Abstract
Neuroscientific research on emotion regulation suggests that the interplay between emotion and cognition may be fundamental to the ability to adaptively regulate emotions. Although emotion and cognition have historically been considered to be in opposition, more recent research suggests that they are also integrated, coordinated, and complementary. In this article, I review studies showing that scalp-recorded event-related potentials (ERPs) reflecting emotion-cognition integration can be used as clinically meaningful indices of emotion regulation in children and adults, and have the potential to serve as biomarkers for emotion regulation and risk for specific affective disorders. Drawing on neuroscience and behavioral research, I propose a model in which ERP measures of emotion-cognition integration rather than opposition is the guiding principle for detecting neural markers for emotion regulation. Suggestions for a future research agenda are then presented.
Emotion regulation is a core developmental acquisition in early and middle childhood: Adaptive emotion regulation promotes child well-being, whereas difficulties with emotion regulation are related to mood disruptions and behavioral problems (Cole, Martin, & Dennis, 2004; Cole, Michel, & Teti, 1994; Eisenberg & Fabes, 1992). Although there is no single agreed upon definition of emotion regulation, broadly speaking emotion regulation refers to the ability to monitor, evaluate, and modify the intensity and temporal dynamics of emotional reactions (Thompson, 1994). In addition, among the core capacities that support emotion regulation are the ability to control attention, decision making, and other cognitive processes that take place in emotionally demanding contexts (Cole et al., 2004; Dennis, Malone, & Chen, 2009b; Lewis, Lamm, Segalowitz, Stieben, & Zelazo, 2006b). Neuroscientific studies in this field have received growing attention because they elucidate the basic processes underlying emotion regulation and because regulatory processes and subtle changes in emotion are often covert and may not be readily detected in observable behavior. Neuroscientific models also emphasize the interplay between emotion and cognition, which has been central to current conceptualizations of emotion regulation (Lévesque et al., 2004; Lewis, 2005; Ochsner & Gross, 2008; Urry et al., 2006).
Despite this emphasis, emotion and cognition historically have been examined separately and as processes that are intrinsically in opposition, a conceptual and methodological divide that still exits. With the onset of sophisticated neuropsychological studies of emotion and emotion regulation, however, there has been growing interest in the inexorable interconnections rather than distinctions between emotion and cognition. Detection of a neural marker for emotion regulation that can capture emotion-cognition interactions can address the gap in our understanding of how emotion and cognition are related to one another in the context of emotion regulation and affective risk and resilience. Such a program of research has the potential to reveal core mechanisms in emotion regulation and inform early detection and intervention for mood problems in childhood.
In this paper, I draw primarily on research using scalp-recorded event-related potentials (ERPs) to present an emotion-cognition integration model for the detection of neurophysiological markers for emotion regulation. I then outline suggestions for a future research agenda in which emotion-cognition integration rather than opposition is the guiding principle for detecting neurophysiological markers for emotion regulation and affective psychopathology.
The Development of Emotion Regulation from a Neuroscience Perspective
Emotion regulation is supported by neural systems involved in emotional reactivity, cognitive control, and the interplay between the two (Derryberry & Rothbart, 1997; Henderson & Wachs, 2007; Luu & Tucker, 2004; Urry et al., 2006). A ventral system underlies emotional arousal and motivational processes (Critchley, 2005; Dolan, 2002), and includes limbic and brainstem regions and medial and orbitofrontal structures of the prefrontal cortex. This system is sensitive to information that is motivationally significant, and thus capitalizes on rapid and relatively automatic evaluative and regulatory processes. In contrast, a dorsal system underlies relatively effortful, executive functions such as attention regulation and cognitive control of reactivity and arousal (Luu, Tucker, & Derryberry, 1998; Ochsner et al., 2004), and includes areas of the dorsolateral and medial frontal cortex. This dorsal network supports the ability to regulate arousal in more deliberate ways and utilizes motivationally relevant information from the ventral network to direct attention and memory and to plan actions.
The anterior cingulate cortex (ACC), located in the medial frontal cortex, appears to be an intermediary between functions of the ventral and dorsal networks, and thus is a key structure in the interactions between emotion and cognition and in emotion regulation (Bush, Luu, & Posner, 2000; Luu & Tucker, 2004). The ACC is active during action and conflict monitoring tasks: tasks that make demands on the control of attention due to the presence of conflicting information, the need for inhibitory control, or the detection of errors (van Veen & Carter, 2002a, 2002b; van Veen, Cohen, Botvinick, Stenger, & Carter, 2001). The ACC is composed of ventral and dorsal segments that are reciprocally active in response to emotional versus cognitive tasks (Bush et al., 2000). Rostral portions of the ACC are engaged during both positive and negative emotionally-charged tasks (Bush et al., 2000) and during emotion regulation and inhibition tasks (Beauregard, Levesque, & Bourgouin, 2001). Given these functions, the ACC is likely critical to adaptive and maladaptive emotion regulation in that the ACC signals the need for flexible self-regulation and coordination, integrating both affective information and executive control processes such as plans, goals, and cognitive control strategies. Thus, the ACC is an intermediary between higher order cognition and emotional arousal (Luu & Tucker, 2004; Paus, 2001).
Compared to the ventral system, the dorsal system has a protracted developmental course (Casey, Getz, & Galvan, 2008). This is evident behaviorally in tasks related to effortful control, inhibitory control, and executive functions, which increase from early childhood through adolescence (Casey et al., 1997b; Diamond & Taylor, 1996; Enns, Brodeur, & Trick, 1998; Kochanska, Coy, & Murray, 2001). These behavioral changes co-occur with important neural changes (Casey, Giedd, & Thomas, 2000). For example, while performing inhibition tasks such as a go/no-go task, children show greater activation of prefrontal regions than do adults (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Durston et al., 2002), suggesting increasing cortical efficiency with age. This is consistent with the view that greater cognitive capacity coincides with gradual loss of synapses along with an apparent strengthening of remaining synaptic connections (Casey et al., 2000; Huttenlocher, 1990). The ACC shows similar anatomical and functional maturation with age, including changes in volume that correlate with response inhibition performance (Casey et al., 2000; Casey, Trainor, Giedd, Vauss, & et al., 1997a) as well as changes in the amplitudes of neurophysiological responses related to action monitoring and inhibitory control functions of the ACC (Lewis et al., 2006b; Segalowitz & Davies, 2004).
