Abstract
Behavioral sensitization is an animal model for aspects of cocaine addiction. Cocaine-sensitized rats exhibit increased AMPA receptor (AMPAR) surface expression in the nucleus accumbens (NAc) which may in turn enhance drug seeking. To identify signaling pathways contributing to AMPAR up-regulation, we measured AMPAR surface expression and signaling pathway activation in the NAc of cocaine-sensitized rats, cocaine-exposed rats that failed to sensitize and saline controls on withdrawal days (WD) 1, 7, and 21. We focused on calcium/calmodulin-dependent protein kinase II (CaMKII), extracellular signal-regulated protein kinase (ERK), and protein kinase A (PKA). In sensitized rats, AMPAR surface expression was elevated on WD7 and WD21 but not WD1. ERK2 activation followed a parallel time-course, suggesting a role in AMPAR up-regulation. Both sensitized and non-sensitized rats exhibited CaMKII activation on WD7, suggesting that CaMKII activation is not sufficient for AMPAR up-regulation. PKA phosphorylation, measured using an antibody recognizing phosphorylated PKA substrates, increased gradually over withdrawal in sensitized rats, from below control levels on WD1 to significantly greater than controls on WD21. Using proteomics, novel sensitization-related PKA substrates were identified, including two structural proteins (CRMP-2 and α-tubulin) that we speculate may link PKA signaling to previously reported dendritic remodeling in NAc neurons of cocaine-sensitized rats.
Keywords: addiction, AMPA receptor, cocaine, nucleus accumbens, rat, sensitization, receptor trafficking
Behavioral sensitization refers to the progressive increase in responsiveness to drugs that develops during their repeated administration and persists long after drug exposure is discontinued. Behavioral sensitization models aspects of psychostimulant addiction because sensitization develops not only to the locomotor-activating effects of psychostimulants, but also to their incentive-motivational effects (Robinson and Berridge 2008). For example, sensitized rats work harder to obtain drugs in self-administration paradigms (Vezina 2004).
Neurons of the nucleus accumbens (NAc) are critical mediators of motivated behavior. They integrate inputs from cortico-limbic regions important for motivation and project to regions involved in motor execution (Kelley 2004). Cortico-limbic inputs excite NAc neurons via α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors (AMPARs; Pennartz et al. 1990; Hu and White 1996) and, not surprisingly, drug seeking is blocked by AMPAR antagonists and elicited by intra-NAc infusion of AMPA (Cornish et al. 1999; Cornish and Kalivas 2000; Di Ciano and Everitt 2001; Suto et al. 2004; Bäckström and Hyytia 2007; Famous et al. 2008; Ping et al. 2008).
Changes in synaptic distribution of AMPARs underlie many forms of plasticity including addiction-related plasticity (Wolf et al. 2004; Kauer and Malenka 2007). Several lines of evidence suggest that AMPAR transmission is enhanced in the NAc of sensitized rats (Bell and Kalivas 1996; Pierce et al. 1996; Churchill et al. 1999; Bell et al. 2000; Suto et al. 2004; Bachtell and Self 2008). This may be due in part to a withdrawal-dependent increase in cell surface and synaptic expression of GluR1/2-containing AMPARs (Boudreau and Wolf 2005; Boudreau et al. 2007; Kourrich et al. 2007; Ghasemzadeh et al. 2009). This effect was not observed in cocaine-exposed rats that failed to sensitize (Boudreau and Wolf 2005). Enhanced AMPAR transmission in the NAc is functionally significant because it has been linked to enhanced drug seeking (Suto et al. 2004; Anderson et al. 2008; Conrad et al. 2008), although its relationship to the expression of locomotor sensitization remains unclear (see Discussion).
The goal of the present study was to investigate three signaling pathways that are implicated in AMPAR trafficking (Derkach et al. 2007) and may contribute to AMPAR plasticity in the NAc of cocaine-sensitized rats: extracellular signal-regulated kinase (ERK), Ca2+-calmodulin-dependent protein kinase II (CaMKII), and cAMP-dependent protein kinase (PKA). By measuring signaling pathway activation in saline controls, sensitized rats and non-sensitized cocaine-exposed rats at three different withdrawal times (1, 7, or 21 days after discontinuing injections), we found that sensitization is characterized by evolving changes in signaling pathway activation over the first 3 weeks of withdrawal. Testing a causal role for any pathway in AMPAR redistribution using pharmacological or molecular inhibitors is difficult because, as shown here, AMPAR redistribution develops slowly. However, to further evaluate candidate pathways, we assessed correlations between signaling pathway activation and AMPAR distribution for individual rats. Finally, we used proteomics to identify novel PKA substrates that exhibit enhanced phosphorylation in the NAc of sensitized rats in late withdrawal.
Materials and methods
Animals and drug treatment
Male Sprague-Dawley rats weighing 250–275 g (Harlan Laboratories, Indianapolis, IN, USA) were group housed with food and water available ad libitum on a 12 h light/dark cycle. Injections and behavioral testing were performed between 11 am and 4 pm. All procedures were approved by the Institutional Animal Care and Use Committee. Rats were assigned to saline and cocaine groups after 10 days in the colony. On the day before the first injection, rats were habituated to the testing procedure by placement in a photocell cage for 20 min (San Diego Instruments, San Diego, CA, USA) followed by a mock injection (syringe without needle touched to abdomen) and 2 h in the photocell cage. On treatment day 1, rats were habituated to photocell cages for 20 min and then injected with cocaine (15 mg/kg, i.p.; provided by NIDA) or saline (1 mL/kg, i.p.). Locomotor activity was measured for 2 h. For the next 5 days, animals received cocaine (30 mg/kg, i.p.) or saline in home cages. On the last day of treatment (day 7), rats received 15 mg/kg cocaine or saline as described for day 1 and locomotor activity was monitored for 2 h. Sensitization was assessed by comparing days 1 and 7 using criteria described previously (Boudreau and Wolf 2005). After 1, 7, or 21 days of withdrawal in home cages, rats were killed for biochemical analysis.
