Abstract
One of the most important tasks of childhood is learning to self-regulate in the presence of negative emotions. Until recently, almost no research has examined the neurophysiological correlates of emotional self-regulation as it develops over childhood and adolescence. We were interested in plotting a fine-grained developmental profile of the neural underpinnings of self-regulation, in the context of negative emotion, for 7- to 14-year-old children. We predicted that children would recruit less cortical activation with age in the service of self-regulation, reflecting increased neural efficiency with development. We also predicted that children would recruit more cortical activation with increased negative emotion, possibly reflecting greater demand on cortical resources. We administered a go/nogo task with an emotion induction block and we measured the amplitude of the N2, an event-related potential associated with inhibitory control, as it varied with block and with age. Furthermore, we estimated activation for a ventral prefrontal region of interest (ROI; suggestive of orbital frontal, ventromedial prefrontal, or rostral anterior cingulate activation) and a dorsomedial prefrontal ROI (suggestive of dorsal anterior cingulate activation) frequently modeled as cortical generators underlying the N2. Results revealed a marginal decrease in mediofrontal scalp activation, but a more pronounced decrease in activation of the ventromedial prefrontal ROI, with age. There were no age-related changes in dorsomedial prefrontal ROI activation. Lastly, as predicted, we found increased ventral prefrontal ROI activation during the negative emotion induction, possibly reflecting greater recruitment of frontocortical resources underlying emotion regulation, but developmental change in this activation was no different than for the other conditions. Thus, both self-regulation in general and emotion regulation in particular recruited less cortical activation with age, suggesting more efficient cortical mechanisms of response inhibition.
Keywords: emotion regulation, response inhibition, N2, event-related potentials, source analysis, go/nogo, emotion induction, ventral prefrontal, dorsomedial prefrontal
A key aspect of emotional development is the capacity to expend effort to control, modify, or inhibit behaviors that are induced by negative emotion. The inhibition of behavioral tendencies associated with negative emotion is often included under the rubric of emotion regulation. In this study, we examined an ERP component, generally associated with response inhibition, before, during, and after, an emotion induction. Thus, differences in the neural correlates of response inhibition during and/or following the induction of negative emotion may tap key features of children's developing capacity for effective emotion regulation.
The development of self-regulation in general and emotion regulation more specifically is thought to be shaped by various factors, such as temperament and parenting styles (for a discussion see Calkins & Fox, 2002). Over the course of development, the child learns to regulate actions that flow from negative emotion, such as hitting or yelling when angry and freezing or fleeing in the presence of fear, first as a result of a supportive caregiver environment and later as a function of voluntary or effortful control (Calkins & Fox, 2002; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Kochanska, Coy, & Murray, 2001; Ruff & Rothbart, 1996). With the development of increasingly sophisticated regulatory abilities, children are able to regulate their actions more efficiently and more effectively in everyday emotional contexts. We were interested in examining the processes underlying these developmental differences.
Recently, researchers have started examining the neural mechanisms underlying regulatory functions. Specific event-related potentials (ERPs), and estimates of the cortical generators underlying these ERP components, have been associated with regulatory processes. One ERP component, the mediofrontal N2, is observed between 200 and 500 ms post-stimulus and is generally associated with action regulation processes, such as inhibitory control (Dimoska, Johnstone, Barry, & Clarke, 2003; Falkenstein, Hoormann, & Hohnsbein, 1999; Jonkman, Lansbergen, & Stauder, 2003; Overtoom et al., 1998; Schmajuk, Liotti, Busse, & Woldorff, 2006; Smith, Johnstone, & Barry, 2004) and conflict monitoring or response selection (Bartholow, Pearson, Dickter, Sher, Fabiani, & Gratton, 2005; Bekker, Kenemans, & Verbaten, 2004; Dimoska, Johnstone, & Barry, 2006; Nieuwenhuis, Yeung, & Cohen, 2004; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003). Furthermore, a number of studies have shown greater N2 activation for infrequent nogo trials than frequent go trials (for example, Bekker, Kenemans, & Verbaten, 2005; Donkers & van Boxtel, 2004; Falkenstein et al., 1999; Nieuwenhuis et al., 2004), suggesting that this increase in activation taps the additional inhibitory or response selection resources required for successful task performance.
Moreover, the N2 may also be sensitive to the processing of emotional information. For example, two studies from our laboratory have shown emotion-related changes in N2 activation within a go/nogo task designed to induce negative emotion (Lewis et al., 2006; Stieben, Lewis, Granic, Zelazo, Segalowitz, Pepler, 2007). This task consisted of three blocks, with participants' gaining points for their successful performance during the first and third blocks but losing their earned points during the middle block. According to a self-report measure administered directly after the task, participants felt increased negative emotions during the emotion induction block compared to the other blocks. (In previous reports we inferred that induced negative emotion carried over into the early parts of the last block). Results revealed greater N2 activation during and/or after the emotion induction block in comparison with the first block. These results indicate that the N2 is also sensitive to emotional processing, which we interpreted to reflect increases in emotion regulation. Moreover, a recent study by Lamm, DeCicco, and Dennis (submitted) directly measured the link between N2 activation and emotion regulation. Specifically, they found that the individuals who suppressed their emotional expressions, interpreted as an emotion regulation strategy, showed a different pattern of N2 activation across easy and more difficult trials than individuals who did not suppress their emotional expressions. Together these studies suggest that the magnitude of N2 activation indexes not only response inhibition, as generally assumed, but also emotion regulation, viewed as greater regulatory efforts to inhibit actions in the presence of negative emotion.
The N2, like other scalp activation patterns, is the sum of all underlying cortical generators active at a particular moment in time; however, activation in some of these regions may not be directly related to response inhibition. For example, a number of studies modeling the activation underlying the N2 (in visual tasks) have shown generators suggestive of occipital, ventral temporal, and parietal activation (for example, Lamm, Zelazo, & Lewis, 2006; Lewis et al., 2006; Stieben et al., 2007) regions not directly related to response inhibition. Given that activation from all these cortical regions appear to contribute to the N2 scalp topography—either by enhancing its polarization (e.g., projecting negative current towards or near the mediofrontal scalp area) or diminishing its polarization (e.g., projecting positive current towards or near the mediofrontal scalp area)—much information can be gained by conducting a source-space analysis to estimate the activation of cortical regions directly related to regulatory functions. Thus, by performing a source-space analysis one can address broad questions of spatial localization while also utilizing the excellent temporal specificity inherent in ERP methods.
