Abstract
Heritable germline epimutations in MSH2 have been reported in a small number of Lynch Syndrome families which lacked germline mutations in the MSH2 gene. It is not known whether somatic MSH2 methylation occurs in MSH2 mutation-positive Lynch Syndrome subjects or sporadic colorectal cancers (CRCs). Therefore, we determined the methylation status of the MSH2 gene in 268 CRC tissues, including 222 sporadic CRCs and 46 tumors that did not express MSH2. We also looked for microsatellite instability (MSI), germline mutations in the MSH2 and EpCAM genes, somatic mutations in BRAF and KRAS, and the CpG island methylator phenotype (CIMP). We observed that somatic MSH2 hypermethylation was present in 24% (11 of 46) of MSH2-deficient (presumed Lynch Syndrome) tumors, while no evidence for MSH2 methylation existed in sporadic CRCs (MSI and MSS) or normal colonic tissues. Seven of 11 (63%) patients with MSH2 methylation harbored simultaneous pathogenic germline mutations in the MSH2 gene. Germline EpCAM deletions were present in 3 of 4 patients with MSH2 methylation but without pathogenic MSH2 germline mutations. The mean methylation scores at CIMP-related markers were significantly higher in Lynch Syndrome tumors with MSH2 methylation than MSH2-unmethylated CRCs. In conclusion, our data provide evidence for frequent MSH2 hypermethylation in Lynch Syndrome tumors with MSH2 deficiency. MSH2 methylation in this subset of individuals is somatic, and may serve as the ‘second hit’ at the wild type allele. High levels of aberrant methylation at CIMP-related markers in MSH2 methylated tumors raises the possibility that MSH2 is a target susceptible to aberrant methylation in Lynch Syndrome.
Keywords: Colon cancer, MSH2, Lynch Syndrome, DNA methylation, EpCAM, DNA mismatch repair
Introduction
Lynch Syndrome (previously called hereditary non-polyposis colorectal cancer or HNPCC) is an autosomal dominant colorectal cancer susceptibility syndrome characterized by germline mutations in DNA mismatch repair (MMR) genes, most frequently in MLH1 and MSH2, and less often in MSH6 and PMS2 (1–3). Mutational inactivation of MMR genes lead to insufficient DNA repair and the development of tumors with high levels of microsatellite instability (MSI-H), which is a characteristic feature of >95% of Lynch Syndrome associated colorectal cancers (CRC) (4, 5). Patients with Lynch Syndrome typically demonstrate a germline mutation and somatic inactivation of the wild type allele of the relevant MMR gene through a second event that is either a mutation or deletion of the wild-type allele.
The MLH1 gene is methylated in ~12% of sporadic CRCs (6), giving rise to a MSI-H phenotype with similar clinico-pathological features as hereditary tumors (7–11). It is believed that these sporadic MSI CRCs evolve through the CpG island methylator phenotype (CIMP) pathway, in which MLH1 is one of multiple different targets of transcriptional inactivation (12–14).
Recent discoveries have suggested a novel paradigm, in which the DNA MMR genes MLH1 and MSH2 can be targets of ‘germline methylation’ in some individuals with Lynch Syndrome (15–18). The first evidence for this came from studies in which MLH1 was found to be methylated in the peripheral blood and other germline tissues in Lynch Syndrome patients who did not carry germline MLH1 mutations (16, 17, 19). More recently, heritable germline epimutations in MSH2 were reported in a few mutation-negative Lynch Syndrome families (18, 20). Subsequent studies have revealed that germline deletions at the 3’-end of the EpCAM gene (formerly called TACSTD1), located immediately upstream of MSH2, is the cause of this heritable somatic epimutation (21).
In spite of the growing interest in ‘germline’ epigenetic regulation of MMR genes in Lynch Syndrome CRC, to the best of our knowledge, no study has investigated the role of ‘somatic’ MSH2 promoter methylation in the pathogenesis of sporadic MSI and MSH2-deficient Lynch Syndrome tumors. In view of this gap in understanding, we studied a group of 46 MSH2-deficient Lynch Syndrome CRCs for germline mutations and the methylation status of the MSH2 gene, and deletions in the EpCAM gene. In addition, we studied MSH2 methylation in a cohort of 222 sporadic CRCs, which included 15 sporadic MSI tumors. Herein, we report that somatic MSH2 methylation is frequent in Lynch Syndrome CRCs, and may constitute the ‘second hit’ required to inactivate the wild-type MSH2 allele. Furthermore, we found excessive methylation at CIMP-related loci in MSH2 methylated Lynch Syndrome CRCs, which suggests that MSH2 is a frequent susceptibility target of aberrant methylation in the colon.
Materials and Methods
Tissue specimens
This study analyzed a cohort of 268 CRCs, which included 222 sporadic cancers and 46 Lynch Syndrome tumors. All 222 sporadic CRCs, which included 15 sporadic MSI cancers were enrolled at the Okayama University Hospital, Okayama, Japan. Tumor tissues from 46 Lynch syndrome CRCs lacking MSH2 expression were obtained from Heidelberg University, Heidelberg, Germany (22). The patients were classified to have a Lynch syndrome associated CRC if either a pathogenic germline mutation was identified in the MSH2 gene or if the patients fulfilled Bethesda/Amsterdam criteria and presented with one or more MSI-H CRCs that lacked expression of the MLH1 or MSH2 protein by immunohistochemistry. Similarly, patients deemed to have a sporadic MSI-positive CRC when they failed to fulfil criteria for hereditary cancer, but showed loss of MLH1 protein expression and associated methylation of the promoter region (Supplementary Table 1). In the cohort of 46 MSH2-deficient CRCs, DNA was available from 35 cases for germline mutation analysis of the MSH2 gene. Germline deletion analysis at the 3’- end of the EpCAM gene was performed on all samples that showed MSH2 methylation (n=11). Patients provided informed consent for use of their tissues, and Institutional Review Boards of both institutions approved this study.
Microsatellite instability analysis
MSI analysis was performed by examination of the NCI-workshop panel of five markers, which included two mononucleotide repeats (BAT25 and BAT26) and three dinucleotide repeat (D2S123, D5S346 and D17S250)(23). Tumors showing allelic shifts in ≥2/5 markers were classified as MSI-H (hereupon referred to as “MSI”), and the rest were classified as MSS. Utilizing this criterion, all 46 MSH2-deficient CRCs were MSI. Of the 222 sporadic CRCs, 15 cases were MSI and the remaining 207 cancers were MSS.
