Abstract
Recent studies have shown that lymphangiogenesis, or the growth of lymphatic vessels, at the periphery of tumors promotes tumor metastasis to lymph nodes. We show here that the fibronectin-binding integrin α4β1 and its ligand fibronectin are novel functional markers of proliferative lymphatic endothelium. Tumors, as well as lymphangiogenic growth factors, such as VEGF-C and VEGF-A, induce lymphatic vessel expression of integrin α4β1. Integrin α4β1 then promotes growth factor and tumor-induced lymphangiogenesis, as genetic loss of integrin α4β1 expression in Tie2Cre+ α4loxp/loxp mice or genetic loss of α4 signaling in α4Y991A knockin mice blocks growth factor and tumor-induced lymphangiogenesis, as well as tumor metastasis to lymph nodes. In addition, antagonists of integrin α4β1 suppress lymphangiogenesis and tumor metastasis. Our studies show that integrin α4β1 and the signals it transduces regulate the adhesion, migration, invasion and survival of proliferating LECs. As suppression of α4β1 expression, signal transduction or function in tumor lymphatic endothelium not only inhibits tumor lymphangiogenesis but also prevents metastatic disease, these results demonstrate that integrin α4β1-mediated tumor lymphangiogenesis promotes metastasis and is a useful target for the suppression of metastatic disease.
Keywords: Lymphangiogenesis, integrin, fibronectin
Introduction
Tumor metastases are a leading cause of cancer-related mortality, and both tumor cell intrinsic and extrinsic factors can promote metastasis (1-3). Metastases can be detected in draining lymph nodes before they are detected in distant organs, and for most tumors, the clinical record suggests that lymph node metastases progress to distant metastases (4). Lymph nodes are thus the initial or frequent sites of metastasis for many tumors, including human pancreatic, gastric, breast, and prostate carcinomas, and melanomas. Tumor lymphangiogenesis, the growth of new lymphatic vessels, has been linked to the formation of lymph node metastases (1-7). Lymphatic capillaries, unlike typical blood capillaries, lack pericytes and a continuous basal lamina. Due to their greater permeability, lymphatic capillaries may be more effective than blood capillaries in allowing passage of tumor cells into and out of vessels. Peritumoral growth of lymphatic vessels has thus been associated with lymphatic metastasis (3, 5-7). Accordingly, increased expression of VEGF-C or VEGF-A, which promotes lymphangiogenesis in primary tumors, correlates closely with increased incidence of regional lymph node and distant metastases in both humans and animals (3, 8-10). Systemic administration of antagonists of the VEGF-C receptor, VEGF-R3, blocks primary tumor lymphangiogenesis and metastasis (11-14).
The recent identification of selective markers of lymphatic endothelial cells (LECs) has allowed identification of mechanisms that regulate lymphangiogenesis. LEC selectively express Lyve-1, a member of the CD44 hyaluronic acid receptor family (15), Prox-1, a lymphatic vessel specific homeobox transcription factor (16) and podoplanin (17). While growth factors and their receptors play critical roles in angiogenesis and lymphangiogenesis, little is known about the roles of the integrin family of cell adhesion proteins in tumor lymphangiogenesis (18). The integrin family of membrane receptors for extracellular matrix (ECM) proteins and immunoglobulin superfamily molecules includes Arg–Gly–Asp (RGD) binding integrin αvβ3, α5β1, αIIbβ3, αvβ6, and α3β1, as well as the Glu-Ile-Leu-Asp-Val (EILDV)-binding integrin α4β1 (19-20). A number of endothelial cell integrins, including α1β1, α2β1, α4β1, α5β1, α6β1, α6β4, α9β1, αvβ3 and αvβ5, have been implicated in the regulation of cell growth, survival and migration during vascular angiogenesis (21). However, little is known about the adhesion mechanisms that regulate pathological lymphangiogenesis, although integrin α9β1 has been shown to promote embryonic development of the lymphatic system (22). In the studies presented here, we found that the fibronectin-binding integrin α4β1 promotes the adhesion and migration of LECs during growth factor and tumor-induced lymphangiogenesis, thereby facilitating tumor metastasis.
Materials and Methods
Reagents
Recombinant human bFGF, VEGF-A and VEGF-C were from R&D Systems (Minneapolis, MN). Rabbit anti-human, rabbit-anti-mouse Lyve-1 antibodies (RDI-102PA50 and RDI-103PA50) and hamster anti-murine podoplanin (103-M40) were from Research Diagnostics Incorporated (Concord, MA). Rat anti-mouse CD31 (MEC 13.3) was from BD Bioscience (San Diego, CA). Rabbit anti-human/mouse podoplanin (D240) was from Biocare Medical LLC (Concord, CA). Murine anti-pan-species Prox-1 (MAB5652, clone 5G10) and anti-human fibronectin (TEV-1) were from Millipore. Goat anti–integrin alpha 4 (sc-6590) was from Santa Cruz Biotechnology. Murine anti-human α4β1 (HP1/2), rat anti-murine α4β1 (PS2) and rat isotype matched control antibody (IgG2b) were gifts from Biogen-Idec (Cambridge, MA and San Diego, CA). Anti-murine VEGFR3 (AFL4) was from eBioscience. Alexa488-conjugated murine anti-pan-cytokeratin (Clone C11) was from Cell Signaling Technology. Donkey anti-goat, rabbit and mouse IgGs conjugated with Alexa Fluors 488, 568 or 647 were from Jackson Immunoresearch. Growth factor depleted Matrigel was from Becton-Dickinson.
Cell Culture
LECs (HMVEC-dLyNeo, Cambrex) were cultured in endothelial growth medium (EGM-2) containing 10% FBS (Cambrex/Lonza). LLC and B16 melanoma cells were obtained from the American Type Culture Collection (ATCC), Panc02 pancreatic ductal carcinoma cells were obtained from the NCI DCTDC Tumor Repository and each was cultured in DMEM containing 10% FBS and antibiotics.