Surface receptor cross-linking
Surface and intracellular GluR1 levels were determined with a bis(sulfosuccinimidyl)suberate (BS3) protein cross-linking assay (Boudreau and Wolf 2005). NAc tissue (core and shell) was dissected from a 2 mm coronal section obtained using a brain matrix and chopped into 400 μm slices using a McIllwain chopper (Vibratome, St. Louis, MO, USA). Slices were added to eppendorf tubes containing ice-cold aCSF spiked with 2 mM BS3 (Pierce Biotechnology, Rockford, IL, USA). After 30 min of cross-linking (4°C), the reaction was quenched with 100 mM glycine (10 min, 4°C). Slices were pelleted by brief centrifugation. Pellets were resuspended in ice-cold lysis buffer containing protease and phosphatase inhibitors and sonicated for 5 s. After brief centrifugation, the supernatant fraction was used for further studies. Protein concentration was determined according to Bradford (1976). Samples were aliquotted (~15 aliquots per rat) and stored at –80°C.
Western blotting
Samples (20–30 μg total protein/lysate) were electrophoresed on 4–15% Tris–HCl (Biorad, Hercules, CA, USA) or 3–8% Tris Acetate (Invitrogen, Carlsbad, CA, USA) SDS-gradient gels. Proteins were transferred to polyvinylidene fluoride membranes for immunoblotting. Membranes were washed in ddH2O, blocked with either 1% goat serum/5% non-fat dry milk or 3% bovine serum albumin in TBS-Tween-20 (TBS-T), pH 7.4, for 1 h at 21°C, and then incubated overnight at 4°C with antibody to GluR1 (1 : 500, Millipore, Billerica, MA, USA). Or, membranes were incubated overnight with phospho-specific antibodies recognizing activated ERK (pERK1/2 1 : 10 000; Millipore) or CaMKII (pCaMKII; 1 : 5000, Phosphosolutions, Aurora, CO, USA) and then stripped (62.5 mM Tris–HCl, pH 6.7, 100 mM β-mercaptoethanol, 2% SDS) before probing with phosphorylation independent antibodies to ERK or αCaMKII (Total ERK1/2, 1 : 20 000, Millipore; Total αCaMKII, 1 : 10 000, Millipore). Membranes were washed extensively with TBS-T, incubated for 60 min with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (1 : 10 000; Millipore) and washed again in TBS-T. Visualization was achieved by chemiluminescence (ECL; GE Healthcare, Piscataway, NJ, USA) using HyperFilm ECL film. Bands of interest were quantified using TotalLab (Nonlinear Dynamics Ltd., Newcastle, UK). Total protein in each lane was determined by staining membranes with Ponceau S (Sigma–Aldrich, St. Louis, MO, USA). To measure PKA activation, we used an antibody recognizing phosphorylated substrates of PKA (Cat. # 9621, 1 : 5000, Cell Signaling Technology, Danvers, MA, USA). This antibody was produced by immunizing rabbits with a synthetic phospho-PKA substrate peptide and purified over an affinity column using the same peptide. A potential concern is overlapping substrate specificity with other Arg-directed protein kinases with similar Arg requirements [e.g., AKT and to a lesser extent protein kinase C (PKC)] relative to the phosphorylated Ser/Thr (e.g., Kennelly and Krebs 1991). To assess this, we compared the PKA substrate antibody with the AKT substrate antibody (Cell Signaling Technology) in western blotting experiments using rat NAc tissue and found that the antibodies produced distinct banding patterns. Of the nine major bands we detected with the PKA substrate antibody, none were detected with the AKT substrate antibody. Furthermore, only the PKA substrate antibody showed increased immunoreactivity by western blotting in tissue that was stimulated with the PKA activator Sp-adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (SpcAMPS) or the D1-like agonist (±)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetra-hydro-1H-3-benzazepine hydrobromide (SKF81297) (data not shown). Similarly, the PKC substrate antibody (Cell Signaling Technology) produced a significantly different banding pattern (data not shown) and the peptide used to raise the PKC substrate antibody did not diminish immunoreactivity detected with the PKA substrate antibody, while the PKA substrate peptide eliminated this immunoreactivity (see Fig. 4). Together, these results indicate specificity of the PKA substrate antibody. Additional validation studies were published recently (Sindreu et al. 2007).
Fig. 4.
Phosphorylation of PKA substrates in the NAc of sensitized rats is decreased in early withdrawal but increased in late withdrawal. (a-left panel) Immunoprobing NAc cross-linked tissue (20 or 10 μg of total protein loaded onto the gel) with the PKA substrate antibody in the presence of peptide vehicle (PKA + Veh.) results in the detection of nine immunoreactive bands. (a-center panel) Immunoreactivity is unchanged by pre-incubation of the PKA substrate antibody with the PKC substrate peptide (PKA + PKC pep.). (a-right panel) Immunoreactivity is eliminated by preincubation with the PKA substrate peptide (PKA + PKA pep.). (b) Representative immunoblot demonstrating the bands scanned to assess overall PKA phosphorylation (OVERALL, left lane) and individual PKA substrates (right lane). (c–e) Overall, PKA substrate phosphorylation is decreased on WD1, unchanged on WD7, and increased on WD21 in sensitized rats. WD 1: SAL (n = 12), SENS (n = 7), NON (n = 9); WD 7: SAL (n = 16), SENS (n = 12), NON (n = 16); WD 21: SAL (n = 15), SENS (n = 10), NON (n = 12). Data (mean+S.E.M.) are normalized to SAL controls. *p < 0.05, **p < 0.01, significantly different from SAL group.
