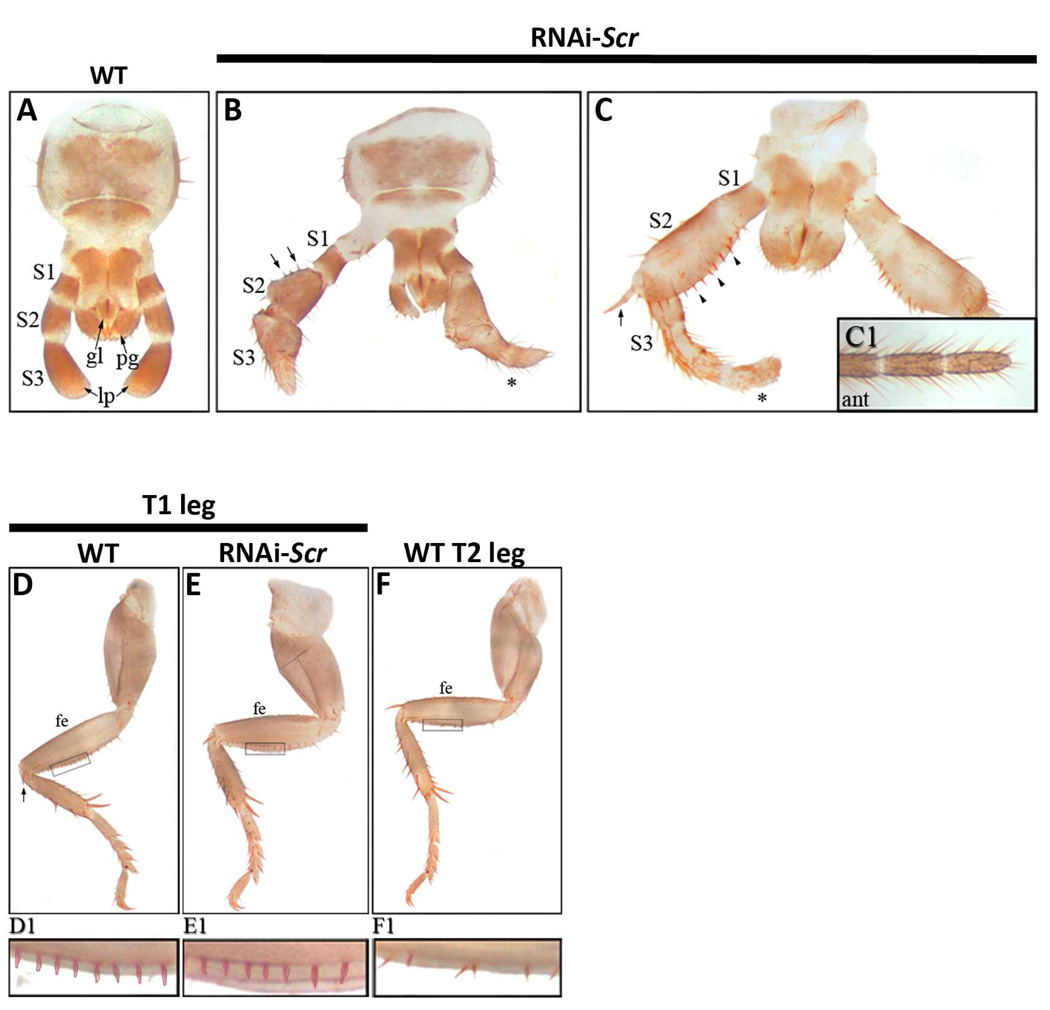
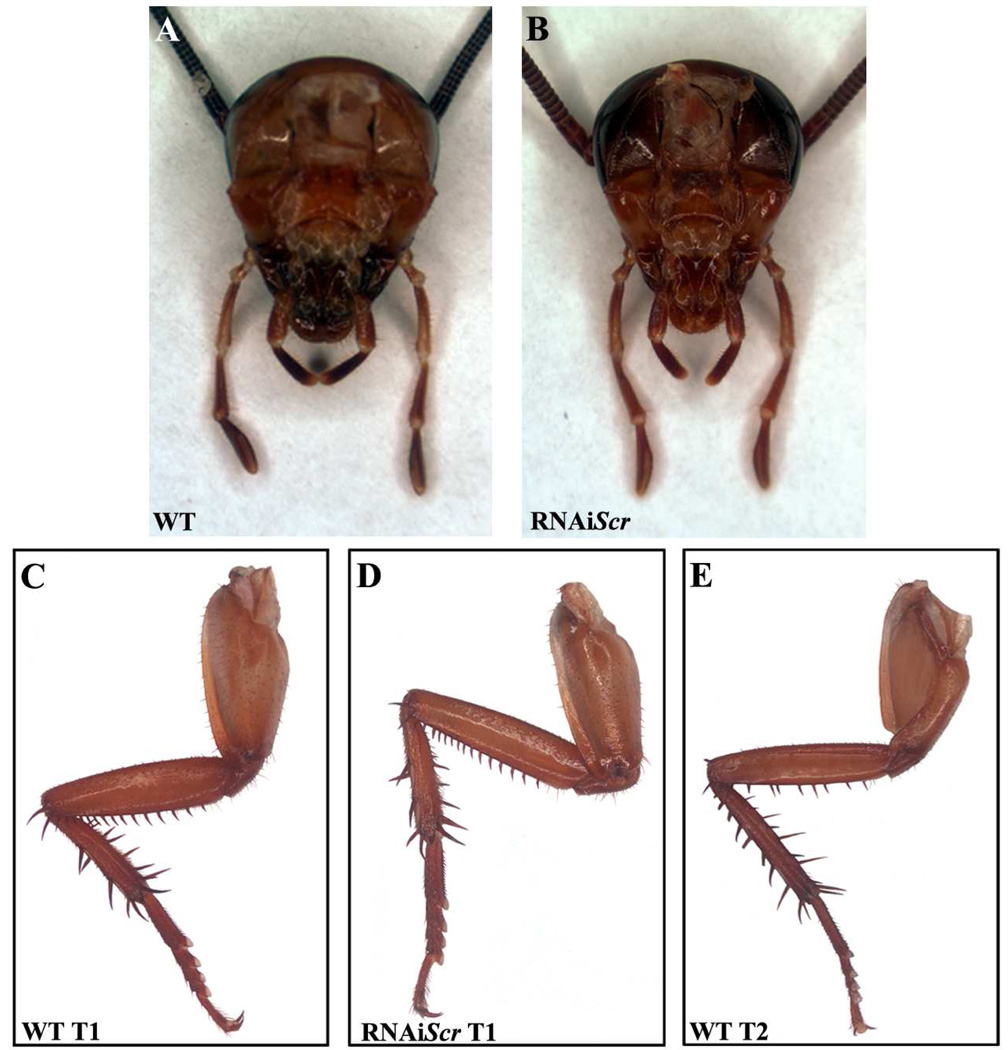
Periplaneta exhibits the ancestral mandibulate or “chewing” mouthparts where the labial segment is fused at the base with each side composed of three “branches” called the glossa (innermost), the paraglossa (middle), and the labial palp (outermost) (Fig. 2A). The labial palp is articulated and composed of three sub-segments that, for ease of description, will be referred to as S1 (most proximal), S2 and S3 (most distal). In moderate RNAiScr phenotypes, the S2 sub-segment of the labial palp develops several large, sclerotized bristles (arrowheads) that are reminiscent of those found on the femur of thoracic legs (Fig. 2B). The S3 sub-segment is also modified and develops numerous bristles and long hairs (Fig. 2B, asterisk) that appear to have a mixed leg/antennal identity. Strong RNAiScr first nymph phenotypes (Fig. 2C) are characterized by a complete transformation in which S2 assumes the identity of the T1 femur complete with a row of organized bristles (arrowheads) along the ventral margin. In addition, a large spur develops at the distal end of S2 (arrow), which is a distinct feature of the femoral segment of wild type thoracic legs. The third sub-segment is also transformed, becoming partially segmented with a mixed leg-antennal identity. Note that the distal-most portion of S3 (Fig. 2C, asterisk) exhibits hairs that are reminiscent of ones found on antennae (Fig. 2C1). At the same time, the morphologies of the glossa and paraglossa remain unaltered even in the strongest observed RNAi phenotypes and suggest that Scr does not play a role in the establishment of these structures. These results indicate that the role of Scr during embryonic development in Periplaneta is restricted to controlling the identity of the labial palps, but not the entire labial appendage.
