Abstract
Elements of the immune system act as intimate regulators of cancer progression, inhibiting early stages of tumor growth, through immunosurveillance while facilitating later stages of tumor progression. Recent findings have revealed that activated CD8 T cells can stimulate mammary epithelial tumor cells to undergo epithelial-mesenchymal transition (EMT) and to acquire the greatly increased tumorigenic capability and chemotherapeutic resistance of breast cancer stem cells (BCSC). These studies provide a window to understanding how BCSC arise and are maintained within tumors, and how to best target these processes for therapeutic benefit.
Background
Epithelial-mesenchymal transition (EMT) is a fundamental biologic process by which epithelial cells detach from the surrounding tissue and acquire characteristics of mesenchymal cells, a unique motile, spindle-shaped cell with end-to-end polarity. Cells which have undergone EMT can migrate out of their epithelial layers to distant points and either remain mesenchymal or redifferentiate into epithelial cells by a process known as mesenchymal-epithelial transition (MET). EMT is best characterized for its role in embryonic development; induction of EMT is necessary for formation of the mesoderm and endoderm during gastrulation and for delamination of the neural crest. Following EMT, disseminated mesenchymal cells act as progenitors for many different tissue types. EMT occurring in epiblast cells at gastrulation, for example, gives rise to skeletal muscle, bone, and connective tissue, and EMT in neural crest epithelia gives rise to glial cells, neurons, melanocytes, cranial bone and connective tissue (1). There is increasing evidence that EMT or EMT-related processes are involved in the underlying pathologies of several disease states including tumor initiation and progression (2). Investigations using culture models have shown that many of the components of the tumor microenvironment are capable of inducing EMT, including transforming growth factor-β (TGFβ), matrix metalloproteinases, and growth factors. Studies using animal models have shown that key transcription factors involved in developmental EMT, including Snail and Twist, are also activated in cancers. Artificial expression of Snail and Twist in epithelial tumor cells induces transition to a mesenchymal phenotype, which is accompanied by increased malignant properties such as invasion and metastasis (3). There is currently considerable debate as to the role of EMT in tumor progression, and more specifically, whether EMT of tumor cells must necessarily result in a complete, irreversible transition to a mesenchymal state, or whether a partial or transient EMT may be more common (4).
Stem cells have long been investigated for their central role in organ development, but recent studies have shown that cells with stem/progenitor characteristics play critical roles as well in tumor formation and progression (5). Stem cells have been characterized as having low proliferative rates, existing as minority populations within tissues in defined niches, and having responses to extracellular stimuli that are distinct from those of the more differentiated cells within the organ (6). Stem cells exhibit self renewal and can divide symmetrically, to produce additional stem cells, or asymmetrically, to generate progenitor cells that can subsequently differentiate into the many different cell types within the organ. While it was long suspected that some cancers may develop from transformed stem/progenitor cells, or that some cancer cells could acquire stem/progenitor characteristics such as limitless proliferation, it was not until 1994 that so-called tumor-initiating cells, also known as cancer stem cells (CSCs), were first identified in human acute myeloid leukemia (7). Subsequent studies have identified CSCs in solid tumors, including breast, brain, colon, and pancreas (reviewed in ref (8)). Investigations of CSCs have taken on increased urgency with the discovery that they are resistant to conventional cancer treatments, suggesting that newer therapies should be directed specifically to the CSC subpopulations to improve outcomes. Identification of CSCs and the determination of their derivation and maintenance within tumors have become important areas of research. A significant advance toward these objectives was provided by a recent series of studies showing that induction of EMT in human or mouse mammary epithelial or epithelial tumor cells can result in the generation of cells with stem cell properties, such as self renewal and resistance to toxicants (9-11).