Cell adhesion assays
Adhesion assays were performed by coating non-tissue culture treated plates with 10μg/ml CS-1 fibronectin or rsVCAM overnight at 4°C. Plates were blocked for 2h with 3% heat denatured Bovine Serum Albumin (BSA). LECs were incubated in plates for 15min at 37°C in the presence of 25μg/mL of isotype matched anti-α5β1 (JBS5), anti-avβ3 (LM609), anti-αvβ5 (P1F6) or anti-α4β1 (HP1/2) antibodies. Plates were washed three times, stained with crystal violet, extracted and absorbance at 560nm was measured. Assays were performed three times with triplicate samples per group.
Migration assays
LECs monolayers were scratched using a 20μL pipette tip. Plates were washed and media containing 100ng/mL VEGF-C and function blocking anti-integrin α4β1, anti-α5β1, anti-αvβ3 or anti-αvβ5 antibodies (25μg/mL) were added for 8-24h. “Wound” closure was quantified from digital images using Metamorph imaging software (Version 6.3r5, Molecular Devices). Experiments were performed three times.
Lymphatic endothelial cell tube formation
5 × 104cells hLECs were added to chamber slides containing Matrigel in the presence of 50ng/ml VEGF-C and medium or 25μg/ml of anti-α4β1 (HP1/2), anti-αvβ5 (P1F6), anti-αvβ3 (LM609), anti-α5β1 (JBS5) and plates were incubated at 37°C for 24h. The mean number of vessel branchpoints +/-SEM was determined for triplicate samples. Experiments were performed three times.
Thoracic duct sprouting assay
Thoracic ducts were carefully dissected from mice and cultured as described (23). Ducts were cut into 1 mm-long rings, embedded in type I collagen gels for 4d in DMEM containing 10% FBS and then fixed in 4% paraformaldehyde. Sprouting area was measured using Metamorph imaging software.
Clinical specimen collection
Patients at the Moores UCSD Cancer Center in La Jolla, CA, underwent breast or gastric surgical treatment using standard techniques. Normal tissue was obtained from patients undergoing breast reduction or prophylactic mastectomy. Specimens were reviewed by a pathologist to assess the surgical margin tissue. Tissues not needed for diagnosis were embedded in OCT for cryosectioning: 35 invasive tumors [24 ductal carcinomas (8 stage I, 9 stage II, 5 stage III, 2 stage IV) and 11 lobular carcinomas (6 stage I, 1 stage 2, 4 stage III)], 5 non-invasive cancer and 15 normal mammary glands.
Immunohistochemistry
Lymphatic vessels were detected by immunostaining of cryosections with 2μg/ml anti-human Lyve-1 (RDI-102PA50), anti-murine Lyve-1 (RDI-103PA50), anti-Prox-1 (MAB5652, clone 5G10), anti-murine podoplanin (103M40) or anti-human podoplanin (D240). Integrin α4β1 was detected with 2μg/ml anti-α4β1 (6590). Lymphatic vessels were quantified in 5-10 microscopic fields per cryosection by automated pixel density determination as the mean number of pixels +/- SEM for each treatment group.
The mean number of mice with metastases in inguinal (LLC), brachial/axillary (PyMT), or hilar (Panc02) lymph nodes was determined by immunostaining cryosections of lymph nodes with 5μg/ml Alexa 488 conjugated anti-murine cytokeratin (C11) in three replicate experiments. B16 melanoma metastases were detected by H&E staining of lymph node sections.
Thick cryosections (20μm for Matrigel plugs and 50μm for tumors) were fixed in 1% paraformaldehyde for 1h at 4°C, washed in PBS for 5min, blocked in 0.3% Triton X-100, 0.2% BSA, 5% normal goat serum, 0.1% NaN3 in PBS for 2h RT, and incubated in 5μg/ml primary antibody overnight at 4°C. Sections were washed four times for 1h each at 4°C, then incubated in Alexa 568 or 488 conjugated donkey anti-rat IgG secondary antibody overnight at 4°C. Sections were washed 4 times for 1h RT, postfixed in 1% paraformaldehyde, rinsed in PBS and coverslips mounted.
Transgenic animals
PyMT+ transgenic female mice were derived as previously described (24). FVB and C57Bl6 mice were from Charles River, and C57Bl6 integrin α4Y991A mice were derived as previously described (25). Male Tie2Cre+ mice (26) from Jackson Laboratories were crossed with female integrin α4loxp/loxp mice (27) and Tie2Cre+ α4loxp/+ progeny were then crossed with α4loxp/loxp mice to obtain sibling Tie2Cre-α4loxp/loxp, Tie2Cre+ α4loxp/+ and Tie2Cre+ α4loxp/loxp mice.
Tumor studies
5×105 LLC or B16 cells were injected subcutaneously into wildtype (WT) or integrin α4Y991A mice in a C57Bl6 background (n=10-12). Animals were sacrificed 3 weeks later. Alternatively, WT mice with 7 day old palpable (30 mm3) tumors were treated by intraperitoneal injections of 200μg/25g body weight of function-blocking anti-integrin α4 (n=10), isotype matched control rat IgG1 (n=10) or saline (n=10) every third day for 2 weeks. Lymphatic vessels and metastases were quantified in tumors and lymph nodes, respectively, in three replicate experiments.
To study orthotopic pancreatic carcinomas, the abdominal cavities of mice (n=10) were opened, and the tails of the pancreas were exteriorized. One million syngeneic Panc02 cells were injected into the pancreatic tail, the pancreas was placed back into the abdominal cavity and the incision was closed. Tumors and hilar lymph nodes were excised after 30d. Experiments were performed three times.
Lymphangiogenesis assays
400μl of ice cold Matrigel containing saline or 400ng of VEGF-C, VEGF-A or bFGF was injected into WT (n=10), α4Y991A (n = 10), Tie2Cre+ α4loxp/loxp (n=4), Tie2Cre+ α4loxp/+ (n=2) or Tie2Cre-α4loxp/loxp (n=4) mice for 10d. In other studies, WT mice were treated by intraperitoneal injection with 200μg/mouse of function-blocking anti-α4β1 (PS2), anti-VEGFR3 (AFL4), PS2 plus AFL4, rsVCAM or isotype control anti-α5β1 antibodies on d1, 3 and 6 (n=10). After 10d, Matrigel plugs were removed, and 5μm sections were immunostained with anti-Lyve-1 antibodies. At least 5 fields/section were analyzed. Experiments were performed at least 3 times.