Fig. 2.
Embryonic RNAiScr phenotypes in the labial palps and T1 legs of Periplaneta. (A–C) Wild type and RNAiScr labial phenotypes. (A) Wild type labium of first nymph. The articulated labial palps are composed of three sub-segments: S1 (proximal), S2 (middle), and S3 (distal). (B) The labial palps of a moderate RNAiScr first nymph. The phenotypic effects are most noticeable in S2 and S3 where large thoracic leg-like ectopic bristles form (black arrowheads). The distal half of S3 develops numerous hairs (*) resembling those covering the antennae; compare to (C1). (C) Strong RNAiScr first nymph showing a transformation of the labial palp into a mixed leg-antennal identity. S2 shows a complete transformation, assuming the identity of the T1 femur. Note the row of bristles (black arrowheads) and the large spur at its distal end (black arrow; compare to 1D, black arrow). S3 transforms into a mixed leg (proximal) and antennal (distal) identity. (C1) Distal tip of a wild type antenna; compare with distal half of transformed S3 in B and C (*). (D–F) Wild type and RNAiScr T1 and T2 leg phenotypes. (D) Wild type T1 leg. (E) T1 leg of RNAiScr first nymph. The femur is characterized by a row of approximately seventeen small, closely organized bristles along the entire length of the ventral side. These features remain unaffected in Scr-depleted nymphs, compare to wild type T1 femur in D. (F) Wild type T2 leg showing similar morphology to T1 legs. The only observable difference is the row of bristles along the ventral margin, which are fewer in number (approximately 10) and more spaced out. (D1–F1) Magnified view of boxes in D–F showing that the T1 leg of RNAiScr first nymphs retains its identity.
Role of Scr in Periplaneta T1 leg development
Unlike the situation observed in Drosophila, Tribolium and Oncopeltus, the T1 legs of Periplaneta first nymphs only slightly differ from their T2 counterparts. Specifically, the femurs of both T1 and T2 legs contain an organized row of bristles along the ventral side, with approximately 17 bristles on T1 legs that are closely spaced together and only approximately 10 bristles on the T2 femur that are spaced further apart (compare Fig. 2D1 to Fig. 2F1). Notably, the T1 femoral bristle pattern in the prothoracic legs of Periplaneta RNAiScr first nymphs is unaffected and the appendages appear wild type (compare Fig. 2D1 and Fig. 2E1). These data show that despite clear T1 leg expression at later stages of development (Fig. 1D; (Passalacqua et al., 2009), Scr has no obvious role in defining the external morphology of this appendage.
Role of Scr in dorsal ridge development
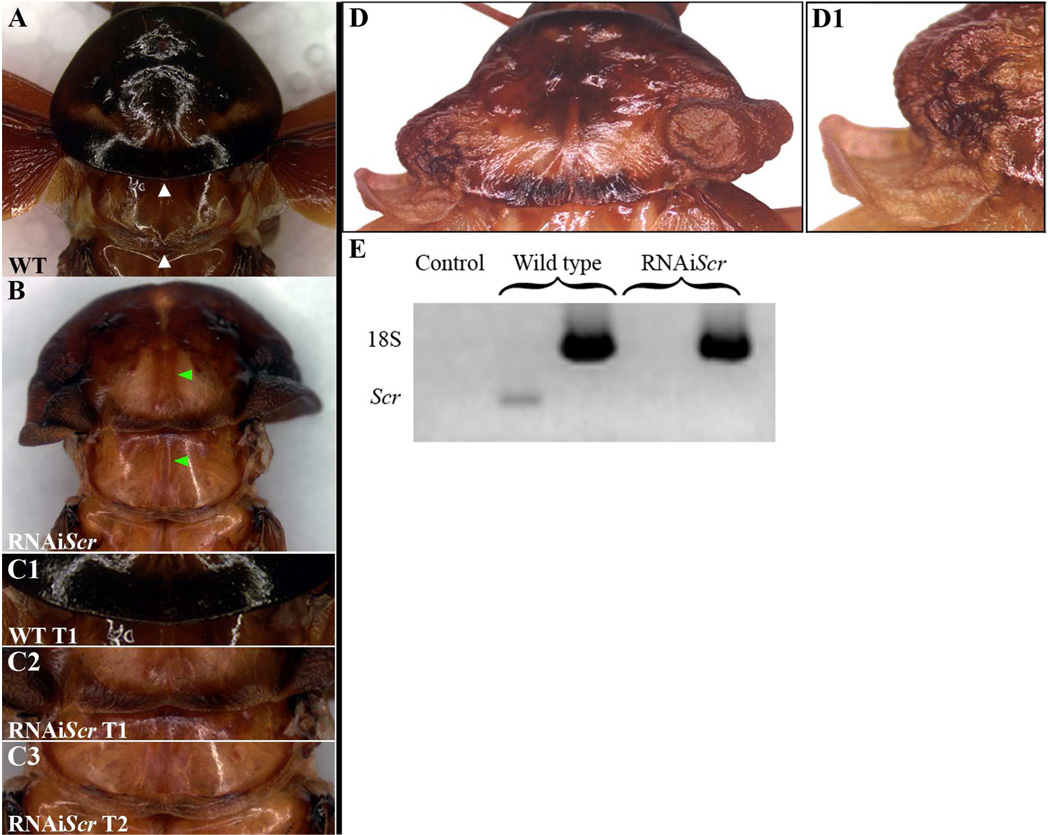
The dorsal ridge is a multipartite structure that forms a division between the head and thoracic region in insects (Rogers and Kaufman, 1996; Shippy et al., 2006). As originally proposed by Rogers and Kaufman, (1996), the dorsal ridge is composed of both gnathal and pregnathal segments and can be divided into two parts, Dr-I and Dr-II. Dr-I is the segmental like entity that forms from the dorsolateral extension of the labial and maxillary segments during dorsal closure, while Dr-II is derived from the dorsal-most portions of the maxillary, mandibular, intercalary and antennal segments (Rogers and Kaufman, 1996). As shown in Fig. 3C–D1, strong RNAiScr Periplaneta first nymphs develop an ectopic supernumerary segment between the head and prothorax that disrupts the ancient boundary that is normally defined by the dorsal ridge. This result indicates that Scr plays a pivotal role in directing the proper establishment of this highly conserved division in Periplaneta.