PKA substrate identification by mass spectrometry
150 μg or 400 μg of NAc tissue prepared in low salt lysis buffer [25 mM HEPES pH 7.4, 150 mM NaCl, 2 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 μM microcystin-LF, 1 μM okadiac acid, 1x protease inhibitor cocktail (EMB Biosciences, San Diego, CA, USA), and 0.1% Nonidet P-40 (EMB Biosciences)] along with See Blue Plus prestained protein standards (Invitrogen) was loaded and electrophoresed on a preparative 20 cm × 20 cm 10% Tris–HCl gel. Half of the gel (samples flanked on both sides with protein standards) was stained with colloidal Coomassie Blue and subsequently with SYPRO Ruby (Sigma-Aldrich). The other half (samples flanked on both sides with standards) was transferred onto a polyvinylidene fluoride membrane for immunoblotting with antibody recognizing phosphorylated PKA substrates (Cell Signaling Technology; see previous section). Stained and immunoblotted images were aligned, crude masses were identified based on mobility in the gel using TotalLab software, and gel bands were excised for proteomic analyses in the Midwest Proteome Center (Rosalind Franklin University of Medicine and Science). Excised PKA substrate bands were processed after reduction (10 mM dithioerythritol) and alkylation (55 mM iodoacetamide) in 50 mM ammonium bicarbonate pH 7.8 at 21°C in the dark. Tryptic digestion occurred overnight (Trypsin Gold, Promega, Madison WI, USA) with an enzyme:substrate ratio of 1 : 80 according to manufacturer's protocols with the above buffer supplemented with 4 mM CaCl2. Samples were then desalted with either Zip-tips (Waters Corp., Milford, MA, USA) or desalted by gel filtration tips for larger fragments.
Mass spectrometry was performed with an ABI-QSTAR XL hybrid quadrupole Q-TOF MS/MS system equipped with a nanoelectrospray source (Applied Biosystems, Framingham, CA, USA) combined with a nanoliquid chromatography (LC Packings, Sunnyvale, CA, USA) front-end used to analyse peptide sequences. The nano-LC utilized a 75 μmID × 100 mm C18 column at a flow rate of 400 nL/min and a linear gradient of 5–40% solvent B (95% acetonitrile, 0.08% trifluoroacetic acid) over 35 min. The m/z response of the mass spectrometer was calibrated daily with manufacturer's standards for an accuracy of < 10 ppm. Sequence coverage by MS/MS was obtained with an automatic search paradigm using MASCOT software (Matrix Science, London, UK) and Protein Prospector (prospector.ucsf.edu). A mass tolerance of 100 ppm and one allowed trypsin miscleavage (for phosphorylated serines and threonines) (Tullai et al. 2000) was utilized. Search parameters allowed for variable methionine oxidations and cysteine carbamidomethylation. Identification was judged by the number of peptides, sequence coverage, MASCOT mowse score and MS/MS spectral quality.
Data analysis
Two-way ANOVA with time as the repeated measure, followed by a Tukey test, was used to compare locomotor activity on days 1 and 7 of behavioral trials. For Westerns, data for experimental groups were normalized to controls and compared using ANOVA followed by a Tukey test. Spearman Rank Order was used to test for correlations between two measures. Significance was set at p < 0.05. For some experimental groups, N-values vary slightly between analyses because of problems with particular lanes in western blots.
Results
Time-course of AMPAR redistribution during cocaine withdrawal
Data from seven behavioral trials (some reported previously; Boudreau and Wolf 2005) were used for the current study. In each trial, rats received seven injections of saline or cocaine (15 mg/kg on treatment days 1 and 7; 30 mg/kg on days 2–6). Locomotor activity was measured on treatment days 1 and 7. As expected from prior studies using this regimen (Pierce et al. 1996; Churchill et al. 1999; Boudreau and Wolf 2005; Boudreau et al. 2007), about half of the cocaine-treated rats developed sensitization based on comparison of treatment days 1 and 7 for saline and cocaine groups (see Boudreau and Wolf 2005 for criteria). Thus, each trial yielded saline controls, sensitized rats and non-sensitized rats.
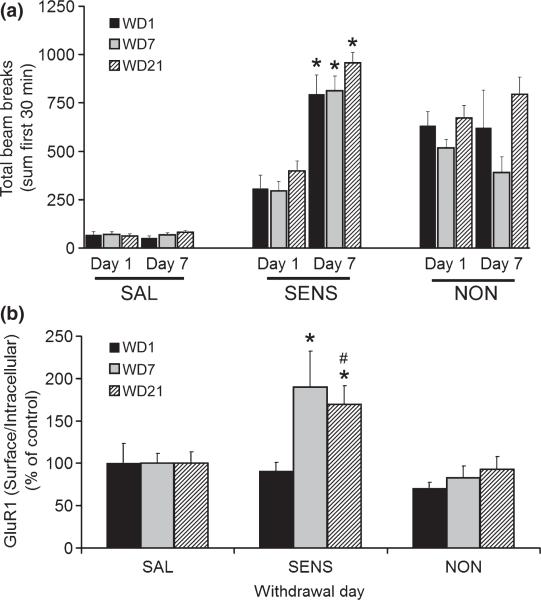
Fig. 1a shows locomotor activity counts on treatment days 1 and 7 for rats destined for biochemical analysis on withdrawal day (WD) 1, WD7, or WD21. Within each group (saline, sensitized and non-sensitized), rats destined for analysis at different withdrawal times had equivalent responses on treatment days 1 and 7. Saline rats showed no change in activity between days 1 and 7, as expected (Fig. 1a left). Sensitized rats showed significantly higher activity on day 7 (Fig. 1a, middle). Non-sensitized rats showed no change between treatment days 1 and 7 (Fig. 1a, right). However, their initial response to cocaine on treatment day 1 was greater than the initial response of rats that developed sensitization, whereas their response on day 7 was lower (p < 0.05 in both cases; comparison of all non-sensitized versus all sensitized rats). We did not measure stereotypy so we cannot rule out the possibility that the “non-sensitized” group failed to exhibit enhanced locomotion on treatment day 7 because they had developed stereotyped behaviors incompatible with locomotion. However, in preliminary studies with this regimen, we did not observe stereotyped behaviors that would preclude locomotion, and results of Sabeti et al. (2003) argued against this explanation for failure of a subgroup of rats to develop locomotor sensitization to cocaine. Furthermore, the non-sensitized rats resembled saline controls more than sensitized rats in their biochemical profile (see subsequent sections and Discussion).