Statistical analysis
All statistical analyses were performed with a two-tailed Student's t-test or ANOVA.
Results
Integrin α4β1 and fibronectin are markers of tumor lymphatic endothelium
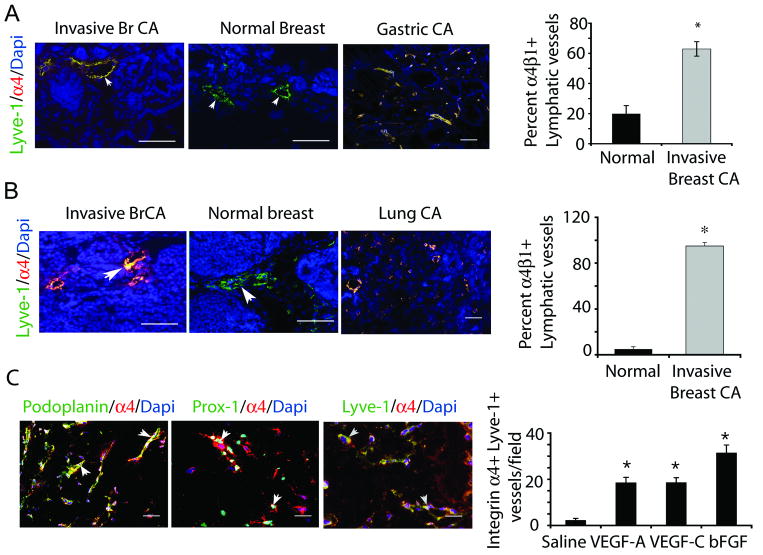
To identify cell adhesion proteins that regulate tumor lymphangiogenesis, we evaluated the expression of several integrins on lymphatic vessels in normal and tumor tissues, including integrin α4β1, α5β1, αvβ3 and αvβ5. These integrins were selected for their well-defined roles in angiogenesis and wound healing (18). We found that only integrin 〈4β®1 a cell surface receptor for fibronectin and VCAM-1, vascular cell adhesion molecule (28-29), was highly upregulated on tumor lymphatic vessels (Figure 1, Supplementary Figure 1-2). Cryosections of breast tissue from patients with or without invasive carcinomas and from patients with gastric tumors were immunostained with antibodies to integrin α4β1 and Lyve-1, podoplanin or Prox-1, well-established markers of lymphatic endothelium (15,17). We found that integrin α4β1 (red) was strongly expressed on Lyve-1, Prox-1 and podoplanin+ lymphatic vessels (green) in mammary tumors but not in normal mammary glands (Figure 1A-B; Supplementary Figures 1-3). Integrin α4β1 was also expressed on lymphatic endothelium in human gastric tumors (Figure 1A). Importantly, expression of integrin α4β1 on lymphatic endothelium indicated the presence of invasive ductal or lobular tumors as more than 60% of tumor lymphatic vessels were integrin positive, while less than 20% of normal lymphatic vessels were integrin positive (Figure 1A).
Figure 1. Integrin α4β1 and fibronectin are markers of proliferative lymphatic endothelium in human and murine tumors.
(A) Left, integrin α4β1 (red), Lyve1 (green) and DAPI (blue) immunostaining of human invasive breast ductal carcinoma, normal breast and gastric tumors. Right, percent integrin α4+ lymphatic vessels +/- SEM per 100× field in mammary tissue from A (n=15 normal, 35 invasive, *p<0.001). (B) Left, integrin α4β1 (red), Lyve1 (green) and DAPI (blue) immunostaining of mammary glands from PyMT- (normal) and PyMT+ (invasive breast carcinoma) mice, and LLC tumors. Right, percent +/- SEM integrin α4+ lymphatic vessels per 100× microscopic field in PyMT- and PyMT+ mammary tissue (n=10, *p<0.001). (C) Left, immunostaining of VEGF-C treated tissue for Lyve-1, podoplanin or Prox-1 (green), integrin α4β1 (red), and DAPI (blue). Right, mean number of integrin α4+ lymphatic vessels/100× microscopic field in saline, bFGF, VEGF-A or VEGF-C saturated Matrigel (n=10, *p<0.001). Scale bars, 50μm.
Integrin α4β1 was also strongly expressed on lymphatic endothelium in tumors from mice with PyMT+ spontaneous breast tumors and was not expressed in normal breast tissue (Figure 1C, Supplementary Figure 2). This integrin was also expressed on lymphatic vessels in Lewis lung carcinoma tumors (Figure 1C). Expression of integrin α4β1 on lymphatic endothelium in murine mammary glands correlated with the presence of invasive ductal tumors, as 100% of lymphatic vessels in murine breast tumors were integrin α4β1 positive while only 5% of lymphatic vessels in normal mouse breast tissue expressed this integrin (Figure 1C).
To determine whether integrin α4β1 expression is induced by purified lymphangiogenic factors, we stimulated lymphangiogenesis in mice by implanting VEGF-C saturated Matrigel plugs and immunostained cryosections of these tissues to detect integrin α4β1 and three independent markers of lymphatic vessels: podoplanin, Lyve-1 and Prox-1 (15-17). Integrin α4β1 expression co-localized extensively with each of these three distinct markers of lymphatic vessels (Figure 1C, left). These results confirm that integrin α4β1 is a marker of proliferative lymphatic vessels. We next investigated which lymphangiogenic factors can induce integrin α4β1 expression in lymphatic vessels. VEGF-C, VEGF-A and bFGF strongly induced α4β1 expression on lymphatic vessels during lymphangiogenesis in vivo (Figure 1C, right). In addition, we found that integrin α4β1 is strongly expressed on the surface of 90% of cultured LECs (Supplementary Figure 3). Thus, integrin α4β1 is a marker of proliferating lymphatic vessels.