A few studies conducted with children and adults have modeled the generators underlying the N2 to areas suggestive of activation in the dorsal anterior cingulate cortex (ACC) and/or activation of ventral prefrontal regions, including the orbitofrontal cortex (OFC), the rostral ACC, and the ventromedial prefrontal cortex (VMPFC; Bekker et al., 2005; Bokura, Yamaguchi, Kobayashi, 2001; Lavric, Pizzagalli, & Forstmeier, 2004; Lamm, Zelazo, & Lewis, 2006; Lewis et al., 2006; Nieuwenhuis et al., 2003; Stieben et al., 2006). Dorsal ACC activation has been linked to various deliberate or “executive” regulatory functions, such as attention regulation (Crottaz-Herbette & Menon, 2006), performance or conflict monitoring (Blasi, Goldberg, Weickert, Das, Kohn, Zoltick et al., 2006; Santesso, Segalowitz, & Schmidt, 2005; for a review see Botvinick, Cohon, & Carter, 2004), and response inhibition (Blasi et al., 2006; Tamm, Menon, Ringel, & Reiss, 2004). In contrast, ventral prefrontal activation has been linked with “ongoing” or relatively automatic regulatory or evaluative processes, such as “in-the-moment” inhibitory control and the evaluation of positive or negative aspects of current or anticipated stimuli (Blasi et al., 2006; Drevets, Videen, Price, Preskorn, Carmichael, & Raichle, 1992; Durston, Mulder, Casey, Ziermans, & van Engeland, 2006; Eshel, Nelson, Blair, Pine, & Ernst, 2007; Kaladjian, Jeanningros, Azorin, Grimault, Anton, & Mazzola-Pomietto, 2007, Ochsner, Ray, Cooper, Robertson, Chopra, Gabrieli et al., 2004; for reviews see Rolls, 2000; Phillips, Ladouceur, & Drevets, 2008). Increases in ventral prefrontal activation have also been associated with increased negative emotion (for example, Baumgartner, Lutz, Schmidt, & Jancke, 2006; Kawasaki et al., 2001) and more specifically emotion regulation (for example, Ochsner et al., 2004; for a review see Quirk & Beer, 2006). Thus, the two prefrontal areas to which N2 activation have consistently been modeled, the ventral PFC and the dorsal ACC, have been linked with affective and non-affective (respectively) regulatory processes.
Having outlined the association between the mediofrontal N2 and emotion regulation, as well as the functional significance of the cortical generators frequently thought to underlie this component, we now discuss the development of the N2 and its hypothesized generators. Developmental studies using continuous performance tasks, including a previous study conducted by our lab, have generally revealed decreases in N2 activation with age (Johnstone, Pleffer, Barry, Clarke, and Smith, 2005; Jonkman, Lansbergen, & Stauder, 2003; Lewis et al., 2006). However, one recent study did not find this pattern of effects (Ladouceur, Dahl, & Carter, 2007). It is not clear why this study revealed different results but it may be related to the task performed. Johnstone et al. (2005), Jonkman et al. (2003), and Lewis et al. (2006) used go/nogo tasks to evoke ERPs, while Ladouceur et al. (2007) used a flanker task. Thus, the majority of studies show that the regulatory functions measured by the N2 require less cortical activation with age, suggesting age-related increases in cortical efficiency (Casey et al. 1997; Casey, Giedd, & Thomas, 2000; Luna et al., 2001). In other words, with age, children may not need to recruit neural populations as extensively in order to effectively perform the same task. However, functional magnetic resonance imagining (fMRI) and positron emission tomography (PET) studies investigating the development of the dorsal ACC and ventral PFC are less consistent. A number of studies show increases in prefrontal activation with age (Adleman et al., 2002; Eshel et al., 2007; Monk et al., 2003; Rubia, Smith, Taylor, & Brammer, 2007; Rubia et al., 2006; van Bogaert, Wikler, Damhaut, Szliwowski, & Goldman, 1998), whereas other studies show decreases (Casey et al. 1997; Marsh et al., 2006; Rubia et al., 2006; Tamm, Menon, & Reiss, 2002), and one study showed a quadratic effect with adolescents revealing greater activation than both children and adults (Luna et al., 2001).
It is not clear why imaging studies differ so much from each other and from the N2 literature. It may be that N2 activation is artificially enhanced by developmental differences in skull thickness. Specifically, older children have thicker skulls (Adeloye, Kattan, Silverman, 1975) that may cause more resistance to current flow. However, Lamm et al. (2006) demonstrated that developmental differences in N2 amplitudes were better predicted by developmental differences in executive function than maturational differences in variables unrelated to performance, such as skull thickness. Nevertheless, these findings leave considerable uncertainty as to the direction of developmental trends in cortical activation underlying self-regulation.
To help clarify the direction of maturational change in prefrontal activation levels, specifically those mediating self-regulation in the context of negative emotion, we examined developmental differences in estimated activation for two prefrontal regions frequently modeled to underlie the N2. Furthermore, to ascertain how regulatory activation patterns change in the context of negative emotion, we used an experimental paradigm intended to capture children's regulatory abilities before, during, and after a negative emotion induction within a go/nogo task. We chose two broad regions of interest (ROIs) that were suggestive of dorsal ACC and ventral PFC (OFC, VMPFC, and/or rostral ACC) activation. However, while other studies have estimated cortical sources at the group level, for example by modeling the average location of cortical dipoles, we used a distributed inverse model to extract estimated ROI activation values for each participant. This allowed us not only to model cortical sources descriptively, as has been done previously (Jonkman, Sniedt, & Kemner, 2007; Lewis et al., 2006), but also to test age and emotion effects statistically. Despite ambiguous findings to date, we predicted a developmental decrease in activation, consistent with the hypothesis of increasing cortical efficiency with age. Since our paradigm was specifically designed to test response inhibition related to negative emotion—as a means of estimating the cortical underpinnings of emotion regulation—we predicted increased ventral prefrontal ROI activation with increased emotion (during and/or after the emotion induction block) and that this activation would decrease with age reflecting more efficient emotion regulation. Lastly, one point of clarification, all brain region results outlined in this paper reflect estimated activation and not actual activation, as is the case with fMRI analyses.