Immunohistochemical staining (IHC) of MLH1 and MSH2 proteins
IHC staining was performed to determine protein expression for the MLH1 and MSH2 proteins in all Lynch syndrome and sporadic MSI cases. IHC staining was performed on formalin-fixed paraffin-embedded tissues, using the tyramide signal amplification biotin system (PerkinElmer, Boston, MA). Briefly, after deparaffinization and rehydration, antigen retrieval was achieved by immersing the tissue sections in citrate buffer (pH 6.0) and exposed to microwave irradiation for 20 minutes. Thereafter, tissue sections were blocked for endogenous peroxidase in phosphate-buffered saline containing 3% H2O2, and the sections were incubated for 3 h with a monoclonal antibody for hMLH1 (clone G 128-728, 1/100, BD PharMingen, San Diego, CA) or hMSH2 (clone G219-1129, 1/3,000, BD PharMingen). Negative control slides were incubated with phosphate buffer instead of a specific antibody. This step was followed by further incubations in secondary antibody (Vector Laboratories, Burlingame, CA), streptavidin-peroxidase and biotinyl tyramide. The final brown coloration for both MMR proteins was developed using diaminobenzidine as a chromogen, and hematoxylin as a nuclear counterstain. Sections with obvious nuclear staining were deemed positive. Tumor tissues were considered negative only when there was a clear evidence for positive staining in the surrounding non-neoplastic tissues including normal colonic epithelium, lymphocytes or stromal cells.
Germline mutation analysis of the MSH2 gene
MSH2 germline mutation analyses were performed by initial prescreening for mutations by denaturing high performance liquid chromatography, followed by mutation confirmation through direct sequencing as described previously (22, 24). A systematic search for large genomic deletions was performed using multiplex ligation-dependent probe amplification (MLPA) according to the manufacturer’s protocol (MRC-Holland, Amsterdam, The Netherlands) (22).
Sodium bisulfite modification and CIMP analyses
Genomic DNA from tumor tissues and the corresponding normal mucosa of all 46 MSH2-deficient Lynch Syndrome CRCs, 15 sporadic MSI CRCs, and 207 sporadic MSS CRCs was available for methylation analyses. Genomic DNA was bisulfite modified to convert all the unmethylated cytosine residues to uracils. Briefly, 0.5–2.0 µg of DNA was denatured with NaOH, treated with sodium bisulfite, and purified using the Wizard DNA Clean-up System (Promega, Madison, WI). The methylation status of MLH1, p16INK4a, p14ARF, MINT1, MINT2, and MINT31 CIMP markers was evaluated by Combined Bisulfite Restriction Analysis (COBRA) as described previously (14). Following densitometric quantification of methylated and unmethylated bands, tumors with ≥5% methylation at each marker were considered methylation-positive, while tumors with low background levels of methylation, which may be present in some normal appearing colorectal mucosa (<5%), were defined as methylation-negative as described previously (14).
Methylation analysis of the MSH2 promoter region
Methylation status of the MSH2 promoter CpG island was investigated by COBRA and bisulfite sequencing procedures. Supplementary Table 2 lists the primer sequences and PCR conditions for both methodologies. PCR reactions for COBRA were carried out on bisulfite-modified template DNA in a 25 µl PCR mixture containing 12.5 µl of HotStarTaq Master Mix kit (Qiagen, Valencia, CA), 0.5 µM of each PCR primer, and approximately 25 ng of bisulfite-modified DNA. The PCR products were digested with TaqI or HpyCH4IV (New England Biolabs Inc, Ipswich, MA) at 65°C or 37°C for 16h, respectively. The digested DNA was separated on 3% agarose gels in 1× TAE buffer and stained with ethidium bromide. A Gel Logic 200 Imaging System (Eastman Kodak Co., Rochester, NY) was used to perform densitometric analyses on all gels. Band intensities were quantified using Kodak 1D analysis software (Eastman Kodak Co., Rochester, NY). The methylation levels (ratios of methylated to unmethylated DNA) were determined from the relative intensities of cut and uncut PCR products to quantify methylation. As with the CIMP markers described previously, tumors with ≥5% MSH2 methylation were considered methylation-positive, while tumors with low background levels of MSH2-methylation (<5%) were defined as methylation-negative. Human normal colonic DNA treated with SssI methylase (New England Biolabs Inc) was used as a positive control for methylated alleles, whereas DNA from normal lymphocytes was used as a control for unmethylated alleles. Water was used as a negative PCR control to monitor for PCR contamination.
To confirm the methylation profiles obtained by COBRA, bisulfite sequencing for the MSH2 promoter region was performed in a subgroup of MSH2 methylated and unmethylated tumors. For bisulfite sequencing, nested PCR was performed in a 25 µl PCR mixture containing 12.5 µl of HotStarTaq Master Mix kit (Qiagen) and appropriate concentrations of PCR primers (primer sequences are shown in Supplementary Table 2). PCR products were purified using a QIAquick PCR purification kit (Qiagen) and sequenced on an ABI 3100-Avant DNA sequencer.
EpCAM deletion analysis
The EpCAM gene was screened for the deletion of exons 3, 8 and 9 using the SALSA MLPA Kit P072-B1 from MRC-Holland (Amsterdam, The Netherlands) according to the manufacturer’s instructions. DNA from a healthy donor without a deletion at the 3’ end of EpCAM was used as a negative control for MLPA analysis.
BRAF and KRAS mutation analyses
Direct sequencing was performed to identify BRAF exon15 (V600E) mutations and KRAS exon 2 (codon 12/13) mutations. PCR for the BRAF and the KRAS genes was carried out in a 2 5 µl PCR mixture containing 12.5 µl of HotStarTaq Master Mix kit (Qiagen) with concentrations of primers, listed in Supplementary Table 1, as previously described (14). PCR products were purified using the QIAquick PCR purification kit (Qiagen) and directly sequenced on an ABI 3100-Avant DNA sequencer.
Statistical analyses
The methylation status of MSH2 and other loci as determined by COBRA was analyzed as a categorical variable (methylated = methylation level ≥5%, unmethylated = methylation level <5%). MSH2-deficient CRCs were divided into subgroups according to MSH2 methylation status, and were analyzed for potential associations with a number of clinico-pathological and epigenetic parameters using the Χ2 test. Methylation scores were calculated based upon the total number of loci methylated at CIMP markers. The differences in mean methylation scores between MSH2 methylated and unmethylated cases were analyzed by the Kruskal-Wallis test. If the Kruskal-Wallis test indicated differences among various CRC subgroups, further pair-wise comparisons for each of the subgroups were performed using the Steel-Dwass test, which is a non-parametric multiple comparison method. All reported P values are 2-sided and a P <.05 was considered statistically significant.