To determine whether LECs also express integrin α4β1 ligands, we immunostained cultured LECs with antibodies directed against the α4β1 ligands fibronectin or VCAM-1. We observed fibronectin expression in cultured LECs by Western blotting and in lymphatic vessels in vivo by immunostaining of PyMT+ spontaneous breast tumors (Supplementary Figure 3). In contrast, we did not observe VCAM-1 expression in LECs by FACs analysis (Supplementary Figure 3) or by Western blotting (not shown). These results demonstrate that integrin α4β1 and its ligand fibronectin are novel markers of proliferative lymphatic endothelium in vitro and in invasive tumors in vivo.
Lymphangiogenesis depends on integrin α4β1 signal transduction
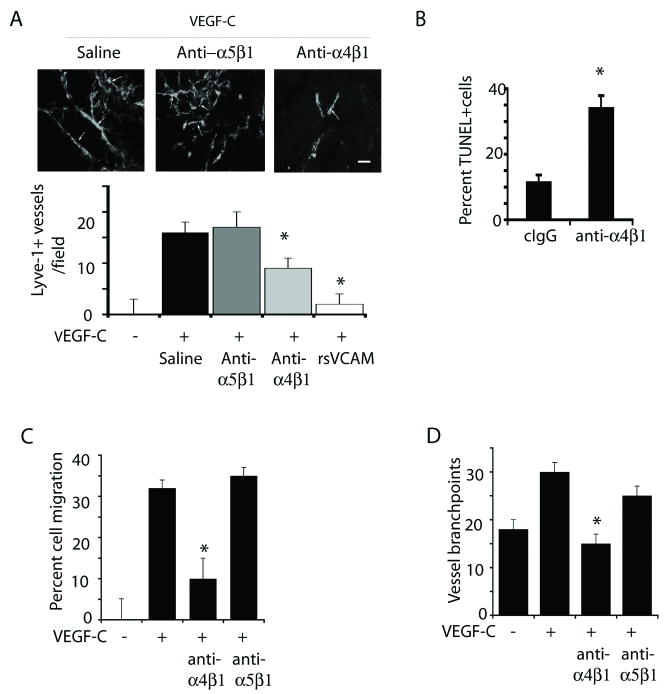
To determine whether integrin α4β1 regulates lymphangiogenesis, we subcutaneously implanted mice with Matrigel saturated with saline, VEGF-C or VEGF-A and treated animals with antagonists of murine integrin α4β1 including function-blocking anti-α4β1 antibodies and recombinant soluble VCAM, and with isotype-matched control antibodies (Figure 2A). While VEGF-C and –A stimulated the growth of new lymphatic vessels in Matrigel, both integrin α4β1 antagonists, but not control antibodies, strongly suppressed lymphangiogenesis in VEGF-C (Figure 2A) and VEGF-A (Supplementary Figure 4) stimulated animals. Notably, antibody antagonists of α4β1 induced apoptosis of LECs in vivo, as detected by TUNEL staining of Lyve-1+ vessels in Matrigel plugs (Figure 2B, Supplementary Figure 5).
Figure 2. Inhibition of integrin α4β1 function blocks lymphatic endothelial cell migration and lymphangiogenesis.
(A) Images and quantification of Lyve-1+ lymphatic vessels/field +/- SEM in VEGF-C saturated Matrigel from saline, anti-α4β1, anti-α5β1 or recombinant soluble VCAM-treated mice from these mice (n=10, *p<0.002). (B) Mean percent TUNEL+Lyve1+ vessels/field +/- SEM, *p < 0.0002, in Matrigel from A. (C) VEGF-C stimulated LEC migration in the presence of medium, anti-α4β1 or anti-α5β1 antibodies (cIgG) (*p < 0.01). (D) LEC in vitro “vessel” formation in the presence of anti-α4β1 or isotype matched control (anti-α5β1) antibodies; mean vessel branchpoints per 100× field +/-SEM (*p < 0.0001)
We also found that integrin α4β1 promotes adhesion and migration of LECs on cellular (CS-1) fibronectin. LECs adhered to CS-1 fibronectin, and antibody antagonists of integrin α4β1 but not antibodies to other integrins, including anti-αvβ3 (Supplementary Figure 5), anti-αvβ5 and anti-α5β1 (not shown), blocked LEC adhesion on CS-1 fibronectin-coated plates. We also observed that VEGF-C stimulated LEC migration, which was blocked by antagonists of integrin α4β1 but not by antibody antagonists of other integrins, including anti-α5β1 (Figure 2C, Supplementary Figure 5). VEGF-C also stimulated LEC invasion and vessel (“tube”) formation in three-dimensional matrices in vitro, which was blocked by antibody antagonists of integrin α4β1 but not by isotype matched antagonists of α5β1 (Figure 2D, Supplementary Figure 5), αvβ3 or αvβ5. Together, these results suggest that integrin α4β1 promotes LEC invasion during lymphangiogenesis in vivo.
