Abstract
Research during the past 20 years on families of children with developmental disabilities has yielded a rich body of knowledge about the stress of parenting a child with DD, and the risk and protective factors that result in profiles of family resilience vs. vulnerability at various stages of the family life course. Virtually all of this research has been based on data collected from self-report measures, and has focused on family interactions and relationships, and the psychosocial well-being of individual family members. The present chapter focuses on different sources of data, namely biomarkers, which have the potential to extend our understanding of the biological mechanisms by which the stress of parenting a child with developmental disabilities can take its toll on parents’ physical and mental health. We focus on two examples: (1) variations in the FMR1 gene, FMRP, and FMR1 messenger RNA in mothers of children with fragile X syndrome and the association of these measures with maternal depression and anxiety; and (2) profiles of cortisol expression in mothers of children with disabilities and the association of cortisol with daily measures of caregiving stress.
Introduction
Childhood developmental disability can place tremendous physical, financial, time, and psychological burdens on the rest of the family (Orsmond, Lin, & Seltzer, 2007; Seltzer, Greenberg, Floyd & Hong, 2004), although profiles of resilience are frequently noted (Glidden & Schoolcraft, 2003; Lounds, Seltzer, Greenberg, & Shattuck, 2007). Previous family research has relied largely on self-report measures of health, psychological well-being, and stressors (Abbeduto, Seltzer, Shattuck, Krauss, Orsmond, & Murphy, 2004; Seltzer, Greenberg, Floyd, Pettee, & Hong, 2001). The pathways of influence between parenting stress and physiological functioning remain virtually unexplored among parents of individuals with developmental disabilities. The study of biomarkers thus has the potential to add to our understanding of caregiving stress by corroborating and validating self-report measures, and by giving insight into the mechanisms by which parenting stress takes a toll on health and well-being.
According to an NIH study group (the Biomarkers Definitions Working Group, 2001), a biomarker is "a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention." Only a few studies on parenting a child with a disability have incorporated biomarkers into the research design. For example, one recent study by Gallagher, Phillips, Drayson, and Carroll (2008) reported that parents of children with developmental disabilities mounted a poor antibody response to pneumococcal vaccination, particularly in the context of high levels of child behavior problems.
Another study that incorporated biomarkers was conducted by Epel and colleagues (2004), who reported that in parents who have a child with autism or other developmental or chronic disability, longer duration of caregiving was associated with greater telomere shortening and elevated oxidative stress, controlling for maternal age. In addition, this study estimated the toll taken by perceived parenting stress, as measured by telomere length, and concluded that cellular aging was accelerated by 9–17 years in the highest stress group, relative to those in the lowest stress group (Epel et al., 2004). These patterns provide strong evidence of the link between the experiences of parents of children with disabilities and their biological functioning.
The purpose of the present chapter is to review research on two other types of biomarkers that have been used to investigate associations between parenting stress and parental psychosocial well-being. We first review research on parents of children with fragile X syndrome and examine how variation in their profiles of the FMR1 gene, FMRP levels, and FMR1 messenger RNA are associated with their levels of depression, anxiety, and other dimensions of psychological well-being. In this example, variation in the biomarker is conceptualized as increasing vulnerability to parenting stress. Next, we examine how daily parenting stress is associated with dysregulation of cortisol, a stress hormone, in parents of children with disabilities. In this example, variation in the biomarker is conceptualized as the consequence of parenting stress. Thus, in this chapter, we aim to review literature on biomarkers as both antecedents to and consequences of the stress that is associated with parenting a child with disabilities.
Fragile X Syndrome and Related Conditions
Fragile X syndrome (FXS) is the leading inherited cause of intellectual disability (Crawford, Acuna, & Sherman, 2001) and the single leading known cause of autism (Demark, Feldman, & Holden, 2003). FXS results from a mutation in the 5′untranslated region of the FMR1 gene located on the X chromosome (Brown, 2002). In the healthy allele, there are approximately 55 or fewer repetitions of the CGG sequence of nucleotides comprising the FMR1 gene (Nolin et al., 1994). In FXS, there is an expansion to 200 or more repetitions. Importantly, lesser variations in the FMR1 gene are also associated with adverse phenotypic consequences. Individuals who have between 55 and 200 CGG repeats in the gene are said to carry the premutation. The premutation can expand to the full mutation when passed on from mother to child (Nolin et al., 1996). In addition, a sizeable proportion of individuals with the premutation display many of the same behavioral features of individuals with FXS, albeit typically in a less severe form (Bailey, Raspa, Olmstead, & Holiday, 2008). The premutation is also associated with elevated risk for two disorders that do not occur in individuals with the FMR1 full mutation: primary ovarian insufficiency (POI), which includes premature menopause, and Fragile X-associated Tremor-Ataxia Syndrome (FXTAS), a late-onset neurodegenerative disorder (Cornish, Turk, & Hagerman, 2008). Families that include one or more child who has FXS or even the FMR1 premutation, therefore, are likely to experience high levels of stress and non-normative life experiences as a (direct or indirect) result of the characteristics and behaviors of their affected children (Murphy & Abbeduto, 2005).
FXS, thus, is a multigenerational disorder and its effects on families do not emanate only from the affected children, but also from parents and grandparents. In the case of a child with FXS, the child will have inherited the problem gene from his or her mother, who would be a carrier of either the premutation or the full mutation (Nolin et al., 1996). In the case of children with the FMR1 premutation, sons will have inherited the gene from their mothers and daughters from either their mother or father (Nolin et al., 1996). (For more details of the inheritance profile of the FMR1 gene, see Hagerman, 1999). Some parents (and grandparents) of children with an expanded FMR1 allele will thus be affected by many of the same challenges as their children, which may well make them less able to deal with life stressors, including those associated with their child’s condition (Esbensen, Seltzer, & Abbeduto, 2007). Consequently, understanding the functioning of any individual in a family affected by an FMR1 expansion will require examination of his or her own genetic status and experiences within the family.
To date, the identification of biomarkers of risk in FXS and related conditions has been conceptualized largely in terms of the prediction of trajectories of cognitive, linguistic, and social-affective development in affected individuals. We believe, however, that it is also useful to view those biomarkers as predictors of risk for the family and for individuals trying to meet various family roles, especially the role of parent, which is the focus of the remainder of this section on FXS and related conditions. In addition to reviewing the published literature, we present new data from our own research linking FMR1 biomarkers and parental well-being measures.
Biochemical alterations and phenotypic correlates
Variation in FMR1 CGG repeat size is a useful biomarker of various types of risk that could affect parents, as it defines differences between “healthy” and “affected” and between full mutation and premutation carriers. At the same time, however, there are a variety of additional “downstream” biochemical processes that may be even better indicators of risk because they not only reflect the effects of the FMR1 expansion, but also the contributions of various background genes and environmental events (Belmonte & Bourgeron, 2006). Thus, we now briefly review studies of the measurable biochemical changes associated with variation in the FMR1 gene and the phenotypic correlates of those variations. Although these studies have not focused specifically on individuals who are parents of affected children, they provide a useful context for understanding parental well-being in families with members who carry an FMR1 expansion. We then review the published studies of psychological well-being in parents, with an emphasis on studies that have included FMR1 biomarkers. Throughout our review, we consider both the full mutation and the premutation case. We do so because although premutation carriers are more likely than full mutation carriers to be having children and raising families, (1) individuals with the full mutation are not sterile and (2) studies in which mothers have been recruited through an affected child have found more than 10% of the mothers to be carriers of the FMR1 full mutation (e.g., Bailey et al., 2008).
FMR1 full mutation
The full mutation typically leads to hypermethylation and transcriptional silencing so that the FMR1 gene does not produce, or produces at greatly reduced levels, the protein (FMRP) it normally would (Oostra &Willemsen, 2003). FMRP is an RNA-binding protein that regulates translation of biochemical “messages” into proteins at the synapse (Jin & Warren, 2003) and thus, it is involved in important ways in experience-dependent neural development and functioning (Klintsova & Greenough, 1999). The function of FMRP appears to be largely inhibitory in that it prevents activity in various biochemical pathways and thereby ensures that neural activation occurs in a “controlled” manner (Cornish et al., 2008). In a sense, reduced FMRP leads to exaggerated biochemical reactions that adversely affect neural function. It is known, for example, that lowering the level of FMRP leads to activity in the mGluR5 pathway that would otherwise be blocked (Huber et al., 2002), which then leads to long-term depression (LTD), or reduced responsiveness to stimuli, in the hippocampus and other brain regions involved in learning and memory (Bear et al., 2004).