Research suggests that the immune system has a role in preventing cancer development. By targeting premalignant and malignant cells in a process called immunosurveillance, immune responses can eliminate cancer cells prior to tumors becoming clinically apparent or can attenuate tumor growth and progression. Investigations of immunosurveillance have established roles for both innate and adaptive immune effectors in preventing spontaneous or chemically induced tumors in mice (12). Immunocompromised mice have increased tumor formation, and tumors emerging in these mice are more immunogenic (i.e. rejected by immune competent mice) than those developing in fully immune competent mice, suggesting that emergent tumors are immunoedited or, in other terms, have a different phenotype due to their prior interactions with immune effectors (13). However, the immune system can also promote tumor development. For example, chronic inflammatory responses mediated by activated B cells and associated antibodies have been directly shown to be critical in the initiation of skin cancer in K14-HPV16 mice (14). Chronic inflammation is often associated with increased cancer risk in humans; patients with inflammatory bowel disease have an increased risk of colorectal cancer, Helicobacter pylori infection is associated with gastric adenocarcinomas and mucosa-associated lymphomas, and chronic hepatitis is associated with hepatocellular carcinoma (15). For patients with chronic inflammatory conditions, therefore, suppressing the immune response can reduce subsequent cancer development. Thus, an important debate has emerged that is attempting to reconcile the tumor-protective and the tumor-promoting roles of the immune system. One possible scenario is that the initial immune response against the tumor is modified by the tumor for promoting its own survival and progression. Another hypothesis takes into account the intrinsic genetic instability of tumor cells, and proposes that the immune system may select for or induce tumor cells that have acquired immune evasive properties. Which scenario is closer to the truth remains to be determined, but what is clear is that understanding tumor and immune system interactions may be an important goal for cancer prevention and treatment.
Immunoediting Promotes Tumor EMT and Acquisition of Breast Cancer Stem Cell Properties
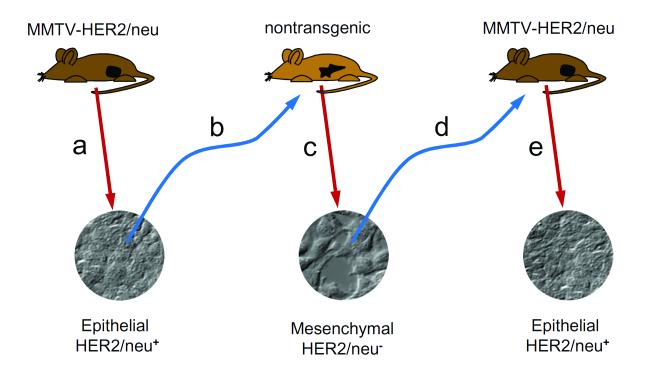
In recent years, the study of tumor immunosurveillance has led to the concept of immunoediting, a model used to describe the dynamic interaction of tumors with the immune system. As put forth by Dr. Robert Schreiber and his colleagues, immunoediting encompasses three phases: elimination, the initial destruction of tumor cells; equilibrium, in which the tumor growth is suppressed; and escape, in which tumors evade immune destruction by becoming less immunogenic or more immune suppressive (12, 13). That mutated or downregulated major histocompatibility molecules is an often observed feature of tumor cells suggests that immunoediting may be a general phenomenon (13). Previously we demonstrated that immunoediting in a mouse model of breast cancer relapse generates tumors that have undergone EMT (16). When epithelial tumors from neu-transgenic mice that express the cell surface rat neu oncogene under control of the mammary epithelial cell-specific mouse mammary tumor virus (MMTV) promoter were transplanted into non-transgenic syngeneic mice, a T cell-dependent rejection occurred, but the mice subsequently relapsed with tumors enriched in neu-negative variant tumor cells that had a mesenchymal phenotype (16, 17) (Figure 1). It appeared that an immunoediting process had either induced the epithelial tumor cells to activate an EMT, or selected for a subpopulation of tumor cells that had already undergone an EMT, so as to downregulate the expression of the epithelial-specific MMTV promoter and consequent loss of expression of the neu oncogene. These results represented the first direct demonstration that EMT may be a potential mechanism of immunoediting in cancer.
