Abstract
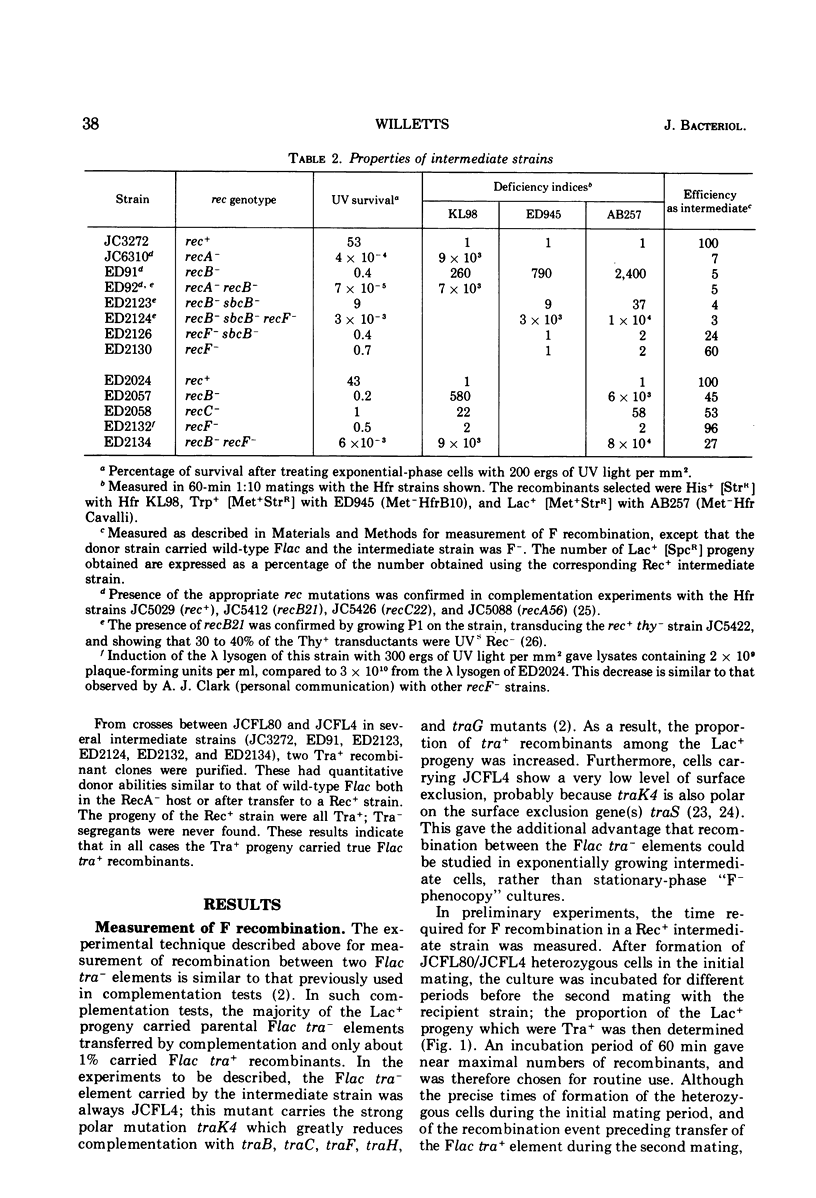
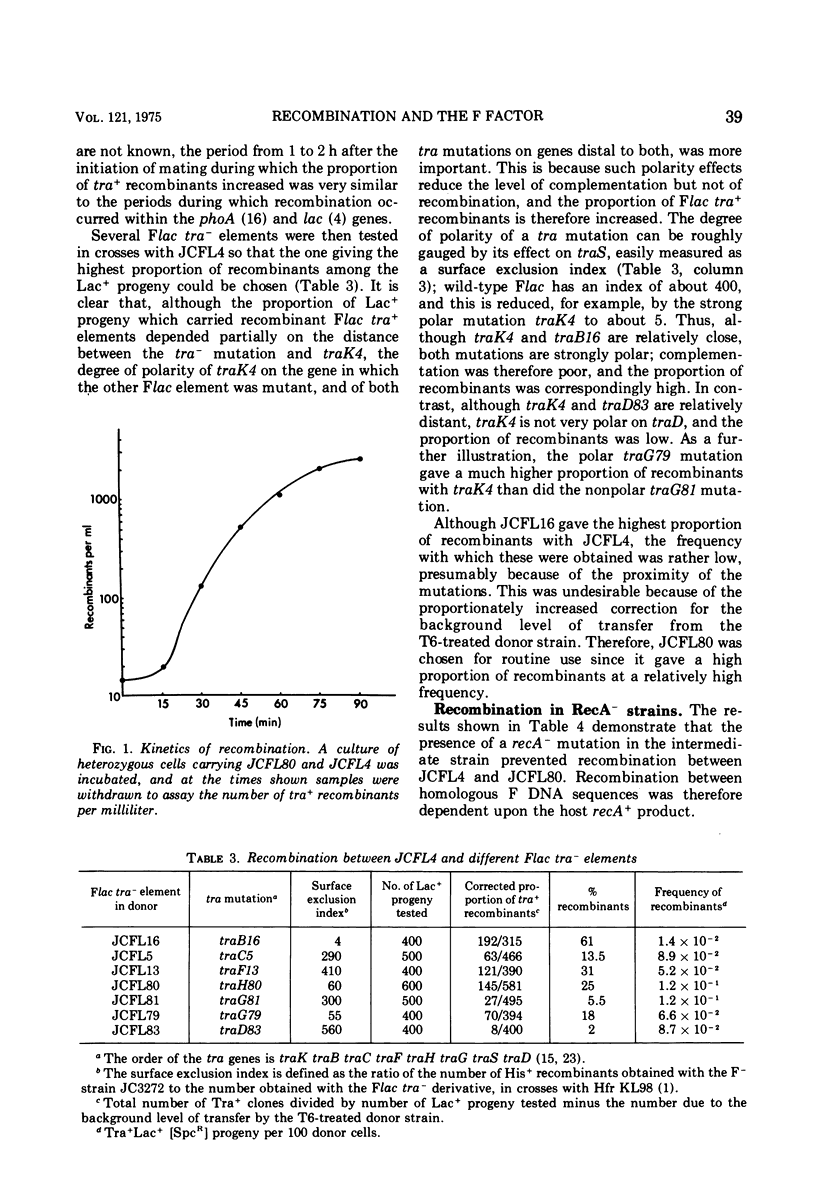
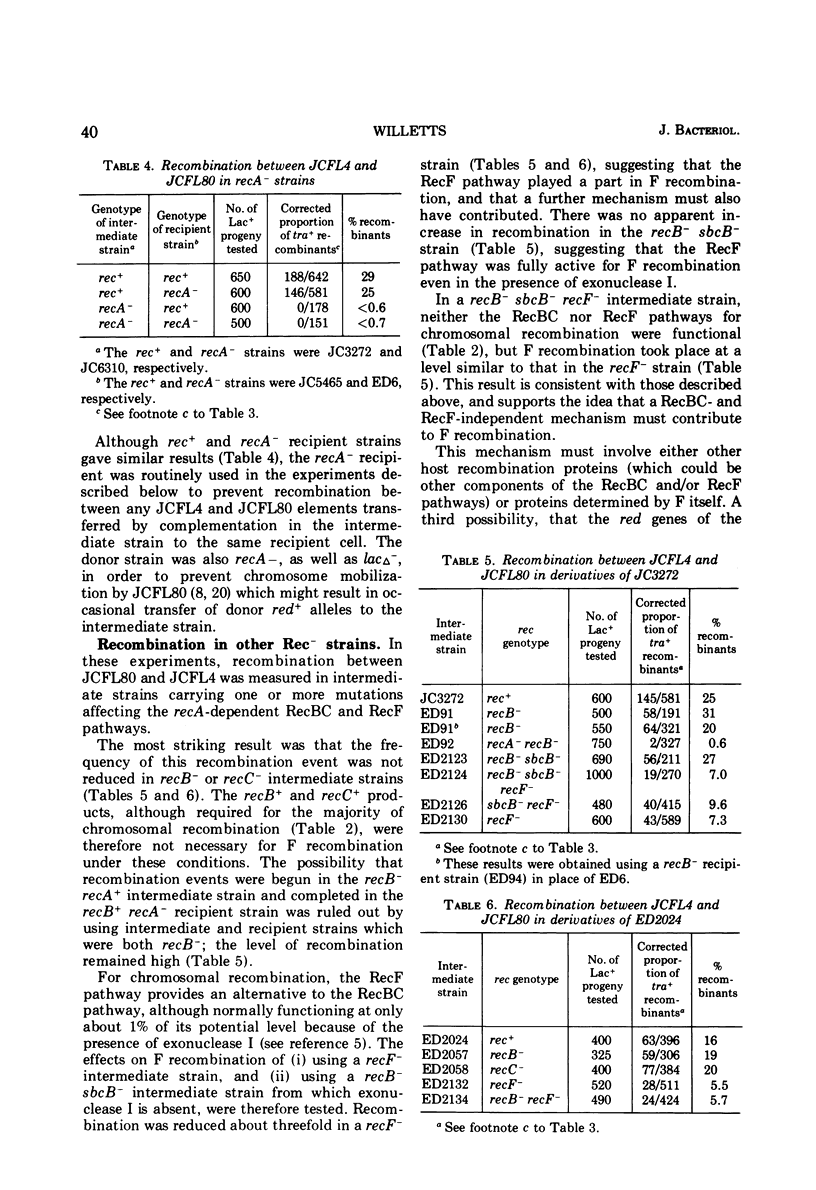
Recombination between two Flac tra minus elements to give Flac tra plus recombinants was measured in Rec plus and Rec minus strains of Escherichia coli K-12. Polar tra mutations were used to increase the proportion of tra plus recombinants among the parental Flac tra minus elements transferred by complementation. The kinetics, measured in a rec plus strain, showed that recombination began about 1 h after the initiation of mating and was completed about 1 h later. Recombination was abolished in a recA minus strain, reduced by two-thirds in a recF minus strain, and unaffected in recB minus and recC minus strains. It is proposed that the part not due to the RecF pathway results from a RecBC- and RecF-independent system for formation of single-stranded joins. One such join could be followed either by transfer and a site-specific recombination event, or by a second single-stranded join and then transfer: in either case replication and inheritance of the recombinant molecule would be dependent upon the F transfer replication system. Chromosome mobilization by an F' element was normal in a recB plus recF minus strain, and was reduced only fourfold in a recB minus recF plus strain: in the latter strain, both the RecF pathway and the system for single-stranded joins may have contributed to mobilization. Measurement of post-conjugational chromosomal recombination in exponential-phase recipient cells carrying surface exclusion-deficient Flac mutants indicated that F does not itself determine a generalized recombination system able to replace the RecA plus product or the RecBC and RecF pathways.