Results
MSH2 is frequently methylated in Lynch Syndrome, but not sporadic, CRCs
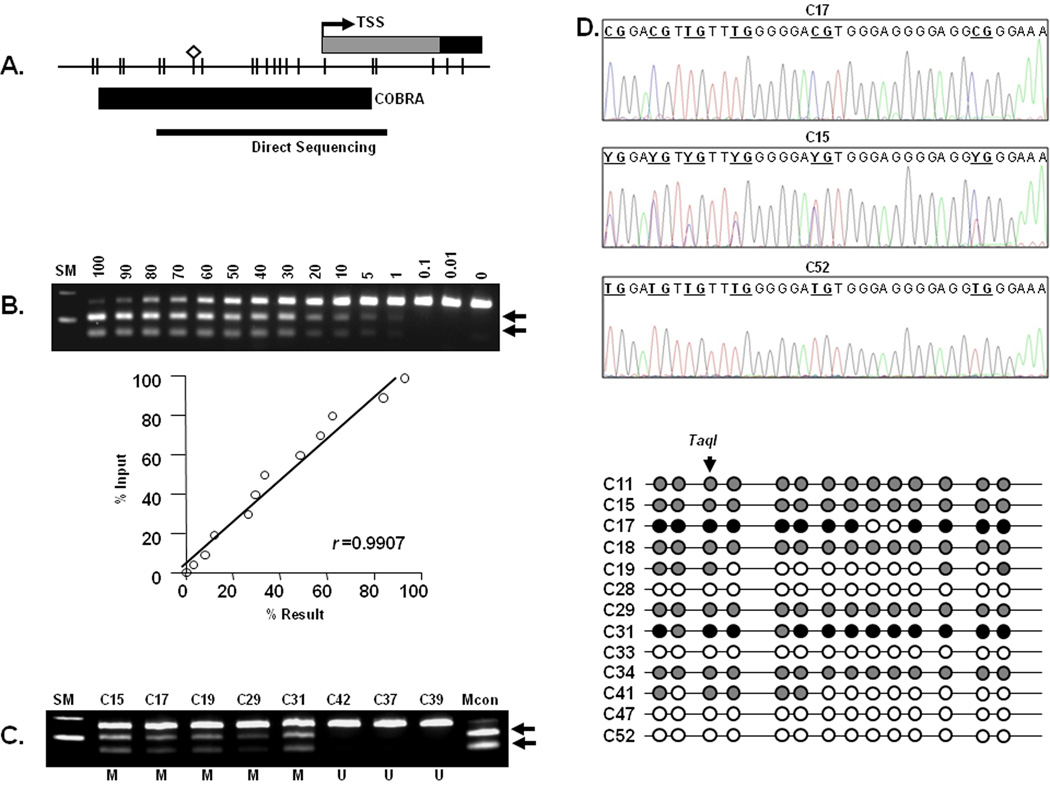
In this study, using COBRA, we investigated the MSH2 methylation status in tumor and non-neoplastic tissues from 46 MSH2-deficient cancers, 15 sporadic MSI patients, and 207 MSS CRCs. The COBRA for MSH2 was designed and optimized to examine methylation levels of a CpG site located at −73 bp from the transcription start site (TSS) of the MSH2 promoter (Figure 1A). This CpG site was identified through bisulfite sequencing analysis of a larger region of the MSH2 promoter, and is within the same segment of the promoter reported to be methylated in a recent publication (18). The methylation levels were quantitated, and the lower limit of measurable methylation was ≥1% (Figures 1B and 1C).
Figure 1.
A. Schematic depiction of the MSH2 CpG island surrounding the transcription start site (TSS, black arrow): CpG dinucleotides are represented as short vertical lines. Location of the combined bisulfite restriction analysis (COBRA) amplicon and bisulfite genomic sequencing PCR products are indicated by thick and thin horizontal lines, respectively. The diamond represents the Taq1 recognition site.
B. Determination of the quantitative accuracy of COBRA for the MSH2 CpG island: As shown in the upper panel, COBRA was performed with the TaqI restriction endonuclease. Genomic DNA treated with SssI methylase (methylated) and normal control lymphocytes (unmethylated) were mixed at various ratios to determine the quantitative nature of the methylation assay. The numbers above each lane indicate the percentage of methylation. Arrows represent methylated alleles that are cleaved by TaqI; SM = size marker.
The lower panel is an illustration of linear regression analysis for the methylation results shown in above panel: The calculated DNA methylation percentages are plotted as a function of the percentage input DNA treated with SssI. The correlation coefficient is 0.9907 for the TaqI digests, indicating that this is a robust assay.
C. Aberrant MSH2 methylation in MSH2-deficient colorectal tumors: The numbers on top of each lane indicate various CRCs. Arrows indicate methylated alleles digested by TaqI. SM, size marker; Mcon, methylated control; M, methylated; U, unmethylated
D. Representative examples of MSH2 promoter bisulfite sequencing: The figures in the upper 3 panels illustrate representative examples of CRCs showing different degrees of methylation by direct bisulfite sequencing: case C17 from a methylated CRC shows methylated cytosines (indicated as CG) that remained cytosines following bisulfite conversion; case C15 with hemi-methylated CpGs (indicated as YG) showing overlapping C and T alleles; and case 52, from an unmethylated CRC showing CpG sites as TG as a result of bisulfite modification
The lower panel summarizes bisulfite sequencing results from 13 cases (9 methylated and 4 unmethylated) at all 14 CpG sites analyzed in the MSH2 promoter: White circles indicate unmethylated CpG sites (e.g. C28 and C33), gray circles indicate partially methylated CpG sites (e.g. C11 and C15) and black circles indicate fully methylated CpG sites (e.g. C17 and C31).
Among the group of 46 MSH2-deficient Lynch syndrome CRCs, 11 (24%) cases showed somatic hypermethylation in the MSH2 promoter (Figure 1D and Table 1). On the other hand, none of the 222 sporadic tumors that included both sporadic MSI cancers and MSS tumors demonstrated MSH2 promoter methylation. Similarly, we did not see any evidence for MSH2 methylation in the entire collection of matching normal mucosal DNA from the 46 MSH2-deficient CRC patients.
Table 1.