Thus, the nature of interactions between the limbic system and higher cortical functions may differ between children and adults and in part explain developmental differences in emotion regulation (Bush et al., 2000; Davidson, 2002; Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003). For example, delayed development of the prefrontal cortex may create a mismatch between a child’s reactivity and capacity for impulse control. This may result not only in difficulties with emotion regulation, but in changes in the activity of neural regions that integrate emotional and cognitive functions, such as the ACC. Neurophysiological measures reflecting activity of the ACC are quite well-understood in adults and children, and thus may be particularly well-suited to the study of development and emotion regulation. This research is reviewed below.
Emotion-Cognition Integration
Emotion and cognition are acknowledged to be closely integrated in emotion regulation (Gross, 1998; Lewis et al., 2006b; Ochsner, Bunge, Gross, & Gabrieli, 2002), but many studies continue to examine emotion as the antithesis of cognition, rather than an integral part of thought processes (c.f., Gray, 2004; Lewis, 2005). Broadly speaking, integration refers to a combination of parts that work together or form a whole that better achieves a common objective or set of objectives. Integration between emotion and cognition further implies that distinct aspects of emotion and cognition, such as specific emotional states or different cognitive control functions, can influence each other in selective ways (Gray, 2004; Gray & Burgess, 2004). For example, neuroimaging research by Gray and colleagues showed that experimentally induced approach- and withdrawal-related emotions has a selective effect on cognitive control functions, such as working memory (Braver, Cohen, & Barch, 2002; Gray, 2001; Gray, 2004): approach-related emotions (amusement) induced by videos enhanced verbal working memory whereas they compromised spatial working memory. Conversely, induced withdrawal-related emotions (fear and anxiety) enhanced spatial working memory but compromised verbal working memory. These findings also illustrate that mild negative emotions, such as anxiety, can actually enhance at least one aspect of cognition and that at some stage of task processing, emotion and cognition equally contribute to behavioral regulation (Gray, 2004). Moreover, this research suggests that specific emotions enhance some cognitive functions but impair others and that there are conditions under which emotion and cognition are in opposition and those in which they are mutually supportive (Dennis, 2006; Derryberry & Rothbart, 1997; Gray, 2004; Luu & Tucker, 2004; Wolfe & Bell, 2007). A range of emotions, cognitive processes, and contexts must be assessed if we are to delineate the specific ways in which integration influences self-regulation.
Research on decision making and memory further highlights the importance of emotion-cognition integration. For example, when emotional functioning is compromised, social reasoning may be impaired. Damasio and colleagues’ studies of patients with lesions to neural networks supporting emotional functioning show that social decision making is severely compromised in these patients (Damasio, Tranel, & Damasio, 1991). Other research shows that economic decision making is actually enhanced among more emotionally reactive individuals (Seo & Barrett, 2007) and that emotion bolsters both memory accuracy and a subjective sense of recollection (Phelps & Sharot, 2008).
The emotion-cognition integration perspective also highlights the potentially adaptive function of negative and positive emotions in relation to the development of emotion regulation. Functional perspectives on emotion posit that negative emotions do not uniformly have a disruptive impact, and instead are fundamentally adaptive if well regulated (Campos, Campos, & Barrett, 1989; Frijda, 1986). From early in development, negative emotions may actually facilitate cognitive processes, attention, and learning, making them adaptive under some conditions (Barrett & Campos, 1987; Carver, 2004). For example, in a study with typically developing children, expressions of anger were temporally associated with increased use of adaptive and context-appropriate actions during emotional challenges (Dennis, Cole, Wiggins, & Zalewski, 2009a). This idea that emotions serve adaptive, organizing behavioral functions tends to be overlooked, despite the fact that functional emotion theory has been embraced by many scholars. Research examining neural processes related to emotion regulation is in the unique position to delineate patterns of neural activity associated with positive and negative emotions and adaptive cognitive control processes. However, few studies have done so within an emotion-cognition framework highlighting the adaptive function of both positive and negative emotions.
Emotion Cognition Integration and the Neural Bases for Emotion Regulation
In addition to mutually supportive patterns of integration, emotions and cognitions are also at times in opposition: in emotionally evocative moments, our ability to control our thoughts and behavior can be diminished (Drevets & Raichle, 1998). Research on neural bases of emotion regulation has focused on this pattern of opposition between emotion and cognition, and thus highlights the cognitive control of emotion as a core index of effective regulation of behavior and emotions (Bishop, Duncan, Brett, & Lawrence, 2004; Ochsner & Gross, 2005; Urry et al., 2006). In particular, cognitive strategies to suppress emotion are examined, such as directing attention to less affectively-charged aspects of a stimulus or by deliberately altering the meaning of a stimulus. For example, reappraisal involves reinterpreting a situation or stimulus before an emotional response is elicited: such as interpreting an impending job interview as an opportunity to display your excellent qualifications versus an intimidating prelude to rejection (Gross & Thompson, 2007). When such cognitive emotion regulation strategies are used, neural regions supporting cognitive control are more active (such as areas of the prefrontal cortex), whereas regions involved in emotional reactivity and processing, such as limbic regions like the amygdala are less active (Ochsner et al., 2004; Urry et al., 2006). Similar patterns of neural activation are elicited when participants categorize or encode unpleasant stimuli on non-affective versus affective dimensions, suggesting that attentional focus also influences recruitment of cognition to modulate emotional processing (Hariri et al., 2003; Keightley et al., 2003; Mathews, Yiend, & Lawrence, 2004). These findings highlight opposition or an inverse relation between emotion and cognition such that “cold” cognitive abilities like attention and executive functions modulate (usually reduce) “hot” affective responses (Ayduk, Mischel, & Downey, 2002). The implicit and often explicit assumption is therefore that the path to mental and emotional health relies upon control processes that dampen down negative emotions. On the other hand, when emotions are “up-regulated”, similar neural regions of the prefrontal cortex are activated while amygdala activity is increased (Ochsner et al., 2004), suggesting that cognitive control effects both the up- and down-regulation of emotion. These findings also hint at the possibility that similar patterns of activation might underlie affective enhancement of cognitive functions – such as when emotional states bolster cognition.
From the perspective of emotion-cognition integration, this “hot versus cold” distinction may be overly simplistic because it solely attributes regulatory control to the prefrontal cortex and strongly highlights conditions under which emotions must be “cooled” by cognitive control mechanisms (e.g., the prefrontal cortex suppresses activity of the amygdala and other limbic regions in support of emotion regulation). Certainly, there are numerous situations in which this pattern supports adaptive behavior. Both positive and negative emotions, however, serve a regulatory function such that reduced or compromised emotional reactivity can have a negative impact on behavioral control (Cole et al., 2004; Damasio et al., 1991; Izard, 2007; Luu & Tucker, 2004; Seo & Barrett, 2007).