In addition to intellectual disability, the FMRP deficit in FXS leads to a characteristic behavioral phenotype, which includes both neurocognitive and social-affective features. In the neurocognitive domain, the full mutation is associated with especially severe delays or impairments in sequential processing (Burack et al., 1999; Dykens et al., 1987), working memory (Ornstein et al., 2008), and attention (Bailey et al., 2008), particularly inhibitory control and inattentiveness (Cornish, et al., 2008). In the social-affective domain, hyperarousal (Wisbeck et al., 2000), hyperactivity (Baumgardner et al., 1995; Dykens, Hodapp, Ort, & Finucane, 1989; Freund, Reiss, & Abrams, 1993; Mazzocco et al., 1993), and anxiety (Bailey et al., 2008), particularly social anxiety (Bregman et al., 1988; Mazzocco et al., 1998), are frequent in individuals with the full mutation. Autistic-like behaviors are also common in FXS (Bailey et al., 1998, 2001; 2000a; Feinstein & Reiss, 1998), with 25% to 50% of affected individuals meeting diagnostic criteria for co-morbid autism (Bailey et al., 2004; Brown et al., 1982; Demark et al., 2003; Hatton et al., 2006; Kaufmann et al., 2004; Lewis et al., 2006; Rogers, Wehner, & Hagerman, 2001; Sabaratnam et al., 2003). Numerous studies have found that variations in both the neurocognitive and social-affective features of the phenotype are correlated with FMRP level among those with the full mutation (e.g., Bailey, Hatton, Skinner, & Mesibov, 2001a; Bailey, Hatton, Tassone, Skinner, & Taylor, 2001b; Cohen et al., 1996; Kwon et al., 2001; Loesch et al., 2002; Loesch et al., 2004; Loesch et al., 2006; Menon et al., 2000), making FMRP level a useful biomarker of risk for individuals with the FMR1 full mutation. In terms of parental well-being, a parents’ own FMRP level could be viewed as a biomarker of the psychological resources available to deal with stressful experiences and thus, as an indicator of vulnerability, or risk for less optimal outcomes.
The full mutation leads to FXS in about 1 in 4000 males and 1 in 6,000 to 8,000 females (Crawford et al., 2001). On average, males and females have similar phenotypes, although with milder effects in females (Bailey et al., 2008). This sex difference in affectedness is the result of the fact that males have a single X chromosome, whereas females have two. Moreover, the process of X inactivation early in embryological development in females results in the “turning off” of one X chromosome in each cell, which effectively reduces the impact of the FMR1 mutation relative to males (Tassone, 2000). The relative proportions of active and inactive mutation-carrying X chromosomes contribute to differences in affectedness among females, making the activation ratio another useful biomarker of vulnerability to parenting stress, albeit only for females.
FMR1 premutation
There is a complex pattern of alterations in several biochemical processes important for neural development in individuals carrying the FMR1 premutation. In particular, there appears to be a decrease in FMRP levels for some, but not all, individuals who have large premutations (i.e., >100 CGG repeats; Tassone et al., 2000). Perhaps more importantly, Tassone and colleagues (2000) have found levels of FMR1 messenger RNA (mRNA) that are 2 to 8 times the levels seen in individuals with the healthy FMR1 allele, with a correlation between mRNA levels and CGG repeat number (Allen et al., 2004). This elevation is thought to lead to RNA toxicity, which in turn has numerous adverse phenotypic consequences (Hagerman & Hagerman, 2004), including the formation of inclusions in astrocytes and neurons in many regions of the brain (Greco et al., 2006). Not surprisingly, such substantial alterations in neural development and function are associated with numerous adverse physical and behavioral outcomes, including some not found in FXS (Cornish et al., 2008).
Nearly one-fourth of women with the premutation are affected by POI, a condition associated with premature menopause (i.e., before age 40) and decreased fertility (Cronister et al., 1991). The condition is also accompanied by increased levels of several hormones and endocrine problems (Welt et al., 2004). Interestingly, the risk of POI has been found in a recent study (Sullivan et al., 2005) to be associated with premutation size in a nonlinear manner, increasing with CGG repeat number up to 100 CGG repeats, but declining a bit thereafter. Thus, there is much to be learned about the relationship between POI and the biomarkers discussed thus far. Moreover, although it is reasonable to suppose that the hormonal changes associated with POI as well as the psychological impact of early menopause and decreased fertility could affect psychological well-being and adaptation to the role of parent of a special needs child, this area has yet to be investigated.
Males and, to a lesser extent, females with the premutation are also at elevated risk during late adulthood for FXTAS (Hagerman & Hagerman, 2004). FXTAS is characterized by intention tremor and ataxia, which become increasingly severe with age (Hagerman et al., 2005). The disorder also has cognitive and social-affective features, including problems in memory and executive function and increased anxiety and disinhibition (Berry Kravis et al., 2007; Cornish et al., 2008; Grigsby et al., 2006). These physical and psychological challenges will no doubt affect the quality of life of individuals with FXTAS; however, the condition might have other indirect effects on the family. The mother of a young child with FXS, for example, might have to deal with the emotional and financial demands of simultaneously caring for an affected parent and this additional “burden” may further limit her ability to deal effectively with the needs and challenges of her own child. Unfortunately, empirical tests of this and other possible indirect effects of FXTAS on families have yet to be conducted.
Other features of the FMR1 premutation phenotype are similar, but less severe, than are those observed in the full mutation case (Bailey et al., 2008). Males with the premutation have problems (relative to typically developing age-matched peers) in several cognitive domains, including executive function, long-term memory, and social perception (Aziz et al., 2003; Cornish et al., 2008; Hessl et al., 2007; Moore et al., 2004). These males are also at elevated risk for various forms of psychopathology, such as ADHD, anxiety, obsessive-compulsive disorders, and autism (Aziz et al., 2003; Goodlin-Jones et al., 2004; Hessl et al., 2005). Females with the premutation, especially those with larger premutations (and thus, lower FMRP and higher FMR1 mRNA levels), are at elevated risk for anxiety, depression, obsessive-compulsive disorder, and features of autism (Goodlin-Jones et al., 2004; Hessl et al., 2005). There is little evidence, however, in support of a cognitive phenotype for premutation females (Allen et al., 2005; Moore et al., 2004; Steyaert et al., 2003). Again, it is reasonable to suppose, although this has yet to be investigated empirically, that these features of the premutation phenotype can limit a parent’s psychological resources and thereby increase his or her vulnerability to parenting stress.
In addition to differences between premutation carriers and individuals with a healthy FMR1 allele, there is considerable variation at both the genetic and behavioral levels among individuals who have the premutation. Moreover, there is strong evidence of correlations between measurable biochemical variables and several important features of the premutation phenotype. FMRP correlates with cognition and brain activation patterns (Loesch et al., 2004; Moore et al., 2004). More recently, FMR1 mRNA levels have been found to correlate with measures of psychopathology in males and females with the premutation (Hessl et al., 2005). CGG repeat size and activation ratio have also been found to correlate with depression and other symptoms of psychopathology in females (Johnston et al., 2001), although recent evidence (described below) suggests that the relationship may be nonlinear. Thus, these variables are likely to be useful indices of vulnerability to stress and lower levels of well-being among parents of individuals with an FMR1 expansion.
Stress and well-being of parents of individuals with FXS
Nearly all of the studies on stress and well-being in parents of individuals with FXS or FMR1-related conditions have focused on mothers. These studies have clearly demonstrated that mothers of children, adolescents, and young adults with FXS display high rates of stress and mental health symptoms and lower quality of life as compared with mothers of similarly aged typically developing individuals, although there is considerable inter-individual variability in the former group. In one such study, Roberts et al. (2008) estimated the rates of psychopathology in 93 mothers of children who carried the premutation and had children with FXS, and compared them with mothers of unaffected children. Th comparison group was selected from the National Comorbidity Survey Replication (NCS-R). Structured psychiatric interviews with the mothers of the children with FXS yielded higher rates of lifetime major depressive disorder, lifetime panic disorder (without agoraphobia), and current agoraphobia without panic disorder than in the NCS-R sample. In another study, Head, Chavis, Serafin, Maddocks, and Abbeduto (2008), conducted clinical interviews with 33 mothers of children with FXS and found a rate of major depressive disorder that was lower than that in the Roberts et al study, but still in excess of that expected for women in the general population. Head et al. also found, however, that the most frequent diagnosis (lifetime and current combined) was anxiety disorder, which was observed in three-quarters of the women, which is well in excess of expectations for the general population. Studies using self-reports of mental health symptoms (e.g., the Symptom Checklist-90-R; SCL-90-R), and measuring additional negative facets (e.g., parenting stress) and positive facets (e.g., optimism) of well-being, have also suggested that a relatively high proportion of biological mothers of individuals with FXS have psychological issues severe enough to warrant a psychiatric diagnosis or professional intervention (e.g., Bailey et al., 2008).
There is also evidence that mothers of individuals with FXS may have more stress and mental health challenges, as a group, than mothers parenting individuals with several other types of developmental disabilities. Abbeduto et al. (2004) found that mothers of adolescents and young adults with FXS were more pessimistic about their child’s future and believed that their children felt less close to them compared to mothers of age-matched individuals with Down syndrome. In addition, the mothers of the youth with Down syndrome displayed better functioning than a comparison group of mothers of age-matched youth with autism on virtually every measure administered in the Abbeduto et al. study. In contrast, the mothers of the youth with FXS seldom differed from the mothers of the youth with autism. In general, mothers of youth with autism have been found to be among the most stressed of those parenting a son or daughter with developmental disabilities (Esbensen et al., 2008). Thus, it appears that mothers of adolescents and young adults with FXS, as a group, fall on the upper end of the risk continuum.