Summary of MSH2 expression, methylation, germline mutations and the associations with other clinical and genetic factors in 46 MSH2-deficient Lynch Syndrome CRCs
| Case | Age | Location | MSH2 Protein expression |
MSH2 Methylation |
Germline MSH2 mutationa |
Germline EpCAM mutationa |
BRAF | KRAS |
|---|---|---|---|---|---|---|---|---|
| C19 | 31 | Distal | — | + | c.840_841insT | — | none | c.35G>A |
| C11 | 40 | unknown | — | + | c.2291G>A | — | none | none |
| C52 | 52 | Proximal | — | + | c.2584G>T | — | none | c.38G>A |
| C18 | 31 | unknown | — | + | c.28C>T | — | none | c.38G>A |
| C29 | 50 | unknown | — | + | c.795delT | — | none | none |
| C41 | 42 | Proximal | — | + | c.2575G>T | — | none | none |
| C34 | 32 | unknown | — | + | deletion (exon 3–7) | n. a. | none | c.38G>A |
| C01 | 53 | unknown | — | — | c.1720C>T | n. a. | none | c.38G>A |
| C12 | 32 | unknown | — | — | c.1906G>C | n. a. | none | none |
| C13 | 67 | unknown | — | — | exon 12, 4bp deletion | n. a. | none | none |
| C14 | 39 | Distal | — | — | c.28C>T | n. a. | none | none |
| C22 | 43 | Proximal | — | — | c.973_974insT | n. a. | none | none |
| C25 | 42 | Distal | — | — | c.972_973ins184 | n. a. | none | c.38G>A |
| C27 | 62 | Proximal | — | — | c.416delA | none | none | |
| C03 | 58 | unknown | — | — | c.942+3A>T | n. a. | none | c.35G>A |
| C32 | 46 | unknown | — | — | c.942+3A>T | n. a. | none | none |
| C35 | 36 | Proximal | — | — | c.942+3A>T | n. a. | none | none |
| C36 | 46 | Proximal | — | — | c.2237_2240delTCAT | n. a. | none | none |
| C37 | 47 | Distal | — | — | c.2584G>T | n. a. | none | none |
| C39 | 37 | Proximal | — | — | deletion (exon 1–2) | n. a. | none | none |
| C04 | 37 | unknown | — | — | c.2006-1G>C | n. a. | none | none |
| C42 | 40 | unknown | — | — | c.1835C>G | n. a. | none | none |
| C08 | 55 | unknown | — | — | c.1077-2A>G | n. a. | none | none |
| C09 | 26 | Distal | — | — | c.30_31insA | n. a. | none | c.38G>A |
| C15 | 28 | unknown | — | + | unclassified variant (c.4G>A) | + | none | none |
| C17 | 45 | unknown | — | + | unclassified variant (c.4G>A) | + | none | none |
| C26 | 39 | unknown | — | — | unclassified variant (c.942G>A) | n. a. | none | none |
| C07 | 33 | unknown | — | — | unclassified variant (c.1316_1318delCTC) | n. a. | none | none |
| C31 | 60 | Proximal | — | + | none | + | none | none |
| C16 | 30 | unknown | — | — | none | n. a. | none | none |
| C02 | 61 | unknown | — | — | none | n. a. | none | none |
| C23 | 45 | Proximal | — | — | none | n. a. | none | none |
| C40 | 26 | unknown | — | — | none | n. a. | none | none |
| C43 | 35 | Distal | — | — | none | n. a. | none | none |
| C44 | 49 | Proximal | — | — | none | n. a. | none | none |
| C47 | 46 | Distal | — | + | n. a. | – | none | c.35G>A |
| C28 | 51 | Proximal | — | — | n. a. | n. a. | none | none |
| C30 | 35 | Proximal | — | — | n. a. | n. a. | none | none |
| C38 | 49 | Proximal | — | — | n. a. | n. a. | none | none |
| C45 | 44 | Proximal | — | — | n. a. | n. a. | none | none |
| C48 | 61 | Distal | — | — | n. a. | n. a. | none | c.38G>A |
| C49 | 45 | Distal | — | — | n. a. | n. a. | none | c.35G>A |
| C05 | 43 | unknown | — | — | n. a. | n. a. | none | none |
| C50 | 69 | Proximal | — | — | n. a. | n. a. | none | none |
| C51 | 65 | Distal | — | — | n. a. | n. a. | none | none |
| C06 | 21 | unknown | — | — | n. a. | n. a. | none | none |
n.a.=not analyzed.
To further support and confirm the methylation data obtained by COBRA, we next performed direct bisulfite sequencing in a subset of 13 MSH2-deficient CRCs (9 cases with MSH2 methylation and 4 cases without MSH2 methylation: Figures 1D). This approach allowed us to further confirm the methylation profile of the 14 CpG sites that are located between the −93bp and +32 bp region of the MSH2 promoter. All 9 CRCs which showed methylation by COBRA also demonstrated widespread MSH2 promoter methylation when analyzed by bisulfite sequencing (Figure 1D). On the contrary, congruent with our COBRA results, none of the MSH2-proficient tumors showed any evidence for MSH2 methylation at any of the CpG dinucleotides within the promoter’s CpG island.
MSH2 promoter methylation may constitute the second hit in MSH2-deficient Lynch Syndrome CRCs
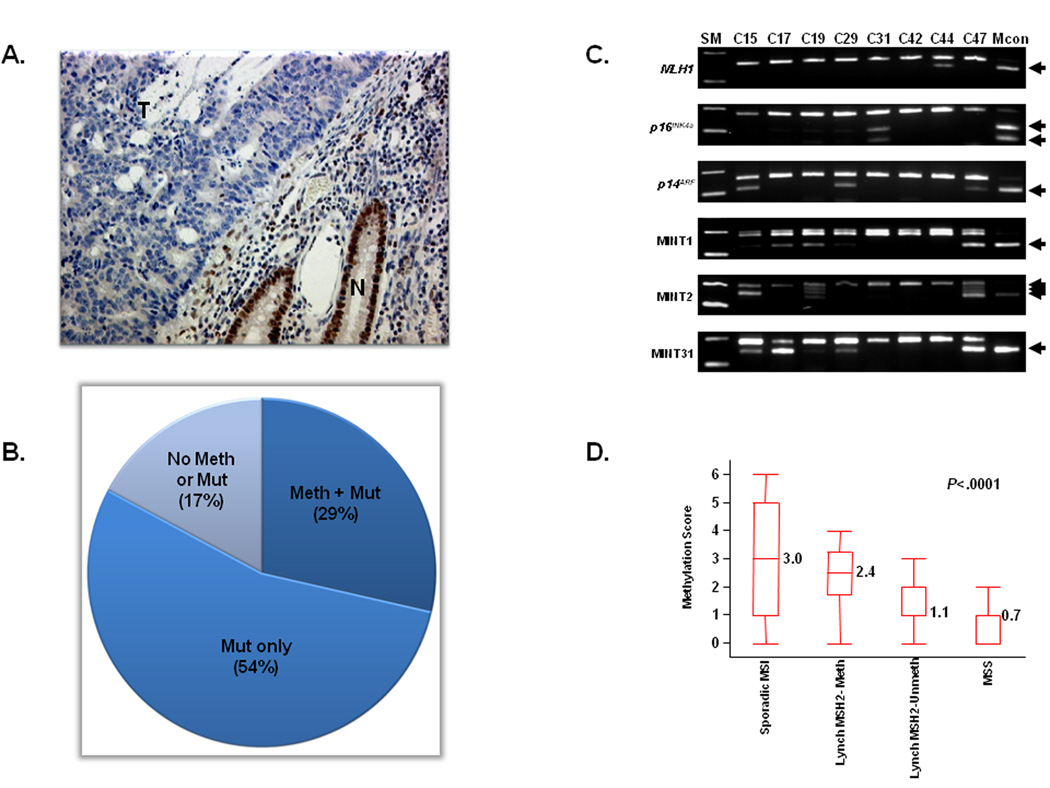
We next questioned the relevance of MSH2 methylation in the context of other genetic alterations in MMR-deficient CRCs. Since evidence for MSH2 methylation was only present in MSH2-deficient tumors, we looked for germline mutations in 35 of 46 patients from which germline DNA was available for mutational analysis. Table 1 summarizes the clinical, genetic and epigenetic data from all 46 MSH2-deficient CRCs, and Figure 2A illustrates a representative example of absent MSH2 expression in a MMR-deficient tumor. Eighty percent of patients (28 of 35) had a germline alteration in the MSH2 gene. Among these, 24 cases had well-established pathogenic mutations, while the remaining 4 cases harbored unclassified variants in the MSH2 gene: two patients with c.4G>A mutations, one with a c.942G>A mutation, and one individual had c.1316_1318delCTC. In silico analysis using the Polyphen prediction tool (http://genetics.bwh.harvard.edu/pph/) revealed c.4G>A mutation to be "possibly damaging", while c.942G>A mutation was considered "silent" (25). Consequently, of the 24 Lynch Syndrome patients with a confirmed germline mutation in MSH2, 7 cases (29%) displayed the simultaneous presence of both a pathogenic germline mutation and somatic promoter methylation of the MSH2 gene, suggesting MSH2 promoter hypermethylation serves as a ‘second hit’ in these tumors.