Fig. 1.
Withdrawal from cocaine is accompanied by increased GluR1 S/I ratios in the NAc of sensitized rats. (a) Rats received seven daily injections of saline (SAL) or cocaine. Cocaine rats were divided into sensitized (SENS) and non-sensitized (NON) groups based on comparison of locomotor activity on the first and last treatment days (Day 1 and Day 7) and then assigned to different groups destined for biochemical analysis on withdrawal day (WD) 1, WD7, or WD21. Data (mean±S.E.M.) show equivalence of groups destined for different withdrawal times and a significant increase in activity over 7 days of cocaine treatment only for SENS rats (*p < 0.01). Results represent the sum of seven independent trials: 2 for WD1 (SAL, n = 12, SENS, n = 8; NON, n = 9); 2 for WD7 (SAL, n = 18; SENS, n = 15; NON, n = 17); and 3 for WD21 (SAL, n = 18; SENS, n = 12; NON, n = 13). (b) GluR1 S/I ratios in SAL, SENS and NON groups analyzed on WD1, WD7, or WD21. Data (mean + SEM) are normalized to the corresponding SAL group. *p < 0.01, #p < 0.05; significantly different from SAL and NON groups, respectively.
NAc tissue was obtained from all rats and cross-linked with BS3, a membrane impermeant cross-linking agent. BS3 cross-links cell surface proteins, increasing their apparent mass, while intracellular proteins remain unmodified, enabling surface (S) and intracellular (I) pools to be distinguished by SDS-PAGE and western blotting. The NAc from one rat yields enough cross-linked tissue for ~15 immunoblots. One aliquot from each rat was used to measure the GluR1 surface/intracellular ratio (S/I), while others were used to assess signaling pathways (see next section). GluR1 was studied because most AMPARs in the NAc contain this subunit (Boudreau et al. 2007; Conrad et al. 2008).
We found that the sensitized group exhibited significantly higher GluR1 S/I ratios than control rats on WD7 and WD21 but not WD1, indicating that AMPAR surface expression increases during the first week of withdrawal and then remains elevated for at least two more weeks (Fig. 1b). Then we used a Spearman Rank Order test to determine if individual rats exhibited correlations between GluR1 S/I and the magnitude of behavioral sensitization (Table 1). On WD1, no positive correlations were found; in fact, nonsensitized rats yielded a significant negative correlation. On WD7, a positive correlation between GluR1 S/I and the magnitude of sensitization existed for all cocaine-exposed rats. When cocaine-exposed rats were divided into sensitized and non-sensitized groups, only sensitized rats showed a positive correlation. Similarly, on WD21, a positive correlation was observed for all cocaine-exposed rats and again, after division into sensitized and non-sensitized groups, only sensitized rats showed a positive correlation. Thus, withdrawal-dependent increases in AMPAR surface expression were observed only in rats that developed locomotor sensitization.
Table 1.
Spearman rank order r and p-values for relationships between the magnitude of sensitization (Day 7/Day 1), GluR1 S/l ratio, pERK2/ERK2, pCaMKII/CaMKII, pCaMKII/total protein, CaMKII/total protein and overall PKA substrate phosphorylation in saline (SAL), all cocaine-exposed (ALL), sensitized (SENS) and non-sensitized (NON) rats on WD1, WD7, and WD21
| Day 7/Day 1 |
GluR1 S/1 |
pERK2/ERK2 |
pCAMKII/CaMKII |
pCAMKII/total protein |
Total CAMKII/ Total Protein |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | SAL | ALL | SENS | NON | SAL | ALL | SENS | NON | SAL | ALL | SENS | NON | SAL | ALL | SENS | NON | SAL | ALL | SENS | NON | SAL | ALL | SENS | NON | ||
| WD 1 | GluFM S/l | r | –0.33 | 0.02 | –0.26 | – 0.89 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| p | 0.33 | 0.94 | 0.66 | 0.00 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| pERK2/ERK2 | r | 0.26 | 0.06 | 0.14 | 0.60 | –0.01 | –0.63 | –0.43 | –0.68 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| p | 0.42 | 0.81 | 0.80 | 0.08 | 0.97 | 0.02 | 0.42 | 0.07 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| pCAMKII/CaMKII | r | 0.13 | – 0.59 | –0.32 | –0.38 | –0.35 | 0.13 | 0.31 | 0.11 | 0.43 | 0.03 | 0.60 | –0.43 | – | – | – | – | – | – | – | – | – | – | – | – | |
