Abstract
Dehydrozingerone analogs and related compounds were screened as potential antitumor promoters by using the in vitro short-term 12-O-tetradecanphorbol-13-acetate (TPA)-induced Epstein-Barr virus early antigen (EBV-EA) activation assay. Among 40 synthesized compounds, the prenylated analogs 16 and 34–36 showed the most significant and promising activity (100% inhibition of activation at 1×10 3 mol ratio/TPA, and 82–80%, 37–35%, 13–11% inhibition at 5×102, 1×102, 1×10 mol ratio/TPA, respectively) in this screening. Their activity profiles were comparable to that of the reference standard curcumin. While a prenyl moiety conferred potent chemopreventive activity, an extended prenyl unit such as a farnesyl moiety did not improve activity. Because in vitro inhibitory effects in this assay generally correlate well with in vivo inhibitory effects on tumor promotion, our results strongly suggested that prenylated 16 and 34–36 are likely to be promising antitumor promoters.
Keywords: dehydrozingerone, antitumor-promoting effect, Epstein-Barr virus, two-stage carcinogenesis
1. Introduction
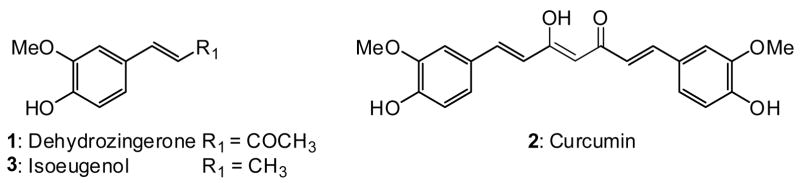
The natural product dehydrozingerone (DZ, 1) is the “half analog” of curcumin (2) (Figure 1), which is known to have potent anti-oxidant, anti-inflammatory, and antitumor promoting (chemopreventive) activities [1,2]. Curcumin inhibits epidermal inflammation in mice and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced tumor promotion in mouse skin [3]. Structure-activity relationship (SAR) correlations of curcumin analogs as antitumor promoting agents have also been investigated [4,5]. Dehydrozingerone (1) and isoeugenol (3), which have similar catechol skeletons, but different alkenyl side chains, had stronger antitumor promotion effects than curcumin [6]. In the search for antitumor promoters from natural sources, the antitumor promoting properties of phenylpropanoids have also been reported [7–9]. The above evidence strongly supports the use of 1 as a lead to develop novel antitumor promoters and to further explore the structural features necessary for chemopreventive activity. Because certain natural products and synthetic compounds containing a prenyl moiety showed strong activity against the Epstein-Barr virus early antigen (EBV-EA) activation induced by TPA in Raji cells [10–14], prenyl derivatives of 1 were thus synthesized and evaluated for in vitro inhibitory activity against EBV-EA. In this paper, we report the synthesis and SAR study of dehydrozingerone analogs as chemopreventive agents.
Figure 1.