Fig. 3.
Dorsal ridge phenotypes of RNAiScr Periplaneta americana first nymphs. (A) Wild type first nymph showing the characteristically large pronotum that conceals most of the head. (A1) Ventral view of the head and thoracic boundary. (B) Lateral view of wild type first nymph. (B1) Magnified view of lateral head and T1 of wild type first nymph shown in (B). Black arrows in A, B, and B1 point to the dorsal ridge (C) Strong RNAiScr nymph phenotype showing the development of a supernumerary segment between the head and the prothorax. (C1) Extra segment viewed from the ventral side (open black arrowheads point to the ectopic segment). (D) Lateral view of RNAiScr first nymph. (D1) Close up of supernumerary segment shown in (D). Black brackets depict the length of the ectopic segment in (D–D1). Abbreviations: ant = antenna; fe = femur; gl = glossa; pg = paraglossa; lp = labial palp; S1 = sub-segment 1 of lp; S2 = sub-segment 2 of lp; S3 = sub-segment 3 of lp.
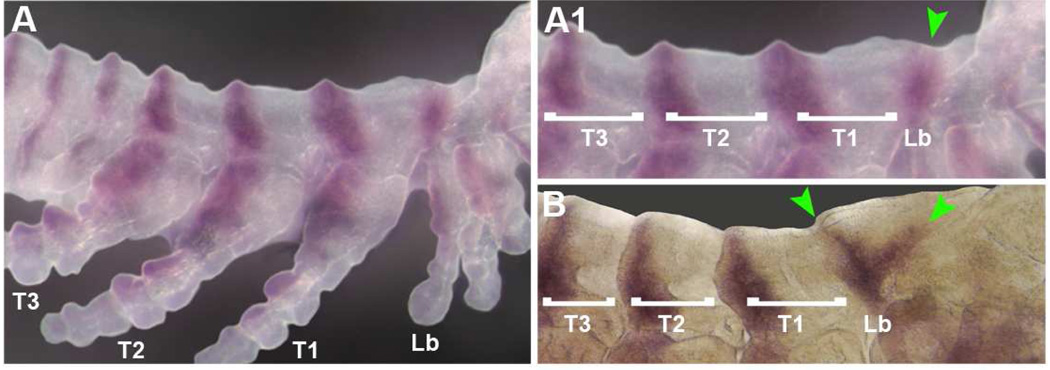
Previous studies in Drosophila and Tribolium have shown that Scr expression co-localizes with that of the segment polarity gene engrailed (en) in the posterior portion of Dr-I and suggests that both Scr an en may play important roles in the proper formation of the labial and maxillary derived portions of the dorsal ridge (Rogers and Kaufman, 1996; Shippy et al., 2006). More specifically, the first evidence of dorsal ridge development is the connection of stripes of en expression in the posterior compartments of the maxillary and labial segments by a second stripe of en expression along the lateral edge of the anterior compartment of the labial segment (Rogers and Kaufman, 1996; Rogers et al., 1997; Shippy et al., 2006). This lateral connection of the labial and maxillary stripes of en expression has been reported for all insects studied to date, including Periplaneta (Marie and Bacon, 2000; Patel et al., 1989; Peterson et al., 1998; Rogers and Kaufman, 1996; Rogers et al., 1997; Shippy et al., 2006). Once connected, this single band of en expression subsequently extends dorsally and ultimately forms the posterior portion of the dorsal ridge (Rogers and Kaufman, 1996; Rogers et al., 1997; Shippy et al., 2006). As depicted in Fig. 4A–A1, this process is conserved in Periplaneta, as a single band of combined maxillary and labial expression extends dorsally to encircle the developing embryo during dorsal closure. However, in RNAiScr embryos, this single band of expression bifurcates during dorsal extension, resulting in the formation of a de novo band of en expression anterior to the labial stripe (Fig. 4B, green arrowheads). The presence of two stripes of en expression correlates with the presence of the two additional segmental grooves that appear on either side of the ectopic supernumerary dorsal segment in strong RNAiScr Periplaneta first nymphs (Fig. 3D1).
Fig. 4.
Engrailed (en) mRNA accumulation in wild type and RNAiScr Periplaneta americana embryos. (A) Wild type embryo showing a combined Mx/Lb stripe of engrailed expression that circumvents the embryo. (A1) Close up of the embryo shown in (A). Green arrowhead points to the single Mx/Lb stripe of en expression. (B) Similarly staged RNAiScr embryo stained for en mRNA accumulation. The single band of Mx/Lb expression bifurcates (green arrowhead) and results in the formation of a de novo band of en expression anterior to the Lb stripe. Abbreviations: Mx = Maxillary, Lb = Labial
II. Post-embryonic functions of Scr in Periplaneta americana
Unfortunately, first nymphs that hatch from maternal RNAiScr injections never successfully complete post-embryonic development and usually die by the third nymphal stage. This result indicates that there may be a critical functional requirement of Scr during the early stages of Periplaneta post-embryogenesis, similar to that what was reported in Oncopeltus (Chesebro et al., 2009). To circumvent this induced lethality, we focused our post-embryonic analysis of Scr function on the final three stages of development. In general, RNAiScr performed at the last nymphal stage resulted in weak to moderate phenotypes, while treatments 2–3 stages prior to adulthood resulted in much stronger phenotypes. These data suggest that the functional requirement of Scr during post-embryonic development in Periplaneta may be cumulative and is reminiscent of the situation observed in Oncopeltus (Chesebro et al., 2009). In order to determine the effectiveness of our RNAi methodology, injected sixth stage nymphs were allowed to molt into the next stage upon which their T1 plates were dissected and evaluated for Scr mRNA. As shown by our RT-PCR analysis in Fig. 6E, the expression of Scr mRNA is abolished in T1 of RNAiScr nymphs and indicates that the observed adult phenotypes can be attributed to the depletion of Scr.
Fig. 6.
Adult RNAiScr phenotypes of the prothoracic (T1) segment in Periplaneta americana. (A) Dorsal view of the prothoracic (T1) and mesothoracic (T2) segments of a wild type adult. The posterior margin of T1 has a rounded, smooth morphology while T2 exhibits a thickening of the cuticle that forms a ridge-like structure (white arrowheads). (B) Dorsal view of the prothoracic (T1) and mesothoracic (T2) segments of a RNAiScr adult. The posterior margin of T1 now exhibits a thickening of the cuticle and starts to assume a T2-like identity. In addition, an ectopic groove appears along the midline of the T1 segment that is normally only found on T2 (green arrowheads). (C1) Close up of the posterior portion of the T1 segment of the wild type adult shown in (A). (C2) Close up of the posterior portion of the T1 segment of the RNAiScr adult shown in (B). Note that the cuticle exhibits a thickening and appears like the ridge-like structure present on T2 (compare to (C3)). (C3) Close up of the posterior portion of the T2 segment of the RNAiScr adult shown in (B). This segment is unaffected and appears wild type (compare to (A)). (D) Strong RNAiScr adult phenotype. Ectopic wing-like tissue develops from the posterior lateral portion of T1. (D1) Close up view of the left ectopic wing-like tissue of the strong RNAiScr adult shown in (D). (E) RT-PCR analysis of Scr mRNA in the prothoacic plates of seventh nymphs. RNAiScr nymphs show a complete depletion of Scr mRNA in T1 as compared to wild type.