| p | 0.67 | 0.02 | 0.44 | 0.32 | 0.31 | 0.67 | 0.56 | 0.78 | 0.18 | 0.90 | 0.24 | 0.26 | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Overall PKA | r | 0.36 | –0.09 | –0.32 | –0.30 | –0.42 | 0.23 | –0.77 | 0.75 | 0.30 | –0.21 | 0.09 | –0.37 | 0.80 | 0.01 | 0.07 | –0.10 | – | – | – | – | – | – | – | – | |
| p | 0.27 | 0.74 | 0.44 | 0.41 | 0.21 | 0.44 | 0.10 | 0.04 | 0.35 | 0.45 | 0.92 | 0.31 | 0.00 | 0.95 | 0.84 | 0.79 | – | – | – | – | – | – | – | – | ||
| WD 7 | GluFM S/l | r | 0.47 | 0.58 | 0.60 | –0.11 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| p | 0.14 | 0.00 | 0.02 | 0.78 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| pERK2/ERK2 | r | 0.41 | 0.16 | 0.32 | 0.04 | 0.53 | 0.44 | 0.43 | 0.43 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| p | 0.10 | 0.46 | 0.27 | 0.89 | 0.03 | 0.04 | 0.14 | 0.30 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| pCAMKII/CaMKII | r | 0.08 | 0.06 | 0.26 | 0.25 | 0.15 | –0.11 | –0.04 | –0.14 | –0.43 | 0.30 | 0.42 | 0.04 | – | – | – | – | – | – | – | – | – | – | – | – | |
| p | 0.77 | 0.77 | 0.36 | 0.47 | 0.57 | 0.65 | 0.89 | 0.72 | 0.09 | 0.18 | 0.19 | 0.89 | – | – | – | – | – | – | – | – | – | – | – | – | ||
| pCAMKII/total protein | r | –0.05 | –0.01 | –0.03 | 0.18 | –0.24 | –0.20 | –0.21 | –0.26 | –0.04 | –0.12 | –0.05 | –0.17 | – | – | – | – | – | – | – | – | – | – | – | – | |
| p | 0.82 | 0.98 | 0.91 | 0.61 | 0.34 | 0.37 | 0.45 | 0.66 | 0.88 | 0.58 | 0.86 | 0.64 | – | – | – | – | – | – | – | – | – | – | – | – | ||
| CaMKII/total protein | r | 0.02 | –0.05 | –0.18 | 0.03 | –0.10 | –0.13 | –0.18 | –0.26 | 0.47 | –0.31 | –0.35 | –0.25 | – | – | – | – | – | – | – | – | – | – | – | – | |
| p | 0.94 | 0.82 | 0.53 | 0.91 | 0.70 | 0.59 | 0.55 | 0.66 | 0.07 | 0.18 | 0.28 | 0.49 | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Overall PKA | r | –0.12 | –0.25 | –0.53 | 0.19 | 0.17 | –0.41 | –0.49 | –0.11 | 0.14 | – 0.43 | –0.34 | –0.50 | 0.39 | 0.13 | 0.05 | 0.20 | 0.14 | 0.55 | 0.58 | 0.45 | 0.15 | 0.43 | 0.50 | 0.42 | |
| p | 0.62 | 0.25 | 0.06 | 0.58 | 0.48 | 0.08 | 0.10 | 0.78 | 0.58 | 0.05 | 0.30 | 0.13 | 0.12 | 0.55 | 0.87 | 0.56 | 0.58 | 0.01 | 0.04 | 0.20 | 0.57 | 0.05 | 0.09 | 0.24 | ||
| WD 21 | GluR1 S/l | r | –0.18 | 0.51 | 0.58 | –0.01 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| p | 0.46 | 0.00 | 0.01 | 0.97 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| pERK2/ERK2 | r | –0.23 | 0.36 | 0.36 | 0.36 | 0.62 | –0.06 | –0.39 | –0.20 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| p | 0.36 | 0.12 | 0.39 | 0.24 | 0.01 | 0.81 | 0.34 | 0.53 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| pCAMKII/CaMKII | r | –0.11 | –0.06 | 0.15 | –0.07 | –0.06 | – 0.47 | – 0.67 | –0.34 | 0.30 | 0.09 | 0.17 | 0.01 | – | – | – | – | – | – | – | – | – | – | – | – | |
| p | 0.66 | 0.79 | 0.68 | 0.82 | 0.79 | 0.03 | 0.04 | 0.26 | 0.22 | 0.71 | 0.66 | 0.96 | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Overall PKA | r | –0.12 | 0.38 | –0.32 | 0.22 | –0.06 | 0.48 | 0.57 | 0.15 | –0.28 | 0.28 | –0.10 | 0.34 | –0.10 | – 0.64 | – 0.75 | – 0.57 | – | – | – | – | – | – | – | – | |
| p | 0.62 | 0.08 | 0.35 | 0.48 | 0.80 | 0.03 | 0.10 | 0.62 | 0.26 | 0.23 | 0.79 | 0.27 | 0.69 | 0.00 | 0.01 | 0.04 | – | – | – | – | – | – | – | – | ||
Shaded boxes indicate statistically significant corrections.
We also compared S, I and total (S + I) GluR1 levels in NAc tissue from sensitized, non-sensitized and saline groups (data not shown, but results for some WD21 trials were reported previously; Boudreau and Wolf 2005). In every group where GluR1 S/I increased, surface GluR1 also increased significantly, whereas intracellular levels tended to decrease, though not significantly. GluR1 total protein levels in sensitized rats were modestly but not significantly increased at all three withdrawal times (115–124% of control). Other studies using similar regimens have detected increases in total GluR1 of similar magnitude that achieved statistical significance (Churchill et al. 1999; Ghasemzadeh et al. 2009).
Next, signaling pathways (CaMKII, ERK and PKA) known to be important for hippocampal AMPAR trafficking (see Introduction) were examined in NAc total tissue homogenates from these same rats (tissue was cross-linked, but this does not affect detection of intracellular antigens).