Structures of Dehydrozingerone (1), Curcumin (2), and Isoeugenol (3)
2. Results and discussion
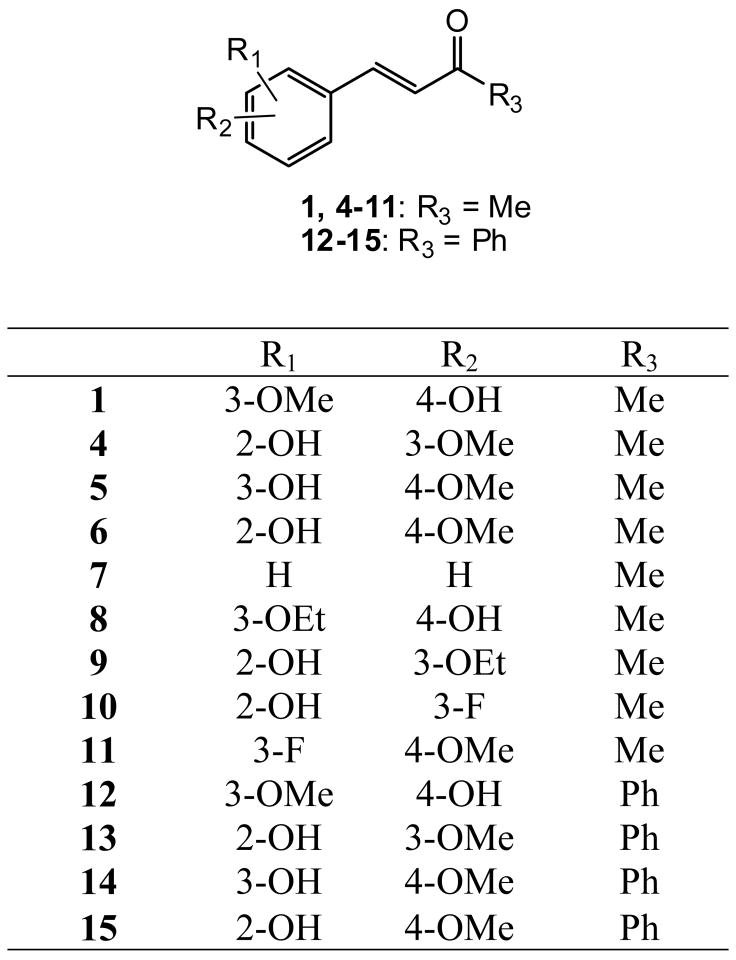
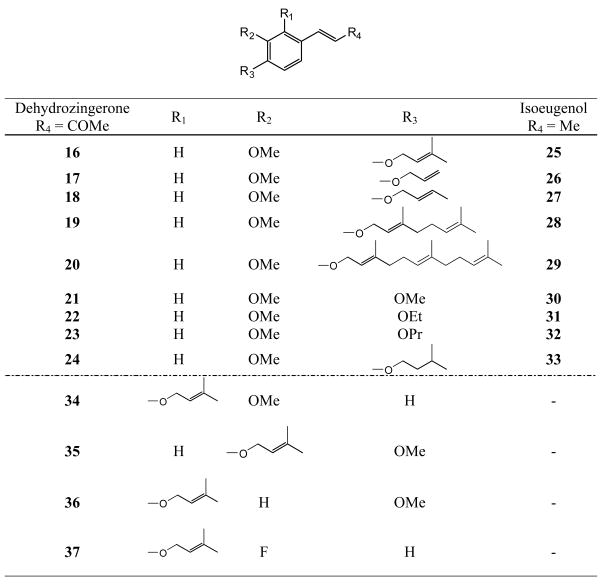
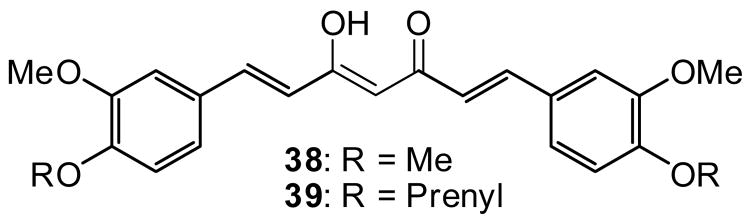
All compounds were previously synthesized [15]. Figure 2 shows the structures of analogs 4–11, which are derivatives of 1, and of the related chalcones 12–15, in which the terminal methyl group is replaced by phenyl. Figure 3 shows the structures of analogs 16–33, in which various types of alkyl and alkenyl groups were added to the C-4’ alcohol of 1 and 3, as well as four additional prenyloxy derivatives 34–37. The structures of methylated and prenylated curcumins (38 and 39, respectively) are shown in Figure 4. All analogs were evaluated in vitro as inhibitors of EBV-EA activation induced by TPA in Raji cells [16–18], and the inhibitory data are shown in Tables 1 and 2. Figure 5 indicates the typical fluorescent finding of EBV-EA activation in Raji cell through the fluorescence microscope.
Figure 2.
Structures of Deydrozingerone Analogs
Figure 3.
Structures of Dehydrozingerone (16–24, 34–37) and Isoeugenol (25–33) Analogs
Figure 4.
Curcumin Analogs 38 and 39
Table 1.