Scr abolition does not affect structures previously established during embryogenesis
Due to their hemimetabolous mode of development, the final morphology of the labial appendage in Periplaneta is established during embryogenesis and only increases in size during post-embryogenesis. Despite the wide range of severity of RNAiScr phenotypes, the labial appendage is never affected and appears wild type (Fig. 5A–B). In addition, there is no change in the morphology of the T1 legs regardless of the stage at which the RNAi treatment was performed (Fig 5C–D). This is consistent with our observation in first nymphs (Fig. 2D–F), suggesting that Scr does not play a role in establishing the external morphology of these appendages during either embryonic or post-embryonic development. This is consistent with results from Oncopeltus (Chesebro et al., 2009), indicating that the post-embryonic abolishment of Scr has little to no effect on structures that are previously established during embryogenesis in hemimetabolous insect species.
Fig. 5.
Comparison of the labial appendages and the thoracic legs of wild type and RNAiScr Periplaneta americana adults. (A) Dissected head of a wild type adult. (B) Dissected head of an RNAiScr adult. Note that the labial appendages are unaffected and appear wild type. (C) Wild type adult T1 leg. (D) T1 leg of an RNAiScr adult. The legs remain unaltered and do not take on a T2 identity. (E) Wild type adult T2 leg.
The role of Scr in the prothorax
In contrast to appendages such as mouthparts and legs, the prothoracic (T1) plate itself displays major morphological alterations with regard to its size and shape throughout post-embryogenesis. As shown in Fig. 6A, the wild type adult pronotum is a greatly enlarged, oval-shaped structure that is morphologically distinct from T2, particularly in the posterior margins. More specifically, the T1 segment has a rounded, smooth morphology while the posterior margin of T2 exhibits a thickening of the cuticle that forms a ridge-like structure (Fig. 6A, white arrowheads). A second unique feature of T2 is the presence of a longitudinal invagination at the point where the left and right plates meet at the midline (Fig. 6B, lower green arrowhead). In strong RNAiScr phenotypes, the T1 segment is transformed toward a T2-like identity as evidenced by the appearance of an ectopic thickening of the cuticle of the posterior margin of this segment (Fig. 6C1–C3). In addition, an ectopic groove appears along the midline of the T1 segment that is normally only seen on T2 (Fig. 6B, green arrowheads). These data mirror what was recently reported in Oncopeltus (Chesebro et al., 2009), and provide independent corroboration that the input of Scr is critical for the proper growth and development of the T1 segment during post-embryonic development in hemimetabolous insect species.
Scr suppresses wing development on the prothorax during post-embryonic development in Periplaneta
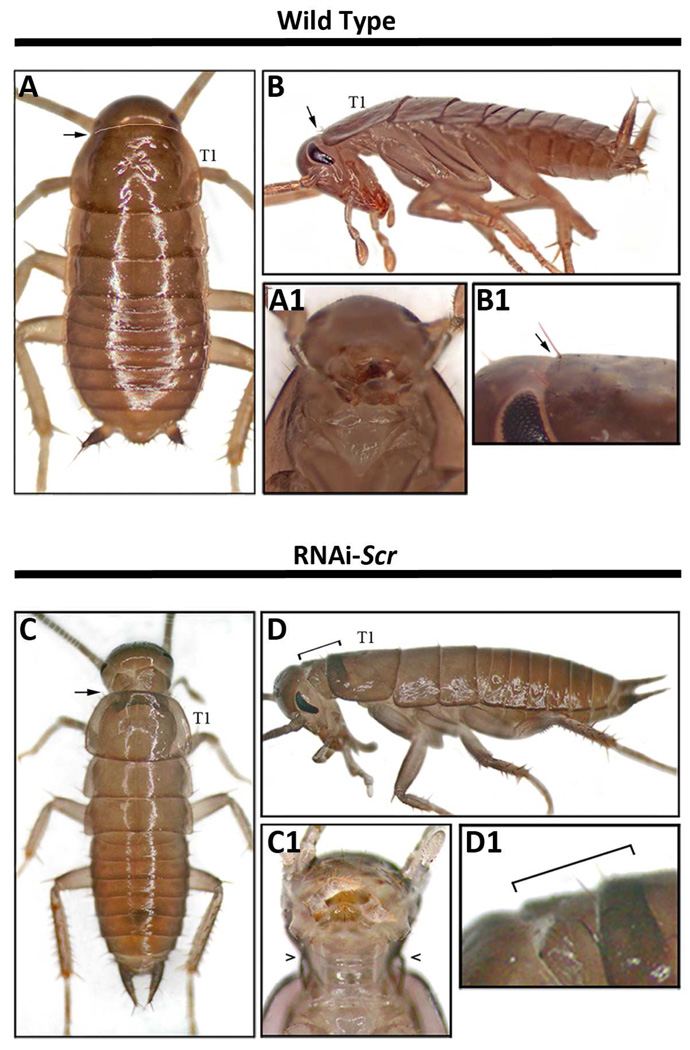
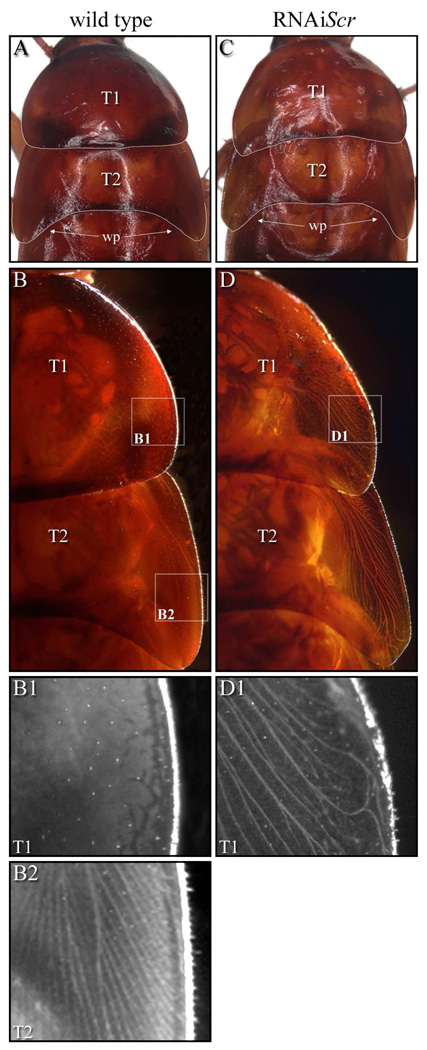
The most noticeable post-embryonic phenotype is the presence of ectopic wings that clearly originate from the paranotal tissue of the prothorax (Fig. 6D–D1). Normally in wild type, wing pads begin to form on the meso- and metathorax (T2 and T3, respectively) two to three nymphal stages preceding adulthood and easily distinguish these two segments from T1, which lacks these structures. By the last nymphal stage, the wing pads on T2 increase in size and point toward the posterior, while T1 remains devoid of wing pads (Fig. 7A). In addition, under indirect lighting conditions wing veins (trachea) become clearly evident in the lateral margins of T2 wing pads (Figs. 7B2) while T1 lacks these structures even at high magnification (Figs. 7B1). Our data show that RNAiScr treatments administered at the last nymphal stage generally result in weak phenotypes. However, RNAi treatments two to three stages before adulthood generally result in much stronger phenotypes as evidenced by the formation of an ectopic wing pad on the prothoracic segment at the last nymphal stage. As shown in Fig. 7C, the ectopic T1wing pad exhibits a posterior-lateral extension of tissue that is reminiscent of the morphology of the mesothoracic wing pad. In addition, this ectopic structure also features the formation of trachea that is normally only found within the lateral regions of the T2 wing pads (Fig. 7D–D1). The fact that earlier RNAiScr treatments generally result in stronger phenotypes, complete with an ectopic wing pad, suggests that the suppression of the wing developmental program on T1 by Scr may be cumulative, and that the input of this gene may be required throughout post-embryogenesis to completely suppress wing growth on this segment. These data may at least partially explain why fully developed wings can never be recapitulated when Scr is depleted at later nymphal stages in both Periplaneta (this study) or in Oncopeltus (Chesebro et al.,2009).