AMPAR redistribution is paralleled by increased ERK2 phosphorylation
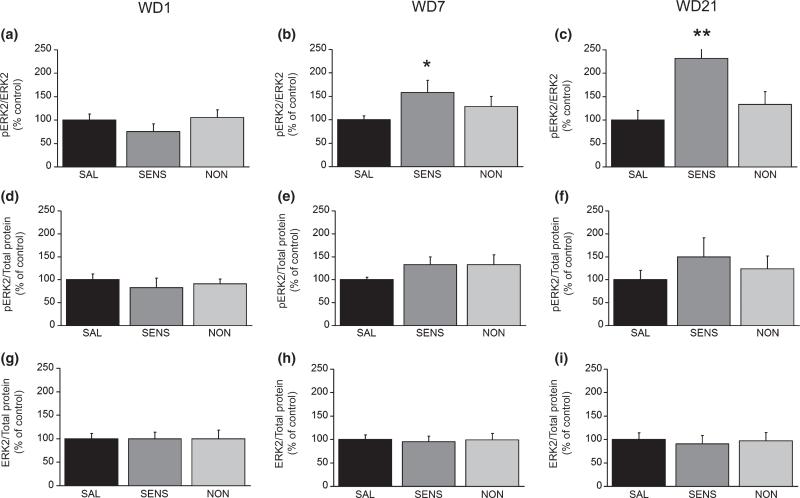
Ras-ERK signaling is implicated in AMPAR synaptic insertion in hippocampal neurons (Zhu et al. 2002), so we examined ERK activation using phospho-specific antibodies. Cocaine withdrawal did not alter pERK1 or total ERK1 (data not shown). For ERK2, no group differences were found on WD1 (Fig. 2a, d, and g). However, on WD7 and WD21, when the GluR1 S/I ratio was increased in sensitized rats, pERK2/ERK2 was also increased in sensitized rats (Fig. 2b and c) whereas total ERK2 protein was unchanged (Fig. 2h and i). ERK2 measurements for non-sensitized rats did not differ from saline controls. Thus, increased pERK2/ERK2 paralleled the increased AMPAR surface expression observed in sensitized rats on WD7 and WD21.
Fig. 2.
ERK2 phosphorylation, like AMPAR surface expression, is increased in the NAc of sensitized rats (SENS) on WD7 and WD21 compared with non-sensitized (NON) and saline (SAL) rats. (a–c) PhosphoERK2 normalized to total ERK2 protein (pERK2/ERK2). (d–f) pERK2 normalized to total protein in the lane (pERK2/total protein). (g–i) Total ERK2 levels normalized to total protein in the lane (ERK2/total protein). WD 1: SAL (n = 12), SENS (n = 7), NON (n = 9); WD 7: SAL (n = 16), SENS (n = 12), NON (n = 16); WD 21: SAL (n = 17), SENS (n = 10), NON (n = 12). Data (mean + SEM) are normalized to SAL controls.*p < 0.05, **p < 0.01, significantly different from SAL group.
We evaluated correlations between pERK2/ERK2 and the GluR1 S/I ratio for individual rats on WD1, WD7 and WD21 (Table 1). A significant positive correlation was observed for saline controls on WD7 and WD21. This may reflect the normal role of ERK2 signaling in AMPAR synaptic incorporation as inferred from results of Zhu et al. (2002). Lack of correlation on WD1 may indicate perturbation of normal relationships shortly after discontinuing the mildly stressful experience of daily saline injections. Among cocaine groups, the only significant relationship was a positive correlation between pERK2/ERK2 and GluR1 S/I for all cocaine-exposed rats on WD7 that was not present when sensitized and non-sensitized groups were assessed separately, perhaps due to lower N-values. Thus, while the increases in GluR1 S/I and pERK2/ERK2 occurred in parallel, the magnitude of these increases was not significantly correlated in sensitized rats, suggesting an indirect relationship between the two.
CaMKII phosphorylation is increased in cocaine-exposed rats on WD7
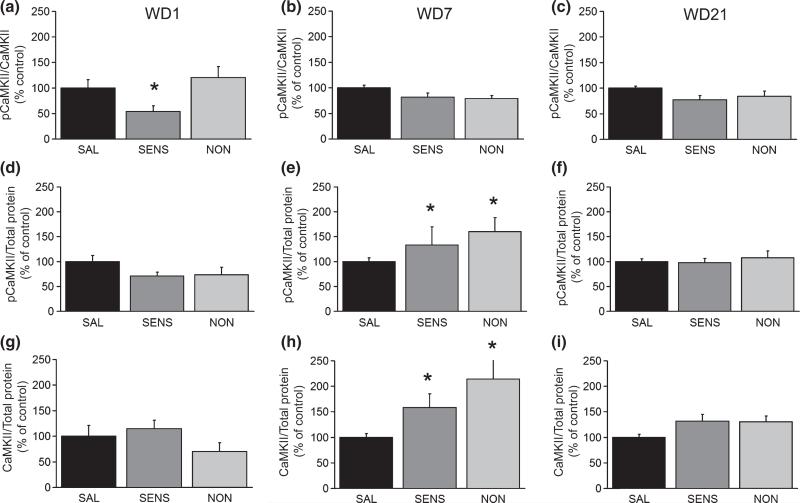
Activation of CaMKII induces AMPAR synaptic insertion during hippocampal LTP (Hayashi et al. 2000), so we assessed CaMKII phosphorylation in our NAc aliquots. Sensitized rats on WD1 exhibited decreased pCaMKII/CaMKII (Fig. 3a) but no change in total CaMKII protein (Fig. 3g). On WD7, pCaMKII and total CaMKII levels increased in both sensitized and non-sensitized rats (Fig. 3e and h). Because both measures increased proportionally, there was no overall change in pCaMKII/CaMKII in either group (Fig. 3b). By WD21, there were no significant between-group differences for any CaMKII parameter (Fig. 3c, f, and i).
Fig. 3.
CaMKII protein and phosphorylation are increased after 7 days of withdrawal in the NAc of sensitized (SENS) and non-sensitized rats (NON) compared with controls (SAL). (a–c) PhosphoCaMKII normalized to total CaMKII protein (pCaMKII/CaMKII). (d–f) pCaMKII normalized to total protein in the lane (pCaMKII/total protein). (g–i) Total CaMKII levels normalized to total protein in the lane (CaMKII/total protein). WD 1: SAL (n = 11), SENS (n = 7), NON (n = 9); WD 7: SAL (n = 16), SENS (n = 12), NON (n = 14); WD 21: SAL (n = 17), SENS (n = 10), NON (n = 10). Data (mean + SEM) are normalized to SAL controls. *p < 0.05, significantly different from SAL group.