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge E. A., Low K. B. Detection of transcribable recombination products following conjugation in rec+, reCB- and recC-strains of Escherichia coli K12. J Mol Biol. 1974 Mar 15;83(4):447–457. doi: 10.1016/0022-2836(74)90506-3. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Clowes R. C., Moody E. E. Chromosomal transfer from "recombination-deficient" strains of Escherichia coli K-12. Genetics. 1966 Apr;53(4):717–726. doi: 10.1093/genetics/53.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Howe T. G. Recombination and complementation between R factors in Escheichia coli K 12. Genet Res. 1971 Dec;18(3):287–297. doi: 10.1017/s0016672300012696. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Howard-Flanders P. Recombinant F' factors from Escherichia coli K-12 strains carrying recB or recC. J Bacteriol. 1972 May;110(2):578–584. doi: 10.1128/jb.110.2.578-584.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Hirota Y. Gene recombination and segregation of resistance factor R in Escherichia coli. J Bacteriol. 1966 Jan;91(1):51–62. doi: 10.1128/jb.91.1.51-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Mitsuhashi S. Drug resistance of enteric bacteria. VII. Recombination of R factors with tetracycline-sensitive mutants. J Bacteriol. 1966 Nov;92(5):1351–1356. doi: 10.1128/jb.92.5.1351-1356.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K., Achtman M., Willetts N. Deletion map of the Escherichia coli K-12 sex factor F: the order of eleven transfer cistrons. J Bacteriol. 1972 Jun;110(3):857–863. doi: 10.1128/jb.110.3.857-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G. P., Siddiqi O. Enzyme synthesis following conjugation and recombination in Escherichia coli. J Mol Biol. 1968 Mar 14;32(2):201–210. doi: 10.1016/0022-2836(68)90004-1. [DOI] [PubMed] [Google Scholar]