Figure 2.
A. MSH2 immuno-histochemical staining: A representative example of a CRC with intact MSH2 expression indicated by brown nuclear staining in the normal epithelial cells (N), while there is loss of MSH2 expression in the tumor tissue (T) in the same patient.
B. Frequencies of germline mutations in MSH2 and EpCAM genes and MSH2 promoter methylation in MSH2-deficient CRCs: The pie chart illustrates data from 35 MSH2-deficient CRCs with a confirmed germline mutation in the MSH2 or EpCAM genes or the presence of MSH2 promoter methylation. Ten cases (29%) showed both germline mutations and promoter methylation, 19 cases (54%) harbored only germline mutations in MSH2, and 6 cases (17%) did not have either a germline mutations in either gene or promoter methylation of MSH2.
C. Representative examples of aberrant methylation in the 6 CIMP-related markers: The number in the top panel indicates various CRCs. Arrows indicate methylated alleles. SM, size marker; Mcon, methylated control
D. Mean methylation scores in various subgroups of CRCs: The mean methylation scores in each subset of CRCs were calculated by analyzing 6 CIMP-related loci (MLH1, p16INK4a, p14ARF, MINT1, MINT2, and MINT31). In the box-plot diagrams, the horizontal line within each box represents the median. The limits of each box are the inter-quartile ranges. The whiskers are the maximum and minimum values. The numbers next to each box denote the mean methylation score. The P values above the square panels were based on Kruskal-Wallis 1-way analyses of variance on ranks. Statistical differences among any two individual groups are shown as pairwise comparisons in Table 4.
EpCAM deletions in somatic MSH2-methylated CRCs
Recent reports have proposed a role for deletion of the 3’-end of the EpCAM gene as a mechanism for MSH2 methylation in Lynch Syndrome subjects who do not have germline mutations in MSH2. In our group of MSH2-deficient Lynch Syndrome CRCs, 7 of 11 cases with MSH2 methylation also had simultaneous pathogenic MSH2 germline mutations in the other allele, while the remaining 4 cases (patients C15, C17, C31 and C47, Table 1) either did not show pathogenic germline mutations, or we did not have sufficient materials to perform mutation analysis. In an attempt to understand the underlying cause for the somatic MSH2 methylation in these tumors, we studied EpCAM deletions in these 11 MSH2 methylation-positive patients. Three of the four patients without pathogenic germline MSH2 mutations showed evidence for EpCAM deletion. This is consistent with the observations from previous studies that deletions in this gene are rare, and represent a mechanism for MSH2 methylation in a small proportion of Lynch Syndrome patients that show ‘germline’ methylation in this MMR gene.
Overall, of the 35 MSH2-deficient Lynch syndrome cases which were analyzed for for germline mutations in MSH2 and EpCAM genes, 10 patients (29%) showed simultaneous presence of MSH2 hypermethylation and mutation in either the MSH2 or EpCAM genes, 19 cases (54%) had only germline MSH2 mutations, while 6 subjects (17%) had neither germline mutations nor MSH2 promoter methylation (Figure 2B). Since 70% (7 of 10) of patients with MSH2 methylation also harbored germline mutations in this gene, this clearly suggest that methylation was the second inactivating event in these tumors.
MSH2 methylated CRCs share features of CIMP CRCs
Since frequent hypermethylation of many genes is one of the characteristic features of tumors with CIMP, we next determined associations between MSH2 hypermethylation and various clinical, genetic and epigenetic factors. Since CIMP is present in a majority of sporadic MSI CRCs (due to MLH1 methylation) and as many as 30–40% of sporadic MSS cancers(13), we also studied detailed associations between sporadic MSI or MSS subgroups of tumors and the MSH2-deficient Lynch Syndrome cases (Table 2 and 3). As expected, patients with sporadic MSI and MSS cancers were significantly older than the MSH2-deficient Lynch Syndrome cases. Sporadic MSI tumors were more frequent in females than males (sporadic MSI, 60%; MSH2-deficient Lynch Syndrome CRCs 33%; MSS, 35%). In addition, 92% of sporadic MSI and 60% of MSH2-deficient Lynch Syndrome CRCs were located in the proximal colon, in contrast to 30% of MSS cancers.
Table 2.
Association between MSH2 methylation and various clinical, genetic and epigenetic factors in MSH2-deficient Lynch syndrome CRCs
| MSH2 Methylation %(n) | |||||
|---|---|---|---|---|---|
| Methylated | Unmethylated | P value | |||
| (n=11) | (n=35) | ||||
| Clinical | Age | ≥65 | 0 (0) | 9 (3) | 0.3152* |
| Factor | <65 | 100 (11) | 91 (32) | ||
| Gender | Female | 18 (2) | 37 (13) | 0.2419* | |
| Male | 82 (9) | 63 (22) | |||
| Location | Proximal | 60 (3) | 60 (12) | 1.0* | |
| Distal | 40 (2) | 40 (8) | |||
| Missing data | (6) | (15) | |||
| Genetic | MSH2/EpCAM Mutation | Mutation | 100 (10) | 76 (19) | 0.3496* |
| Factor | Status | No mutation | 0 (0) | 24 (6) | |
| Not analyzed | (1) | (10) | |||
| BRAF/KRAS | BRAF mutant | 0 (0) | 0 (0) | 0.1001* | |
| Mutation | KRAS mutant | 45 (5) | 17 (6) | ||
| Status | Both wild type | 55 (6) | 83 (29) | ||
| Epigenetic | MLH1 | Methylated | 0 (0) | 6 (2) | 0.4176* |
| Factor | Unmethylated | 100 (11) | 94 (33) | ||
| p16INK4a‡ | Methylated | 20 (2) | 6 (2) | 0.1722* | |
| Unmethylated | 80 (0) | 94 (32) | |||
| p14ARF‡ | Methylated | 50 (5) | 21 (7) | 0.0664* | |
| Unmethylated | 50 (5) | 79 (27) | |||
| MINT1‡ | Methylated | 60 (6) | 26 (9) | 0.0493* | |
| Unmethylated | 40 (4) | 74 (25) | |||
| MINT2‡ | Methylated | 60 (6) | 6 (2) | <0.0001* | |
| Unmethylated | 40 (4) | 94 (32) | |||
| MINT31‡ | Methylated | 50 (5) | 47 (16) | 0.8700* | |
| Unmethylated | 50 (5) | 53 (18) | |||
P values were calculated by the X2 test.