Consistent with this view, the affective evaluation hypothesis (Luu & Tucker, 2004) argues that executive control and affective functions are mutually regulated in order to direct and control behavior. In particular, the model proposes that conflict and error monitoring and other activities of the anterior cingulate cortex (ACC) intrinsically involve affective set points against which actions are monitored, and thus these cognitive control functions are constrained by emotional individual differences, contexts, and goals. Thus, affective processes are necessary for intact self-regulation. This view is rooted in cybernetic principles of homeostasis, set points, and feedback systems (Nauta, 1971; Pribram, 1960) and is consistent with dynamic systems ideas (Lewis, 2005). Given the massive interconnectivity between the frontal lobe and limbic networks, including hypothalamic and brainstem regions, action plans represented in the cortex can be evaluated in terms of motivational and affective significance by limbic networks (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001). Thus, in a feed-forward fashion, emotional processing centers create expectancies and evaluative set-points that are critical for ongoing feedback during action monitoring. A unitary focus on the regulatory role of prefrontally-mediated cognitive functions, without consideration of the role of affective motive set-points to guide plans and actions, is thus limited because it captures only one basis for regulatory control. This limitation might be most salient when considering affective psychopathologies in which emotional dysregulation is a key symptom.
This perspective has received relatively little empirical attention, in part because it runs counter to the prevailing view in cognitive neuroscience that cognition regulates emotion. Research using ERPs related to action monitoring, however, may provide a fertile testing ground for examining the heterogeneous impact of affect on cognition. For example, the error-related negativity (ERN) is an ERP generated in the medial frontal cortex and is thought to reflect error processing (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Gehring, Goss, Coles, & Meyer, 1993; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001). The ERN is a sharp negative deflection peaking within about 100 ms after an incorrect response at fronto-central electrode sites. The ERN reflects the relatively automatic process of checking actual behavior against an internal goal or standard. The ERN, as output of an error detection system, may guide emotion regulation by marking the cognitive and emotional significance of a deviation from an anticipated response (Luu & Tucker, 2004).
The ERN is enhanced among individuals showing anxiety - such as obsessive compulsive disorder or obsessive compulsive traits (Gehring, Himle, & Nisenson, 2000; Hajcak, Franklin, Foa, & Simons, 2008; Hajcak & Simons, 2002; Johannes et al., 2001) and generalized anxiety disorder and excessive worry (Hajcak, McDonald, & Simons, 2003). This enhancement of the ERN has also been documented in anxious children (Ladouceur, Dahl, Birmaher, Ryan, & Axelson, 2006a) and among adults made to feel more self-conscious about their performance (Hajcak, Moser, Yeung, & Simons, 2005). Conversely, anxiolytic medications suppress ERN amplitude (Johannes et al., 2001).
In contrast, studies that examine error processing in emotional contexts suggest additional patterns. For example, one study found that when errors were made in the context of threat-relevant faces (anger faces) a high state anxiety group showed reduced ERNs (Compton et al., 2007). Another study showed that when a conflict monitoring task (a flanker task) was superimposed over neutral, pleasant, and unpleasant pictures, ERNs peaked later and were smaller in the context of unpleasant compared to pleasant backgrounds (Larson, Perlstein, Stigge-Kaufman, Kelly, & Dotson, 2006). Taken together, ERN studies suggest that affective processes – specifically affective traits and emotional contexts – modulate the amount of cognitive resources devoted to error monitoring. This could in turn have a direct impact on emotion regulatory competence, in particular the ability to control attention and monitor errors to influence behavior in emotional contexts. The association between clinical anxiety and enhanced ERNs also suggests that emotion-cognition integration does not simply follow a “see-saw” pattern – when emotionality “goes up” cognitive processes “go down.” Instead, some emotional traits up-regulate the allocation of cognitive resources, perhaps by changing the detection threshold and increasing sensitivity for cognitive operations like conflict monitoring (Carter et al., 1998b; Luu & Tucker, 2004).
These studies highlight that emotional conditions and traits modulate basic cognitive processes, and that negative emotions are well-integrated into the functioning of cognitive control networks, such as those including the medial and prefrontal cortex (Luu & Tucker, 2004). These results provide a strong basis upon which to develop hypotheses concerning patterns of emotion-cognition integration (both mutually supportive and adversarial) that underlie emotional and self regulation.
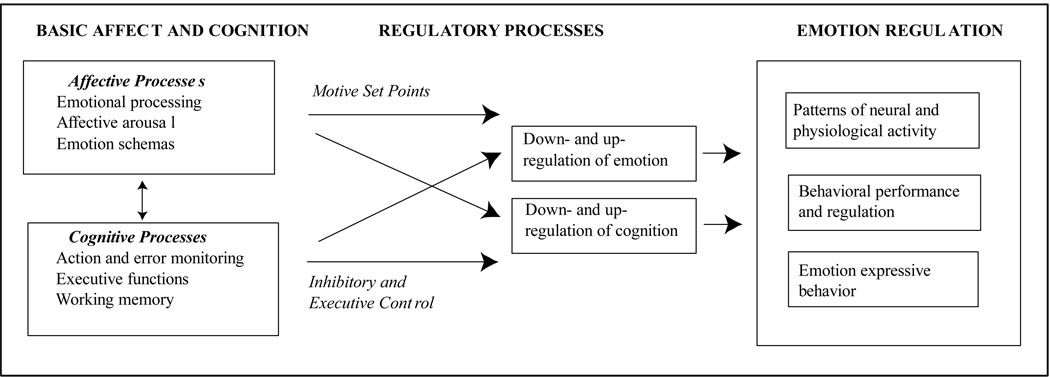
Figure 1 portrays a model of emotion-cognition integration in which both affective and cognitive processes interact to accomplish the up- and down-regulation of emotion and cognition, which are expressed in terms of patterns of neural and physiological activation, behavioral performance, and emotion expressive behaviors. This model highlights that emotion and cognition must be considered together rather than in isolation and that both affective and cognitive processes can exert control and establish constraints on emotion regulation. This is a general model, so it is for future research to determine whether the interactions between specific aspects of cognitive control (e.g., action monitoring or cognitive inhibition) and specific types of reactivity (e.g., approach or avoidance) lead to distinct emotion regulatory outcomes. In the next section, research relevant to this model is presented. These studies identify ERPs that index the first stage of the model (the interplay between emotion and cognition), and in turn relate these ERPs to regulatory processes and emotion regulation outcomes.
Figure 1.