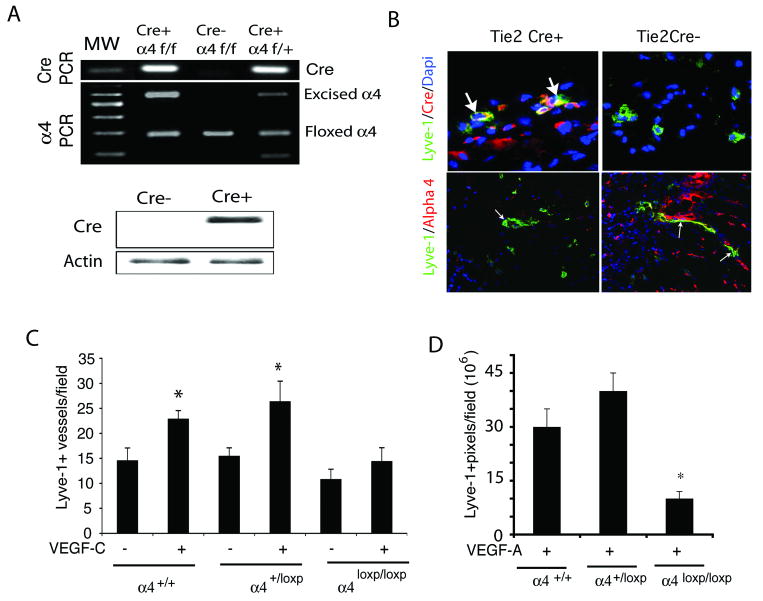
To explore the role of α4β1 in lymphangiogenesis further, we examined lymphangiogenesis in mice deficient for expression of integrin α4β1 in LECs using an in vivo Matrigel plug lymphangiogenesis assay. As integrin α4-/- mice die at E11.5 due to cardiac malformations (30), we examined lymphangiogenesis in a tissue-specific integrin deletion mutant, the Tie2Cre α4loxp/loxp mouse. We crossed Tie2Cre mice, which express Cre under the influence of the Tie2 promoter in endothelial cells, with integrin α4loxp/loxp mice (27). Cre+ α4loxp/loxp mice were identified by genomic PCR from tail DNA for Cre and integrin α4 alleles (Figure 3A, upper). Cre expression in Tie2Cre+ α4loxp/loxp mice in vivo was detected by Western blotting of lung tissue lysates from Tie2Cre+ α4loxp/loxp and Tie2Cre-α4loxp/loxp mice (Figure 3A, lower). Cre expression was also demonstrated in LECs in vivo by immunostaining of VEGF-C saturated Matrigel plugs to detect Cre and Lyve-1 (Figure 3B, upper). Integrin α4 expression on Lyve-1+ lymphatic vessels was reduced in Tie2Cre+ α4loxp/loxp animals compared to that of Tie2Cre-animals (Figure 3B, lower). To explore the role of integrin α4β1 in lymphangiogenesis, we stimulated Tie2Cre+ α4loxp/+, Tie2Cre-α4loxp/loxp and Tie2Cre+ α4loxp/loxp littermates by implanting mice with Matrigel containing saline, VEGF-C (Figure 3C) or VEGF-A (Figure 3D). We found that lymphangiogenesis was induced in Tie2Cre+ α4loxp/+ and Tie2Cre-α4loxp/loxp mice, but not in Tie2Cre+ α4loxp/loxp mice. Together, these results indicate that integrin α4β1 expression is required for lymphangiogenesis.
Figure 3. Inhibition of VEGF-C lymphangiogenesis in integrin α4 mutant animals.
(A) Upper, Genomic PCR analysis of Tie2Cre(+)α4loxp/loxp, Tie2Cre(+)α4loxp/+ and Tie2Cre(-) α4loxp/loxp mice for Cre-recombinase (100bp), intact integrin α4 (180bp), floxed α4 (280bp) and excised α4 (600bp). Lower, Western blotting of Cre-recombinase (38 kD) and beta-actin (42kD) in lung lysates from Tie2Cre(+)α4loxp/loxp and Tie2Cre(-) α4loxp/loxp mice. (B) Upper, Cryosections of VEGF-C saturated Matrigel plugs in Tie2Cre(+)α4loxp/loxp and Tie2Cre (-) α4loxp/loxp mice immunostained to detect Cre (red) and Lyve-1+ expression (green) and counterstained with DAPI (blue). Cre+ vessels are indicated by arrows. Lower, Cryosections of VEGF-C saturated Matrigel plugs in Tie2Cre(+) and Tie2Cre (-) mice immunostained to detect integrin α4 (red) and Lyve-1 (green) positive vessels (arrows). (C-D) Mean Lyve1+ lymphatic vessels/field +/- SEM in Matrigel plugs from (C) VEGF-C or (D) VEGF-A stimulated Tie2Cre(+)α4loxp/loxp, Tie2Cre(+)α4loxp/+ and Tie2Cre(-) α4loxp/loxp mice (*p<0.007).
To determine how integrin α4β1 regulates lymphangiogenesis, we evaluated the interactions of VEGF-C and α4β1 in vitro. VEGF-C strongly stimulated integrin α4β1 mediated LEC adhesion and promoted integrin α4β1 association with the adaptor protein paxillin within focal adhesions at the leading edges of cells, as determined by immunocytochemistry and co-immunoprecipitation studies (Figure 4A, Supplementary Figure 6). As VEGF-C promotes integrin α4β1 expression in vivo (Figure 1) and activity in vitro (Figure 4A), it is likely that VEGF-C and integrin α4β1 function in the same molecular pathway. Importantly, while antagonists of integrin α4β1 and VEGF-R3 both substantially inhibit lymphangiogenesis, their effects are neither additive nor synergistic, suggesting that these two molecules function in the same molecular pathway (Supplementary Figure 7).
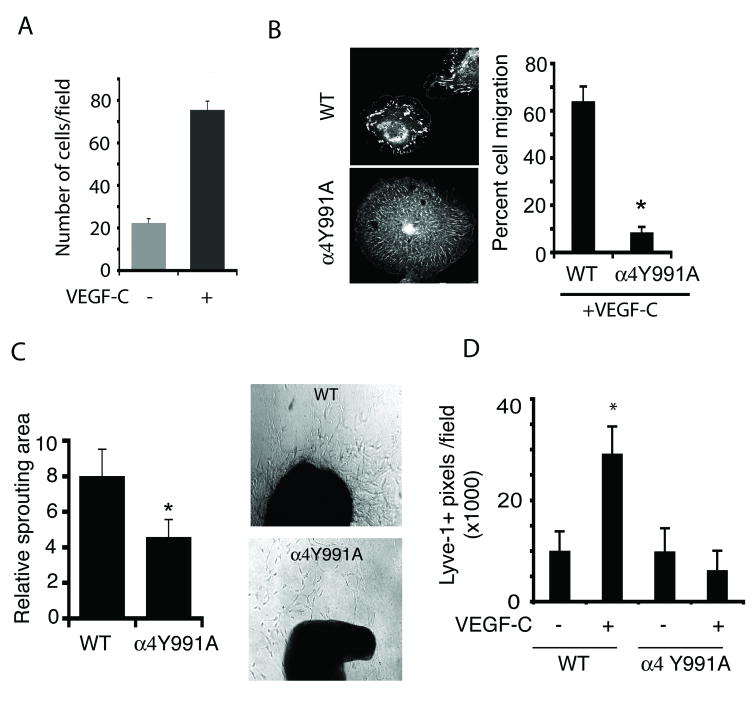
Figure 4. Integrin α4Y991A mutation suppresses LEC invasion and lymphangiogenesis.