In addition to documenting the extent of psychological challenges among mothers parenting sons and daughters with FXS, researchers have begun to address the sources of these challenges. In fact, there is now compelling evidence that, in addition to elevated risk conferred by the biomarkers of FXS, maternal psychological distress and well-being can be traced, at least in part, to characteristics of the son or daughter with FXS, most notably the extent of challenging behavior. Indeed, there is evidence that symptoms of depression (Abbeduto et al., 2004; Bailey et al., 2008; Murphy et al., 2008), anxiety (Murphy et al., 2008; Roberts et al., 2008), and parenting stress (Bailey et al., 2008; Johnston et al., 2003) are predicted by child challenging behavior, as are more general measures of maternal well-being, such as optimism and quality of life (Bailey et al., 2008). Although other personal and contextual factors, such as number of affected children, parent education, and income also contribute to the prediction of such measures, child challenging behavior consistently emerges as a strong predictor of psychological distress for mothers of individuals with FXS (Esbensen et al., 2008), as it does for mothers parenting children with other developmental disabilities (Minnes, 1998).
In contrast to the many studies examining child and other environmental contributions to maternal psychological well-being for mothers carrying an FMR1 expansion, few parenting studies have included relevant maternal biomarkers. Moreover, most studies addressing the contribution of maternal genotype to well-being have relied on CGG repeat size as the only biomarker of interest, and with inconsistent results. Johnston et al. (2001) found a positive correlation between self-reported symptoms of depression on the SCL-90-R)and CGG repeat number in a sample of mothers carrying the premutation who had children with FXS. In contrast, Bailey et al. (2008) administered a large battery of self-report measures and conducted clinical interviews to assess the well-being of 108 mothers who carried the premutation or full mutation and who had a child with FXS. Bailey did not find any contribution of maternal CGG repeat to maternal well-being. In particular, there was no difference between premutation and full mutation mothers in scores on the well-being measures, and no correlation between maternal CGG repeat number and well-being for the premutation mothers.
Abbeduto and colleagues have recently examined the relationship between FMR1 allele size and mental health symptoms in a small sample (n = 27) of mothers of adolescents and young adults with FXS. Characteristics of the sample are provided in Table 1. All were biological mothers identified through their adolescent or young adult son or daughter with FXS. The sample of mothers was largely White, in their 40s, married, and carried the premutation. The mothers completed the SCL-90-R, which yields several T scores, including for depression and anxiety. The target child’s father or teacher or both completed the Problem Behavior Scale of the Scales of Independent Behavior-Revised, which assesses behavior problems. It should be noted that these mothers were relatively well functioning according to their SCL-90-R scores, as only 8 met the definition of “caseness” (i.e., a T score of 63 or above) for depression and 3 for anxiety, although other data we collected suggested that many more had dealt with mental health problems at previous points in their lives. Similarly, the mean score of the adolescents and young adults with FXS fell at the edge of the “normal” range on the Problem Behavior Scale, suggesting that, as a group, they too were functioning relatively well.
Table 1.
Families of Adolescents or Young Adults with FXS: Selected Characteristics of Mothers, Children, and Families
| Characteristic | Mean | SD | Min. | Max. |
|---|---|---|---|---|
| Maternal | ||||
| No. CGG Repeats | 98.7 | 24.7 | 70 | 155 |
| Age (in years) | 45.3 | 6.6 | 33.5 | 61.7 |
| IQa | 109.4 | 13.6 | 86 | 134 |
| Educationb | 5.6 | 1.7 | 3 | 8 |
| Child | ||||
| Challenging Behaviorc | −9.1 | 9.6 | −40 | 2 |
| Family | ||||
| Incomed | 7.9 | 2.7 | 3 | 11 |
| No. Children | 2.3 | 1.2 | 1 | 5 |
| No. Children w/ DD | 1.6 | 0.6 | 1 | 3 |
| % Single-Parent | 22 | -- | -- | -- |
Based on administration of the Kaufmann Brief Intelligence Test.
Based on a rating scale of from 1 (Grade 8 or less) to 8 (advanced graduate degree), with a rating of 6 signifying a college graduate.
Based on scores from the Problem Behavior scale of the Scales of Independent Behavior-Revised. Scores derived from administrations to teacher and/or father. Lower scores reflect greater problems with challenging behaviors. Not available for 3 children.
Based on a rating scale of from 1 (annual income $10,000 or less) to 16 (annual income $150,000 or more) in $10,000 increments. Not available for 3 children. Not available for on family.
As can be seen in Table 2, SCL-90 depression scores were significantly correlated with several maternal characteristics, child challenging behavior, and family characteristics. Anxiety scores were correlated with a more narrow set of variables, suggesting the possibility of different causal pathways for depression and anxiety in this population. Most importantly for present purposes, maternal FMR1 allele size (i.e., CGG repeat length) was correlated with both maternal depression and anxiety, consistent with the notion of a genetic susceptibility to some types of mental health problems. Surprisingly, however, the correlation between allele size and SCL-90-R scores was negative; that is, larger FMR1 expansions in the premutation range were associated with fewer and/or less intense symptoms of depression and anxiety. A negative relationship also emerged when we compared allele size in mothers meeting criteria for caseness against those not meeting criteria, although only for depression, t (1, 24.06) = 2.54, p = .018.
Table 2.
Families of Adolescents or Young Adults with FXS: Bivariate Correlations between Maternal Mental Health Measures and Selected Characteristics of Mothers, Children, and Families
| Characteristic | Depression (SCL-90-R) |
Anxiety (SCL-90-R) |
|---|---|---|
| Maternal | ||
| No. CGG Repeats | −.58**** | −.49*** |
| Age (in years) | −.34* | −.27 |
| IQa | −.42** | −.41** |
| Educationb | −.42** | −.30 |
| Child | ||
| Challenging Behaviorc | −.35* | −.31 |
| Family | ||
| Incomed | −.41** | −.26 |
| No. Children | −.10 | −.06 |
| No. Children w/ DD | −.03 | .00 |
Based on administration of the Kaufmann Brief Intelligence Test.
Based on a rating scale of from 1 (Grade 8 or less) to 8 (advanced graduate degree), with a rating of 6 signifying a college graduate.
Based on scores from the Problem Behavior scale of the Scales of Independent Behavior-Revised. Scores derived from administrations to teacher and/or father. Lower scores reflect greater problems with challenging behaviors. Not available for 3 children.
Based on a rating scale of from 1 (annual income $10,000 or less) to 16 (annual income $150,000 or more) in $10,000 increments. Not available for 3 children. Not available for on family.
p≤ .10,
p≤ .05,
p≤ .01,
p≤ .005, with all tests two-tailed
We explored the relationships displayed in Table 2 in a series of regression analyses and found that maternal CGG repeat number and child challenging behavior made independent contributions to maternal health symptoms. In a regression that included four predictor variables (maternal CGG repeat number and IQ, child challenging behavior, and family income) and SCL-90-R anxiety scores as the dependent variable, the β was -.51 for maternal allele size, t = 2.6, p = .018, and -.41, t = 2.3, p = .033 for child behavior. In the same analysis for SCL-90-R depression scores, the β was -.57 for maternal allele size, t = 3.1, p = .006, and -.46, t = 2.7, p = .016 for child behavior. Regressions including additional maternal and family variables did not change the results appreciably. Thus, smaller maternal premutations and more serious child challenging behaviors predicted worse maternal mental health. Moreover, the negative relationship between mental health and CGG repeat number was not explained by correlated differences on any of the other variables, although the small sample size precluded an examination of all variables of interest or their interactions.
Our findings are consistent with a model in which the FMR1 premutation is thought to confer increased risk for mental health problems over and above the contribution of child challenging behaviors and other factors “external to the mother.” Nevertheless, our findings regarding premutation size are surprising in that they suggest that it is only the smaller premutations that increase vulnerability to psychological distress. This interpretation must be considered speculative until the biological mechanisms underlying differences in risk and premutation size are more fully understood. Caution is also required because our finding is at odds with those of Johnston et al. (2001) who found a positive correlation between maternal repeat size and symptoms of depression. Nevertheless, it is important to note that Roberts et al. (2008) also found a negative correlation between mental health problems and allele size in their larger sample of biological mothers of children with FXS, suggesting that our findings did not emerge because of some unidentified idiosyncratic feature of our sample. Roberts et al. suggested a protective function of the larger FMR1 premutations.
Ultimately, understanding the pathways from maternal genetic status to mental health outcomes will require that researchers move beyond CGG repeat number and instead rely on biomarkers that capture more “downstream” biochemical processes (e.g., FMRP and mRNA levels), reflecting the influence of other background genes and environmental events as well as the FMR1 mutation. Moreover, it is likely that the use of a combination of several biomarkers may well be most informative, as each reflects somewhat different biochemical processes and thus, each may make a unique contribution to maternal mental health. This possibility is illustrated in a recent study by Hessl et al. (2005), who found a positive correlation between FMR1 mRNA levels and self-reported anxiety measured by the SCL-90-R for a sample of women with the premutation (largely mothers of children with FXS); however, this correlation emerged only for women who had activation ratios reflecting a higher proportion of active X chromosomes containing the premutation (rather than the healthy allele). Such findings serve as a reminder of the complexity of development, even in the case of a singe-gene disorder (Belmonte & Bourgeron, 2006) and thus, of the need to create a comprehensive battery of well-characterized and understood biomarkers when attempting to evaluate risk.