Method
Participants
Participants were 85 English-speaking children aged 7 to 16 years, with normal or corrected-to-normal vision, and free of any psychiatric diagnoses. Twenty nine children were eliminated due to low trial counts leaving a sample of 56 children (more boys than girls were eliminated from the study, χ2(1) = 4.11, p = .04, but there was no significant age difference between children who were eliminated and children who were included). The age span was subdivided into five periods of 2 years each. The oldest age group, the 15- to 16-year olds, had too few participants and therefore was not analyzed, leaving a final sample size of 49 (27 boys; see Table 1 for number of participants comprising each group). Participants were recruited from a large urban center through advertisements in a city-wide newspaper and paid $40.00 and a toy or gift certificate for their participation. Ethical approval of the project was obtained from the University of Toronto and the Hospital for Sick Children in Toronto.
Table 1.
Number of participants comprising each age group.
| Group | N |
|---|---|
| 7-8 | 08 |
| 9-10 | 15 |
| 11-12 | 13 |
| 13-14 | 13 |
| Total | 49 |
Procedure
Children were accompanied to the laboratory by a parent. Following a brief introduction to the testing environment, electrode sensor nets, and recording system, parental consent and child assent were obtained. Parents were then seated in a nearby room. Children were informed that they could win a prize for playing the EEG computer game and were shown two toy bins. One of the bins contained small, undesirable toys such as small plastic cars, whereas the second bin contained a wide selection of more desirable, age-appropriate toys such as large action figures, stuffed animals, games, and $10.00 gift certificates from a local music store. The children were informed that, with successful performance (accumulation of points) in the game, they would be able to choose their desired prize, but that less successful performance would limit their choice to the less desirable toy bin. Children were then seated in front of a computer monitor with the distance and alignment to the monitor controlled by the use of a chin rest. The electrode sensor net was applied. Children were instructed to make responses during the game by clicking a button on the response pad with the index finger of their dominant hand (writing hand). They were given a practice block of 30 trials, with the option to repeat the practice block, to ensure proficiency with the task.
ERP paradigm
Task
The emotion induction go/nogo task that was used in the present study was partly adapted from a task developed by Garavan, Ross, and Stein (1999), and was presented using E-Prime software (Psychological Software Tools, Pittsburgh, PA). In standard go/nogo paradigms, participants are required to press a button as fast as possible given a particular category of stimuli (the “go” condition) and withhold responding given another category of stimuli (the “nogo” condition). Participants in this study were instructed to click the button for each letter presented but to avoid clicking when a letter was repeated a second time in succession. Different pairs of letters were used for each block (block A: x, y; block B: o, p; block C: u, d) to enhance novelty without modifying the level of difficulty and to facilitate guided recall during a self-report scale administered to all participants. The nogo error rate for the task was maintained at 50% ± 10% by dynamically adjusting the stimulus duration and thus the inter-trial interval. Stimulus duration was increased with each erroneous response made on nogo trials. However, decreases in stimulus duration only occurred when the nogo trial followed a correct go trial. This constraint was incorporated to prevent stimulus duration adjustments due to chronic non-responding. The dynamic adjustment of the stimulus duration was intended to provide the same level of challenge for all participants at all ages. Because even with this dynamic adjustment of stimulus duration there may still have been slight variations in task difficulty between age groups and blocks, we also entered stimulus duration as a covariate to all analyses. The dynamic adjustment of stimulus duration was also applied to obtain a sufficient number of correct nogo trials for ERP averaging. Error feedback was provided by a red bar in the middle of the screen following incorrect responses, omitted responses, and late responses.
Children were presented with a practice block and three blocks of trials (blocks A, B, and C). In blocks A and C children gained points. These blocks were structurally identical, each consisting of 200 trials, including 66 nogo trials, in pseudorandom sequence. Children lost points consistently during block B, due to changes in the point-adjustment algorithm. By the end of this block, children lost all or almost all of their points. The loss of points was intended to induce negative emotions, such as anxiety, sadness, anger, and frustration. To limit the duration of children's distress, block B consisted of only 150 trials, including 40 nogo trials. With a return to the more generous algorithm in block C, children regained their points to win the desired prize. For each block, their accumulated points were displayed every 5 to 25 trials in the center of the computer screen. The displayed points were in red if points were lost since the last reward window and in green if points were gained. Additionally, if points were lost, an unpleasant “buzzer” sound was delivered to highlight the fact that they were performing poorly. Furthermore, if points were gained, a pleasant “tinkling” sound was delivered to highlight the fact that they were performing well. Points were added for correct nogo responses and deducted for response errors on both go and nogo trials. Children were reminded at the beginning of the task, and the onset of each block, that a high number of points were required to win the “big prize.”
Self-report emotion-induction check
The emotion induction scheme was assessed with a subjective rating scale administered directly after the go/nogo task. An 8.5 by 11 inch card with animated faces representing five different emotions was presented to the children. The five categories were upset, mad, nervous, satisfied, and excited. Children were asked to rate the intensity of each of these emotions on a 10-point Likert scale for each of the three blocks. Cards showing animated emotion faces of different intensities were used to help children identify the intensity of their emotions. Furthermore, to help children recall how they felt in each of the blocks, researchers indicated which letter combination was used for each block (e.g., x/y in block A, o/p in block B).
Analyses
EEG data collection and analysis
EEG was recorded using a 128-channel Geodesic Sensor Net and sampled at 250 Hz, using EGI software (Net Station; Electrical Geodesic, Inc., Eugene, OR). Data acquisition was started after all impedances for all EEG channels were reduced to below 50 kΩ. All channels were referenced to Cz (channel 129) during recording and later rereferenced against an average reference corrected for the polar average reference effect (PARE correction; Junghoefer, Elbert, Tucker, & Braun, 1999). Eye blink and eye movement artifacts (70 μV threshold), signals exceeding 200 μV, and fast transits exceeding 100 μV were removed during the averaging process. Data was filtered using a FIR bandpass filter with a lowpass frequency of 30 Hz and a highpass frequency of 1 Hz. Correct nogo data were segmented into epochs from 400 ms before to 1000 ms after stimulus onset and baseline corrected for the 400 ms preceding the stimulus. Correct nogo trials that did not have a correct go trial preceding and following them were removed, because they most likely reflected attentional lapses or chronic nonresponding, and would thus pollute the averaged ERP. Because of this cleaning process, the mean number of trials comprising correct nogo ERPs was 24.46 (ranging from 8 to 55 trials). To avoid the confounding effect of trial count on amplitude values, amplitude analyses were conducted with trial count as a covariate. The nogo N2 was scored as the largest negative deflection with a mediofrontal topography between 200 and 500 ms post-stimulus. Given that frequent go stimuli have consistently revealed less activation than infrequent nogo stimuli (for example, Bekker et al., 2005; Donkers & van Boxtel, 2004; Falkenstein et al., 1999; Nieuwenhuis et al., 2004); that nogo minus go activation is confounded by motor activation; and because subtracting go activation from nogo activation for a rapid task may actually subtract out some of the regulatory processing that we wanted to evaluate, we chose not to analyze go activation or nogo-go activation. All ERP data were scored by two independent coders and discrepancies were inspected and adjusted by a third coder.