Table 3.
Frequency of DNA methylation at each epigenetic Marker in CRCs and its association with MSH2 expression and MSI Status
| MSH2 Deficient CRC | MSH2 Proficient CRC | |||||
|---|---|---|---|---|---|---|
| Lynch Syndrome (n=46) |
Sporadic MSI (n=15) |
MSS (n=207) |
P value | |||
| Clinical | Age | ≥65 | 7 (3) | 80 (12) | 51 (105) | <0.0001 a |
| Factor | <65 | 93 (43) | 20 (3) | 49 (102) | <0.0001 b | |
| 0.0283 c | ||||||
| Gender | Female | 33 (15) | 60 (9) | 35 (73) | 0.0372 a | |
| Male | 67 (31) | 40 (6) | 65 (134) | 0.864b | ||
| 0.0553c | ||||||
| Location | Proximal | 60 (15) | 92 (12) | 30 (60) | 0.0597a | |
| Distal | 40 (10) | 8 (1) | 70 (143) | 0.0022 b | ||
| Missing data | (21) | (2) | (4) | <0.0001 c | ||
| Genetic | KRAS/BRAF | BRAF mutant | 0 (0) | 67 (10) | 5 (10) | <0.0001 a |
| Factor | Mutation | KRAS mutant | 24 (11) | 0 (0) | 35 (73) | 0.0724b |
| Status | Both wild type | 76 (35) | 33 (5) | 60 (124) | <0.0001 c | |
| Epigenetic | MSH2 | Methylated | 24 (11) | 0 (0) | 0 (0) | 0.0364 a |
| Factor | Unmethylated | 76 (35) | 100 (15) | 100 (207) | <0.0001 b | |
| 1.0c | ||||||
| MLH1 | Methylated | 4 (2) | 60 (9) | 0 (0) | <0.0001 a | |
| Unmethylated | 96 (44) | 40 (6) | 100 (207) | 0.00259 b | ||
| <0.0001 c | ||||||
| p16INK4a * | Methylated | 9 (4) | 53 (8) | 16 (33) | 0.000237 a | |
| Unmethylated | 91 (40) | 47 (7) | 84 (174) | 0.2444b | ||
| 0.0003 c | ||||||
| p14ARF * | Methylated | 27 (12) | 33 (5) | 8 (16) | 0.6545a | |
| Unmethylated | 73 (32) | 67 (10) | 92 (191) | 0.00018 b | ||
| 0.0011 c | ||||||
| MINT1* | Methylated | 34 (15) | 40 (6) | 9 (19) | 0.6798a | |
| Unmethylated | 66 (29) | 60 (9) | 91 (188) | <0.0001 b | ||
| 0.0003 c | ||||||
| MINT2* | Methylated | 18 (8) | 53 (8) | 20 (41) | 0.00818a | |
| Unmethylated | 82 (36) | 47 (7) | 80 (166) | 0.8049b | ||
| 0.0025 c | ||||||
| MINT31* | Methylated | 48 (21) | 60 (9) | 19 (40) | 0.4116a | |
| Unmethylated | 52 (23) | 40 (6) | 81 (167) | <0.0001 b | ||
| 0.0002 c | ||||||
All P values were calculated by the Χ2 test.
P values were calculated between MSH2 deficiency vs. sporadic MSI
P values were calculated between MSH2 deficiency vs. MSS cancers.
P values were calculated between sporadic MSI vs. MSS.
Two cases were not analyzed in MSH2 deficiency cases.
BRAF mutations were frequently present in sporadic MSI tumors (67%), but were seldom present in MSS tumors (5%), and did not occur at all in MSH2-deficient Lynch Syndrome CRCs (0%). However, KRAS mutations were never present in sporadic MSI cancers (0%), whereas 24% of MSH2-deficient Lynch Syndrome and 35% of MSS tumors harbored KRAS mutations.
We next investigated the methylation status of 6 CIMP-related loci (MLH1, p16INK4a, p14ARF, MINT1, MINT2, and MINT31; Figure 2C and Table 3) in all 268 CRCs, which included all MMR-deficient and –proficient CRCs. Not surprisingly, most sporadic MSI CRCs displayed a significant degree of methylation at all CIMP markers. Interestingly, we observed marked methylation at most CIMP-related markers in the MSH2 methylated tumors, which was statistically significant at the MINT1 and MINT2 loci, when compared with MSH2 unmethylated cancers (Table 3). Of note, none of the MSH2-methylated cancers showed MLH1 methylation, raising the possibility that inactivation of either MMR gene has similar functional consequences. For a better understanding of the role of MSH2 methylation in the context of CIMP and sporadic MSI, we calculated the combined mean methylation scores based upon the number of CIMP-related markers methylated in each of the subgroups. As shown in Figure 2D, the mean methylation score was highest in sporadic MSI tumors (3.0; 95% confidence interval [CI], 1.9–4.1), followed by MSH2-methylated Lynch Syndrome tumors (2.4; 95%CI, 1.5–3.3), MSH2-unmethylated Lynch Syndrome tumors (1.1; 95%CI, 0.9–1.4), and was lowest in MSS tumors (0.7; 95%CI, 0.6–0.9). When we performed nonparametric multiple pairwise comparisons for the mean methylation scores in various subgroups of CRCs (Table 4), we noted that while the mean methylation scores were statistically different in each pairwise comparison, the scores were very similar when the results were compared between sporadic MSI and MSH2-methylated cancers. Collectively, the somatic MSH2 methylation observed in Lynch Syndrome tumors indicates that MSH2 is an important target of aberrant methylation, and that this is an important consideration in the pathogenesis of CRCs in Lynch Syndrome-MSH2 type.
Table 4.