It is also important to reiterate that other factors, such as child challenging behavior, also contribute to maternal mental health. Indeed, contextual factors, such as number of affected children in the family and family income, as well as maternal characteristics, such as education, contribute as well (Abbeduto et al., 2004). It is also the case that other genes make independent contributions to mental health and may well interact with the FMR1 gene to affect risk. Thus, maternal FMR1 status is only part of the picture needed to understand an individual’s risk for mental health challenges or the most effective path to prevention or treatment.
In concluding, it is interesting to consider a study by Franke et al. (1998), which demonstrates a particularly creative approach to investigating the role of biological variables in the psychological distress and well-being of mothers of individuals with FXS. These investigators included mothers who themselves carried the FMR1 premutation and relied on diagnostic interviews and observation to determine whether they met criteria for various psychiatric disorders. Franke et al. also included several control groups of women (e.g., mothers of children with autism, premutation women without affected children) in an attempt to parse out the contributions of maternal genetic status, parenting per se, and parenting a child with fragile X syndrome. In general, the women who carried the premutation and who had children with FXS were found to be at greatest risk for several psychiatric conditions. They were the most likely to be diagnosed with an anxiety disorder or a major depressive episode, and they were more likely to be so diagnosed than were women who had the premutation, but had no affected children. Again, such findings suggest that, although the biochemical alterations associated with the FMR1 expansion do increase the risk of mental health challenges, there are many other factors that contribute as well, including, of course, those associated with parenting a son or daughter with FXS.
Limitations of the FMR1 biomarkers
It is important to acknowledge that although significant correlations between the FMR1-related biomarkers and measures of neurocognitive and social-affective functioning have been found in numerous studies, the correlations have typically accounted for only a rather small proportion of phenotypic variance. These modest correlations may reflect the fact that the biomarkers are calculated only from peripheral blood rather than from neural tissue, which obviously cannot be sampled except under “unusual” circumstances, such as from postmortem tissue under autopsy. Although estimates of FMRP and other FMR1 biomarkers from lymphocytes can be assumed to be virtually identical to their distribution in brain for males with the full mutation, they can provide only approximations for females and mosaic males (Brown, 2002). In studies of mothers of affected children, then, the biomarkers contain considerable error, a problem that is compounded by the small numbers of participants in most studies. It is likely, therefore, that the current set of biomarkers available in human studies will seriously underestimate the contribution of genetic variation in stress and well-being among parents of individuals with FXS and related conditions.
Summary and directions for future research
There is considerable evidence from decades of research that the biomarkers we have considered are broadly predictive of “affectedness” in individuals with an FMR1 expansion. Those with a full mutation typically display a characteristic phenotype that includes high rates of intellectual disabilities and social-affective problems, including anxiety and autism. Individuals who carry the premutation are at risk for milder cognitive and social-affective symptoms, but also for conditions, such as POI and FXTAS, that do not occur in the full mutation case. The biomarkers we have considered also appear to be predictive of psychological well-being in women who carry the premutation and are raising sons and daughters with FXS, although the relationships among the biomarkers and psychological symptoms are complex and inconsistent across studies. In the case of these mothers, it appears that they have poorer mental health outcomes, as a group, because of a genetic vulnerability to mental health problems and are less well-equipped to deal with the stresses of life, including those that arise (directly and indirectly) from parenting a child with challenging behavior.
Knowing that the FMR1 expansion produces a vulnerability to psychological stress in mothers, however, is only the beginning of an explanation. Additional research is needed to determine more fully the causal pathways and mediators involved in producing mental health outcomes for these mothers. There is a need for research at multiple levels of analysis, from that focusing on biochemical processes at the synapse and the structural and functional integrity of neural systems, to that focused on the ways in which the psychological and biological characteristics of a woman who carries the problematic allele affects her reactions to stress at various points in development both before and after the birth of her affected child, as well as the ways in which those reactions are tempered by the broader context in which she lives.
Finally, there is a need for research on other members of the family and the ways in which the FMR1 biomarkers can help us understand family risk more broadly. How do fathers who carry the FMR1 premutation deal with parenting stress? Do they display the same vulnerabilities as mothers? What of siblings who carry the premutation? Are they less able to deal with challenges within the family relative to siblings who carry the healthy allele? As we address these questions and continue to learn more about the pathways from gene to behavior in FXS we may be able to move beyond conceptions of risk for individuals and toward conceptions of risk for families that take into account the genetic and psychological vulnerabilities of all family members and the dynamic relationships among them.
Cortisol Profiles in Parents of Children with Disabilities
Whereas the biomarkers of FXS and the premutation appear to increase the vulnerability of parents to poor mental health outcomes and reduced ability to deal with caregiving stress, other biomarkers are useful indicators of the consequences of parenting children with disabilities. One such biomarker that has been shown to be a sensitive measure of the effects of stress is cortisol, which is an indicator of the activity of the hypothalamic-pituitary-adrenocortical (HPA) axis. A large body of research has demonstrated that disruption of the HPA axis is associated with physical and mental health problems (Gunnar & Vasquez, 2001; Heim, Newport, Heit, Graham, Wilcox, Bonsall, et al., 2000). Cortisol plays a vital role in linking stress exposure to health problems (McEwen, 1998). However, prior to our own research (Seltzer, Almeida, Greenberg, Stavla, Stawski, Hong, et al., in press), this pathway had not been examined in parents of children with disabilities. Therefore, in this section of the chapter, we review the literature on cortisol and stress, drawing from studies of other sub-groups of the population, and then we present data from our program of research, which has examined stress and cortisol expression in parents of children with disabilities.
Stress and cortisol
Cortisol normally peaks shortly after waking in the morning and then gradually declines throughout the rest of the day. Diurnal cortisol (i.e., the pattern of cortisol expressed throughout the day) provides a window into individuals’ chronobiology (Keenan, Licinio, & Veldhuis, 2001). The early morning and evening levels of cortisol reflect daily engagement and disengagement, respectively, of the brain with peripheral physiology, and hence with the external environment. Failure to activate the HPA axis in the morning may indicate difficulty in responding to the ordinary challenges that are faced every day. Failure to deactivate the HPA axis in the evening may indicate difficulty in disengaging from external demands, leading to inhibition of restoration and recovery processes associated with sleep (Sapolsky, Krey, & McEwen, 1986). (Note that the phrases “failure to activate” and “failure to deactivate” do not imply that cortisol is under the intentional control of the individual; rather these phrases reflect physiological processes.)
Short-term increases in cortisol are thought to reflect a ‘normal’ physiological response to stressor exposure (Sapolsky et al., 1986). However, individual differences as well as variation in the nature of stressors may influence the magnitude of such responses, leading to exaggerated (hyper) or diminished (hypo) responsiveness. The impact of variation in cortisol stress reactivity is thought to accumulate over time in response to repeated or chronic stressor exposure, thereby leading to persistently high or low levels of circulating cortisol (which in turn can influence multiple aspects of physiological functioning). Both hyper- and hypo-responsive cortisol stress reactivity are symptomatic of poor physical health, generally interpreted as wear-and-tear on the HPA-axis (Kiecolt-Glaser et al., 1986; Segerstrom & Miller, 2004). The measurement of daily cortisol rhythms may provide the best window into stress physiology, yielding information about overall levels and fluctuations in cortisol levels across the day, and the association of these characteristics of cortisol with exposure to stressful experiences and individual/contextual factors.
Daily stresses have been shown to be important predictors of individual and family functioning (Crnic & Greenberg, 1990; DeLongis, Folkman, & Lazarus, 1988). Studying cortisol expression in parents of children with disabilities offers a new opportunity to examine how daily life experiences influence daily physiology and are associated with indicators of health and well-being.
Research has shown that individuals who experience acute stressors display elevations in cortisol levels at waking and 30 minutes after waking as compared to individuals who do not experience acute stress (Dickerson & Kemeny, 2004). For example, Kirschbaum and colleagues (1993) demonstrated that when research participants were given a laboratory task such as having to give a speech or perform mental arithmetic, this led to a two- to four-fold elevation in cortisol levels above their baseline level.
However, a different pattern of cortisol expression is evident in the context of chronic stress. Although acute stress leads to elevations in cortisol, hypoactivity of the HPA axis has been documented in the face of chronic stress, such as unemployment, bereavement, environmental disasters, chronic fatigue syndrome, fibromyalgia, PTSD, and parenting children with cancer (Baum, Schaeffer, & Lake, 1985; Demitrack et al., 1991; Griep, Boersma, & de Kloet, 1993; Jacobs et al., 1987; Meewisse, Reitsma, Vries, Gernsons, & Olff, 2007; Miller, Chen, & Zhou, 2007; Ockenfels et al. 1995; Scott & Dinan, 1998). Pruessner, Hellhammer, and Kirschbaum (1999) found that teachers scoring high on burnout showed lower overall cortisol secretion relative to peers who are low on burnout. Adam and Gunnar (2001) found that mothers who worked more hours and had more children at home had lower morning cortisol values and a less pronounced decline in cortisol levels across the day than mothers working fewer hours and having fewer children. Similarly, in a study of parents of children with cancer, Miller, Cohen, and Ritchley (2002) found that these parents had lower levels of cortisol secretion one hour post-awakening than parents of healthy children, and showed a flatter diurnal decline in cortisol. In a meta-analysis of 37 studies of 828 people with PTSD and 800 controls, Meewisse et al. (2007) found that individuals with PTSD had significantly lower levels of cortisol than controls who had not been exposed to trauma.