Source-space analysis
We used a distributed inverse model—uses the change in activation from one electrode to another (in this case 128 electrodes)—to calculate the source-space activation because this type of algorithm estimates activation voxel-by-voxel and sample-by-sample and the user does not have to “fit” any dipoles. We were thereby able to limit the influence of user bias. Specifically, we utilized an algorithm called LAURA (local autoregressive average), a constraint applied to the minimum-norm method which minimizes the discrepancy between values of adjacent voxels (to achieve the most realistic model) within the GeoSource interface (Electrical Geodesic, Inc., Eugene, OR; for a review of these constraints and other minimum norm solutions, see Michel, Murray, Lantz, Gonzalez, Spinelli, & Grave de Peralta, 2004). Furthermore, we used a regularization constant (indicating how much noise is modelled) of 10 −3. This amount of regularization revealed current flow patterns that matched (visual inspection) the grand-averaged scalp topography (collapsing across all blocks and groups) better than other levels.
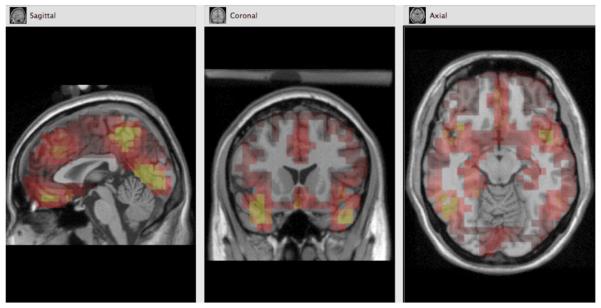
Morphology-based regions of interest (ROIs) were generated using the Montreal Neurological Institute (MNI) average adult MRI. The ventral prefrontal ROI, shown in Figure 1, approximates activation in the VMPFC, OFC, and rostral ACC. The dorsomedial prefrontal ROI, also shown in Figure 1, approximates activation in the dorsal ACC. Each ROI was comprised of a subset of dipoles (or voxels). Source waveform amplitudes (nA) for all dipoles within an ROI were extracted for 400 ms before stimulus onset to 500 ms after stimulus onset and baseline corrected using the 400 ms before stimulus onset. To ensure that each participant's maximal activation was analyzed, we chose the voxel and moment in time (within the 300 ms marked by the N2) that showed the most activation for each ROI.
Figure 1.
Morphology-based regions of interest (ROIs) generated using the Montreal Neurological Institute (MNI) average adult MRI.
However, applying the same inverse solution to all children and using this procedure to evaluate developmental differences in cortical efficiency may be problematic. Currently there is much debate as to the degree to which factors such as skull thickness impede the flow of cortical activation and how this resistance in current flow changes throughout development. Specifically, because older children have thicker skulls (Adeloye et al., 1975) causing greater current resistance and thus less scalp activation, developmental differences in source-space activation may appear greater than they actually are. Given that reliable age-specific head models (providing not only information on skull shape but also developmental differences in current resistance) have not been developed yet, we could only approximate the effect of developmental differences in current resistance on estimated cortical activation. We did this by measuring each participant's baseline activation and then adding this as a covariate to our ANOVAs. Specifically, we entered each ROIs maximal activation for the 100 ms before stimulus onset. We chose this time period because it should have been free of activation differences evoked by the previous trial. This procedure should have also eliminated differences in resting cortical activation which otherwise may have influenced task specific developmental differences, for example, younger children may have greater resting activation. Thus, by applying this procedure to all our ROI analyses, we should have eliminated developmental differences in activation not related to task demands. However, this procedure would not have eliminated any potential age-related source localization problems, such as activation in younger children appearing slightly more superficial due to less current dispersion brought about by thinner skulls. To minimize this potential problem, we analyzed broad ROIs that not only encompassed the specific activation of interest (as revealed in the grand-averaged data) but also adjacent areas, thereby analyzing a large enough area so that differences in maximal activation for all age groups were captured.
Results
Go/nogo behavioral data analyses
A 4 (age group) × 3 (block) repeated-measures ANOVA was conducted for go response times and go/nogo performance accuracy with sex and stimulus duration as covariates. The response time measure was straightforward. However, because perseverative responding leads to high accuracy on go trials and low accuracy on nogo trials, whereas chronic nonresponding leads to high accuracy on nogo trials and low accuracy on go trials, each of these measures is misleading on its own. Therefore, to better reflect the overall quality of performance, we analyzed accuracy scores averaged across both trial types.
Response time results showed a main effect of age, F(3, 41) = 5.79, p = .002, while performance accuracy scores did not, F(3, 41) = .57, ns. Planned contrasts, collapsing across blocks, revealed slower response times for the 7-8-year olds than the 11-12 (p = .001) and 13-14-year olds (p = .002), and slower response times for the 9-10-year olds than the 11-12 (p = .008) and 13-14-year olds (p = .009). Both response times and performance accuracy scores revealed main effects of block: response times showed a linear main effect of block, F(1, 41) = 4.96, p = .03, while performance accuracy showed a quadratic main effect of block, F(1, 41) = 18.93, p < .001. Planned contrasts revealed slower response times for block A than blocks B and C (p < .001), lower accuracy scores for block B than blocks A and C (p < .001), and lower accuracy scores for block A than block C (p < .001). In sum, children revealed slow but relatively accurate responding in block A, fast and less accurate responding in block B, and fast and accurate responding in block C. Moreover, children revealed faster responding with age but no change in accuracy. These results were as expected: it was assumed that children's performance would speed up as they got older and more proficient with discriminating stimuli and pressing buttons. Furthermore, the dynamic adjustment procedure successfully adjusted stimulus presentation rate so as to maintain an approximately constant error rate.