Pair-wise comparisons of methylation scores with between sporadic MSI and MSH2-deficient Lynch syndrome CRCs and MSH2 methylation status
| No. | Mean Methylation Score (95%CI) | |
|---|---|---|
| Sporadic MSI | 15 | 3.00 (1.89–4.11) |
| Lynch Syndrome with MSH2 methylation | 10 | 2.40 (1.50–3.30) |
| Lynch Syndrome with MSH2 unmethylation | 34 | 1.12 (0.86–1.37) |
| MSS | 207 | 0.72 (0.58–0.86) |
| Pairwise Comparison | P value* |
|---|---|
| Sporadic MSI vs. Lynch Syndrome with MSH2 Methylation | 0.8684 |
| Sporadic MSI vs. Lynch Syndrome with MSH2 Unmethylation | 0.0044 |
| Sporadic MSI vs. MSS | <0.0001 |
| Lynch Syndrome with MSH2 Methylation vs. Lynch Syndrome with MSH2 Unmethylation | 0.0033 |
| Lynch Syndrome with MSH2 Methylation vs. MSS | 0.0001 |
| Lynch Syndrome with MSH2 Unmethylation vs. MSS | 0.0024 |
P values were based on Steel-Dwass test.
Discussion
Recent evidence for germline MSH2 methylation in mutation-negative Lynch Syndrome patients with CRC prompted us to investigate whether MSH2 may also be a target of somatic hypermethylation in the CRC tissues of patients with Lynch Syndrome-MSH2 type. For this study, we analyzed a collection of 268 CRCs, which included 46 MSH2-deficient presumed Lynch Syndrome CRCs, 15 sporadic MSI CRCs, and 207 sporadic MSS CRCs. Prior to methylation analysis, we performed MSH2 germline mutational analysis and noted that 80% of MSH2-deficient presumed Lynch patients harbored germline mutations in this gene (thus proving Lynch Syndrome-MSH2 type). However, our subsequent analysis provides evidence that 24% (11/46) of MSH2-deficient Lynch Syndrome patients display MSH2 hypermethylation in their tumor tissues. Moreover, 63% (7/11) of MSH2 methylated CRCs had a simultaneous pathogenic germline MSH2 mutation, suggesting that methylation may be the required second inactivating event in these tumors. No evidence for MSH2 methylation was observed in normal tissues available for analysis or any of the sporadic CRCs, indicating that this epigenetic alteration occurs in a disease-specific manner. Additionally, while interrogating associations between MSH2 methylation and methylation at multiple CIMP-related markers, we discovered that MSH2-methylated tumors also possessed markedly higher levels of promoter methylation, suggesting that the MSH2 promoter may be a particular target of aberrant methylation in Lynch Syndrome CRCs.
Germline mutations in the DNA MMR genes MLH1 and MSH2 are the most frequent causes of Lynch Syndrome. However, in order for a tumor to arise in these individuals, the other, wild-type allele needs to be inactivated according to Knudson’s “two-hit” hypothesis. This second hit can be a genetic alteration resulting in a deletion or somatic mutation (26, 27), or can be an epigenetic alteration, a mechanism that has not been rigorously investigated in Lynch Syndrome tumors. Epigenetic inactivation of MLH1 is the primary cause of sporadic MSI CRCs (which make up at least 12% of all CRCs), and germline epimutations in MLH1 gene have been described in some Lynch Syndrome patients (16, 17, 19). Likewise, recent evidence indicates that the MSH2 gene is another target of germline epimutations in some MSH2 mutation-negative Lynch Syndrome individuals (21, 28). However, it is not clear whether MSH2 methylation is strictly a germline event, or whether it can occur on a somatic basis.
Almost a decade ago, the first efforts to study the epigenetic regulation of the MSH2 gene in CRC produced negative results, and investigators failed to observe evidence for MSH2 methylation in a small cohort of sporadic primary CRCs(10). Since this report, no study has investigated MSH2 methylation in a large group of colorectal tumors and normal colonic mucosal tissues. Similar to the previous report, our study did not find MSH2 methylation in any sporadic MSI CRCs or normal colonic tissues. However, we found that 24% (11 of 46) of the MSH2-deficient Lynch Syndrome cases showed aberrant methylation in the MSH2 promoter. Interestingly, of the 24 MSH2-deficient Lynch Syndrome tumors with a confirmed pathogenic germline mutation, 29% of the tumors had a simultaneous germline mutation and promoter methylation, suggesting that methylation is the “second hit”, fulfilling Knudson’s two-hit hypothesis in these MSH2-deficient Lynch Syndrome CRCs.
Our results suggest that the MSH2-methylation observed in our collection of Lynch Syndrome tumors is a somatic event which is present in ~30% of MSH2-deficient Lynch syndrome CRCs, and is distinct from the previous reports where it was shown to be a heritable germline MSH2 epimutation (21). There are several logical explanations for this new paradigm. First, the evidence for MSH2 methylation in our collection of Lynch Syndrome CRCs was primarily present in patients with germline mutations in MSH2, rather than in germline mutation negative cases as reported previously (20, 21). The essential observation is that MSH2 methylation co-existed with a germline mutation in 63% of our Lynch Syndrome-MSH2 type CRCs. Second, in our collection of Lynch Syndrome patients with MSH2 deficiency, we did not find this epigenetic defect in the matched normal mucosa of MSH2-methylated tumors, arguing against a germline defect in these individuals. Third, we detected EpCAM deletions in only 3 of 11 patients presenting with MSH2 hypermethylation, which further supports the notion that MSH2 hypermethylation in our patients occurred in a somatic manner, and through a different mechanism than what has been previously reported (20, 21).
This concept derives further support from our data that sought associations between MSH2 hypermethylation and aberrant methylation of six classical CIMP-related markers (MLH1, p16INK4a, p14ARF, MINT1, MINT2, and MINT31) in MSH2-deficient Lynch Syndrome CRCs and sporadic MSI tumors. In this regard, our collection of tumors showed lower frequency (7%) of MSI-positive tumors. Although the frequency of MSI CRCs in our study were somewhat lower compared to 12–15% rates reported in Caucasian populations, our results are in general agreement with lower MSI frequencies typically observed in the Japanese and Spanish populations. To our surprise, we found that the mean methylation scores at CIMP-related markers were higher in MSH2-methylated Lynch Syndrome tumors compared with unmethylated tumors. Pairwise comparison analysis further revealed that although slightly higher, there were no significant differences in mean methylation scores between sporadic MSI (i.e., MLH1-methylated) and MSH2-methylated (Lynch Syndrome) CRCs. These data underscore the contribution of aberrant methylation to the evolution of Lynch Syndrome adenomas (29) and CRCs (14). Of note, the high levels of methylation observed in sporadic MSI and MSH2-methylated Lynch syndrome tumors, where the MLH1 and MSH2 genes serve as targets of aberrant methylation respectively, indicate the functional significance of somatic epigenetic inactivation of these genes in the pathogenesis of two completely different subtypes of CRC.