Thus, cortisol shows a different pattern with chronic than acute stress: acute stress is associated with sharper elevations in the morning rise of cortisol, whereas chronic stress is associated with a flatter pattern of low levels of cortisol expression throughout the day, i.e., a lower morning rise and a less pronounced decline at the end of the day.
Apart from our program of research (described in the next section), no previous study has extended the investigation of stress effects on cortisol dysregulation to parents dealing with the demands of caring for a child with disabilities. However, based on past research on other populations experiencing chronic stress, we hypothesized that parents of children with disabilities would exhibit patterns of hypoactivation of cortisol.
Measurement of daily stress and the diurnal rhythm of cortisol
Our research protocol for the measurement of daily stress and salivary cortisol is based on the methods developed by Almeida (Almeida et al., 2002) for the National Study of Daily Experiences (NSDE), one of the projects that comprise the National Survey of Midlife in the United States (MIDUS; Carol Ryff, PI). MIDUS is a national probability sample of English-speaking, non-institutionalized adults who were aged 25 to 74 in 1994 (MIDUS I; Brim, Ryff, & Kessler, 2004). Follow-up data were collected from 2003 to 2005 (MIDUS II; n = 4032).
A subset of MIDUS II sample members was also included in the National Study of Daily Experiences (NSDE; David Almeida, PI), which is the source of data for the Daily Diary Study analyses we present in this chapter. The NSDE consists of 15- to 25- minute telephone interviews at the end of each of eight consecutive days. The NSDE daily telephone interview includes questions about daily experiences in the past 24 hours concerning the number of stressors and positive events, and daily measures of positive and negative affect (Almeida et al., 2002).
As part of the NSDE, salivary cortisol samples are collected 16 times (i.e., four times each day on days 2 through 5 of the eight-day study). Respondents receive a Home Saliva Collection Kit one week prior to their initial phone call. Sixteen numbered and color-coded “salivettes” are included in the collection kit, each containing a small absorbent wad, about 3/4 of an inch long, as well a detailed instruction sheet. In addition to written instructions, telephone interviewers review the collection procedures and answer any questions.
The four saliva samples collected each day are scheduled to provide data about the characteristic diurnal rhythm of cortisol: one upon wakening, one 30 minutes after getting out of bed, one before lunch, and one at bed time. Data on the exact time respondents provided each saliva sample are obtained from the nightly telephone interviews and on a paper-pencil log sent with the collection kit. In addition, approximately 25% of the respondents received a “smart box” to store their salivettes. These boxes contain a computer chip that recorded the time respondents open and close the box. The correlations between self-reported times and the times obtained from the “smart box” ranged from .75 for the evening occasion to .95 for the morning, substantiating the reliability of the self-reported times of saliva collection.
Measures of salivary cortisol derived from the samples include the absolute values at each of the four collection times (upon awakening, 30 minutes later, before lunch, before bedtime), as well as two parameters of diurnal rhythm: morning rise and daily decline. Morning rise is an indicator of how high an individual’s cortisol rises, measured from awakening to 30 minutes after awakening. Daily decline refers to the slope from the typically highest point in the day, measured at 30 minutes after awakening, through the collection before bed.
Study samples: Parents of children with disabilities and comparison group parents
All parents in the MIDUS study were asked if any of their children had a developmental or a mental health problem, and if so, which child had the condition and the name of the particular diagnosis the child had received. Approximately one in ten (10.5%) MIDUS participants responded affirmatively, of whom nearly half (46.3%) had a child with a developmental problem, about the same number (42.7%) had a child with a mental health problem, and the remaining 11% had a child with another type of neurological disability.
A sub-sample of the MIDUS II participants who also participated in the NSDE (n = 806 at the time of the present analysis) had a child with a developmental or mental health problem (n = 82). About half (47.6%) had developmental disorders and the others (52.4%) had mental health diagnoses. Among the developmental disorders were intellectual disability (mental retardation), cerebral palsy, Down syndrome, hydrocephalus, muscular dystrophy, pervasive developmental disorders, specific genetic disorders (e.g., cri du chat syndrome), ADHD, seizure disorders, traumatic brain injury, etc. Among the mental health diagnoses were schizophrenia, bipolar disorder, depression, anxiety disorders, eating disorders, alcohol and drug abuse, etc. Thus, the present sample was characterized by a heterogeneous set of disabilities.
We selected as a comparison group a sample of NSDE respondents who had at least one living child, but no child with a disability or chronic health condition, and who never provided care to a family member. For this comparison group, we selected the 82 individuals most similar to the parents of children with a disability with respect to parent gender, parent age, number of children in the household, child age, whether the target child lives with the parent, parent marital status, and parent educational attainment. (See Seltzer et al., in press, for details of the methods and findings). Table 3 portrays the characteristics of the sample of parents of children with disabilities and the comparison group, and shows that the two groups were very similar.
Table 3.
Parents of Children with Disabilities: Descriptive statistics (mean with standard deviation in parenthesis) of parents of children with disabilities (n = 82) and comparison group parents (n = 82).
| Variables | Parents of children with disabilities |
Comparison group |
|---|---|---|
| Parent’s Characteristics | ||
| Age | 57.4 (13.0) | 57.4 (13.1) |
| Gender (1 = female/ 0 = male) | .59 (.50) | .59 (.50) |
| Race (1 = non-Hispanic white / 0= others) | .96 (.19) | .97 (.16) |
| Marital status (1 = married / 0 = not married) | .79 (.41) | .84 (.37) |
| Employment status | .57 (.50) | .61 (.49) |
| (1 = employed; 0 = not employed) | ||
| Years of education | 14.4 (2.65) | 14.5 (2.35) |
| Total household income | $74,400 (49,800) | $78,300 (50,100) |
| Number of children | 3.29 (1.91) | 3.21 (1.26) |
| Child’s Characteristics | ||
| Age | 29.3 (13.4) | 29.9 (13.4) |
| Gender (1 = female/ 0 = male) | .40 (.49) | .40 (.49) |
| Living with parents (1 = yes/ 0 = no) | .41 (.50) | .32 (.47) |
As shown in Table 3, the sons and daughters in both groups were nearly 30 years of age and their parents were in their late 50s, on average. Most of the parents were mothers (almost 60%), and nearly all were non-Hispanic whites. The two groups were similar with respect to marital status (about 80% were married) and employment status (about 60% were employed), and both groups averaged about two years of education beyond high school. The one variable on which the two groups differed was the percentage who had children still living at home, with the comparison group less likely to have coresident children than the group of parents of individuals with disabilities (32% vs. 41%), which is to be expected given the ability differences between the two groups of children.
Daily stress in parents of children with disabilities and the comparison group
As described in Seltzer et al. (in press), this sample of parents of children with disabilities diverged considerably in daily experiences from parents in the comparison group, despite demographic similarity. As shown in Table 4, parents of children with disabilities reported a significantly elevated number of days during the Daily Diary Study when they had arguments and an elevated number of days when they experienced tense moments when they could have had an argument but chose not to do so, relative to the comparison group. The former also reported experiencing a greater number of stressors each day, a greater number of days when they experienced at least one stressor, a greater severity of stressors, and a greater number of stressors that occurred at home, than the comparison group. The parents of children with disabilities also reported significantly elevated levels of negative affect, reflecting more anxiety and depression on a daily basis, than the comparison group, and a marginally lower level of positive affect.
Table 4.
Parents of Children with Disabilities: Mean comparisons between parents of children with disabilities (n = 82) and comparison group parents (n = 82) on type and severity of stressors, mood, and symptoms.
| Variables | Parents of children with disabilities |
Comparison group |
|||
|---|---|---|---|---|---|
| M | SD | M | SD | t-test | |
| Stressors: | |||||
| Argumentsa | 0.13 | 0.15 | 0.08 | 0.12 | 2.36* |
| Avoided argumentsa | 0.18 | 0.17 | 0.13 | 0.13 | 2.21* |
| Number of stressors/day (mean) | 0.74 | 0.64 | 0.52 | 0.42 | 2.60** |
| Days with any stressors (%) | 0.50 | 0.26 | 0.40 | 0.25 | 2.49** |
| Work stressorsa | 0.07 | 0.10 | 0.08 | 0.14 | −0.57 |
| Home stressorsa | 0.13 | 0.14 | 0.09 | 0.11 | 2.30* |
| Network Stressorsa,b | 0.02 | 0.07 | 0.01 | 0.03 | 1.28 |
| Severity of stressors (mean) c | 2.51 | 1.32 | 2.09 | 1.00 | 2.27* |
| Positive Events: | |||||
| Number of positive events/day (mean) | 1.09 | 0.66 | 1.04 | 0.63 | 0.49 |
| Days with any positive event (%) | 0.69 | 0.28 | 0.69 | 0.26 | −0.13 |
| Affect: | |||||
| Negative affectd | 0.20 | 0.18 | 0.14 | 0.15 | 2.17* |
| Positive affecte | 2.57 | 0.73 | 2.78 | 0.66 | −1.88+ |
reflects the percent of days in the Daily Diary Study when the type of stress was reported.
defined as stress in the lives of individuals in the respondent’s social support network
severity was rated from “not at all stressful’ to “very stressful”.
the negative affect scale (10 items) measured anxiety, hostility and depression on a 5-point scale from “none of the time” to “all of the time”.
the positive affect scale (10 items) measured enthusiasm, alertness, and vitality. The rating scale was the same as for negative affect.
p=. 06
p< .05,
p< .01.