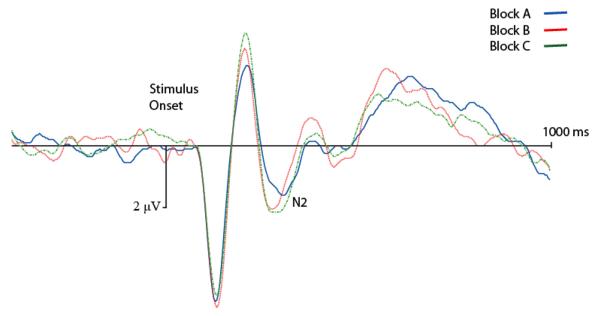
Scalp waveform analyses
All analyses of N2 amplitudes were conducted on correct nogo stimulus-locked waveforms for electrode Fz (where grand averaged ERPs revealed maximal scalp activation). Figure 2 shows grand-averaged waveforms collapsing across groups. These waveforms reveal N1, P2, and N2 components, as well as a late frontal P3 component, that are all distinct and well-formed. This figure is presented to show the morphology of the waveforms and not to highlight group or block differences, since grand-averaged waveforms can be visually deceptive and give the appearance of differences that are not actually present.
Figure 2.
Grand-averaged ERP waveforms at site Fz, collapsing across age groups. Activation is shown as negative down.
A 4 (age group) × 3 (block) repeated-measures ANOVA was conducted for N2 amplitudes with sex, stimulus duration, and trial count as covariates. Results did not reveal a main effect of age, F(3, 40) = 1.70, ns. Since these results are inconsistent with our previous results, using a very similar task (Lewis et al., 2006), and with numerous other studies, we investigated whether differences in P2 amplitudes confounded the effect. The P2 is a prominent frontal component, generally larger than the N2, occurring just prior to the N2. The P2's downward-going slope (after the peak) often overlaps with the latency range of the N2 and may therefore “lift” or make more positive the activation values for the N2. To eliminate this “lifting” effect, we regressed out the variance associated with the P2. The residualized N2 amplitudes were then analyzed. We did not eliminate variance associated with the classic parietal P3 since this component generally does not show a lot of activation in frontal electrodes. N2 amplitudes, with P2 activation removed, revealed a main effect of age, F(3, 40) = 2.84, p = .05, but no main effect of block, F(1, 40) = 1.30, ns, or block-by-age interaction, F(3, 40) = 2.12, ns. Contrasts, corrected for multiple comparisons and collapsed across blocks, revealed less activation (less negative amplitudes) for the 11-12-year olds than the 7-8-year olds (p = .05). Thus, older children revealed marginally smaller N2 amplitudes than younger children (see Figure 3).
Figure 3.
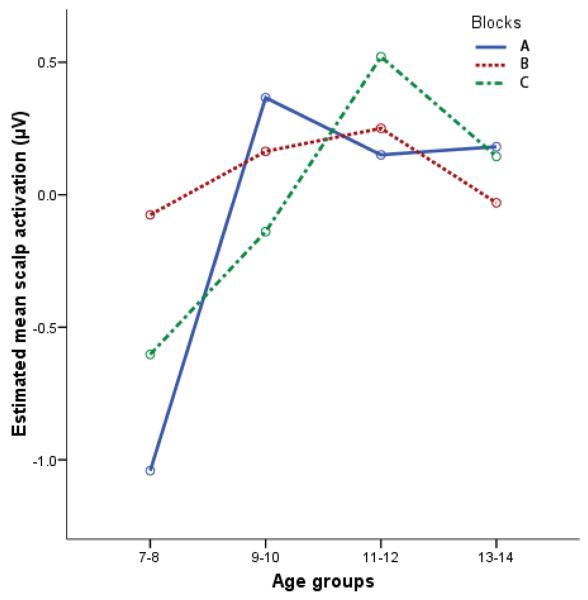
Age differences in scalp activation (site Fz), with variance associated with P2 activation removed, by block. Activation values are presented as standardized residuals. Greatest magnitude of activation is down.
Source-space analyses
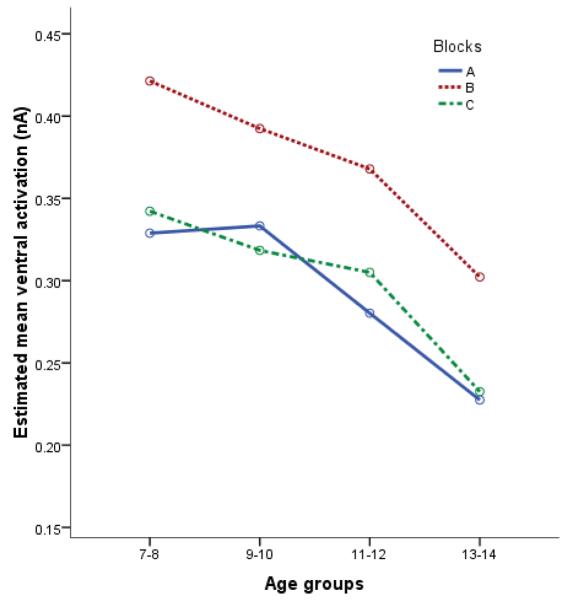
We conducted separate 4 (age group) × 3 (block) repeated-measures ANOVAs, with sex, stimulus duration, and trial count as covariates, for the ventral prefrontal and dorsomedial prefrontal ROIs. Furthermore, as outlined in the Methods section, we entered a region-specific measure of baseline activation as a covariate to all source-space analyses to eliminate non-task-related activation differences. As depicted in Figure 4, results for the ventral prefrontal ROI showed a main effect of age, F(3, 39) = 3.38, p = .03. We also found a quadratic main effect of block, at the level of a trend, F(1, 39) = 3.17, p = .08, but no age-by-block interaction, F(3, 39) = .49, ns. Collapsing across blocks, planned contrasts revealed less ventral prefrontal ROI activation for the 13-14-year olds than the 7-8 (p = .008), 9-10 (p = .008), and 11-12-year olds (p = .05). Furthermore, collapsing across age, planned contrasts revealed more ventral prefrontal ROI activation in block B than in blocks A (p < .001) and C (p < .001). Thus, consistent with our hypotheses, we found decreased ventral prefrontal ROI activation with age and greater ventral prefrontal ROI activation for the emotion induction block compared to the other blocks. However, as is revealed by Figure 5, we did not find any significant dorsomedial prefrontal ROI effects: no main effect of age, F(3, 39) = .80, ns, no main effect of block, F(1, 39) = .69, ns, and no age-by-block interaction, F(3, 39) =.62, ns.
Figure 4.
Age differences in ventral prefrontal ROI activation by block. Greatest magnitude of activation is up.