Relative ratioa of EBV-EA activation with respect to positive control in presence of dehydrozingerone analogs.
| Compound | Percentage EBV-EA positive cells |
IC50 | |||
|---|---|---|---|---|---|
| Compound concentration (mol ratio/TPAb) | |||||
| 1000 | 500 | 100 | 10 | ||
| Dehydrozingerone (1)c | 0 (70) c | 44.2 | 75.5 | 95.4 | 372 |
| 4 | 0 (70) | 46.7 | 77.7 | 97.6 | 380 |
| 5 | 0 (70) | 44.1 | 74.6 | 94.3 | 369 |
| 6 | 0 (70) | 45.8 | 76.9 | 96.3 | 378 |
| 7 | 0 (70) | 48.6 | 78.1 | 98.9 | 378 |
| 8 | 0 (70) | 42.2 | 73.7 | 93.1 | 368 |
| 9 | 0 (70) | 43.9 | 74.8 | 95.3 | 370 |
| 10 | 0 (60) | 51.3 | 79.4 | 98.0 | 385 |
| 11 | 0 (60) | 53.4 | 81.0 | 99.0 | 389 |
| 12 | 0 (60) | 37.2 | 79.4 | 100 | 379 |
| 13 | 0 (60) | 38.6 | 81.5 | 100 | 381 |
| 14 | 0 (60) | 37.9 | 80.7 | 100 | 380 |
| 15 | 0 (60) | 37.2 | 80.3 | 100 | 380 |
Values represent percentages relative to the positive control value (100%).
TPA concentration is 20 ng/mL (32 pmol/mL).
Values in parentheses are viability percentages of Raji cells.
Table 2.
Relative ratioa of EBV-EA activation with respect to positive control in presence of dehydrozingerone analogs and related compounds.
| Compound | Percentage EBV-EA positive cells |
IC50 | |||
|---|---|---|---|---|---|
| Compound concentration (mol ratio/TPAb) | |||||
| 1000 | 500 | 100 | 10 | ||
| Dehydrozingerone (1)c | 0±0.5 (70)d | 44.2±1.9 | 75.5±2.6 | 95.4±0.2 | 372 |
| 16 | 0±0.2 (60) | 19.0±1.3 | 65.2±2.0 | 88.7±0.4 | 216 |
| 17 | 0±0.4 (60) | 21.9±1.5 | 67.8±2.1 | 90.0±0.2 | 234 |
| 18 | 0±0.3 (60) | 20.7±1.5 | 66.9±2.1 | 89.3±0.3 | 220 |
| 19 | 0±0.4 (60) | 23.5±1.5 | 71.0±2.3 | 95.1±0.1 | 242 |
| 20 | 0±0.4 (60) | 24.9±1.3 | 73.9±2.3 | 97.7±0.2 | 250 |
| 21 | 0±0.4 (60) | 22.8±1.3 | 70.9±2.0 | 93.7±0.3 | 239 |
| 22 | 0±0.6 (60) | 38.5±1.7 | 72.0±2.3 | 92.1±0.2 | 368 |
| 23 | 0±0.2 (60) | 21.5±1.2 | 68.9±2.1 | 91.7±0.3 | 238 |
| 24 | 0±0.3 (60) | 20.1±1.3 | 66.2±2.0 | 89.0±0.3 | 220 |
| Isoeugenol (3)c | 16.7±1.5 (70) | 53.5±1.9 | 81.0±2.7 | 100±0.1 | 490 |
| 25 | 0±0.2 (60) | 19.9±1.1 | 66.0±1.9 | 89.5±0.4 | 219 |
| 26 | 0±0.4 (60) | 22.8±1.4 | 68.0±2.2 | 92.7±0.2 | 236 |
| 27 | 0±0.3 (60) | 21.8±1.3 | 67.3±2.1 | 90.3±0.3 | 234 |
| 28 | NAe | - | |||
| 29 | 0±0.5 (60) | 26.3±1.4 | 75.3±2.3 | 98.8±0.1 | 261 |
| 30 | 0±0.4 (60) | 23.6±1.5 | 72.0±2.3 | 95.6±0.2 | 246 |
| 31 | NA | - | |||
| 32 | 0±0.4 (60) | 22.7±1.3 | 69.3±2.3 | 92.8±0.1 | 240 |
| 33 | 0±0.4 (60) | 21.2±1.4 | 67.3±2.1 | 91.5±0.2 | 237 |
| 34 | 0±0.2 (60) | 19.1±1.1 | 64.8±2.0 | 88.0±0.4 | 222 |
| 35 | 0±0.2 (60) | 18.0±1.0 | 63.2±2.0 | 86.8±0.3 | 207 |
| 36 | 0±0.2 (60) | 19.7±1.1. | 63.2±2.0 | 86.8±0.3 | 219 |
| 37 | 0±0.2 (60) | 22.8±1.3 | 67.7±2.1 | 90.5±0.3 | 231 |
| Curcumin (2)c | 0±0.4 (60) | 21.1±1.1 | 80.1±2.4 | 100±0.1 | 379 |
| 38 | 0±0.2 (60) | 19.6±1.1 | 76.5±2.4 | 98.5±0.1 | 253 |
| 39 | 0±0.1 (60) | 17.3±0.9 | 72.6±2.1 | 92.4±0.3 | 240 |