However, the parents of children with disabilities did not differ from the comparison group in all respects; they were not different in the number of days when they experienced a stressor at work or when members of their social support network experienced stress, and they reported an equal number of positive events and days with a positive event during the 8-day Diary Study.
Thus, parents of children with disabilities had daily lives that were similar to the norm in their experience of positive events, stress at work, and stress experienced by their social support network. However, their lives were characterized by elevated levels of negative affect, stress at home, arguments, tense moments, and several other measures of stress that were assessed during the 8-day diary study. We next asked whether there is a “biological signature” of this level of daily stress, namely whether parents of children with disabilities differed from the comparison group in their level and pattern of cortisol expression.
Cortisol expression in parents of children with disabilities and the comparison group
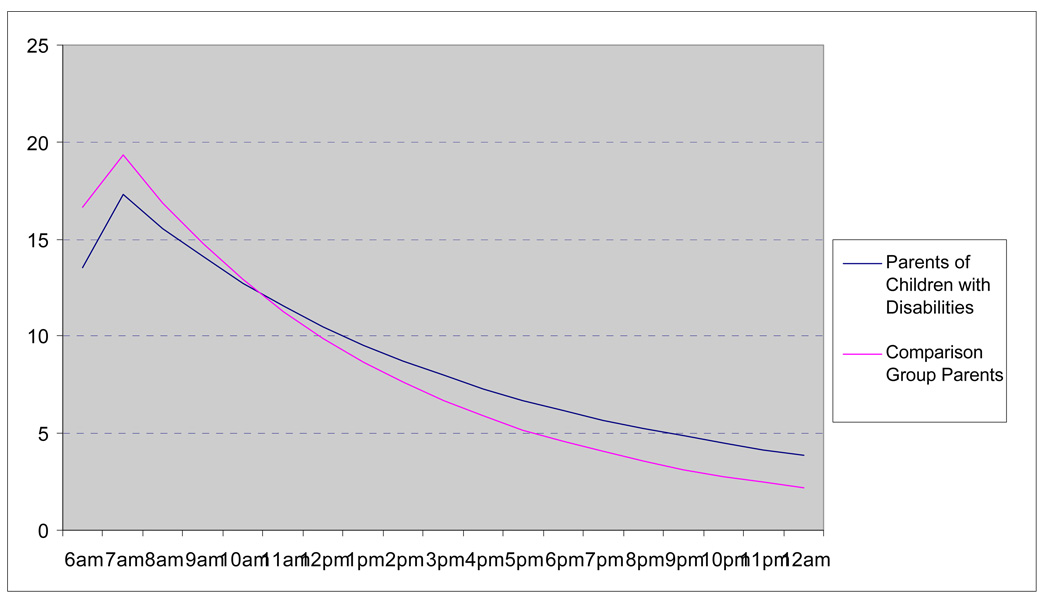
Using multilevel modeling, we examined group differences in the diurnal rhythm of cortisol. We found that parents of children with disabilities and comparison parents did not differ significantly in the slope of the morning rise, but parents of children with disabilities exhibited significantly less pronounced daily decline slopes (see Figure 1; the full results of the multi-level models are available in Seltzer et al., in press). This pattern indicates that parents of children with disabilities are significantly less likely to deactivate the HPA axis at the end of the day than their counterparts in the comparison group, suggesting inhibition of restoration and recovery processes for parents of children with disabilities. This pattern remained significant even after controlling for the residential status of the child.
Figure 1.
Diurnal rhythm of cortisol in parents of children with disabilities and comparison group parents.
We also examined whether the amount of time parents spent with their children on a given day predicted variation in diurnal pattern of cortisol and other indicators of daily psychological well-being. Specifically, we investigated if there were within-person associations between time spent with children, on the one hand, and negative affect and the cortisol measures, on the other, and compared parents of children with disabilities and unaffected parents on these measures. For this analysis, we focused only on the co-resident subgroup to ensure a closer association between daily contact with children and parental psychological and biological response.
We found that there was a significant interaction between parental status (having a child with a disability versus having unaffected children) and time spent with co-resident children, with respect to parental well-being outcomes and cortisol expression (see Seltzer et al., in press for the data). On days when they spent more time with their children, parents of children with disabilities reported significantly higher levels of negative affect compared to days when they spent less time with their children, whereas parents in the comparison group did not evidence a difference in negative affect based on the amount of time they spent with their children. In addition, parents of children with disabilities had a less pronounced daily decline of cortisol expression on days when they spent more time with their children as compared to days they spent less time, whereas the opposite pattern was evident for the parents in the comparison group. These findings suggest that parents of children with disabilities were less likely to deactivate the HPA axis during days when they spent more time with their children than on days when they spent less time with their children.
Based on these analyses, we have tentatively concluded that there indeed is a biological signature of parenting a child with disabilities. Such parents experience elevated levels of stress and are less likely to show the characteristic daily decline pattern in cortisol expression, particularly on days when they spend more time with their coresident children. These findings suggest that, at the end of the day, the brain is less likely to be disengaged from peripheral physiology in parents of children with disabilities than in parents whose children do not have disabilities. However, in this analysis, parents of children with disabilities did not differ from the norm in the slope of their morning rise of cortisol, suggesting that they “gear up” for the day’s challenges as well as their peers who do not have children with disabilities. This pattern of normative daily rise but flatter daily decline is only partially characteristic of a classic chronic stress response.
One explanation for this partial chronic stress pattern concerns the heterogeneity of the diagnoses represented in the sample. Some of the diagnoses are chronic and long-lasting, while others are more transitory. Furthermore, some of the diagnoses reflect developmental problems, whereas others reflect mental health problems. The heterogeneity in child diagnosis encompasses diverse behavioral phenotypes, which likely have diverse effects on parents’ daily lives and biological responses. The heterogeneity of the sample with respect to the types of child disabilities is one important limitation of the present study. The fact that the sample was drawn from a nationally representative study is one of its most important strengths.
Summary and directions for future research
Thus far, our research incorporating the biomarker of cortisol into studies of parents of children with disabilities has revealed two preliminary conclusions. First, parenting a child with a disability leaves a biological signature and cortisol is one biomarker that detects this signature. Specifically, we observed differences between parents of children with disabilities and parents of unaffected children in one important aspect of their physiological response to stress, namely deactivation of the HPA axis at the end of the day. Parents of children with disabilities were significantly less likely to deactivate their HPA axis at the end of the day than unaffected parents, and this was particularly the case on days when they spent more time with their children. These findings may suggest a pile-up of stress during the day.
Second, we believe that it will be profitable to disaggregate samples of parents of children with disabilities according to the specific diagnosis of their child. We are currently applying this same daily diary and cortisol collection methodology in studies focusing on distinct groups defined by the specific developmental disability of their child – autism, fragile X syndrome, and Down syndrome. Past research (e.g., Abbeduto et al., 2004; Dykens, Hodapp, & Finucane, 2000; Ly & Hodapp, 2002) has shown that these three groups of mothers differ in their level of self-reported parenting stress, with mothers of individuals with autism reporting the highest level of parenting stress, mothers of individuals with Down syndrome reporting the lowest levels of parenting stress, and mothers of individuals with fragile X syndrome close to the level experienced by those whose child has autism. By extending this line of comparative self-report research to include the biomarker of cortisol, we will be able to determine the extent to which self-reported differences in stress correlate with the biological data.
These studies are currently ongoing. Within the sample of mothers of individuals with fragile X syndrome, we will be particularly interested to determine whether the biomarkers of fragile X alter the pattern of stress reactivity as evidenced in their cortisol expression patterns.
Summary and Conclusions: Next Steps in Research on Biomarkers in Families of Individuals with Developmental Disabilities
In this chapter, we have highlighted only a small subset of the potential array of biomarkers that might prove to be fruitful in the investigation of the biopsychosocial impact of parenting children with developmental disabilities. Therefore, one important agenda for future research is to expand the range of biomarkers incorporated in family research in the field of developmental disabilities. Past research on other populations points the way toward biomarkers that would potentially be profitable in advancing developmental disabilities family research.