Fig. 7.
Morphology of Periplaneta wild type and RNAiScr seventh instars. (A–B2) Wild type. (C–D1) RNAiScr. (A) Wild type seventh nymph pronotum (T1) and mesonotum (T2). Note the large wing pads (arrows) on the lateral margins of T2 making this segment morphologically distinct from T1. (B) Dorsal image of wild type T1 and T2 illuminated by indirect light. The branching wing veins (trachea) along the lateral margins of T2 are quite evident, while trachea are not observed in the pronotum. (B1) Magnified image of lateral margin of T1 (upper box in B1). Note the absence of wing veins. (B2) Magnified image of lateral margin of T2 (lower box in B). Branched veins are clearly present in the developing wing pad. (C) T1 and T2 of an Scr-RNAi seventh nymph. Compared to wild type, the morphology of T1 is altered due to the development of ectopic wing pads at the lateral margins of this segment. (D) Close-up view of lateral margins of T1 and T2 illuminated with indirect light showing the development of trachea in the ectopic wing pads. (D1) The development of veins in T1 is unmistakable (compare to B1). Note, however, that the developing trachea are not identical to those in wild type T2, suggesting an incomplete transformation of T1 toward T2.
Legend: T1 = prothorax; T2 = mesothorax; wp = wing pad.
Discussion
Studies in Drosophila, Tribolium, and Oncopeltus have shown that Scr functions in two distinct body regions (head and thorax), playing critical roles in establishing identity to the labial segment, suppressing wing growth on the prothoracic (T1) segment and directing the formation of T1 leg combs (Beeman et al., 1993; Beeman et al., 1989; Chesebro et al., 2009; Curtis et al., 2001; DeCamillis et al., 2001; Hughes and Kaufman, 2000; Pattatucci et al., 1991; Shippy et al., 2006; Struhl, 1982; Wakimoto et al., 1984). Scr expression analyses have also been performed in a wide range of insect species ranging from (listed early to late-branching): Zygentoma, Orthoptera, Dictyoptera, Hemiptera, Coleoptera, and Diptera (Curtis et al., 2001; Mahaffey and Kaufman, 1987; Passalacqua et al., 2009; Rogers et al., 1997; Zhang et al., 2005). As a result, a rather large, comprehensive data set of Scr expression is available and has been used to gain an insight into how the roles of this gene may have changed throughout insect evolution. However, it is necessary to provide support for hypotheses drawn from such studies with functional data. The present study imparts novel insights into this very issue as Periplaneta represents the most basal insect lineage in which a detailed functional analysis of Scr has been performed.
Role of Scr in labial development
As shown in Fig. 2C, strong Periplaneta RNAiScr phenotypes result in a labial appendage with a mixed leg/antennal identity. More specifically, the middle (S2) sub-segment of the labial palp is clearly transformed into a femur while the distal most sub-segment of this appendage (S3) is more reminiscent of an antennae based its on morphology, bristle patterning and the lack of claws. Note, however, that the inner-most portions of the labium (glossa and paraglossa) are unaffected even in the strongest RNAiScr phenotypes. This result is intriguing, as Scr is expressed throughout the labial segment at both early and mid embryonic stages of development (Fig. 1B–C, Passalacqua et al., 2009). It is only at late stages of development that Scr signal fades from the proximal portions of the labial appendage and localizes in the distal-most portions that will eventually form the elongated palps (Fig. 1D, Passalacqua et al., 2009). These data suggest that Scr expression at later stages of development is critical for the proper formation of the labial palps and that the earlier segmental expression in the labium has no function in the development of the glossa and paraglossa. It therefore appears that additional genes have to be involved in directing the formation of these two proximal structures during Periplaneta embryogenesis.
A second intriguing aspect of the labial Pa-Scr phenotype is the clear morphological distinction between the middle (S2) sub-segment of the palp that is leg-like and the distal (S3) one that has an antennal identity. The commonly accepted paradigm is that insect appendages assume an antennal identity only in a hox-free state (Percival-Smith et al., 1997; Struhl, 1981). Previous functional analyses of hox gene function in the labial segments of Drosophila, Tribolium, and Oncopeltus have all shown that this paradigm is conserved within these lineages (Brown et al., 2000; Curtis et al., 2001; DeCamillis et al., 2001; Hughes and Kaufman, 2000; Percival-Smith et al., 1997; Stuart et al., 1991). More specifically, in Drosophila and Oncopeltus, both Scr and another hox gene proboscipedia (pb) are co-expressed in the labium during embryonic development (Pattatucci et al., 1991; Percival-Smith et al., 1997; Rogers et al., 1997; Rogers et al., 2002; Struhl, 1982). Accordingly, it is only when both Scr and pb are simultaneously depleted that the labial appendage assumes an antennal identity in these species (Hughes and Kaufman, 2000; Percival-Smith et al., 1997). Similarly, the Tribolium orthologs of Scr (Cx) and pb (maxillopedia, mxp) are also co-expressed in the labium during embryonic development (Curtis et al., 2001; DeCamillis et al., 2001; Shippy et al., 2000a; Shippy et al., 2000b; Shippy et al., 2006). In this species, it has been determined that Cx positively regulates mxp in this segment (DeCamillis et al., 2001). As a consequence, the single depletion of Cx causes the labium to develop in a hox-free state and assumes an antennal identity. Based on the fact that the distal most sub-segment of the labial palp of RNAiScr Periplaneta first nymphs develops as antennae (Fig. 2C–C1), it is tempting to speculate that a similar Scr/pb regulatory relationship may also exist in this species as it does in Tribolium. However, such a putative Scr-pb regulatory relationship does not account for the fact that the middle (S2) portion of the labial palp is transformed into a femur in RNAiScr Periplaneta first nymphs (Fig. 2C). The fact that at later stages of embryogenesis Scr is only expressed in the S3 region of the labial palps (Fig. 1D) suggests that the S2 sub-segment of this appendage would likely retain pb expression and function. Studies in Drosophila have shown that the ectopic expression of pb results in the transformation of legs into maxillary or labial palps (Aplin and Kaufman, 1997) and that the sole expression of pb leads to maxillary palp identity (Percival-Smith et al., 1997). According to these data, the proposed residual pb expression in the S2 sub-segment of the labial palp of RNAiScr Periplaneta embryos should cause this region to develop as a mouthpart and not a femur. This result suggests that additional genes are required to establish labial identity in the cockroach as compared to flies.