We also examined possible correlations for individual rats between CaMKII measurements and either the magnitude of sensitization or GluR1 S/I (Table 1). On WD1, pCaMKII/CaMKII was negatively correlated with the magnitude of sensitization for all cocaine-exposed rats but not saline controls, consistent with between-group results showing decreased pCaMKII/CaMKII in sensitized rats (Fig. 3a). No relationship was found between pCaMKII/CaMKII and GluR1 S/I for any group. On WD7, no significant correlations were found between any CaMKII measure and the magnitude of sensitization or GluR1 S/I, despite between-group differences for some CaMKII measures on WD7 as described above (Fig. 3e and h). Finally, on WD21, pCaMKII/CaMKII was negatively correlated with the GluR1 S/I ratio for all cocaine-exposed rats and sensitized rats, but not for non-sensitized rats.
In hippocampal neurons, Ras-ERK activation is downstream of CaMKII in the pathway leading to AMPAR synaptic insertion (Zhu et al. 2002), so we also evaluated correlations between pCaMKII/CaMKII and pERK2/ERK2 for individual rats (Table 1). Although no statistically significant correlations were found, there was a trend towards a positive correlation in saline rats at all withdrawal times (p = 0.09–0.22) and in sensitized rats on WD1 and WD7 (p = 0.24 and 0.19, respectively).
In summary, a transient increase in CaMKII signaling may contribute to AMPAR up-regulation on WD7 but it is not sufficient, as CaMKII is activated in non-sensitized rats on WD7 and they do not develop AMPAR up-regulation.
Phosphorylation of PKA substrates increases in sensitized rats during late withdrawal
Protein kinase A signaling is implicated in AMPAR trafficking to the cell surface (Chao et al. 2002a, b; Esteban et al. 2003; Mangiavacchi and Wolf 2004; Sun et al. 2005, 2008; Gao et al. 2006; Oh et al. 2006; Man et al. 2007). We assessed PKA indirectly by immunoprobing with an antibody recognizing the phosphorylated consensus site for PKA phosphorylation. Antibody specificity was confirmed in control experiments (see Materials and methods) including peptide competition experiments demonstrating that immunoreactivity detected with the PKA substrate antibody is eliminated by the PKA substrate peptide used to raise the antibody but not a PKC substrate peptide (Fig. 4a). Under our conditions, nine major PKA substrate bands ranging from ~90 kDa to ~39 kDa were detected in NAc tissue using the phosphorylated PKA substrate antibody (Fig. 4b).
We began by quantifying overall PKA substrate phosphorylation (sum of the nine bands) in NAc tissue from the same rats analyzed for other measures. In sensitized rats, overall PKA substrate phosphorylation was significantly decreased on WD1, unchanged on WD7, and significantly increased on WD21 compared with saline controls, indicating that it lagged temporally behind AMPAR up-regulation during withdrawal (Fig. 4c–e).
Next, we evaluated correlations between overall PKA phosphorylation and other measures for individual rats. Results are shown in Table 1. No significant correlations between overall PKA substrate phosphorylation and GluR1 S/I were found for sensitized rats. However, on WD1 and WD7, there were trends towards an inverse relationship (r = –0.77 and –0.49, respectively; p = 0.1 for both days), which switched towards a positive relationship (r = 0.57, p = 0.1) on WD21. This shift, combined with a gradual increase in PKA substrate phosphorylation as withdrawal progresses (Fig. 4c–e), is consistent with a possible role for PKA in maintaining AMPAR up-regulation in sensitized rats late in withdrawal.
The withdrawal-dependent increase in overall PKA phosphorylation (Fig. 4) was paralleled by results obtained when each of the nine prevalent PKA substrate bands were analyzed individually for the sensitized group (Fig. 5). On WD1, phosphorylation of PKA substrates 66, 46, and 42 kDa was significantly decreased in sensitized rats and a trend in the same direction was observed for several other bands (Fig. 5d, g, and h). On WD7, phosphorylation of several PKA substrates appeared to be recovering in the direction of control levels (Fig. 5a, d, f, and g). On WD21, the phosphorylation of PKA substrates 90, 66, 54, 46, 42, and 39 kDa was significantly increased compared with saline controls (Fig. 5a, d, and f–i). Thus, we can identify specific substrate proteins that contribute to the gradual increase in overall PKA substrate phosphorylation that occurs in the NAc between WD1 and WD21 in sensitized rats (Fig. 4c–e). No change in the phosphorylation of any of the nine prevalent PKA substrate bands was observed in non-sensitized rats compared with controls at any withdrawal time (data not shown).
Fig. 5.
Phosphorylation of individual PKA substrates increases slowly over the course of withdrawal in the NAc of cocaine-sensitized rats. Panels a–i show data for nine prevalent PKA substrate bands. WD 1: n = 7; WD 7: n = 7; WD 21: n = 10. Data (mean + SEM) are normalized to total protein in the lane and presented as percent of respective SAL controls. *p < 0.01, #p < 0.05; significantly different from SAL and NON groups, respectively. N.D., Not determined.
Next, we tested possible correlations between each PKA substrate band and GluR1 S/I or the magnitude of sensitization (day 7/day 1). We restricted the analysis to WD21 because this is the only withdrawal time when sensitized rats showed overall elevation of PKA substrate phosphorylation (Fig. 4). Of the individual PKA substrates showing elevated phosphorylation in sensitized rats on WD21 (Fig. 5), only PKA substrate band 39 kDA exhibited positive correlations with the other measures. Phosphorylation of this substrate on WD21 correlated with GluR1 S/I in sensitized rats (r = 0.5, p < 0.05) and with both GluR1 S/I and the magnitude of sensitization in all cocaine-exposed rats (r = 0.5, p < 0.05 and r = 0.44, p < 0.05, respectively). These relationships were not present at earlier withdrawal times (data not shown).