(A) Left, LEC adhesion to rsVCAM in the absence (gray) or presence (black) of 300ng/ml VEGF-C. (B) Images, Paxillin localization in VEGF-C stimulated WT and α4Y991A LECs. Graph, VEGF-C stimulated WT and α4Y991A LEC cell migration. (C). Left, mean area of microvessel sprouting from WT or α4Y991A thoracic duct explants (n=6), *p < 0.04. Right, brightfield images of explants. (D) Mean Lyve1+ pixels/field+/-SEM in saline and VEGF-C saturated Matrigel implanted in WT and α4Y991A mice, *p<0.001.
To explore the importance of integrin α4β1 signaling in LECs, we isolated LECs from mice with an integrin α4Y991A knockin mutation (25). This mutation in the cytoplasmic tail of integrin α4β1 disrupts integrin α4β1-mediated association with paxillin and talin (31-33), and blocks α4β1 mediated leukocyte adhesion (33). Although LECs isolated from WT and integrin α4Y991A mice expressed similar levels of integrin α4β1 (Supplementary Figure 6), LECs from α4Y991A mice did not polarize or develop mature paxillin-containing focal adhesions when adhering to CS-1 fibronectin (Figure 4B, left). Importantly, α4Y991A LECs failed to migrate in response to VEGF-C (Figure 4B, right). Additionally, VEGF-C stimulated ex vivo lymphatic vessel sprouting from isolated thoracic ducts (large lymphatic vessels) when isolated from WT but not α4Y991A animals (Figure 4C; Supplementary Figure 6). Finally, VEGF-C stimulated lymphangiogenesis in Matrigel plugs in vivo was completely inhibited in integrin α4Y991A mice (Figure 4D). In fact, integrin α4β1 association with paxillin was suppressed in VEGF-C containing Matrigel plugs from α4Y991A mice and few α4+paxillin+ vessels with well-formed lumen were observed in mutant mice (Supplementary Figure 6). These results indicate that integrin α4β1 expression and signal transduction are required for LEC migration and invasive responses to lymphangiogenesis factors in vitro and during in vivo lymphangiogenesis.
Integrin α4β1 promotes tumor lymphatic metastasis
As integrin α4β1 promotes growth factor-induced lymphangiogenesis in vivo, we asked whether integrin α4β1 could promote tumor lymphangiogenesis and subsequent metastasis to lymph nodes. To test this possibility, we implanted integrin α4-negative Lewis lung carcinoma (LLC) or B16 melanoma cells subcutaneously into syngeneic mice and treated them with intravascular injections of saline, function-blocking anti-α4β1 or isotype matched control antibodies. Tumors and draining inguinal lymph nodes were removed after twenty-one days and analyzed for tumor lymphangiogenesis and metastasis to lymph nodes. We found that antagonists of integrin α4β1 significantly suppressed lymphangiogenesis (Figure 5A) and metastasis (Figure 5B) in LLC and B16 melanoma tumors, as analyzed by cytokeratin immunostaining (LLC), H&E staining or macroscopic analysis (B16) (Supplementary Figure 8). These studies indicate that integrin α4β1 may play a key role in promoting lymphangiogenesis and thereby tumor metastasis to lymph nodes. These studies also suggest that integrin α4β1 antagonists maybe useful in suppressing lymphatic metastasis by inhibiting tumor lymphangiogenesis.
Figure 5. Inhibition of tumor lymphangiogenesis and tumor metastasis in integrin α4 mutant animals.
(A) Mean Lyve-1+ pixels/field +/- SEM (n=10, *P<0.03) in tumors from mice with LLC or B16 melanoma tumors that were treated with saline, anti-α4β1 and isotype-matched control antibodies. (B) Average percentage of mice +/- SEM with lymph node metastases from A (n=10 per each of 3 studies, *p<0.03). (C) Average number of Lyve-1+ pixels/100× field in LLC or Panc02 tumors grown in WT and α4Y991A mice (n=10, *p<0.05). (D) Mean percent mice with lymph node metastases +/- SEM from C (n=10, *p<0.05).
To determine whether integrin signaling plays a role in tumor lymphangiogenesis and metastasis in vivo, LLC cells were subcutaneously implanted into wild type and integrin α4Y991A mice. Three weeks later, tumors and draining inguinal lymph nodes were removed and analyzed in thick (50μm) and thin (5μm) sections for tumor lymphangiogenesis and metastasis to lymph nodes. We found that tumor-induced lymphangiogenesis was substantially suppressed in α4Y991A mice (Figure 5C, Supplementary Figure 8). Similar reductions in lymphangiogenesis were observed when Panc02 pancreatic carcinoma tumor cells were implanted orthotopically in the pancreas of α4Y991A mutant mice (Figure 5C, Supplementary Figure 8). Importantly, cytokeratin positive tumor metastasis to draining lymph nodes was also suppressed in integrin α4Y991A mutant mice (Figure 5D, Supplementary Figure 8). Additionally, spontaneous metastases of Panc02 cells to other organs were also reduced in α4Y991A mice (Supplementary Figure 9). These studies indicate that integrin α4β1 signaling plays an important role in promoting lymphangiogenesis and thereby tumor metastasis to lymph nodes and other tissues. Taken together, these results indicate that integrin α4β1-mediated tumor lymphangiogenesis is associated with tumor metastasis.