- Moody E. E., Hayes W. Chromosome transfer by autonomous transmissible plasmids: the role of the bacterial recombination (rec) system. J Bacteriol. 1972 Jul;111(1):80–85. doi: 10.1128/jb.111.1.80-85.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siccardi A. G. Effect of R factors and other plasmids on ultraviolet susceptibility and host cell reactivation property of Escherichia coli. J Bacteriol. 1969 Oct;100(1):337–346. doi: 10.1128/jb.100.1.337-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin A., Kushner S. R., Clark A. J. Genetic analysis of mutations indirectly suppressing recB and recC mutations. Genetics. 1972 Oct;72(2):105–115. [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M. Chromosome transfer from F-lac+ strains of Escherichia coli K-12 mutant at recA, recB, or recC. J Bacteriol. 1969 May;98(2):599–604. doi: 10.1128/jb.98.2.599-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Location of the origin of transfer of the sex factor F. J Bacteriol. 1972 Nov;112(2):773–778. doi: 10.1128/jb.112.2.773-778.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Achtman M. Genetic analysis of transfer by the Escherichia coli sex factor F, using P1 transductional complementation. J Bacteriol. 1972 Jun;110(3):843–851. doi: 10.1128/jb.110.3.843-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. Mapping loci for surface exclusion and incompatibility on the F factor of Escherichia coli K-12. J Bacteriol. 1974 Jun;118(3):778–782. doi: 10.1128/jb.118.3.778-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. The genetics of transmissible plasmids. Annu Rev Genet. 1972;6:257–268. doi: 10.1146/annurev.ge.06.120172.001353. [DOI] [PubMed] [Google Scholar]