For example, biomarkers that comprise the composite index of allostatic load are receiving increasing attention in research aiming to gain a greater understanding of the mechanisms by which stress takes not only a psychosocial but also a biological toll and ultimately impacts health (McEwen, 1998, 2000; Singer & Ryff, 2001). Allostatic load is an index that assesses the physiological costs of chronic exposure to stress. It is used to explain how frequent activation of the body's stress response can damage health in the long run. Allostatic load is generally measured through a composite index of indicators of cumulative strain on several organ systems and tissues. Although, on average, elevated levels of stress are associated with high levels of allostatic load, there is great diversity in individual response to stress, including presumably in response to the challenge of parenting a child with a disability. By incorporating measures such as allostatic load into research on parenting children with disabilities, it would be possible to examine not only how parenting a child with developmental disabilities increases parents’ vulnerability to health-related problems but may also suggest pathways to resiliency in such parents.
In addition, future research should prioritize longitudinal studies that have the potential to clarify the long-term impact of childhood developmental disability, and variations in parental risk, on parents’ health across the life course. Virtually all studies to date have examined only concurrent relationships between biomarkers and psychological outcomes. As a result, we do not know which factors move an individual from having a biological vulnerability to actually having an anxiety disorder, depression, or other adverse mental health outcome. Knowledge of such “triggering” factors will be critical for preventing adverse outcomes in these mothers. It is likely that these factors will include both maternal background genes and exposure to various stressors over the life course.
In addition, studies will need to employ interdisciplinary methodologies that allow for the examination of dynamic and complex effects of caregiving on the family. Current methods often do not account for the direct, indirect, and interactive effects of childhood developmental disability on the family. Future research should provide a critical link between subjective measures of parenting stress and objective measures of parents’ physiological response in order to improve understanding of both disease risk among parent caregivers and the implications of parental psychobiology for the quality of life of children with developmental disabilities. Furthermore, quantitative studies, which examine the complex interrelationships between the physiological, behavioral, and social factors that contribute to caregiver vulnerability and resiliency, as well as qualitative studies, which examine the “lived experience” of parents of children with developmental disabilities, will be essential to develop interventions to improve the well-being of such families.
Finally, allostatic load, cortisol, and other biomarkers that serve as indices of the effects of caregiving stress on parents have the potential to be useful in evaluating the effects of various psychosocial and pharmacological treatments. In fact, these biomarkers may be especially sensitive indicators of treatment effectiveness as they reflect changes in adaptation to stressors that are likely to precede changes in measurable psychological and behavioral outcomes. That is, successful interventions will enable parents to better cope with stress, with better coping eventually leading to altered physiological reactions to stress, and ultimately a to reduction in mental and physical health symptoms.
Acknowledgements
This research was supported by grants from the National Institute of Child Health and Human Development R01HD024356 and R03HD048884, L. Abbeduto, PI; P30 HD03352, M.M. Seltzer, PI) and the National Institute on Aging (P01 AG020166, C.D. Ryff, PI, and R01AG019239, D, Almeida, PI) to conduct a longitudinal follow-up of the MIDUS (Midlife in the US) investigation. The original MIDUS study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. We also acknowledge the contributions of Jyoti Savla, Robert Stawski, and Julie Lounds Taylor to the research on parenting and cortisol.
References
- Abbeduto L, Seltzer MM, Shattuck P, Krauss M, Orsmond G, Murphy M. Psychological well-being and coping in mothers of youths with autism, Down syndrome, or fragile X syndrome. American Journal on Mental Retardation. 2004;109:237–254. doi: 10.1352/0895-8017(2004)109<237:PWACIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Allen EG, Sherman S, Abramowitz A, Leslie M, Novak G, Rusin M, et al. Examination of the effect of the polymorphic CGG repeat in the FMR1 gene on cognitive performance. Behavioral Genetics. 2005;35:435–445. doi: 10.1007/s10519-005-2792-4. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The Daily Inventory of Stressful Events: An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Aziz M, Stathopolu E, Callias M, Taylor C, Turk J, Oostra BA, et al. Clinical features of boys with fragile X premutations and intermediate alleles. American Journal of Medical Genetics - Part B. 2003;121B:119–127. doi: 10.1002/ajmg.b.20030. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr., Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30:49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr., Hatton DD, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal on Mental Retardation. 1998;103:29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr., Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders. 2001a;31:165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Tassone F, Skinner M, Taylor AK. Variability in FMRP and early development in males with fragile X syndrome. American Journal on Mental Retardation. 2001b;106:16–27. doi: 10.1352/0895-8017(2001)106<0016:VIFAED>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr., Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics. 2008;146A:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Roberts JE, Hooper SR, Mirrett PL, Roberts JE, Schaaf JM. Research on fragile X syndrome and autism. Implications for the study of genes, environments, and developmental language disorders. In: Rice M, Warren SF, editors. Genes, environments, and language disorders. Mahwah, NH: Lawrence Erlbaum; 2004. pp. 121–150. [Google Scholar]
- Baum A, Schaeffer MA, Lake RC. Psychological and endocrinological correlates of chronic stress at Three Mile Island. In: Williams RB, editor. Perspectives on behavioral medicine, Vol. 2: Neuroendocrine control and behavior. NY: Academic Press; 1985. pp. 201–217. [Google Scholar]
- Baumgardner TL, Reiss AL, Freund LS, Abrams MT. Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics. 1995;95:744–752. [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosciences. 2004;27 doi: 10.1016/j.tins.2004.04.009. 370-277. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience. 2006;9:1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Goetz CG, Leehey MM, Hagerman RJ, Zhang L, Li L, et al. Neuropathic features in fragile X premutation carriers. American Journal of Medical Genetics. 2207;143:19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Bregman JD, Leckman JF, Ort SI. Fragile X syndrome: Genetic predisposition to psychopathology. Journal of Autism and Developmental Disorders. 1988;18:343–354. doi: 10.1007/BF02212191. [DOI] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. The MIDUS National Survey: An overview. In: Brim OG, Ryff CD, Kessler RC, editors. How Healthy Are We: A National Study of Well-Being at Midlife. Chicago, IL: University of Chicago Press; 2004. pp. 1–36. [Google Scholar]
- Brown WT. The molecular biology of the fragile X mutation. In: Hagerman R, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment and research. 3rd ed. Baltimore: Johns Hopkins University Press; 2002. pp. 110–135. [Google Scholar]
- Brown WT, Friedman E, Jenkins EC, Brooks J, Wisniewski K, Raguthu S, French JH. Association of fragile X with autism. Lancet. 1982:100. doi: 10.1016/s0140-6736(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Nolin SL, Sudhalter V, Ding X, Dobkin CS, Brown WT. Mosaicism for the FMR1 gene influences adaptive skills development in fragile X-affected males. American Journal of Medical Genetics. 1996;64:365–369. doi: 10.1002/(SICI)1096-8628(19960809)64:2<365::AID-AJMG26>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Munir F, Cross G. Differential impact of the FMR-1 full mutation on memory and attention functioning: A neuropsychological perspective. Journal of Cognitive Neuroscience. 2001;13:144–150. doi: 10.1162/089892901564126. [DOI] [PubMed] [Google Scholar]
- Cornish KJ, Turk J, Hagerman RJ. Annotation: The fragile X continuum: New advances and perspectives. Journal of Intellectual Disability Research. 2008;52:469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crnic KA, Greenberg MT. Minor parenting stresses with young children. Child Development. 1990;61:1628–1637. doi: 10.1111/j.1467-8624.1990.tb02889.x. [DOI] [PubMed] [Google Scholar]
- Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ. Heterozygous fragile X female: Historical, physical, cognitive, and cytogenetic features. American Journal of Medical Genetics. 1991;38:269–274. doi: 10.1002/ajmg.1320380221. [DOI] [PubMed] [Google Scholar]
- DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: Psychological and social resources as mediators. Journal of Personality and Social Psychology. 1988;54:486–495. doi: 10.1037//0022-3514.54.3.486. [DOI] [PubMed] [Google Scholar]
- Demark J, Feldman M, Holden J. Behavioral relationship between autism and fragile X syndrome. American Journal on Mental Retardation. 2003;108:314–326. doi: 10.1352/0895-8017(2003)108<314:BRBAAF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJP, et al. Evidence for impaired activation of the Hypothalamic-Pituitary-Adrenal Axis in patients with chronic fatigue syndrome. Journal of Clinical Endocrinology and Metabolism. 1991;73:1224–1234. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute strsessors and cortisol response: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dykens E, Hodapp R, Ort S, Finucane B. The trajectory of cognitive development in males with fragile X syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:422–426. doi: 10.1097/00004583-198905000-00020. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen A, Seltzer MM, Abbeduto L. Family well-being in Down syndrome and fragile X syndrome. In: Roberts JE, Warren SF, Chapman RS, editors. Speech and language development and interventions in Down syndrome and fragile X syndrome. Baltimore: Brookes; 2007. pp. 275–295. [Google Scholar]