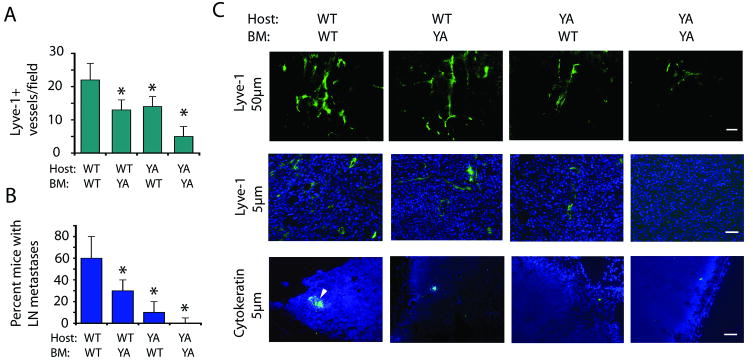
Our studies demonstrate that integrin α4β1 promotes LEC migration, sprouting and vessel formation in vitro as well as growth factor induced lymphangiogenesis and survival in vivo. These results strongly suggest that integrin α4β1 promotes tumor-induced lymphangiogenesis. However, as integrin α4β1 is also expressed on vascular endothelial cells (34) and immune cells (35), which have been shown to promote angiogenesis, lymphangiogenesis, tumor growth and metastasis by expressing pro-angiogenic factors (36), it is not absolutely clear whether integrin α4β1 on LECs promotes tumor lymphangiogenesis and lymphatic metastasis. To decipher the relative roles of vascular, LEC and hematopoietic cell integrin α4β1 in lymphangiogenesis, we examined tumor growth, angiogenesis, lymphangiogenesis, inflammation and metastasis in animals transplanted with bone marrow transplanted from WT and α4Y991A mice (Figure 6, Supplementary Figure 10). Integrin α4Y991A mice were transplanted with WT or α4Y991A bone marrow, while WT mice were transplanted with α4Y991A or WT bone marrow and LLC cells were subsequently implanted. Tumor lymphangiogenesis and metastasis were suppressed by 50% and 80%, respectively, in α4Y991A mice with WT bone marrow, indicating that lymphangiogenesis depends significantly on host integrin α4β1 (Figure 6A-C). However, angiogenesis and tumor growth were not affected in α4Y991A mice with WT BM, indicating that host endothelial cells do not require integrin α4β1 function (Supplementary Figure 10). We did find that lymphangiogenesis and metastasis (as well as angiogenesis and tumor growth) were suppressed by 50% in WT mice with α4Y991A bone marrow (Figure 6, Supplementary Figure 10), indicating that lymphangiogenesis results from the combined effects of bone marrow and host endothelial cell contributions. Importantly, our studies indicate that LEC rather than vascular cell integrin α4 promotes tumor lymphangiogenesis, as tumor angiogenesis and growth are not reduced in α4Y991A hosts yet are reduced in animals with α4Y991A bone marrow. Therefore, our combined results indicate that LEC integrin α4β1 promotes tumor lymphangiogenesis and metastasis, while hematopoietic cell integrin α4β1 may contribute to lymphangiogenesis and metastasis by promoting tumor angiogenesis and growth. Taken together, our in vitro and in vivo studies indicate that LEC integrin α4β1 plays an essential role in promoting tumor lymphangiogenesis and metastasis.
Figure 6. Host contributions of α4β1 integrin to tumor lymphangiogenesis and metastasis.
(A-C) LLC tumors were implanted in integrin α4Y991A mice transplanted with WT or α4Y991A bone marrow and in WT mice transplanted with α4Y991A or WT bone marrow. (A) Mean Lyve-1+ vessels/ field+/- SEM (n=10, *p<0.05). (B) Average percent mice with lymph node metastases +/- SEM (n=10, *p<0.05). (C) Lyve-1 immunostaining of 20μm tumor cryosections; Lyve-1/DAPI immunostaining of 5μm tumor sections; and cytokeratin (green)/DAPI (blue) immunostaining of 5μm lymph node cryosections from A-B. Scale bars indicate 50μm.
Discussion
Recent studies have shown that lymphangiogenesis develops in primary tumors or in the peritumoral space and promotes lymphatic metastasis, as expression of VEGF-A or -C stimulates tumor lymphangiogenesis and metastasis (3, 5-10), while antagonists of VEGF-C or VEGF-R3 suppress these events (11-14). The studies presented here indicate that the LEC integrin α4β1 plays a critical role in tumor lymphangiogenesis and metastasis by promoting LEC migration and survival in vivo.
Our studies show that integrin α4β1, rather than integrin α5β1, αvβ5 and αvβ3, is expressed on lymphatic endothelium in spontaneous and experimental tumors and in response to purified lymphangiogenic growth factors. While other integrins may also participate in the regulation of tumor lymphangiogenesis, limited information is available about which integrins can regulate this process. Integrin α9β1 promotes developmental lymphangiogenesis as integrin alpha 9 null mice exhibit chylothorax, an accumulation of milk in the abdomen of newborn mice which results from improperly functioning lymphatic vessels (22,37), while antagonists of integrin α5β1 blocked inflammatory lymphangiogenesis in the eye and trachea (38-39). However, little is known about roles that these integrins may play in tumor lymphangiogenesis.
Four lines of evidence indicate that LEC integrin α4β1 plays a direct role in regulating lymphangiogenesis. First, integrin α4β1 is poorly expressed in normal lymphatic vessels, but is upregulated during lymphangiogenesis in vivo. Second, antagonists of integrin α4β1 suppress VEGF-C and tumor-induced lymphangiogenesis, as well as tumor metastasis to lymph nodes. Third, lymphangiogenesis is suppressed in Tie2Creα4loxp/loxp animals, which are defective in endothelial cell expression of integrin α4 and in α4Y991A animals, which exhibit defective LEC integrin α4 migration and invasion. Additionally, bone marrow transplant studies confirm that host integrin α4 is required for tumor lymphangiogenesis and metastasis but not as important for tumor angiogenesis and growth.
Numerous studies have indicated that tumor lymphangiogenesis promotes lymphatic metastases by providing a direct conduit for tumor cell escape to nearby draining lymph nodes (3, 5-10). These studies also indicate that breast, prostate, pancreatic and melanoma metastases to distant organs generally arise indirectly from lymphatic metastasis, as prophylactic removal of lymph nodes can prevent widespread disease (4). Although Wong et al. found that knockdown of VEGF-C expression in tumor cells can suppresses tumor lymphangiogenesis without affecting metastasis to lymph nodes (40), other studies indicate that VEGF-C increases delivery of tumor cells to lymph nodes via the lymphatics (41). While it is possible that in some tumor systems, de novo lymphangiogenesis is not required for tumor metastasis, most studies clearly show that tumor lymphangiogenesis does help promote tumor metastasis.