Although these findings provide new insights into the molecular pathogenesis of Lynch Syndrome CRCs, there are limitations to the interpretation of our work that may require attention in future investigations. We have studied a reasonably large collection of MSH2-deficient Lynch Syndrome CRCs, however, studies with larger numbers of documented Lynch Syndrome-MSH2 type are required to validate our results. Although our data suggest that somatic methylation of MSH2 can provide the second hit in Lynch Syndrome CRCs, due to lack of adequate materials and technical limitations, we were unable to prove whether the germline mutations and methylation occurred on two separate alleles in the MSH2 gene. The timing of somatic MSH2 methylation is unknown, and future studies of MSH2-deficient adenomas in patients with known germline mutations may reveal if it is an early event.
In conclusion, this study provides previously unrecognized evidence for relatively frequent aberrant methylation of the MSH2 gene promoter in the CRCs of patients with Lynch Syndrome-MSH2 type. More importantly, we discovered that the aberrant MSH2 methylation in these tumors was not a germline event, but evolved in a somatic manner. Furthermore, similar to MLH1 methylation in sporadic MSI and CIMP-positive CRCs, the existence of somatic MSH2 methylation in some proportion of Lynch Syndrome CRCs that do not express MSH2 protein may serve as a surrogate marker for aberrant methylation, and hereditary CRC. Considering the technical and scientific challenges in identifying novel germline mutations in the MSH2 gene that might help explain MSH2 deficiency in individuals without an identifiable germline mutation in that gene, our data suggest that the detection of MSH2 methylation may be useful in properly identifying and classifying such individuals. Finally, since epigenetic events are potentially reversible, the early diagnosis of MSH2 methylation in suspected Lynch Syndrome patients may have prognostic implications, a concept that mandates further exploration in the future.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by NIH grants CA72851 and CA129286 and funds from the Baylor Research Institute to CRB and AG.
Abbreviations
- MMR
DNA mismatch repair
- CRC
colorectal cancer
- CIMP
CpG island methylator phenotype
- MSI
microsatellite instability
- HNPCC
hereditary nonpolyposis colorectal cancer
Footnotes
Disclosures: None of the authors have any potential conflicts to disclose.
Reference List
- 1.Lynch HT, de la CA. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 2.Peltomaki P, de la Chapelle A. Mutations predisposing to hereditary nonpolyposis colorectal cancer. Adv Cancer Res. 1997;71:93–119. doi: 10.1016/s0065-230x(08)60097-4. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos N, Lindblom A. Molecular basis of HNPCC: mutations of MMR genes. Hum Mutat. 1997;10:89–99. doi: 10.1002/(SICI)1098-1004(1997)10:2<89::AID-HUMU1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Aaltonen LA, Peltomaki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 5.Peltomaki P, Lothe RA, Aaltonen LA, et al. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993;53:5853–5855. [PubMed] [Google Scholar]
- 6.Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- 7.Liu B, Nicolaides NC, Markowitz S, et al. Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nat Genet. 1995;9:48–55. doi: 10.1038/ng0195-48. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaides NC, Papadopoulos N, Liu B, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371:75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- 9.Leach FS, Nicolaides NC, Papadopoulos N, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- 10.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagasaka T, Sasamoto H, Notohara K, et al. Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing different patterns of DNA methylation. J Clin Oncol. 2004;22:4584–4594. doi: 10.1200/JCO.2004.02.154. [DOI] [PubMed] [Google Scholar]
- 12.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goel A, Nagasaka T, Arnold CN, et al. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology. 2007;132:127–138. doi: 10.1053/j.gastro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Nagasaka T, Koi M, Kloor M, et al. Mutations in both KRAS and BRAF may contribute to the methylator phenotype in colon cancer. Gastroenterology. 2008;134:1950–1960. doi: 10.1053/j.gastro.2008.02.094. 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suter CM, Martin DI. Inherited epimutation or a haplotypic basis for the propensity to silence? Nat Genet. 2007;39:573. doi: 10.1038/ng0507-573a. [DOI] [PubMed] [Google Scholar]
- 16.Hitchins MP, Wong JJ, Suthers G, et al. Inheritance of a cancer-associated MLH1 germ-line epimutation. N Engl J Med. 2007;356:697–705. doi: 10.1056/NEJMoa064522. [DOI] [PubMed] [Google Scholar]
- 17.Suter CM, Martin DI, Ward RL. Germline epimutation of MLH1 in individuals with multiple cancers. Nat Genet. 2004;36:497–501. doi: 10.1038/ng1342. [DOI] [PubMed] [Google Scholar]
- 18.Chan TL, Yuen ST, Kong CK, et al. Heritable germline epimutation of MSH2 in a family with hereditary nonpolyposis colorectal cancer. Nat Genet. 2006 doi: 10.1038/ng1866. [DOI] [PubMed] [Google Scholar]
- 19.Gazzoli I, Loda M, Garber J, Syngal S, Kolodner RD. A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the MLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res. 2002;62:3925–3928. [PubMed] [Google Scholar]
- 20.Kovacs ME, Papp J, Szentirmay Z, Otto S, Olah E. Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to Lynch syndrome. Hum Mutat. 2009;30:197–203. doi: 10.1002/humu.20942. [DOI] [PubMed] [Google Scholar]
- 21.Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet. 2009;41:112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 22.Mangold E, Pagenstecher C, Friedl W, et al. Spectrum and frequencies of mutations in MSH2 and MLH1 identified in 1,721 German families suspected of hereditary nonpolyposis colorectal cancer. Int J Cancer. 2005;116:692–702. doi: 10.1002/ijc.20863. [DOI] [PubMed] [Google Scholar]
- 23.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 24.Goecke T, Schulmann K, Engel C, et al. Genotype-phenotype comparison of German MLH1 and MSH2 mutation carriers clinically affected with Lynch syndrome: a report by the German HNPCC Consortium. J Clin Oncol. 2006;24:4285–4292. doi: 10.1200/JCO.2005.03.7333. [DOI] [PubMed] [Google Scholar]
- 25.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuen ST, Chan TL, Ho JW, et al. Germline, somatic and epigenetic events underlying mismatch repair deficiency in colorectal and HNPCC-related cancers. Oncogene. 2002;21:7585–7592. doi: 10.1038/sj.onc.1205968. [DOI] [PubMed] [Google Scholar]
- 27.Tannergard P, Liu T, Weger A, Nordenskjold M, Lindblom A. Tumorigenesis in colorectal tumors from patients with hereditary non-polyposis colorectal cancer. Hum Genet. 1997;101:51–55. doi: 10.1007/s004390050585. [DOI] [PubMed] [Google Scholar]
- 28.Niessen RC, Hofstra RM, Westers H, et al. Germline hypermethylation of MLH1 and EPCAM deletions are a frequent cause of Lynch syndrome. Genes Chromosomes Cancer. 2009;48:737–744. doi: 10.1002/gcc.20678. [DOI] [PubMed] [Google Scholar]
- 29.Kaz A, Kim YH, Dzieciatkowski S, et al. Evidence for the role of aberrant DNA methylation in the pathogenesis of Lynch syndrome adenomas. Int J Cancer. 2007;120:1922–1929. doi: 10.1002/ijc.22544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.