Figure 1. Immunoediting induces reversible EMT.
(a) Tumors that develop in MMTV-HER2/neu transgenic mice have an epithelial morphology and express the HER2/neu surface antigen. (b) Reinjection of these tumor cells into isogenic wild-type mice results in eventual tumors that (c) have a mesenhymal morphology and do not express HER2/neu. (d) Reinjection of these tumor cells into MMTV-HER2/neu mice results in regrowth of tumors that have reverted to the original epithelial morphology and HER2/neu expression pattern.
Subsequent investigations revealed that CD8 T cells were required for outgrowth of the neu-negative mesenchymal variants suggesting local induction of EMT rather than selection (11). Specifically, depletion of CD8 T cells prior to the injection of neu-positive tumor cells did not prevent the initial tumor rejection but these mice failed to relapse. Furthermore, CD8 T cells isolated from mice primed with neu-positive tumor cells were able to induce antigen loss when co-cultured with neu-positive tumor cells. Detailed subsequent characterization of tumor cells isolated from relapsed mice demonstrated that these tumors had characteristics of breast cancer stem cells (BCSCs), as indicated by the cell surface CD24−/loCD44+ marker profile (18) and enhanced mammosphere formation (18). These CD24−/lo CD44+ mesenchymal tumor cells also showed potent tumorigenicity, effectively forming tumors with 1000-10,000 fold fewer cells than with the neu-positive epithelial cells. Additionally, mesenchymal tumor cells had elevated expression of drug transporters, DNA repair enzymes and enhanced resistance to chemotherapy and radiation. One important characteristic of these BCSC-like tumor cells is that they gave rise in vivo to tumors with a heterogeneous tumor population consisting of both CD24− and CD24+ tumor cells. Histopathologic analysis of tumors formed by injection of neu-negative cells showed a predominant epithelial phenotype with the reacquisition of neu expression, suggesting that the immune-induced EMT was fully reversible through the reverse process, MET. Thus, in contrast to their typically ascribed protective role, CD8 T cells were capable of inducing tumors to undergo EMT and to acquire BCSC properties and a more aggressive tumor phenotype.
Selective Targeting of the Immune System for Therapeutic Benefit
How do CD8 T cells induce EMT? Previous studies have shown that exposure to TGFβ or transfection with EMT-inducing transcription factors results in the conversion of cultured human mammary epithelial cells to stem-like cells (9, 10). Immune cells are known to produce a diverse array of EMT mediators, including TGFβ and tumor necrosis factor alpha (TNFα). TGFβ is produced by CD4 T regulatory cells and chronically-stimulated effector CD8 T cells (19) and TNFα is produced by activated macrophages and pro-inflammatory T cells (20). Another possible mediator of EMT is interleukin-like EMT inducer (ILEI) which is expressed in lymphocytes associated with chronic inflammation (21). Local inflammatory responses are also associated with EMT. For example, increasing numbers of leukocytes (macrophages and T cells) infiltrating the kidney after acute unilateral ureteral obstruction in neonatal mice correlated with increased EMT (22). Chronic renal allograft dysfunction is also associated with elevated intraepithelial T cells present in renal tubules which were frequently associated with mesenchymal marker positive cells, and coculture of human renal cortical epithelial cells with a human intraepithelial T cell line for 72 hrs led to increased mesenchymal marker expression as a consequence of T cell-produced TGFβ(23). Overall, the evidence suggests that immune effectors can induce EMT following an acute or chronic inflammatory response. Likely, CD8 T cells trafficking into the tumor microenvironment can produce direct mediators of EMT or alternatively, can produce other cytokines or chemokines (e.g. MCP-1) that can attract in other immune effectors (e.g. macrophages) which provide the stimuli.