A model of emotion-cognition integration in relation to emotion regulation
Using ERPs to Elucidate Emotion-Cognition Integration Related to the Development of Emotion Regulation
In behavioral research, children’s problems with emotion regulation are typically identified by observing their responses to an emotional challenge, such as frustrations, delays of gratification, and difficult or impossible tasks (Cole et al., 2004). Children who show negative affect during such emotional challenges are often characterized as showing poor emotion regulation. However, this methodology, while providing rich information about children’s behavioral responses when faced with emotional dilemmas, is problematic in several ways. First, it runs the risk of labeling what may be appropriate negative emotional responses to challenges as poor emotion regulation. Second, measuring emotion expressive behavior does not necessarily capture regulatory processes. Third, this methodology cannot clearly reveal the nature of emotion regulation as it relates to emotion-cognition integration because it fails to elucidate processes that happen too rapidly or subtly to be expressed in overt behavior. Fourth, this approach does not consider the ways in which both positive and negative emotions can promote adaptive cognitive responses or emotion regulation. Therefore, there is a great need to identify neural processes related to child emotion regulation that also capture the interplay between emotion and cognition.
ERPs may provide a particularly useful measure of rapid affective and cognitive processes relevant to emotion regulation (Banaschewsk & Brandeis, 2007; Lewis et al., 2006b). Compared to other neuroimaging techniques like fMRI, ERPs have a superior temporal resolution on the order of milliseconds. Thus, ERPs can capture both extremely rapid changes in neural processing as well as more slowly unfolding processes over the course of several seconds. In addition, given the relative ease of administration for young children and low cost compared to functional neuroimaging, ERPs are particularly well-suited to examining neural markers for emotion regulation in the developing brain. Finally, ERPs have been successfully used to measure the degree to which emotion engages a given cognitive process, cognitive demands modulate emotional processes, and other emotion-cognition interactions (Cacioppo & Berntson, 1994; Davidson, 1998; Schupp et al., 2002; Schupp, Junghöfer, Weike, & Hamm, 2003).
There is not, however, a well-articulated measurement strategy for identifying neural markers for emotion regulation, particularly in children. The extant research suggests two important types of candidate ERP markers, both of which also reflect emotion-cognition integration: those that reflect attentional control under emotional demands and those that reflect attentional processing of negative emotional stimuli. This body of research is small but growing, and has the potential to provide an objective measure of emotion-cognition integration related to emotion regulation that could complement existing tools for evaluating risk for affective psychopathology and the efficacy of treatments for these disturbances. Below I review developmental studies using ERPs reflecting attentional control under emotional demands and attentional processing of negative emotion to demonstrate that the neural responses of children showing adaptive emotion regulation differ from those showing maladaptive emotion regulation and mood disturbances. I then consider how these ERP markers should be interpreted in the context of developing neural systems.
Attentional control under emotional demands
The ability to perform efficiently on cognitive and attentional tasks under emotional processing demands may reflect core regulatory capacities that support the development of adaptive emotion regulation. As noted above, the ACC is a key brain structure underlying cognitive control and the interplay between emotion and cognition (Allman et al., 2001; Bush et al., 2000; van Veen & Carter, 2002a; Yamasaki, LaBar, & McCarthy, 2002). A growing number of studies of child emotion regulation have targeted ERPs related to activity of the ACC, the N2 and the ERN, to track how attention is modulated under emotional conditions. The N2 occurs over the frontal midline regions 200 to 350 ms following a stimulus (Gehring & Willoughby, 2002; van Veen & Carter, 2002a). Initially, the N2 was thought to reflect successful inhibition of a prepotent response, but now is thought to primarily reflect conflict and action monitoring (Luu, Flaisch, & Tucker, 2000; Nieuwenhuis, Yeung, Van Den Wildenberg, & Ridderinkhof, 2003). That is, it is implicated in tasks requiring monitoring of “crosstalk”, or conflicting information or response options, and is thought to signal the extent to which attentional control is required (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Carter et al., 1998a; van Veen et al., 2001). The N2 and other medial frontal negativities may therefore reflect a gating mechanism in the medial frontal cortex through which motivationally-significant information gains access to prefrontally-mediated cognitive control systems (Potts, Martin, Burton, & Montague, 2006; Todd, Lewis, Meusel, & Zelazo, 2008). Recent work further suggests that in children, the posterior N2 may also reflect this inhibitory function due to relative immaturity of the prefrontal cortex (Ciesielski, Harris, & Cofer, 2004).
In a study of normatively developing school-aged children, we detected a frontal negative component (Nc) in response to task-irrelevant, distracting emotional faces prior to performance on an attention task. We reasoned that, like the N2, the Nc would index the ability to recruit cognitive control under emotional demands, and thus we predicted that the Nc would concurrently predict child emotion regulation capacities. Emotion regulation was measured in two ways: as maternal report of child emotional dysregulation and as attention interference effects following emotional distracters. We found that larger Nc amplitudes to distracters were correlated with reduced attention interference effects, whereas an earlier component related to attention gating, the P1, was associated with reduced maternal report of child dysregulation (Dennis et al., 2009b). Overall, these findings suggest that these ERPs reflect individual differences in attention and cognitive control under emotional demands that relate to emotion regulation. Moreover, it suggests specific rather than global patterns of integration: the modulation of distinct cognitive processes indexed by the Nc and P1 were differentially associated with two measures of emotion regulation.
However, this study did not examine individual differences. ERPs associated with action monitoring, such as the ERN, are sensitive to affective individual differences: ERNs are enhanced among anxious children (Ladouceur et al., 2006b), whereas childhood attention deficit hyperactivity disorder is associated with reduced ERN amplitudes, suggesting that ERN deficits may be associated with difficulty initiating or maintaining response inhibition (Pliska, Liotti, & Woldorff, 2000; Yong-Liang et al., 2000). In this special issue, Henderson and Ladouceur and colleagues take the consideration of individual differences in relation to action monitoring ERPs (the ERN and N2) one step further by directly examining the interplay between emotional reactivity and cognitive control. For example, Henderson examined typically developing children performing a modified flanker task. Greater shyness was associated with poor social-emotional outcomes primarily among children with relatively enhanced N2 responses. In a similar vein, Ladouceur examined typically developing adolescents performing a flanker task, but measured negative affectivity and self-report of attentional control. Youth high in negative affect and high in attentional control showed increased N2 amplitude and a trend toward increased ERN amplitudes. These increased ERP magnitudes were interpreted as reflecting excessive or less efficient action monitoring. Thus, these studies suggest that larger amplitude ERP components related to action monitoring may be associated with less adaptive outcomes, but primarily among those with greater anxiety-related characteristics like shyness and negative affectivity.