Scr does not play a role in the development of T1 legs in Periplaneta americana
In the insect lineages Drosophila, Tribolium and Oncopeltus, Scr directs the formation of a T1-specific structure (combs) that clearly differentiates them from their T2 counterparts (Beeman et al., 1989; Chesebro et al., 2009; Hughes and Kaufman, 2000; Pattatucci et al., 1991). However, the majority of insect lineages do not bear any unique features on their T1 legs and as a result, are morphologically very similar to their T2 legs. One such example is the cricket species Acheta domestica. Interestingly, Scr is clearly expressed in the prothoracic legs at both the mRNA (Rogers et al., 1997) and the protein (Passalacqua et al., 2009) level in this species despite the fact that they are morphologically indistinguishable from their T2 counterparts. Similarly, Scr is also expressed in the T1 legs of Periplaneta (Fig. 1D; Passalacqua et al., 2009) which are morphologically very similar to those that appear on T2. These observations led to the proposition that the posterior expansion of Scr into the T1 legs of more basal insect lineages may have preceded any apparent gain of function during evolution (Passalacqua et al., 2009; Rogers et al., 1997). Consistent with this scenario, the depletion of Scr has absolutely no effect on defining the external morphology of T1 legs of Periplaneta first nymphs or adults (Fig. 2E, Fig. 5D). However, it is possible that Scr may play some role other than defining external morphology of T1 legs, such as in sensory organ differentiation and/or the positioning of PNS neurons.
Role of Scr in Dorsal Ridge Development
The dorsal ridge is a multipartite structure that forms a distinct boundary between the head and thorax of most, if not all insect species (Rogers and Kaufman, 1996; Shippy et al., 2006). Studies in several insect groups including Drosophila and Triboilum have shown that two genes, Scr and the segment polarity gene engrailed (en) are important in the formation of this structure during embryogenesis (Peterson et al., 1998; Rogers and Kaufman, 1996; Rogers et al., 1997; Shippy et al., 2006). The present study reveals that these same two genes play a critical role in the normal growth and development of the dorsal ridge in Periplaneta as well. More specifically, the embryonic abolition of Scr results in the formation of an ectopic supernumerary segment between the head and prothoracic (T1) segments (Fig. 3C–D1). Consistent with this phenotype, the connected stripes of maxillary and labial en expression bifurcate during dorsal extension in RNAiScr Periplaneta embryos (Fig. 4B, green arrowheads), and ultimately form the de novo boundaries of the ectopic supernumerary segment. Interestingly, while functional analyses of the Tribolium Scr ortholog Cx result in an identical phenotype (Shippy et al., 2006), analogous studies in both Drosophila and Oncopeltus do not (Chesebro et al., 2009; Hughes and Kaufman, 2000; Pattatucci et al., 1991; Struhl, 1982). These data reveal the presence of lineage-specific variation in the genetic mechanisms that controls the formation of the dorsal ridge. In a similar fashion, a recent report on the functional role of paired domain gene nubbin (nub) in Oncopeltus has shown that this gene has a novel role in the establishment of the limbless abdomen by up-regulating the homeotic gene abdominal-A (abd-A) in this species (Hrycaj et al., 2008). Identical experiments performed in Drosophila indicate that no such regulatory relationship between nub and abd-A exist in this species (Hrycaj et al., 2008). These results therefore provide a second instance in which variation exists in the regulation in the development of a key insect trait. Future analyses of both Scr and nub in other more basal insect lineages will therefore be able to shed light onto the ancestral genetic ground state that governs the formation of such ancient features.
Insect wing origins
One of the most important innovations in the evolution of the insect body plan was the appearance of articulated, fully functional wings. A remaining fundamental question is to determine the origin and development of articulated wings. While it is generally accepted that insect wings originated only once (i.e. are monophyletic), the morphological origins of these structures remain an unresolved, highly contested debate that has been ongoing for over a century. There are two main theories regarding the evolution of these structures. The paranotal theory suggests that insect wings evolved from fixed extensions of the thoracic terga originally used for gliding from tall terrestrial vegetation (Grimaldi and Engel, 2005; Hamilton, 1971; Quartau, 1986; Snodgrass, 1935). In contrast, the exite or gill theory proposes that wings are derived from the dorsal structures of multibranched ancestral appendages that probably functioned as gills in aquatic environments (Grimaldi and Engel, 2005; Kukalova-Peck, 1991; Wigglesworth, 1973). While both theories have gained an equal amount of support over the past several decades, the use of traditional anatomical, histological and embryological approaches has been unable to provide a definitive answer to the question of insect wing origins (Grimaldi and Engel, 2005; Hamilton, 1971; Kukalova-Peck, 1978; Kukalova-Peck, 1991; Quartau, 1986; Ross, 1964; Snodgrass, 1935; Wigglesworth, 1973).
More recently, modern molecular techniques have been employed in an attempt to distinguish between the two hypotheses. One such study showed that two known Drosophila wing genes are expressed in the dorsal lobe (distal epipodite) of the multibrached limbs of crustaceans and therefore, is consistent with the exite theory of wing origins (Averof and Cohen, 1997). The caveat of this study however, is that the inferences obtained are indirect since no extant insect species possesses multibranched appendages. Hence, such indirect comparative analyses of gene expression lack the means to definitively prove true homologies of divergent structures due to the fact that individual genes can acquire different roles in different developmental contexts (Averof and Cohen, 1997; Choe and Brown, 2007; Choe et al., 2006; de Jong et al., 1989; Hrycaj et al., 2008; Liu and Kaufman, 2005; Patel et al., 1992; Schroder, 2003; Stuart et al., 1991).
An alternative approach is to study the histological and genetic origins of insect wings in a system in which the ancestral form can be recapitulated. According to fossil evidence, extinct pterygotes exhibited wings on every thoracic and abdominal segment (Carroll et al., 1995; Kukalova-Peck, 1978; Tanaka and Ito, 1997). Expression and functional analyses have since established that the subsequent acquisition of Scr in the prothorax of modern winged insect lineages gained a novel role in suppressing the ancestral wing developmental program on this segment (Beeman et al., 1989; Carroll et al., 1995; Chesebro et al., 2009; Rogers et al., 1997). Scr analyses in two hemimetabolous lineages, Oncopeltus (Chesebro et al., 2009), Periplaneta (this study), and in the holometabolous species Tribolium (Beeman et al., 1989; Tomoyasu et al., 2005) and Drosophila (Rogers and Kaufman, 1997) therefore recreate an ancestral condition by relaxing the normally suppressed ancient wing developmental program on the prothorax. As shown Fig. 6D–D1, the abolishment of Scr results in the growth of ectopic T1 wings that originate from the posterior lateral terga of the prothoracic plate. In addition, SCR protein is expressed in the dorsal lateral region of the prothorax of all modern winged species at late stages of embryogenesis (Passalacqua et al., 2009). This finding pinpoints the exact area where Scr is acting to suppress the ancestral wing developmental program on T1. Hence, by using Scr signal as a proxy, we can show that wing primordia are localized to the dorsal lateral region of the prothorax.