The identification of cells within tumors that possess BCSC characteristics has resulted in increased efforts to identify treatments that selectively target this subpopulation of cells. It is likely that traditional treatments for breast cancer such as surgery, chemotherapy, and radiation, which target the bulk of the tumor, may leave behind the more malignant CSCs capable of reseeding tumors and giving rise to subsequent metastases (24). While identification of therapies that selectively target BCSCs is an important goal, a parallel and perhaps equally efficacious approach would be to target the mechanisms of plasticity that generate and maintain the BCSC population in tumors. For example, since immunity is able to induce BCSCs, one approach would be to define and to target the specific immune effectors of this process. While we have identified activated CD8 T cells as the critical effectors of EMT in mouse mammary tumor cells, it is not possible to generally target CD8 T cells given their important role in protection against infection. Further, as alluded to above, it is likely that additional immune components participate in tumor evolution in vivo. Skewing of the macrophage response within the tumor microenvironment from a M2 (tumor-promoting) to M1 (tumor-eradicating) phenotype may have the potential to reduce tumor invasion and metastases. Having a Th2 or regulatory T cell (Treg) polarized CD4 T cell response may promote breast cancer metastases suggesting that agents (e.g. vaccines) that shift from Th2/Treg to an anti-tumor Th1 response may be useful (25). Another approach to inhibit EMT-associated tumor progression is to target the pathways involved in the induction of EMT that specifically lead to the acquisition of BCSC characteristics, as recently demonstrated for inhibitors of EMT pathways mediated by TGFβ or Hedgehog pathways (26, 27). The immune system has long been viewed as a co-conspirator with developing tumors; more recent data has shown that it can also selectively target tumor cells at early stages of cancer progression. An important goal now is to identify how to reduce the tumor promotion abilities of the immune system while preserving or increasing its ability to eliminate tumor cells.
Acknowledgements
Research support: A gift from Martha and Bruce Atwater (KLK). Grants from National Institutes of Health R01-CA122086 (DCR), R01-CA113861 (KLK), K01-CA100764 (KLK), P50-CA116201 (KLK, DCR) and from the Susan B. Komen Foundation grant FAS0703855 (DCR).
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed
References
- 1.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–83. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–49. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–83. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 5.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–20. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 7.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 8.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. Journal of mammary gland biology and neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 9.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santisteban M, Reiman JM, Asiedu MK, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69:2887–95. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 13.Reiman JM, Kmieciak M, Manjili MH, Knutson KL. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–87. doi: 10.1016/j.semcancer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–33. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Lu H, Stone B, et al. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–33. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 17.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol. 2007;37:675–85. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahic M, Henjum K, Yaqub S, et al. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. European journal of immunology. 2008;38:640–6. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 20.Bates RC, Mercurio AM. Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell. 2003;14:1790–800. doi: 10.1091/mbc.E02-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waerner T, Alacakaptan M, Tamir I, et al. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer cell. 2006;10:227–39. doi: 10.1016/j.ccr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Lange-Sperandio B, Trautmann A, Eickelberg O, et al. Leukocytes induce epithelial to mesenchymal transition after unilateral ureteral obstruction in neonatal mice. Am J Pathol. 2007;171:861–71. doi: 10.2353/ajpath.2007.061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson H, Ali S, McDonnell BJ, Burt AD, Kirby JA. Chronic renal allograft dysfunction: the role of T cell-mediated tubular epithelial to mesenchymal cell transition. J Am Soc Nephrol. 2004;15:390–7. doi: 10.1097/01.asn.0000108521.39082.e1. [DOI] [PubMed] [Google Scholar]
- 24.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan XB, Long ZJ, Yan M, et al. Inhibition of Aurora-A suppresses epithelial-mesenchymal transition and invasion by downregulating MAPK in nasopharyngeal carcinoma cells. Carcinogenesis. 2008;29:1930–7. doi: 10.1093/carcin/bgn176. [DOI] [PubMed] [Google Scholar]