These studies provide a novel and innovative perspective on the interplay between negative affect and cognition; but they did not create an emotional context in which to measure ERPs related to action monitoring, and thus did not directly assess attention under emotional demands. In a study with adults varying in normative trait anxiety, we examined the N2 in an emotional context: a flanker task preceded by threat-relevant (fear) or non-threat-relevant (sad, happy, and neutral) emotional distracters (Dennis & Chen, 2009). We found that high trait anxiety was associated with larger N2 amplitudes during congruent (low conflict) trials following fearful faces, which suggests excessive conflict monitoring in a threat-relevant context. Moreover, those high trait anxious participants who showed the largest N2 amplitudes showed the worst attention performance. In this study, as in the studies by Henderson and Ladouceur and colleagues, it was the interplay between affective individual differences and cognitive or attentional control, rather than each individually, that explained the most variance in outcomes.
In other research examining attention under emotional conditions, Lewis and colleagues assessed ERPs in children completing a go/no-go task under emotional demands (losing points towards obtaining a valued prize) (Lewis, Granic, & Lamm, 2006a; Lewis et al., 2006b). For typically developing children, N2 amplitudes were larger after losing points compared to when they gained points, suggesting that cognitive control processes were recruited more heavily in emotionally challenging conditions (Lewis et al., 2006b). In a study with children showing disruptions in emotion regulation, such as clinically elevated internalizing and externalizing problems, greater N2 amplitudes while losing points were correlated with more flexible interpersonal behavior during emotionally challenging interactions between children and their mothers (Lewis et al., 2006a). Another study using this task examined whether the N2 and ERN could distinguish between subtypes of aggressive children and typically developing children (Stieben et al., 2007). Compared to typical children, children showing both internalizing and externalizing problems showed enhanced N2 amplitudes during and even following point loss, whereas ERN amplitudes were greatest for control children and smallest for externalizing children, with children showing both internalizing and externalizing problems falling in between (Stieben et al., 2007). Overall, these studies suggest that the ability to recruit cognitive control under emotionally demanding conditions may reflect a core regulatory capacity that tends to be greater among typically developing children compared to those showing developmental disruptions.
Attentional Processing of Negative Emotion
The ability to modulate attention to and perceptual processing of negative emotional stimuli may be a core capacity underlying the development of emotion regulation. Recent work suggests that the late positive potential (LPP) may be a useful neural marker for attention to emotion. The LPP reflects facilitated attention to emotion, such that it is greater in response to emotional versus neutral stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000) and is reduced following use of cognitive emotion regulation strategies such as reappraisal, which reduces the negative emotional impact of unpleasant stimuli (Foti & Hajcak, 2008; Hajcak, Moser, & Simons, 2006). However, the LPP has rarely been examined in children. In two recent studies with normatively developing children, however, we demonstrated that the LPP operates in ways similar to studies with adults (Hajcak & Dennis, 2009), and that the LPP in children is sensitive to cognitive emotion regulation (Dennis & Hajcak, in press). For example, we found that the LPP was smaller following the directed use of reappraisal – in this case, reinterpreting an unpleasant picture in more neutral terms (Dennis & Hajcak, in press). However, some children did not show expected reductions in the LPP after reappraisals. Reduced modulation of the LPP by reappraisal suggests that one’s ability to effectively use reappraisal as an emotional regulatory strategy to lessen the negative impact of an unpleasant picture is also reduced. Indeed, in this study, reduced modulation of the LPP by reappraisal at an early time window (300–600 ms) was associated with maternal report of greater anxious-depressed symptoms, whereas reduced modulation of the LPP at later stages (up until 2000 ms) was associated with maternal report of greater emotional dysregulation, somatic complaints, and behavioral withdrawal.
These results provide preliminary evidence that modulation of the LPP by cognitive emotion regulation may represent a clinically relevant neural marker for emotion regulation and dysregulation of affect and behavior. Results also suggest that timing of this modulation is critical. Enhanced emotional processing in the earliest and most automatic stages of emotional processing (the early window) may underlie mood-related symptoms; in contrast, more elaborated and conscious emotional processing in later time windows may be more relevant to symptoms involving behavioral regulation (somatic complaints and withdrawal). This is consistent with research with adults showing that anxiety is associated with an attentional bias towards threat-relevant emotional information in early stages of attentional orienting, whereas selective attention to negative emotional information in depression is found more consistently at later stages of attention (with a stimulus duration of at least 1,000 ms) (e.g., Gotlib, Krasnoperova, Yue, & Joormann, 2004). Thus, distinctions between stages of attentional processing might be particularly important for understanding associations between neural markers for emotion regulation and specific mood and affect regulatory problems (Bradley, Mogg, & Lee, 1997).
Based on studies examining both attentional control under emotional demands and attentional processing of negative emotions, Table 1 summarizes ERP responses that may be markers for adaptive emotion regulation. Although findings are preliminary, these studies with children suggest several possibilities. First, when inhibitory control is required in emotional contexts, enhanced ERPs related to cognitive control are associated with more adaptive emotion regulation; but only when individual differences in affective style are not taken into account. For anxiety-related characteristics, like shyness, negative affectivity, and trait anxiety, enhanced ERPs related to cognitive control may be associated with more regulatory problems. Thus, for this family of electrocortical responses, what counts as “balanced” or adaptive recruitment of cognitive control depends on individual characteristics. Finally, when emotional processing changes via cognitive reappraisal is the target task, reduced ERPs related to attention to negative emotion are associated with more adaptive emotion regulation – although it is also possible that future research will find that this pattern also depends on individual differences in affective style and traits. Taken together, these studies provide a foundation for future research examining patterns of adaptive emotion-cognition integration that promote emotion regulation in childhood. It will be important in future research to systematically evaluate whether these patterns differ for children who show specific mood disruptions or temperamental traits, such as shyness, behavioral inhibition, anxiety, or depression. In particular, enhanced N2 and ERN in anxious children may reflect over-control or hyper vigilance, and thus be associated with less adaptive social-emotional outcomes.
Table 1.