Values represent percentages relative to the positive control value (100%).
TPA concentration is 20 ng/mL (32 pmol/mL).
Positive control
Values in parentheses are viability percentages of Raji cells.
Not applicable
Figure 5. Typical fluorescent findings of EBV-EA activation.
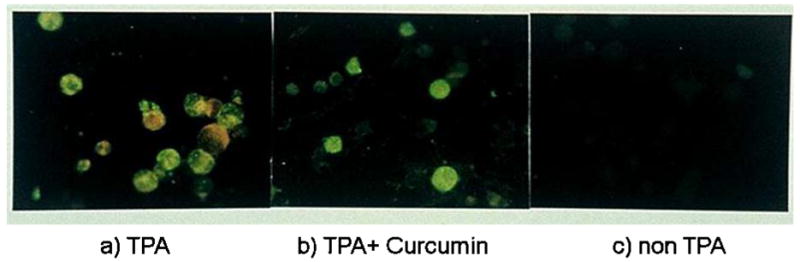
a) EBV-EA expression by treatment with TPA; b) EBV-EA expression was reduced by Curcumin; c) No EBV-EA expression was observed without TPA.
Compounds with 3,4-disubstituted benzalacetone structures (1, 5, and 8) showed slightly better activity than compounds with other disubstitution patterns (e.g. 2,3- or 2,4-). The data for 12–15 indicated that a phenyl group at C4 decreased the activity. No remarkable difference was observed between methoxy and ethoxy groups (1 vs. 8 and 4 vs. 9). However, by comparing 10 and 11 with 1 and 4–9, the presence of fluorine on the benzene ring might decrease the activity.
Compounds 16–24 (dehydrozingerone analogs), 25–33 (isoeugenol analogs), 34–37 (prenylated analogs), and 38 and 39 (curcumin analogs) were also tested using an in vitro synergistic assay on EBV-EA activation induced by TPA. The inhibitory effects of tested compounds and the associated viability of Raji cells are shown in Table 2. Curcumin and 3 were used as positive controls. In this assay, all compounds showed inhibitory effects on EBV-EA activation without high cytotoxicity on Raji cells. At high concentrations (1×103 mol ratio), dehydrozingerone (16–24 and 34–37), isoeugenol (25–33), and curcumin (38, 39) derivatives showed 100% inhibition, and at lower concentrations, were as or more potent than the parent compounds. The prenylated analogs, 16 and 25, showed significant potency compared with other alkylated analogs in the respective series (see 16–24 for dehydrozingerone analogs, and 25–33 for isoeugenol analogs). Prenylated dehydrozingerone analogs 34–37 showed comparable activity with 16, which showed the best activity in the alkylated series. These findings support the reported conclusions that a prenyl moiety is important for optimal inhibitory effects on EBV-EA activation [7–9]. Compound 37 was less active than 16 and 34–36, indicating that fluorine does not affect the activity. Although analog 19 containing a geranyl group (two prenyl units), was more active than 20 and 29 with farnesyl groups (three prenyl units), it was less active compared with other analogs in the dehydrozingerone series. Compounds 17, 18, 22–24 and 26, 27, 31, 32, which contain allyl, 2-butenyl, ethyl, propyl, and isopentyl substituents, respectively, showed similar activity, while methylated compounds 21 and 30 showed slightly lower activity. When analogs with structurally similar alkyl and alkenyl groups were compared, (16 vs 24, 17 and 18 vs 23), the presence of a double bond did not seem to affect the activity.