In a strict sense, these combined data swing the pendulum back in support of the paranotal theory. However, it is important to note that while these data are consistent with this hypothesis, they do not effectively disprove the exite theory. In essence, while the present results unambiguously show that ectopic wings arise from the dorsal lateral portion of the pronotum, what remains to be determined is the cellular origin of the tissue itself. Based on its position, it is tempting to postulate that the ectopic T1 wing tissue is of paranotal origin. And yet, it is conceivable to imagine a scenario where the exopodite tissue in crustaceans was reabsorbed and migrated dorsally in the ancestor of modern winged insects to its current position on the pronotum. Future studies should therefore focus on performing critical hypothesis-testing experiments that can provide support for a single theory. For example, determining that crustacean epipod-specific genes are expressed in regions outside of the observed embryonic Scr pronotal domain would identify tissues that are homologous to exites. The distinction of such tissue from the pronotal domain would provide direct evidence against the exite theory. Such experiments, coupled with studies analyzing the histological origins of ectopic wing tissue in RNAiScr Oncopeltus or Periplaneta individuals can provide a direct manner for investigating the evolution of insect wings.
Acknowledgements
We would like to thank E.M. Golenberg for the critical reading of the manuscript. We also thank Debbie Andrew for generously providing the SCR antibody used in this study and J.P. Couso for the Periplaneta Engrailed (en) clone. We also thank two anonymous reviewers whose comments greatly improved the manuscript. This work was supported by NIH grant GM071927 to A.P. and a Wayne State University wide Thomas C. Rumble Fellowship to S.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abzhanov A, Kaufman TC. Novel regulation of the homeotic gene Scr associated with a crustacean leg-to-maxilliped appendage transformation. Development. 1999;126:1121–1128. doi: 10.1242/dev.126.6.1121. [DOI] [PubMed] [Google Scholar]
- Angelini DR, Kaufman TC. Comparative developmental genetics and the evolution of arthropod body plans. Annu Rev Genet. 2005a;39:95–119. doi: 10.1146/annurev.genet.39.073003.112310. [DOI] [PubMed] [Google Scholar]
- Angelini DR, Kaufman TC. Insect appendages and comparative ontogenetics. Dev Biol. 2005b;286:57–77. doi: 10.1016/j.ydbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Angelini DR, Liu PZ, Hughes CL, Kaufman TC. Hox gene function and interaction in the milkweed bug Oncopeltus fasciatus (Hemiptera) Dev Biol. 2005;287:440–455. doi: 10.1016/j.ydbio.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Aplin AC, Kaufman TC. Homeotic transformation of legs to mouthparts by proboscipedia expression in Drosophila imaginal discs. Mech Dev. 1997;62:51–60. doi: 10.1016/s0925-4773(96)00649-1. [DOI] [PubMed] [Google Scholar]
- Averof M, Cohen SM. Evolutionary origin of insect wings from ancestral gills. Nature. 1997;385:627–630. doi: 10.1038/385627a0. [DOI] [PubMed] [Google Scholar]
- Beeman RW, Stuart JJ, Brown SJ, Denell RE. Structure and function of the homeotic gene complex (HOM-C) in the beetle, Tribolium castaneum. Bioessays. 1993;15:439–444. doi: 10.1002/bies.950150702. [DOI] [PubMed] [Google Scholar]
- Beeman RW, Stuart JJ, Haas MS, Denell RE. Genetic analysis of the homeotic gene complex (HOM-C) in the beetle Tribolium castaneum. Dev Biol. 1989;133:196–209. doi: 10.1016/0012-1606(89)90311-4. [DOI] [PubMed] [Google Scholar]
- Bell WJ, Adiyodi K. The American Cockroach. New York, NY: Chapman and Hall; 1982. [Google Scholar]
- Brown S, DeCamillis M, Gonzalez-Charneco K, Denell M, Beeman R, Nie W, Denell R. Implications of the Tribolium Deformed mutant phenotype for the evolution of Hox gene function. Proc Natl Acad Sci U S A. 2000;97:4510–4514. doi: 10.1073/pnas.97.9.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB, Weatherbee SD, Langeland JA. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375:58–61. doi: 10.1038/375058a0. [DOI] [PubMed] [Google Scholar]
- Chesebro J, Hrycaj S, Mahfooz N, Popadić A. Diverging functions of Scr between embryonic and post-embryonic development in a hemimetabolous insect, Oncopeltus fasciatus. Dev Biol. 2009;329:142–151. doi: 10.1016/j.ydbio.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe CP, Brown SJ. Evolutionary flexibility of pair-rule patterning revealed by functional analysis of secondary pair-rule genes, paired and sloppy-paired in the short-germ insect, Tribolium castaneum. Dev Biol. 2007;302:281–294. doi: 10.1016/j.ydbio.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe CP, Miller SC, Brown SJ. A pair-rule gene circuit defines segments sequentially in the short-germ insect Tribolium castaneum. Proc Natl Acad Sci U S A. 2006;103:6560–6564. doi: 10.1073/pnas.0510440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CD, Brisson JA, DeCamillis MA, Shippy TD, Brown SJ, Denell RE. Molecular characterization of Cephalothorax, the Tribolium ortholog of Sex combs reduced. Genesis. 2001;30:12–20. doi: 10.1002/gene.1027. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Hendriks W, Mulders JW, Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989;14:365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]
- DeCamillis MA, Lewis DL, Brown SJ, Beeman RW, Denell RE. Interactions of the Tribolium Sex combs reduced and proboscipedia orthologs in embryonic labial development. Genetics. 2001;159:1643–1648. doi: 10.1093/genetics/159.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giribet G, Edgecombe GD, Wheeler WC. Arthropod phylogeny based on eight molecular loci and morphology. Nature. 2001;413:157–161. doi: 10.1038/35093097. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Engel M. Evolution of the Insects. New York: Cambridge University Press; 2005. [Google Scholar]
- Hamilton KGA. The insect wing Part I. Origin and development of wings from notal lobes. Journal of the Kansas Entomological Society. 1971;44:421–433. [Google Scholar]
- Hrycaj S, Mihajlovic M, Mahfooz N, Couso JP, Popadić A. RNAi analysis of nubbin embryonic functions in a hemimetabolous insect, Oncopeltus fasciatus. Evol Dev. 2008;10:705–716. doi: 10.1111/j.1525-142X.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Kaufman TC. RNAi analysis of Deformed, proboscipedia and Sex combs reduced in the milkweed bug Oncopeltus fasciatus: novel roles for Hox genes in the hemipteran head. Development. 2000;127:3683–3694. doi: 10.1242/dev.127.17.3683. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Kaufman TC. Hox genes and the evolution of the arthropod body plan. Evol Dev. 2002;4:459–499. doi: 10.1046/j.1525-142x.2002.02034.x. [DOI] [PubMed] [Google Scholar]
- Kukalova-Peck J. Origin and evolution of insect wings and their relation to metamorphosis as documented by the fossil record. Journal of Morphology. 1978;156:53–125. doi: 10.1002/jmor.1051560104. [DOI] [PubMed] [Google Scholar]