ERP Markers for Adaptive Emotion Regulation
| Adaptive Emotion Regulation | ||
|---|---|---|
| Attention under Emotional Demands | Attentional Processing of Negative Emotions | |
| ERPs | ERPs related to activity of the medial frontal cortex (e.g., N2, ERN, Nc and other medial frontal negativities) |
ERPs reflecting attention to and perceptual processing of emotional stimuli (e.g., LPP) |
| Adaptive Function |
“Balanced” recruitment of cognitive control and inhibition of attention to emotion |
Reduced emotional processing |
Developmental Considerations in the Study of ERPs Related to Emotion-Cognition Integration
The nature of developing neural systems must be taken into account when examining emotion-cognition integration. For example, neural efficiency refers to the ability to use fewer neural resources (i.e., reduced activity) to successfully perform increasingly difficult tasks. Some studies have shown less prefrontal activation in adults than in children performing comparable attention control tasks (Casey et al., 1997b; Durston et al., 2002), and both the frontal N2 and frontal P3 during an emotionally charged inhibitory control task (the go/no-go) decrease in amplitude and latency with age (Lamm, Zelazo, & Lewis, 2006; Lewis et al., 2006b). Both of these findings suggest greater neural efficiency in adulthood. In a study examining the N2 in adults in response to emotional distracters (Dennis & Chen, 2007), greater N2 amplitudes were associated with increased attention interference following emotional distracters. This directly contrasts with the finding for the same task in children (Dennis et al., 2009b). Children compared to adults therefore may require increased neural resources to control attention and process emotional information given relatively immature cortical development (Casey et al., 2000; Segalowitz & Davies, 2004). On the other hand, the nature of developmental changes may not be linear, and may vary across ERPs and experimental contexts. Studies using a flanker task show that the amplitudes of the ERN increase with age (Davies, Segalowitz, & Gavin, 2004; Segalowitz & Davies, 2004), but may again decrease among older adults (Mathewson, Dywan, & Segalowitz, 2005). Another study found that both ERN and N2 amplitudes during a flanker task were greater in the adult and late adolescent groups than in the early adolescent group (Ladouceur, Dahl, & Carter, 2007).
Another developmental consideration is that the scalp distribution and presumed neural sources of ERPs have been shown to shift with age. In a study of the N2 and P3 during an emotionally charged inhibitory control tasks, source modeling indicated more central-posterior activation in younger children, giving way to medial–dorsal activation as children matured, suggestive of increased reliance on cognitive control regions such as the dorsal ACC (Lewis et al., 2006b). This finding of developmental frontalization is consistent with other recent neuroimaging and neurophysiological research (Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002; Ciesielski et al., 2004). Thus, it is important to track changes in the amplitude, latency, and frontalization of electrocortical changes which likely reflect increased cortical maturation and efficiency, and which are thought to support emotion regulatory capacities and flexibility across the lifespan.
Suggestions for a Future Research Agenda
Despite significant progress in understanding neural bases for emotion regulation and the role of emotion-cognition integration, many methodological and empirical challenges remain, particularly in developmental neuroscience. Once basic markers for emotion –cognition integration and emotion regulation are further identified, several key empirical steps, if pursued, will yield information that has the potential to deepen our understanding of mechanisms in emotion regulation and how disruptions in specific aspects of emotion regulation may represent a risk factor for distinct types of psychopathology. It is critical that these questions be asked in a developmental context, with a focus on assessing age-related changes in the relations between ERP markers and clinically relevant behaviors and symptoms. An understanding of neural markers for emotion regulation can reveal how emotion is processed and used to support goal-directed behavior in the developing brain, and detect early risk for affective problems even in the absence of overt behavioral signs. To clearly identify these neural markers, I make several suggestions for future research below, including the need to rethink models of emotion-cognition interplay, refine methodological approaches, and consider a range of individual differences.
Challenge predominant models of emotion-cognition interplay
The ability to reduce emotional responses may be one key marker for adaptive emotion regulation (Cole et al., 2004; Gross, 2007). However, studies supporting this notion often examine a restricted range of emotion regulation strategies, such as cognitive reappraisal, and do not explore the context within which emotions are regulated. Additional paradigms should be considered, particularly ones that explore conditions under which emotional processing facilities or directs cognition. For example, tasks should be developed in which emotional information is relevant to task performance or decision making, or in which emotional reactions are important sources of feedback that guide adaptive behavioral options. Another important goal for future research is to track parametric changes in emotion in relation to task performance. For example, an optimal arousal perspectives suggest that performance is facilitated only when arousal is at an optimal level; too much or too little can compromise cognitive and behavioral abilities, consistent with the Yerkes-Dodson principle (Yerkes & Dodson, 1908).
Methodology: Standardized tasks and databases
We are only beginning to understand what represents normative neural responses to emotional demands in children, and several studies highlight that changes continue into adulthood (Mathewson et al., 2005; Segalowitz & Davies, 2004). A battery of emotion regulation assessment tasks should be developed similar to those used to assess the development of cognitive control (Casey, Tottenham, & Fossella, 2002) or child temperament (Goldsmith & Rothbart, 1992). This is an obvious step if we are to increase generalizability of findings across contexts and labs. This also will allow us to systematically assess the psychometric properties of such an assessment battery.
Methodology: Assess associations between ERPs and complex behaviors
Studies that do examine relations between neural responses and behavior typically measure behavior in terms of reaction times during computerized tasks. Such information provides important insights, but as of yet, few studies have examined ERP markers for emotion regulation in relation to more complex emotional behaviors. Exceptions to this include studies of ERP markers for emotion regulation in relation to patterns of mother-child interaction (Lewis et al., 2006a) and relation to maternal report of mood disruptions (Dennis et al., 2009b; Henderson et al., 2006). These data allow us to not only to evaluate neural mechanisms related to normative development, but enhance identification of the clinical relevance of ERPs for the development of mood and anxiety symptoms, as well as adjustment to emotional demands.
Individual differences: Risk and resilience
Examination of normative patterns of neural markers for emotion-cognition integration and emotion regulation must be complemented with careful consideration of individual differences that could be relevant for the development of mood and behavioral problems. To do this, we must pursue ERP markers not for emotion regulation in general, but in relation to distinct child regulatory and mood problems. For example, anxious individuals may show attentional biases towards unpleasant stimuli at very early stages of processing, whereas depressed individuals and those at risk for depression may show such attention biases at later, more elaborated stages of processing (Gotlib et al., 2004; Joormann, Talbot, & Gotlib, 2007; Mogg, Bradley, Williams, & Mathews, 1993). ERPs, because of their excellent temporal resolution, can be used to index these important distinctions. In addition, identifying ERPs related to emotion regulation and clinical status has the potential to reflect biological mediators of emotion regulation that may indicate who is most at risk or most likely to benefit from psychological treatment.