In summary, prenylated dehydrozingerone 16 and its analogs 34–36 showed the most significant and promising activity in this screening (100% inhibition of activation at 1×103 mol ratio/TPA, and 82–80%, 37–35%, 13–11% inhibition at 5×102, 1×102, 1×10 mol ratio/TPA, respectively). While a prenyl moiety conferred potent chemopreventive activity, an extended prenyl unit such a farnesyl moiety did not improve activity. Hydrophobicity might be important for inhibition of TPA-induced EBV-EA activation. Because in vitro inhibitory effects in this assay generally correlate well with in vivo inhibitory effects on tumor promotion [4,5,19,20], our results suggested that 16 and 34–36 are promising antitumor promoters and further in vivo investigations are now in progress.
3. Experimental
3.1 In vitro EBV-EA activation experiments
EBV-EA positive serum from a patient with nasopharyngeal carcinoma (NPC) was a gift from Professor H. Hattori, Department of Otorhinolaryngology, Kobe University. The EBV genome carrying lymphoblastoid cells (Raji cells derived from Burkitt’s lymphoma) were cultured in 10% fetal bovine serum (FBS) in RPMI-1640 medium (Sigma R8758, USA). Spontaneous activation of EBV-EA in our subline of Raji cells was less than 0.1%. The inhibition of EBV-EA activation was assayed using Raji cells (virus non-producer type) as described below. The cells were incubated at 37°C for 48 h in 1 mL of medium containing n-butyric acid (4 mM), TPA [32 pM = 20 ng in 2 μL dimethyl sulfoxide (DMSO)] and various amounts of the test compounds dissolved in 2 μL of DMSO. Smears were made from the cell suspension. The EBV-EA inducing cells were stained by the means of an indirect immunofluorescence technique. In each assay, at least 500 cells were counted, and the number of stained cells (positive cells) was recorded. Triplicate assays were performed for each compound. The average EBV-EA induction of the test compound was expressed as a ratio relative to the control experiment (100%), which was carried out with n-butyric acid (4 mM) plus TPA (32 pM). EBV-EA induction was ordinarily around 35%. The viability of treated Raji cells was assayed by the Trypan blue staining method. The cell viability of the TPA positive control was greater than 80%. Therefore, only these compounds that induced less than 80% (% of control) of the EBV-active cells (those with a cell viability of more than 60%) were considered able to inhibit the activation caused by promoter substances.
Acknowledgments
This investigation was supported in part by a grant CA 17625 from the National Cancer Institute awarded to K. H. Lee. This study was also supported in part by a grant from the Ministry of Education, Sciences, Sports and Cultures, and Ministry of Health and Welfare, Japan (Kyoto).