Figure 5.
Age differences in dorsomedial prefrontal ROI activation by block. Greatest magnitude of activation is up.
Emotion-scale analyses
To assess whether the negative emotion induction was effective, we analyzed the self-report emotion-scale data. Given that we were not interested in individual differences, just block effects, we dropped all between group variables, both as independent variables, i.e., Group, and covariates, i.e., sex, from the analysis. Results revealed a quadratic main effect of block, F(1, 48) = 120.73, p < .001. Planned contrasts revealed that bock B, the emotion induction block, was perceived as significantly more negative than blocks A and C (p < .001). Thus, a negative emotion induction in block B was confirmed by children's self-report.
Discussion
The present study investigated developmental differences in scalp and source activation underlying self-regulation in the context of negative emotion. We predicted that older children would be able to expend less cortical activation in the service of response inhibition. We also predicted that children would have to recruit more cortical activity to inhibit their responding in the presence of negative emotion.
Scalp effects
Consistent with our hypothesis and a number of other studies (Johnstone et al., 2005; Jonkman et al., 2003; Lewis et al., 2006), we found age-related decreases in N2 scalp activation. However, this pattern of results was only found after controlling for the “lifting” effect caused by P2 activation, and even then the statistical effect was only marginal. Thus, these results provide fairly weak evidence that cortical activity underlying children's self-regulation (both emotion related and relatively unemotional) becomes more efficient with age. However, given that N2 scalp activation is “diluted” by activation from generators not directly related to regulatory functions, such as activation suggestive of occipital and ventral temporal activation (see Figure 6), we conducted source-space analyses to better estimate activation differences for regions previously linked with response inhibition and emotional processes, such as emotion regulation.
Figure 6.
Example of grand-averaged estimated cortical activation underlying the N2 (screenshot taken 400 ms after stimulus onset), collapsing across blocks and age groups.
Source-space analyses
In this study, we statistically examined the age-related activation differences for two ROIs, both prespecified as estimates for cortical structures associated with response inhibition: a dorsomedial prefrontal ROI (estimating dorsal ACC activation) and a ventral prefrontal ROI (estimating OFC, VMPFC, and/or rostral ACC activation). Previous studies have developed source models for different age groups (Jonkman et al., 2007; Lewis et al., 2006), but to the best of our knowledge, these modeled age differences have never previously been examined statistically.
Developmental effects
As predicted, we found significantly less ventral prefrontal ROI activation with age (for all three blocks) suggestive of less activity in the ventral portion of the PFC. These results are consistent with a number of fMRI studies showing age-related decreases in activation associated with inhibitory control. For example, Marsh et al. (2006) found decreased frontal (BA 10) and rostral ACC activation with age using a Stroop task; Rubia et al. (2006) found decreased OFC activation with age using a motor Stroop task (participants were asked to respond to congruent or incongruent directional markers); and Casey et al. (1997) found decreased ventral prefrontal (comprised of inferior frontal, middle frontal, OFC, and ACC) activation with age using a go/nogo task.
Furthermore, given this pattern of ventral prefrontal ROI activation and the fact that responding became faster (with no change in performance accuracy) with age, these results are consistent with the hypothesis that cortical mechanisms of inhibitory control become more efficient with development (Booth et al. 2003; Casey et al., 2000; Casey et al. 1997; Luna et al. 2001). The ventral prefrontal region has a prolonged trajectory of developmental change, for example, pruning and myelination processes continue into early adulthood (Giedd et al., 1999; Gogtay et al., 2004). Thus, older children may require less ventral prefrontal processing to effectively inhibit a prepotent tendency to respond to cues. When these cues are presented in the context of negative emotion, the mechanism of response inhibition may be precisely the mechanism recruited for emotion regulation, i.e., the self-regulation of an emotionally motivated action. This interpretation may explain why the age-related reduction in activation of the ventral ROI for the emotion induction block paralleled the other less emotional blocks (but with consistently more activation; see Figure 4).
Interestingly, dorsal prefrontal regions also have an extended developmental trajectory (Gogtay et al., 2004); however, we did not find developmental differences in activation for our dorsomedial prefrontal ROI. Our pattern of results—evoked by an emotionally challenging task—may be due to ventral prefrontal regions being associated with more emotional processing (Bush, Luu, & Posner, 2000; Drevets & Raichle, 1995; Phillips, Drevets, Rauch, & Lane, 2003; Price, Carmichael, & Drevets, 1996; Whalen et al., 1998) while dorsomedial prefrontal activation is associated with more cognitive processing (Bush et al., 2000; Bush, Whalen, Rosen, Jenike, McInerney, & Rauch, 1998; Carter et al., 2000; Kerns et al., 2004; Krawczyk, 2002; for reviews see Bush et al., 2000; Drevets & Raichle, 1998; Eshel et al., 2007; Kesek, Zelazo, & Lewis, 2009). Consistent with these studies, we did find increased ventral prefrontal ROI activation but not dorsomedial prefrontal ROI activation for the negative emotion induction block. However, the absence of developmental effects for the dorsomedial prefrontal ROI cannot be explained by the emotional nature of the task since block A—requiring relatively unemotional response inhibition—should in that case have revealed decreased dorsomedial prefrontal ROI activation with age. However, this pattern of results was not found. In fact, no significant increases or decreases in age-related changes in dorsomedial prefrontal ROI activation was found for this block. Thus, the presence of ventral prefrontal and the absence of dorsomedial prefrontal ROI activation changes with age cannot be explained by this model.
However, it may be that the dorsomedial prefrontal region develops slightly later (Gogtay et al., 2004; for reviews see Kesek et al., 2009; Todd & Lewis, 2008) and thus, we were not able to capture developmental effects within the age range under study. Shaw and colleagues (2008) recently found that ACC grey matter volumes only started to decrease—primarily due to pruning and myelination—around 14 years of age while OFC grey matter volumes start decreasing as early as 8.6 years of age. Furthermore, Kesek et al. (2009) proposed that dorsally-mediated regulatory functions depend on the development of ventrally-mediated functions. Thus, the difference we observed between dorsomedial prefrontal and ventral prefrontal activation patterns could be explained by differences in the developmental trajectories of these regions.