- Franke P, Maier W, Hautzinger M, Weiffenbach O, Gansicke M, Iwers B, et al. Fragile-X carrier females: Evidence for a distinct psychopathological phenotype? American Journal of Medical Genetics. 1996;64:334–339. doi: 10.1002/(SICI)1096-8628(19960809)64:2<334::AID-AJMG20>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Freund LS, Reiss AL. Cognitive profiles associated with the fra(X) syndrome in males and females. American Journal of Medical Genetics. 1991;38:542–547. doi: 10.1002/ajmg.1320380409. [DOI] [PubMed] [Google Scholar]
- Freund LS, Reiss AL, Abrams MT. Psychiatric disorders associated with fragile X in the young female. Pediatrics. 1993;91:321–329. [PubMed] [Google Scholar]
- Gallagher S, Phillips AC, Drayson MT, Carroll D. Parental caregivers of children with developmental disabilities mount a poor antibody response to pneumococcal vaccination. Brain, Behavior, and Immunity. 2008 doi: 10.1016/j.bbi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Glidden LM, Schoolcraft SA. Depression: Its trajectory and correlates in mothers rearing children with intellectual disability. Journal of Intellectual Disability Research. 2003;47:250–263. doi: 10.1046/j.1365-2788.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Goodlin-Jones BL, Tassone F, Gane LW, Hagerman RJ. Autism spectrum disorder and the fragile X premutation. Journal of Developmental and Behavioral Pediatrics. 2004;25:392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Griep EN, Boersma JW, de Kloet RE. Altered reactivity of the hypothalamic-pituitary-adrenal axis in the primary fibromyalgia syndrome. Journal of Rheumatology. 1993;20:469–474. [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) Journal of Neurological Science. 2006;25:227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vasquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Neurodevelopmental disorders. Oxford: Oxford University Press; 1999. [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile X premutation: A maturing perspective. American Journal of Human Genetics. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: A model for autism and neurodegeneration. Current Opinion in Psychiatry. 2005;18:490–496. doi: 10.1097/01.yco.0000179485.39520.b0. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr., Roberts J, et al. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics. 2006;140:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport J, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. The Journal of the American Medical Assoication. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hessl D, Ribvera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, et al. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130:404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey M, Gane LW, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. American Journal of Medical Genetics. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proceedings of the National Academy of Sciences. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SC, Mason J, Kosten TR, Kasl SV, Ostfeld AM, Wahby V. Urinary free cortisol and separation anxiety early in the course of bereavement and threatened loss. Biological Psychiatry. 1987;22:148–152. doi: 10.1016/0006-3223(87)90225-3. [DOI] [PubMed] [Google Scholar]
- Johnston C, Eliez S, Dyer-Friedman J, et al. Neurobehavioral phenotype in carriers of the fragile X premutation. American Journal of Medical Genetics. 2001;103:314–319. [PubMed] [Google Scholar]
- Johnston C, Hessl D, Blasey c, Eliez S, Erba H, Dyer-Friedman J, et al. Factors associated with parenting stress in mothers of children with fragile X syndrome. Journal of Developmental and Behavioral Pediatrics. 2003;24:267–275. doi: 10.1097/00004703-200308000-00008. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau ASM, Bukelis I, Tierney E, Gray RM, et al. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proceedings of the National Academy of Sciences. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Strain EC, Stout JC, Tarr KL, Holliday JE, et al. Modulation of cellular immunity in medical students. Journal of Behavioral Medicine. 1986;9:5–21. doi: 10.1007/BF00844640. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klintsova A, Greenough W. Synaptic plasticity in cortical systems. Current Opinion in Neurobiology. 1999;9:203–208. doi: 10.1016/s0959-4388(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Kwon H, Menon V, Eliez S, Warsofsky IS, White CD, Dyer-Friedman J, et al. Functional neuroanatomy of visuospatial working memory in fragile X syndrome: Relation to behavioral and molecular measures. American Journal of Psychiatry. 2001;158:1040–1051. doi: 10.1176/appi.ajp.158.7.1040. [DOI] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, et al. Cognitive, language, and social-cognitive skills of individuals with fragile X syndrome with and without autism. Journal of Intellectual Disability Research. 2006;50:532–545. doi: 10.1111/j.1365-2788.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Bui QM, Epstein RM, Taylor AK, Hagerman RJ. Effects of the deficits of fragile X mental retardation protein on cognitive status of fragile X males and females assessed by robust pedigree analysis. Developmental and Behavioral Pediatrics. 2002;23:416–423. doi: 10.1097/00004703-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Mental Retardation Developmental Disabilities Research Reviews. 2004;10:31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, et al. Molecular and cognitive predictors of the continuum of autistic behaviors in fragile X. Neuroscience Biobehavioral Review. 2007;31:315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounds J, Seltzer MM, Greenberg JS, Shattuck P. Transition and change in adolescents and young adults with autism: Longitudinal effects on maternal well-being. American Journal on Mental Retardation. 2007;112:401–417. doi: 10.1352/0895-8017(2007)112[401:TACIAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Mazzocco MMM, Pennington B, Hagerman RJ. The neurocognitive phenotype of female carriers of fragile X: Further evidence for specificity. Journal of Development and Behavioral Pediatrics. 1993;14:328–335. [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, De Vries GJ, Gersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Menon V, Kwon H, Eliez S, Taylor AK, Reiss AL. Functional brain activation during cognition is related to FMR1 gene epression. Brain Research. 2000;877:367–370. doi: 10.1016/s0006-8993(00)02617-2. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Daly EM, Schmitz N, Tassone F, Tysoe C, Hagerman RJ, et al. A neuropsychological investigation of male premutation carriers of fragile X syndrome. Neuropsychologia. 2004;42:1934–1937. doi: 10.1016/j.neuropsychologia.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Abbeduto L. Indirect genetic effects and the early language development of children with genetic mental retardation syndromes: The role of joint attention. Infants and Young Children. 2005;18:47–59. [Google Scholar]
- Nolin SL, Glicksman A, Houck GE, Jr., et al. Mosaicism in fragile X affected males. American Journal of Medical Genetics. 1994;51:509–512. doi: 10.1002/ajmg.1320510444. [DOI] [PubMed] [Google Scholar]
- Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AS. Effect of chronic stress associated with unemployment on salivary cortisol: Overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosomatic Medicine. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Oostra B, Willemsen R. A fragile balance: FMRI expression levels. Human Molecular Genetics. 2003;12:249–257. doi: 10.1093/hmg/ddg298. [DOI] [PubMed] [Google Scholar]
- Orland KM, Griffith R, Abbeduto L, Brown WT, Dobkin CS. Maternal emotional well-being in mothers of adolescents with fragile X syndrome; Poster presented at the International Fragile X Conference; St. Louis, MO. 2008. Jul July, [Google Scholar]
- Ornstein PA, Schaaf JM, Hooper SR, Hatton D, Mirrett P, Bailey DB., Jr. Memory skills of boys with fragile X syndrome. American Journal on Mental Retardation. 2008;113:453–465. doi: 10.1352/2008.113:453-465. [DOI] [PubMed] [Google Scholar]
- Orsmond GI, Lin LY, Seltzer MM. Mothers of adolescents and adults with autism: The contribution of disability in siblings to maternal well-being and family adjustment. Intellectual and Developmental Disabilities. 2007;45:257–270. doi: 10.1352/1934-9556(2007)45[257:MOAAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Bailey DB, Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, et al. Mood and anxiety disorders in females with the FMR1 premutation. American Journal of Medical Genetics, B Neuropsychiatric Genetics. doi: 10.1002/ajmg.b.30786. (in press) [DOI] [PubMed] [Google Scholar]
- Rogers S, Wehner E, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2001;22:409–418. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Sabaratnam M, Murthy NV, Wijeratne A, Buckingham A, Payne S. Autistic-like behaviour profile and psychiatric morbidity in Fragile X Syndrome: a prospective ten-year follow-up study. European Child and Adolescent Psychiatry. 2003;12:172–177. doi: 10.1007/s00787-003-0333-3. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Scott LV, Dinan TG. Urinary free cortisol excretion in chronic fatigue syndrome, major depression and in healthy volunteers. Journal of Affective Disorders. 1998;47:49–54. doi: 10.1016/s0165-0327(97)00101-8. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Stawski RS, Hong J, et al. Psychosocial and biological markers of daily lives of midlife parents of children with disabilities. Journal of Health and Social Behavior. doi: 10.1177/002214650905000101. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Floyd FJ, Pettee Y, Hong J. Life course impacts of parenting a child with a disability. American Journal of Mental Retardation. 2001;106:265–286. doi: 10.1352/0895-8017(2001)106<0265:LCIOPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Singer BH, Ryff CD, editors. New horizons in health: An integrative approach. Washington, DC: National Academy Press; [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. Association of FMR1 repeat size with ovarian dysfunction. Human Reproduction. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. American Journal of Medical Genetics. 2000;97:195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. The Journal of Clinical Endocrinology and Metabolism. 2004;89:4569–4574. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- Wisbeck J, Huffman L, Freund L, Gunnar M, Davis E, Reiss A. Cortisol and social stressors in children with fragile X: A pilot study. Journal of Developmental and Behavioral Pediatrics. 2000;21:278–282. doi: 10.1097/00004703-200008000-00004. [DOI] [PubMed] [Google Scholar]