It is yet not clear whether integrin α4β1 also plays a role in the development of the lymphatic system. Integrin α4 null mice die before lymphatic vessels are established (30). Tie2Cre+α4loxp/loxp mutant animals exhibit no defects in development, but these mice also exhibited mosaic Cre expression in endothelial cells. Integrin α4Y991A mice also exhibit no developmental defects. As integrin α9β1 and α4β1 both bind to CS-1 fibronectin and VCAM-1, it is possible that integrin α9β1 can compensate for the loss of α4 during lymphatic development and may play a role in tumor lymphangiogenesis. Future studies will clarify the relative roles of these two integrins during developmental and tumor lymphangiogenesis. In conclusion, our studies demonstrate the important role of the integrin α4β1 in lymphangiogenesis and suggest that antagonists of this integrin may be useful in the clinical setting to suppress the spread of tumors through the lymphatic system.
Supplementary Material
Acknowledgments
We thank Xiaodan Song and Hongjun Peng for excellent technical assistance. All animal studies were approved by the University of California, San Diego Institutional Animal Care and Use Committee and human tissue studies were approved by the UCSD Institutional Review Board. These studies used the Moores UCSD Cancer Center Flow Cytometry and Histology Shared Resources.
This work was supported by NIH grants CA83133 (JAV), CA126820 (JAV), AR27214 (MHG), NIH CA118182 (LGE), DOD W81XWH-06-1-052 (SLB), NIH CA119335-03 (SLB), and postdoctoral fellowships from California Breast Cancer Research Program (BGS), NIH Kirschstein-NRSA (CJA), Pancreatic Action Network-AACR (PF) and California Tobacco Related Disease Research Program (MCS).
References
- 1.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–8. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 4.Leong SP, Cady B, Jablons DM, et al. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25:221–32. doi: 10.1007/s10555-006-8502-8. [DOI] [PubMed] [Google Scholar]
- 5.Roma AA, Magi-Galluzzi C, Kral MA, Jin TT, Klein EA, Zhou M. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod Pathol. 2006;19:392–398. doi: 10.1038/modpathol.3800546. [DOI] [PubMed] [Google Scholar]
- 6.Massi D, Puig S, Franchi A, et al. Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: a case-control study. J Clin Path. 2006;59:166–73. doi: 10.1136/jcp.2005.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadras SS, Lange-Asschenfeldt B, Velasco P, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 8.Bando H, Weich HA, Horiguchi S, Funata N, Ogawa T, Toi M. The association between vascular endothelial growth factor-C, its corresponding receptor, VEGFR-3, and prognosis in primary breast cancer: A study with 193 cases. Oncol Rep. 2006;15:653–59. [PubMed] [Google Scholar]
- 9.Jennbacken K, Vallbo C, Wang W, Damber JE. Expression of vascular endothelial growth factor C (VEGF-C) and VEGF receptor-3 in human prostate cancer is associated with regional lymph node metastasis. Prostate. 2005;65:110–16. doi: 10.1002/pros.20276. [DOI] [PubMed] [Google Scholar]
- 10.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–99. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Varney ML, Backora MW, et al. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–11. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Rajantie I, Pajusola K, et al. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–46. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Lalani AS, Harding TC, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–9. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 14.Roberts N, Kloos B, Cassella M, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–7. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 15.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 17.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–94. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–88. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 20.Komoriya A, Green LJ, Mervic M, et al. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem. 1991;266:15075–79. [PubMed] [Google Scholar]
- 21.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–65. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang XZ, Wu JF, Ferrando R, et al. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–15. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruyère F, Melen-Lamalle L, Blacher S, et al. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods. 2008;5:431–7. doi: 10.1038/nmeth.1205. [DOI] [PubMed] [Google Scholar]
- 24.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Féral CC, Rose DM, Han J, et al. Blocking the alpha 4 integrin-paxillin interaction selectively impairs mononuclear leukocyte recruitment to an inflammatory site. J Clin Invest. 2006;116:715–23. doi: 10.1172/JCI26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–42. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 27.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–60. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- 29.Elices MJ, Osborn L, Takada Y, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–84. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 30.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–60. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Kiosses WB, Rose DM, et al. A fragment of paxillin binds the alpha 4 integrin cytoplasmic domain (tail) and selectively inhibits alpha 4-mediated cell migration. J Biol Chem. 2002;277:20887–94. doi: 10.1074/jbc.M110928200. [DOI] [PubMed] [Google Scholar]
- 32.Alon R, Feigelson SW, Manevich E, et al. Alpha4beta1-dependent adhesion strengthening under mechanical strain is regulated by paxillin association with the alpha4-cytoplasmic domain. J Cell Biol. 2005;171:1073–84. doi: 10.1083/jcb.200503155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manevich E, Grabovsky V, Feigelson SW, Alon R. Talin 1 and paxillin facilitate distinct steps in rapid VLA-4-mediated adhesion strengthening to vascular cell adhesion molecule 1. J Biol Chem. 2007;282:25338–48. doi: 10.1074/jbc.M700089200. [DOI] [PubMed] [Google Scholar]
- 34.Garmy-Susini B, Jin H, Zhu Y, Sung RJ, Hwang R, Varner J. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–51. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem. 2000;275:23409–12. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- 36.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazigou E, Xie S, Chen C, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–86. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietrich T, Onderka J, Bock F, et al. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171:361–72. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okazaki T, Ni A, Ayeni OA, et al. Alpha5beta1 Integrin blockade inhibits lymphangiogenesis in airway inflammation. Am J Pathol. 2009;174:2378–87. doi: 10.2353/ajpath.2009.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–98. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- 41.Hoshida T, Isaka N, Hagendoorn J, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–75. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.