However, in the search for ERP markers for specific mood problems, another aspect of individual differences must be taken into account – whether an ERP marker that indicates a maladaptive response in one individual could reflect adaptive responses in another individual. For example, the affective evaluation hypothesis (Luu & Tucker, 2004) suggests that individual variations in mood such as trait anxiety might constrain whether heightened or dampened action and error-monitoring is associated with adaptive outcomes. That is, for an anxious individual, heightened cognitive control might actually compensate for behavioral disruptions linked to performance anxiety, whereas for a low anxious individual, up-regulation of cognitive control may recruit too many neural resources, thus interfering with the ability to perform a task efficiently (Dennis & Chen, 2007; Dennis & Chen, 2009). A range of methodologies would help clarify this issue, including tracking whether task performance varies with parametric changes in emotion, and whether these associations vary across individuals.
Another important within- and between-person individual difference is how children modulate cognition across a range of affective conditions and task demands. For example, a child who can only recruit neural resources supporting cognitive control during task-relevant affective contexts may show strengths and vulnerabilities that are quite distinct from those of a child who can also recruit cognitive control during distracting, task-irrelevant contexts. Such questions about individual differences have rarely been posed, and few if any studies have systematically examined between-, within-, and cross-context individual differences in relation to neural markers for emotion regulation.
Individual differences: Affective chronometry
Affective chronometry refers to the dynamics of emotional responding (e.g., latency, peak, and decay of emotional responses). There may be important individual differences not only across individuals, but within individuals in terms of how each emotion unfolds over time and interacts with cognitive processes. For example, a pattern of short latency, moderate intensity, and slow decay of joy may protect individuals from developing mood problems even if they show short latency, high intensity, and slow decay of negative emotions such as fear or sadness. Such affective profiles, either protective or risk inducing, would not exert their influence on emotion regulation in a vacuum. Rather, the impact of affective style is also linked to how these emotional characteristics influence and are influenced by cognitive control, attention, memory, and other cognitive processes. ERPs might be particularly useful for addressing these possible patterns because they capture affective and cognitive processes that occur within a few milliseconds, or that unfold more gradually over time – even in the absence of observable behavior (Banaschewsk & Brandeis, 2007).
Summary
There is growing evidence that the interplay between emotion and cognition may be fundamental to the ability to adaptively regulate emotions. Current research, however, has tended to be constrained by the assumption that emotion and cognition are primarily in opposition and that “cool” cognition must control “hot” affect in order to regulate emotions. A small but growing body of research has begun to examine those conditions under which emotion and cognition are instead closely coordinated, integrated, and complementary, or in which affective processes are regulatory. In this article, I drew upon neuroscience, behavioral, and developmental research to articulate an emotion-cognition integration model for the detection of neurophysiological markers for emotion regulation. This body of research is still preliminary, but has made significant progress in laying out the foundations for future studies identifying biomarkers for emotion regulation and affective psychopathology.
Acknowledgements
Preparation of this article was supported by National Institutes of Health (NIH) Grants 5K01 MH075764, 5T34, and GM007823. This publication was also made possible by Grant RR03037 from the National Center for Research Resources (NCRR), a component of the NIH.
References
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The Anterior Cingulate Cortex: The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- Ayduk O, Mischel W, Downey G. Attentional mechanisms linking rejection to hostile reactivity: The role of "hot" vs. "cool" focus. Psychological Science. 2002;13:443–448. doi: 10.1111/1467-9280.00478. [DOI] [PubMed] [Google Scholar]
- Banaschewsk T, Brandeis D. Annotation: What electrical brain activity tells us about brain function that other techniques cannot tell us--A child psychiatric perspective. Journal of Child Psychology and Psychiatry. 2007;48:415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Barrett KC, Campos JJ. Perspectives on emotional development II: A functionalist approach to emotions. In: Osofsky JD, editor. Handbook of infant development. 2nd ed. Oxford, England: John Wiley & Sons; 1987. pp. 555–578. [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occuring dysphoria. Behaviour Research & Therapy. 1997;35:911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Barch DM. The role of the prefrontal cortex in normal and disordered cognitive control: A cognitive neuroscience perspective. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford: Oxford University Press; 2002. pp. 428–448. [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frotnal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE. Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in the anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: A critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. [Google Scholar]
- Campos JJ, Campos RG, Barrett KC. Emergent themes in the study of emotional development and emotion regulation. Developmental Psychology. 1989;25:394–402. [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998a;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998b;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carver CS. Self-regulation of action and affect. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory, and applications. New York, NY, US: Guilford Press; 2004. pp. 13–39. [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Developmental Psychobiology. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, et al. The role of the anterior cingulate in automatic and controlled processes: A developmental neuroanatomical study. Developmental Psychobiology. 1997a;30:61–69. [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-go task. Journal of Cognitive Neuroscience. 1997b;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Harris RJ, Cofer LF. Posterior brain ERP patterns related to the go/no-go task in children. Psychophysiology. 2004;41:882–892. doi: 10.1111/j.1469-8986.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cole PM, Michel MK, Teti LOD. The development of emotion regulation and dysregulation: A clinical perspective. Monographs of the Society for Research in Child Development. 1994;59:73. [PubMed] [Google Scholar]
- Compton RJ, Carp J, Chaddock L, Fineman SL, Quandt LC, Ratliff JB. Anxiety and error monitoring: Increased error sensitivity or altered expectations? Brain and Cognition. 2007;64:247–256. doi: 10.1016/j.bandc.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Somatic markers and the guidance of behavior: Theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal Lobe Function and Dysfunction. New York: Oxford University Press; 1991. pp. 217–229. [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–330. [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Dennis TA. Emotional self regulation in preschoolers: The interplay of temperamental approach reactivity and control processes. Developmental Psychology. 2006;42:84–97. doi: 10.1037/0012-1649.42.1.84. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Chen C. Neurophysiological mechanisms in the emotional modulation of attention: The balance between threat sensitivity and attentional control. Biological Psychology. 2007;76:1–10. doi: 10.1016/j.biopsycho.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Chen C. Trait anxiety and conflict monitoring following threat: An ERP study. Psychophysiology. 2009;46:122–131. doi: 10.1111/j.1469-8986.2008.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Cole PM, Wiggins C, Zalewski MT. The functional organization of preschool age children's emotion expressions and actions in challenging situations. Emotion. 2009a;9:520–530. doi: 10.1037/a0016514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. Directed interpretations modulate the electrocortical response to arousing negative pictures in children. Special Issue on Emotion Regulation, Risk, and Psychopathology: Journal of Child Psychology and Psychiatry. (in press). [Google Scholar]