References
- 1.Sugiyama Y, Kawakishi S, Osawa T. Biochem Pharmacol. 1996;52:519. doi: 10.1016/0006-2952(96)00302-4. [DOI] [PubMed] [Google Scholar]
- 2.Rudy AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Cancer Lett. 1995;94:79. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 3.Huang MT, Smart RC, Wong CQ, Conney AH. Cancer Res. 1988;48:5941. [PubMed] [Google Scholar]
- 4.Ishida J, Kozuka M, Wang H, Konoshima T, Tokuda H, Okuda M, Mou XY, Nishino H, Sakurai N, Lee KH, Nagai M. Cancer Lett. 2000;159:135. doi: 10.1016/s0304-3835(00)00538-3. [DOI] [PubMed] [Google Scholar]
- 5.Ishida J, Kozuka M, Tokuda H, Nishino H, Nagumo S, Lee KH. Bioorg Med Chem. 2002;10:3361. doi: 10.1016/s0968-0896(02)00164-5. [DOI] [PubMed] [Google Scholar]
- 6.Motohashi N, Yamagami C, Tokuda H, Konoshima T, Okuda Y, Mukainaka T, Nishino H, Saito Y. Cancer Lett. 1998;134:37. doi: 10.1016/s0304-3835(98)00239-0. [DOI] [PubMed] [Google Scholar]
- 7.Ito C, Itoigawa M, Furukawa H, Ichiishi E, Mukainaka T, Okuda M, Ogata M, Tokuda H, Nishino H. Cancer Lett. 1999;142:49. doi: 10.1016/s0304-3835(99)00147-0. [DOI] [PubMed] [Google Scholar]
- 8.Ito C, Itoigawa M, Otsuka T, Tokuda H, Nishino H, Furukawa H. J Nat Prod. 2000;63:1344. doi: 10.1021/np0000318. [DOI] [PubMed] [Google Scholar]
- 9.Itoigawa M, Ito C, Tokuda H, Enjo F, Nishino H, Furukawa H. Cancer lett. 2004;214:165. doi: 10.1016/j.canlet.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Ito C, Itoigawa M, Takakura T, Ruangrungsi N, Enjo F, Tokuda H, Nishino H, Furukawa H. J Nat Prod. 2003;66:200. doi: 10.1021/np020290s. [DOI] [PubMed] [Google Scholar]
- 11.Ito C, Itoigawa M, Kojima N, Tokuda H, Hirata T, NIshino H, Furukawa H. J Nat Prod. 2004;67:1125. doi: 10.1021/np030554q. [DOI] [PubMed] [Google Scholar]
- 12.Akihisa T, Tokuda H, Ukiya M, Iizuka M, Schneider S, Ogasawara K, Mukainaka T, Iwatsuki K, Suzuki T, Nishino H. Cancer Lett. 2003;201:133. doi: 10.1016/s0304-3835(03)00466-x. [DOI] [PubMed] [Google Scholar]
- 13.Ito C, Itoigawa M, Miyamoto Y, Onoda S, Rao KS, Mykainaka T, Tokuda H, Nishino H, Furukawa H. J Nat Prod. 2003;66:206. doi: 10.1021/np020372g. [DOI] [PubMed] [Google Scholar]
- 14.Itoigawa M, Ito C, Wu TS, Enjo F, Tokuda H, Nishino H, Furukawa H. Cancer Lett. 2003;193:133. doi: 10.1016/s0304-3835(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 15.Tatsuzaki J, Bastow KF, Nakagawa-Goto K, Nakamura S, Itokawa H, Lee KH. J Nat Prod. 2006;69:1445. doi: 10.1021/np060252z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takasaki M, Konoshima T, Fujitani K, Yoshida S, Nishimura H, Tokuda H, Nishino H, Iwashima A, Kozuka M. Chem Pharm Bull (Tokyo) 1990;38:2737. doi: 10.1248/cpb.38.2737. [DOI] [PubMed] [Google Scholar]
- 17.Tokuda H, Ohigashi H, Koshimizu K, Ito Y. Cancer Lett. 1986;33:279. doi: 10.1016/0304-3835(86)90067-4. [DOI] [PubMed] [Google Scholar]
- 18.Henle G, Henle W. J Bacteriol. 1966;91:1248. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konoshima T, Takasaki M, Tatsumoto T, Kozuka M, Kasai R, Tanaka O, Nie R, Tokuda H, NIshino H, Iwashima A. Biol Pharm Bull. 1994;17:668–671. doi: 10.1248/bpb.17.668. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai N, Kozuka M, Tokuda H, Nobukuni Y, Takayasu J, Nishino H, Kusano A, Kusano G, Nagai M, Sakurai Y, Lee KH. Bioorg Med Chem. 2003;11:1137. doi: 10.1016/s0968-0896(02)00432-7. [DOI] [PubMed] [Google Scholar]