Another possibility is that the type of regulation required by our task dictated the observed pattern of activation. Specifically, it may be that relatively rapid, automatic response inhibition, as required by this go/nogo task, recruits more stimulus-bound controls associated with ventral prefrontal activation (e.g., Kaladjian et al., 2007; Konishi, Nakajima, Uchida, Kikyo, Kameyama, & Miyashita, 1999; Liddle, Kiehl, & Smith, 2001). Dorsomedial prefrontal activation, on the other hand, is often associated with more effortful and deliberate modes of self-regulation, such as those required for cognitive reappraisal (Ochsner et al., 2004) and conflict monitoring (e.g., Stroop and Flanker tasks; Bush et al., 1998; Fan, McCandless, Fossella, Flombaum, & Posner, 2005).
In summary, the age-related pattern of cortical activation found in this study may be due to the age range of children tested and/or the type of self-regulation required by our task. In the future, it would be useful to replicate the present design using a larger age range, in order to test these interpretations.
Emotion effects
As mentioned earlier, we found an increase in ventral prefrontal ROI activation for the emotion induction block compared to the other two blocks. Changes in prefrontal activation, in relation to emotion, have been linked to a number of emotion evaluation and emotion regulation processes. First, several imaging studies have shown changes in ventral prefrontal activation in relation to the experience or evaluation of emotion. For example, Baumgartner, Lutz, Schmidt, & Jancke (2006) found increased ventromedial prefrontal activation when adults viewed emotionally salient pictures (International Affective Picture System; IAPS) with dramatic musical excerpts compared to pictures without music. This increase in activation corresponded with subjective ratings of increased emotional experience. Furthermore, Turner et al. (2007) found reduced ventral PFC activation in relation to subjective ratings of experienced positive emotion while viewing emotionally salient pictures (IAPS) in patients with cerebellum lesion. Lastly, Phan, Britton, Taylor, Fig, and Liberzon (2006) found that ventral PFC activation modulated subjective ratings of experienced emotion while viewing emotionally salient pictures (IAPS) for Vietnam veterans and normal controls. In sum, these studies all revealed changes in ventral PFC activation in relation to experienced emotion. Thus, our participants may have shown increased ventral prefrontal activation in the emotion induction block due their experience of negative emotion. This interpretation is supported by our emotional experience self-report data. Children reported feeling more negative emotions during the emotion induction block compared to the other two blocks.
Second, changes in ventral prefrontal activation have been related to aspects of emotion regulation, such as the down-regulation of amygdala activation. For example, Ochsner and colleagues (2004) found that medial prefrontal and amygdala activation contributed to decreases in negative affect during a reappraisal task. Furthermore, a number of functional connectivity studies have found high levels of coactivation between ventral prefrontal and amygdala activity relating to changes in negative affect (e.g., Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Hare, Tottenham, Galvan, Voss, Glover, & Casey, 2008; Pezawas et al., 2005). For example, Banks and colleagues found amygdala-OFC coupling in adults during a reappraisal task, and that the strength of this coupling predicted successful emotion regulation (Banks et al., 2007). Furthermore, Hare et al. (2008) found that stronger functional connectivity between ventral PFC and the amygdala in children and adults (age ranging from 7 to 32 years) predicted greater habituation of amygdala activation during an emotional face go/nogo task. Lastly, Pezawas et al. (2005) found coactivation between rostral ACC and amygdala activation underlying decreases in negative affect in adults viewing fearful stimuli. In sum, ventral PFC activation may modulate amygdala activation and thereby decrease negative affect. Thus, our children may have shown increased ventral PFC activation during the emotion induction block as a consequence of the need to regulate their increased negative emotions.
As outlined above, the increase in ventral prefrontal ROI activation that we found for the emotion induction block may have been caused by a number of emotion-related processes, such as the evaluation and/or regulation of emotion. Unfortunately the design of our task does not allow us to ascertain the specific underling processes contributing to this increased activation. However, given the evidence for ventral prefrontal activation underlying emotion regulation (e.g., Ochsner et al., 2004) as well as the link between N2 activation and emotion regulation (e.g., Lamm et al., submitted; Lewis et al., 2006), our pattern of results may indeed have tapped increased emotion regulation in the emotion induction block.
Limitations
Although the paradigm used in this study has shown some promising results for several investigative teams, a number of shortcomings limit the confidence with which the results can be interpreted. First, the go/nogo task we used for this study had only approximately 33 correct nogo trials for blocks A and C, and 20 for block B (before artifact correction). We kept the trial count low because we wanted to maintain emotional involvement for children and adolescents, whose attention span and motivation may quickly dissipate. However, especially for participants with a large number of artifacts, our ERPs were averaged over relatively few trials. Thus, any residual artifactual activation may have carried more weight than if we had presented more trials.
Low trial counts are also associated with another limitation. Specifically, ERPs comprised of a small number of trials may have inflated amplitudes. It is not clear how many trials are required to prevent this effect. Therefore, block-specific trial counts were added as covariates in all scalp and source-space analyses.
Another limitation of this study, and of ERP source analyses in general, is related to the estimation of source-space activity. The calculation of source-space activation is based on an inverse model. Because the number of possible solutions is far greater than the number of preset constraints, the problem is considered “ill posed.” Therefore, additional logical constraints have to be applied. Some of the frequently used logical constraints are incorporated in the LORETA and LAURA algorithms (for a review of these constraints and other minimum norm solutions see Michel et al., 2004). However, each method of constraining the inverse solution may produce slightly different results. Therefore it is important to evaluate the “fit” between the inverse model and the scalp topography. Furthermore, it is important to apply a number of constraints (including a number of regularization constants—how noise is addressed) to determine which inverse solution has the best fit before extracting data. This method of comparing inverse solutions to the topography was applied a number of times before source-space data were extracted using LAURA constraints with a relatively conservative regularization constant. However, because of the limitations inherent in all source-space analyses, replication is required before the results can be considered reliable.
Furthermore, the broad ROIs used in this study were generated based on cortical information from the Montreal Neurological Institute average adult MRI. In other words, estimated source-space current was superimposed on an adult MRI rather than an age-specific MRI or the child's own MRI. We did not have resources to ascertain each child's MRI nor have age-specific MRI head models been generate at this time (not available yet). Thus, even though superimposing children's cortical activation over an adult MRI is by no means ideal, this is the current standard. In the future, this study should be replicated using either the child's own MRI (which is the best scenario) or age-specific MRI head models.
Lastly, because source-space analysis uses the amount of activation change between adjacent electrodes to estimate the location and degree of activation, it is susceptible to erroneous results due to noisy channels. This noise would not be apparent in one voxel (and hence not easy to spot), it would be spread out across the entire model; thus, only very thorough cleaning of the data can prevent this problem. Despite us applying all artifacting criteria available within Net Station (Electrical Geodesic, Inc., Eugene, OR), results should be replicated before being considered reliable.