- Kukalova-Peck J. Fossil history and the evolution of hexapod structures. In: Naumann ID, editor. The Insects of Australia: a Textbook for Students and Research Workers. Vol. I. Ithica, New York: Cornell University Press; 1991. pp. 141–179. [Google Scholar]
- Li H, Popadić A. Analysis of nubbin expression patterns in insects. Evol Dev. 2004;6:310–324. doi: 10.1111/j.1525-142X.2004.04039.x. [DOI] [PubMed] [Google Scholar]
- Liu PZ, Kaufman TC. even-skipped is not a pair-rule gene but has segmental and gap-like functions in Oncopeltus fasciatus, an intermediate germband insect. Development. 2005;132:2081–2092. doi: 10.1242/dev.01807. [DOI] [PubMed] [Google Scholar]
- Mahaffey JW, Kaufman TC. Distribution of the Sex combs reduced gene products in Drosophila melanogaster. Genetics. 1987;117:51–60. doi: 10.1093/genetics/117.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfooz N, Turchyn N, Mihajlovic M, Hrycaj S, Popadić A. Ubx regulates differential enlargement and diversification of insect hind legs. PLoS ONE. 2007;2:e866. doi: 10.1371/journal.pone.0000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfooz NS, Li H, Popadić A. Differential expression patterns of the hox gene are associated with differential growth of insect hind legs. Proc Natl Acad Sci U S A. 2004;101:4877–4882. doi: 10.1073/pnas.0401216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie B, Bacon JP. Two engrailed-related genes in the cockroach: cloning, phylogenetic analysis, expression and isolation of splice variants. Dev Genes Evol. 2000;210:436–448. doi: 10.1007/s004270000082. [DOI] [PubMed] [Google Scholar]
- Passalacqua KD, Hrycaj S, Mahfooz N, Popadić A. Evolving expression patterns of the homeotic gene Scr in insects. The International Journal of Developmental Biology. 2009b doi: 10.1387/ijdb.082839kp. doi: 10.1387/ijdb082839kc. [DOI] [PubMed] [Google Scholar]
- Patel NH, Ball EE, Goodman CS. Changing role of even-skipped during the evolution of insect pattern formation. Nature. 1992;357:339–342. doi: 10.1038/357339a0. [DOI] [PubMed] [Google Scholar]
- Patel NH, Kornberg TB, Goodman CS. Expression of engrailed during segmentation in grasshopper and crayfish. Development. 1989;107:201–212. doi: 10.1242/dev.107.2.201. [DOI] [PubMed] [Google Scholar]
- Pattatucci AM, Kaufman TC. The homeotic gene Sex combs reduced of Drosophila melanogaster is differentially regulated in the embryonic and imaginal stages of development. Genetics. 1991;129:443–461. doi: 10.1093/genetics/129.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattatucci AM, Otteson DC, Kaufman TC. A functional and structural analysis of the Sex combs reduced locus of Drosophila melanogaster. Genetics. 1991;129:423–441. doi: 10.1093/genetics/129.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival-Smith A, Weber J, Gilfoyle E, Wilson P. Genetic characterization of the role of the two HOX proteins, Proboscipedia and Sex Combs Reduced, in determination of adult antennal, tarsal, maxillary palp and proboscis identities in Drosophila melanogaster. Development. 1997;124:5049–5062. doi: 10.1242/dev.124.24.5049. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Popadić A, Kaufman TC. The expression of two engrailed-related genes in an apterygote insect and a phylogenetic analysis of insect engrailed-related genes. Dev Genes Evol. 1998;208:547–557. doi: 10.1007/s004270050214. [DOI] [PubMed] [Google Scholar]
- Quartau JA. An overview of the paranotal theory on the origin of insect wings. Publicacoes do instituto de Zoologia "Dr Augusto Nobre", Faculdade de Ciencias do Porto. 1986;194:1–42. [Google Scholar]
- Rogers BT, Kaufman TC. Structure of the insect head as revealed by the EN protein pattern in developing embryos. Development. 1996;122:3419–3432. doi: 10.1242/dev.122.11.3419. [DOI] [PubMed] [Google Scholar]
- Rogers BT, Peterson MD, Kaufman TC. Evolution of the insect body plan as revealed by the Sex combs reduced expression pattern. Development. 1997;124:149–157. doi: 10.1242/dev.124.1.149. [DOI] [PubMed] [Google Scholar]
- Rogers BT, Peterson MD, Kaufman TC. The development and evolution of insect mouthparts as revealed by the expression patterns of gnathocephalic genes. Evol Dev. 2002;4:96–110. doi: 10.1046/j.1525-142x.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- Ross MH. Pronotal wings in Blattella germanica (L.) and their possible evolutionary significance. American Midland Naturalist. 1964;71:161–180. [Google Scholar]
- Schroder R. The genes orthodenticle and hunchback substitute for bicoid in the beetle Tribolium. Nature. 2003;422:621–625. doi: 10.1038/nature01536. [DOI] [PubMed] [Google Scholar]
- Shippy TD, Brown SJ, Denell RE. Maxillopedia is the Tribolium ortholog of proboscipedia. Evol Dev. 2000a;2:145–151. doi: 10.1046/j.1525-142x.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Shippy TD, Guo J, Brown SJ, Beeman RW, Denell RE. Analysis of maxillopedia expression pattern and larval cuticular phenotype in wild-type and mutant tribolium. Genetics. 2000b;155:721–731. doi: 10.1093/genetics/155.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippy TD, Rogers CD, Beeman RW, Brown SJ, Denell RE. The Tribolium castaneum ortholog of Sex combs reduced controls dorsal ridge development. Genetics. 2006;174:297–307. doi: 10.1534/genetics.106.058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass RE. Principles of Insect Morphology. New York: McGraw-Hill; 1935. [Google Scholar]
- Struhl G. A homoeotic mutation transforming leg to antenna in Drosophila. Nature. 1981;292:635–638. doi: 10.1038/292635a0. [DOI] [PubMed] [Google Scholar]
- Struhl G. Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci U S A. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JJ, Brown SJ, Beeman RW, Denell RE. A deficiency of the homeotic complex of the beetle Tribolium. Nature. 1991;350:72–354. doi: 10.1038/350072a0. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Ito T. Studies of the genetics of Prowing (Pw): a primitive homeotic mutant of the German cockroach, Blatella germanica. Zoological Science. 1997:339–346. [Google Scholar]
- Tomoyasu Y, Wheeler SR, Denell RE. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature. 2005;433:643–647. doi: 10.1038/nature03272. [DOI] [PubMed] [Google Scholar]
- Wakimoto BT, Turner FR, Kaufman TC. Defects in embryogenesis in mutants associated with the antennapedia gene complex of Drosophila melanogaster. Dev Biol. 1984;102:147–172. doi: 10.1016/0012-1606(84)90182-9. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB. Evolution of insect wings and flight. Nature. 1973;246:127–129. [Google Scholar]
- Zhang H, Shinmyo Y, Mito T, Miyawaki K, Sarashina I, Ohuchi H, Noji S. Expression patterns of the homeotic genes Scr, Antp, Ubx, and abd-A during embryogenesis of the cricket Gryllus bimaculatus. Gene Expr Patterns. 2005;5:491–502. doi: 10.1016/j.modgep.2004.12.006. [DOI] [PubMed] [Google Scholar]