- Dennis TA, Malone M, Chen C. Emotional face processing and emotion regulation in children: An ERP study. Developmental Neuropsychology. 2009b;34:1–18. doi: 10.1080/87565640802564887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development & Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Diamond A, Taylor C. Development of an aspect of executive control: Development of the abilities to remember what I said and to 'do as I say, not as I do'. Developmental Psychobiology. 1996;29:315–334. doi: 10.1002/(SICI)1098-2302(199605)29:4<315::AID-DEV2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition & Emotion. 1998;12:353–385. [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. [Google Scholar]
- Eisenberg N, Fabes RA. Emotion, regulation, and the development of social competence. In: Clark MS, editor. Emotion and social behavior. Thousand Oaks, CA: Sage Publications, Inc.; 1992. pp. 119–150. [Google Scholar]
- Enns JT, Brodeur DA, Trick LM. Selective attention over the lifespan: Behavioral measures. In: Richards J, editor. Cognitive neuroscience of attention: A developmental perspective. Manwah, NJ: Lawrence Erlbaum; 1998. pp. 393–418. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography & Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20:977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Frijda NH. The emotions. New York, NY: Cambridge University Press; 1986. [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. Laboratory Temperament Assessment Battery. Eugene: University of Oregon; 1992. [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113:127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: approach-withdrawal states double-dissociate spatial from verbal two-back task performance. Journal Of Experimental Psychology General. 2001;130:436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gray JR. Integration of emotion and cognitive control. Current Directions in Psychological Science. 2004;13:46–48. [Google Scholar]
- Gray JR, Burgess GC. Personality differences in cognitive control? BAS, processing efficiency, and the prefrontal cortex. Journal of Research in Personality. 2004;38:35–36. [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. [Google Scholar]
- Gross JJ, Thompson R. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to Affect: Appraisal Strategies Modulate the Electrocortical Response to Arousing Pictures. Emotion. 2006;6:517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Research. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Henderson H, Schwartz C, Mundy P, Burnette C, Sutton S, Zahka N, et al. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain and Cognition. 2006;61:96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Wachs TD. Temperament theory and the study of cognition-emotion interactions across development. Developmental Review. 2007;27:396–427. [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Izard CE. Emotion feelings stem from evolution and neurobiological development, not from conceptual acts: Corrections for Barrett et al. (2007) Perspectives on Psychological Science. 2007;2:404–405. doi: 10.1111/j.1745-6916.2007.00053.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, et al. Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging. 2001;108:101–110. doi: 10.1016/s0925-4927(01)00117-2. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Winocur G, Graham SJ, Mayberg HS, Hevenor SJ, Grady CL. An fMRI study investigating cognitive modulation of brain regions associated with emotional processing of visual stimuli. Neuropsychologia. 2003;41:585–596. doi: 10.1016/s0028-3932(02)00199-9. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Murray KT. The development of self-regulation in the first four years of life. Child Development. 2001;72:1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Ryan ND, Axelson DA. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006a;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, et al. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. Journal Of Child Psychology And Psychiatry, And Allied Disciplines. 2006b;47:1107–1115. doi: 10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Perlstein WM, Stigge-Kaufman D, Kelly KG, Dotson VM. Affective context-induced modulation of the error-related negativity. Neuroreport: For Rapid Communication of Neuroscience Research. 2006;17:329–333. doi: 10.1097/01.wnr.0000199461.01542.db. [DOI] [PubMed] [Google Scholar]
- Lévesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lewis MD. Bridging emotion theory and neurobiology through dynamic systems modeling. Behavioral and Brain Sciences. 2005;28:169–245. doi: 10.1017/s0140525x0500004x. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Granic I, Lamm C. Behavioral differences in aggressive children linked with neural mechanisms of emotion regulation. Annals of the New York Academy of Sciences. 2006a;1094:164–177. doi: 10.1196/annals.1376.017. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006b;18:430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Self-regulation by the medial frontal cortex: Limbic representation of motive set-points. In: Beauregard M, editor. Consciousness, emotional self-regulation and the brain. Amsterdam, Netherlands: John Benjamins Publishing Company; 2004. pp. 123–161. [Google Scholar]
- Luu P, Tucker DM, Derryberry D. Anxiety and the motivational basis of working memory. Cognitive Therapy and Research. 1998;22:577–594. [Google Scholar]
- Mathews A, Yiend J, Lawrence AD. Individual differences in the modulation of fear-related brain activation by attentional control. Journal of Cognitive Neuroscience. 2004;16:1683–1694. doi: 10.1162/0898929042947810. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. BiologicalPsychology. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology. 1993;102:304–311. doi: 10.1037//0021-843x.102.2.304. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. The problem of the frontal lobe: A reinterpretation. Journal of Psychiatric Research. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nature Reviews. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Sharot T. How (and Why) Emotion Enhances the Subjective Sense of Recollection. Current Directions in Psychological Science. 2008;17:147–152. doi: 10.1111/j.1467-8721.2008.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliska SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: Event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biological Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: The medial frontal cortex and the allocation of processing resources. Journal of Cognitive Neuroscience. 2006;18:1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Pribram KH. A review of theory in physiological psychology. Annual Review of Psychology. 1960;11:1–40. [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley M, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2002;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. Neuroreport: For Rapid Communication of Neuroscience Research. 2003;14:1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain and Cognition. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Seo M, Barrett LF. Being emotional during decision making, good or bad? An empirical investigation. Academy of Management Journal. 2007;50:923–940. doi: 10.5465/amj.2007.26279217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. Monographs of the Society for Research in Child Development. 1994;59:25. [PubMed] [Google Scholar]
- Todd RM, Lewis MD, Meusel L-A, Zelazo PD. The time course of social-emotional processing in early childhood: ERP responses to facial affect and personal familiarity in a go-nogo task. Neuropsychologia. 2008;46:595–613. doi: 10.1016/j.neuropsychologia.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002a;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002b;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Wolfe CD, Bell MA. The integration of cognition and emotion during infancy and early childhood: Regulatory processes associated with the development of working memory. Brain and Cognition. 2007;65:3–13. doi: 10.1016/j.bandc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The Relation of Strength of Stimulus to Rapidity of Habit Formation. Journal of Comparative Neurology & Psychology. 1908;18:459–482. [Google Scholar]
- Yong-Liang G, Robaey P, Karayanidis F, Bourassa M, Pelletier G, Geoffroy G. ERPs and behavioural inhibition in a Go/No-go task in children with attention-deficit hyperactivity disorder. Brain and Cognition. 2000;43:215–220. [PubMed] [Google Scholar]