Conclusion
In summary, this study found age-related decreases in ventral prefrontal activation, but not dorsomedial prefrontal activation, when children were regulating their actions by inhibiting prepotent responses. Furthermore, consistent with other studies, we report increased ventral prefrontal activation but not dorsomedial prefrontal activation for the emotion induction block. This pattern of activation may be explained by increased emotion regulation. Thus, both self-regulation in general and emotion regulation in particular recruited less cortical activation with age, suggesting more efficient cortical mechanisms of response inhibition.
Acknowledgments
We gratefully acknowledge the financial support provided by grant No. 1 R21 MH67357-01 from the Developmental Psychopathology and Prevention Research branch of the National Institute of Mental Health (NIMH), as well as support from the Canadian Institutes for Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). We are also grateful for support provided (to PDZ) by the Canadian Foundation for Innovation.
References
- Adeloye A, Kattan KR, Silverman FN. Thickness of the normal skull in the American Blacks and Whites. American Journal of Physical Anthropology. 1975;43:23–30. doi: 10.1002/ajpa.1330430105. [DOI] [PubMed] [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop Color-Word Task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, Gratton G. Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology. 2005;42:33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Lutz K, Schmidt CF, Jancke L. The emotional power of music: How music enhances the feeling of affective pictures. Brain Research. 2006;1075:151–164. doi: 10.1016/j.brainres.2005.12.065. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Verbaten MN. Source analysis of the N2 in a cued go/nogo task. Cognitive Brain Research. 2005;22:221–231. doi: 10.1016/j.cogbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Verbaten MN. Electrophysiological correlates of attention, inhibition, sensitivity, and bias in a continuous performance task. Clinical Neurophysiology. 2004;115:2001–2013. doi: 10.1016/j.clinph.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, et al. Brain regions underlying response inhibition and interference monitoring and suppression. European Journal of Neuroscience. 2006;23:1658–1664. doi: 10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a go/nogo task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/s1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Neural development of selective attention and response inhibition. NeuroImage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulated cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: An interference task specialized for functional neuroimaging—validation study with functional MRI. Human Brain Mapping. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and Psychopathology. 2002;14:477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AM, III, Botvinick MM, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. Journal of Cognitive Neuroscience. 2006;18:766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. 4th Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ. The auditory-evoked N2 and P3 components in the stop-signal task: indices of inhibition, response-conflict or error-detection? Brain and Cognition. 2006;62:98–112. doi: 10.1016/j.bandc.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone SJ, Barry RJ, Clarke AR. Inhibitory motor control in children with attention-deficit/hyperactivity disorder: event-related potentials in the stop-signal paradigm. Biological Psychiatry. 2003;54:1345–1354. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- Donkers FCL, van Boxtel GJM. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. PET imaging studies of human emotional disorders. In: Gazzaniga MS, editor. The cognitive neurosciences. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar deression. The Journal of Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulated cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in go/nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Science USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Mayahi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, Smith JL. Development of inhibitory processing during the go/nogo task. Journal of Psychophysiology. 2005;19:11–23. [Google Scholar]
- Jonkman LM, Lansbergen M, Stauder JEA. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Sniedt FLF, Kemner C. Source localization of the nogo-N2: a developmental study. Clinical Neurophysiology. 2007;118:1069–1077. doi: 10.1016/j.clinph.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Junghoefer M, Elbert T, Tucker DM, Braun C. The polar average referenced effect: A bias in estimating the head surface integral in EEG recording. Electroencephalography and Clinical Neurophysiology. 1999;110:1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophrenia Research. 2007;97:184–193. doi: 10.1016/j.schres.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kawasaki, et al. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. NatureNeuroscience. 2001;4:15–16. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kesek A, Zelazo PD, Lewis MD. The development of executive cognitive funciton and emotion regulation in adolescence. In: Allen N, Sheeber L, editors. Adolescent emotional development and the emoergence of depressive disorders. Cambridge University Press; New York: 2009. [Google Scholar]
- Kochanska G, Coy K, Murray K. The development of self-regulation across the first four years of life. Child Development. 2001;72:1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience and Biobehavioral Reviews. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lamm C, DeCicco J, Dennis TA. Neural Correlates of Emotion Regulation: An ERP Study. Social Neuroscience. submitted. [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, Forstmeier S. When ‘go’ and ‘nogo’ are equally frequent: ERP components and cortical tomography. European Journal of Neuroscience. 2004;20:2483–2488. doi: 10.1111/j.1460-9568.2004.03683.x. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18:430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A Developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clinical Neurophysiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Cohen J. Stimulus modality, perceptual overlap, and the go/nogo N2. Psychophysiology. 2004;41:157–160. doi: 10.1046/j.1469-8986.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhob RK. Electrophysiological correlates of anterior cingulate function in a go/nogo task: effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioural Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Overtoom CCE, Verbaten MN, Kemner C, Kenemans JL, van Engeland H, Buitelaar JK, et al. Associations between event-related potentials and measures of attention and inhibition in the continuous performance task in children with ADHD and normal controls. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37:977–985. doi: 10.1097/00004583-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and the medial prefrontal cortex: a substrate for emotional behavior? Progress in Brain Research. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff H, Rothbart MK. Attention in early development. Oxford University Press; New York: 1996. [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. ERP correlates of error monitoring in 10-year olds are related to socialization. Biological Psychology. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, Woldorff MG. Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia. 2006;44:384–395. doi: 10.1016/j.neuropsychologia.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Inhibitory processing during the go/nogo task: An ERP analysis of children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2004;115:1320–1331. doi: 10.1016/j.clinph.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Ringel J, Reiss AL. Event-related fMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- Todd RM, Lewis MD. Self-regulation in the developing brain. In: Reed J, Rogers JW, editors. Child neuropsychology: Concepts, theory, and practice. Blackwell; London: 2008. pp. 285–315. [Google Scholar]
- Turner BM, Paradiso S, Marvel CL, Pierson R, Ponto LLB, Hichwa RD, et al. The cerebellum and emotional experience. Neuropsychologia. 2007;45:1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bogaert P, Wikler D, Damhaut P, Szliwowski HB, Goldman S. Regional changes in glucose metabolism during brain development from the age of 6 years. NeuroImage. 1998;8:62–68. doi: 10.1006/nimg.1998.0346. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]