We also tested possible correlations between individual PKA substrate phosphorylation and pERK2/ERK in sensitized rats on WD21, based on our observation that both measures were elevated in the sensitized group on WD21 (Figs 2 and 4). Only PKA substrate band 54 kDa showed a positive correlation (r = 0.69, p < 0.05). Finally, because pCaMKII/total protein and CaMKII/total protein increased on WD7 in sensitized and non-sensitized groups (Fig. 3e and h), while PKA substrate phosphorylation was “in transition” at this withdrawal time (Figs 4 and 5), we evaluated possible correlations on WD7 between these CaMKII measures and PKA substrate phosphorylation. pCaMKII/total protein showed significant positive correlations with seven PKA substrates for individual cocaine-exposed rats on WD7 [substrates 90, 78, 74, 42, and 39 kDa (p < 0.01) and 66, 54, and 46 kDa (p < 0.05)]. For all of these bands, significant positive correlations (90, 78, 74, 42, 39 kDa; p < 0.05) or trends (66, 54, 46 kDa; p-values < 0.12) were also found when sensitized rats were assessed separately. In non-sensitized rats, only one significant correlation (39 kDa; p < 0.05) and one trend (42 kDa; p = 0.12) were found. Thus, while pCaMKII/total protein was increased in both sensitized and non-sensitized groups on WD7 (Fig. 3e), only sensitized rats also exhibited correlations on WD7 between pCaMKII/total protein and PKA phosphorylation of multiple substrates (as well as overall PKA phosphorylation; see Table 1), and only sensitized rats showed AMPAR up-regulation. Total CaMKII protein, also elevated in both sensitized and non-sensitized groups on WD7 (Fig. 3h), was positively correlated with phosphorylation of certain substrates (90, 74, 42, 39 kDa; p < 0.05) for individual rats in the cocaine-exposed group on WD7. However, none of these correlations persisted when sensitized rats were analyzed separately (data not shown).
Proteomic identification of PKA substrate proteins of 90, 66, 54, and 39 kDa
We selected four substrate proteins for additional experiments (90, 66, 54 and 39 kDA) based on significant increases in their phosphorylation in sensitized rats on WD21 (Fig. 5) and withdrawal-dependent correlations with other measures (see previous section). As a first step towards identifying these PKA substrates, we performed proteomic analyses on excised bands from colloidal Coomassie- and SYPRO Ruby-stained 1 dimensional SDS-PAGE gels with subsequent identification of bands by mass spectrometry. The starting material was NAc tissue obtained from two adult, drug-naïve rats. Bands to be excised were identified by aligning stained gels and western blots probed with antibody recognizing phosphorylated PKA substrates. First, we analyzed a gel loaded with 400 μg of NAc tissue (Run A). Electrospray ionization MS/MS (ESI-MSn) analyses of the excised and protease-cleaved bands at ~90, ~66, ~54, and ~39 kDa revealed several interesting high score hits (Run A; Table 2). Of these, heat shock protein of 90 kDa (HSP90) α and HSP90 β were identified for the band excised at ~90 kDa, HSP of 70 kDa (HSP70) and Collapsin Response Mediator Protein-2 (CRMP-2) were identified for the band excised at ~66 kDa, α-tubulin was identified as the highest probability hit for the band excised at ~54 kDa band, and guanine nucleotide binding protein alpha-o (Goα) subunit 2 (Goα2) was identified as highest probability hit for the band excised at ~39 kDa (Table 2). Next, we performed a more focused, higher resolution and sensitive ESI-MSn analysis of a second sample run on a one-dimensional gel, this time loading ~150 μg of NAc protein (Run B). Of the high probability hits for the ~66 kDa band from Run A, CRMP-2 was again identified while HSP70 was not (Run B; Table 2). Again, we found α-tubulin and Goα2 to be the highest probability hits for the 54 kDa and the 39 kDa excised bands, respectively (Table 2).
Table 2.
Mass spectrometry results for bands excised at 90, 66, 54, and 39 kDa corresponding to the molecular weights of individual PKA substrate bands (PKA Sub.) from Coomassie stained one-dimensional gels
| PKA Sub. (kDa) | Name | No. of fragments | Score |
|---|---|---|---|
| 90 | Heat shock protein 90 kDa, betaa | 11 | 269 |
| Heat shock protein 90 kDa, alphab | 10 | 211 | |
| 66 | Heat shock 70 kDa protein 8a | 9 | 261 |
| Collapsin response mediator protein-2a | 8 | 173 | |
| Collapsin response mediator protein-2b | 3 | 137 | |
| 54 | αTubulina | 21 | 439 |
| αTubulinb | 3 | 70 | |
| 39 | Guanine nucleotide-bindinga | 3 | 67 |
| Protein Go, alpha subunit 2 | |||
| Guanine nucleotide-bindingb | 3 | 67 | |
| Protein Go, alpha subunit 2 |
Results from Run A (400 μg NAc protein load).
Results from Run B (150 μg NAc protein load). Peptide fragments did not overlap with any other identified proteins. Mowse score values (Score) reflect the probability that the peptide fragments correspond to the identified protein.
Bioinformatics examination of the amino acid sequences with Net PhosK v.1.0 (http://www.cbs.dtu.dk/services/netPhosK) or Phosphosite v.2.0 (http://www.phosphosite.org) revealed that HSP90, CRMP-2, α-tubulin, and Goα2 each contained putative PKA phosphorylation site(s), supporting the hypothesis that these proteins contributed to immunoreactivity detected with the PKA substrate antibody. Another aliquot of the same tissue used for runs A and B was separated by two-dimensional gel electrophoresis to further spatially resolve the proteins and assess the population of phosphorylated species, and then immunoblotted with PKA substrate antibody. This revealed that there were multiple phosphorylation sites with different isoelectric points, consistent with differential and combinatorial phosphorylation sites in the 90, 66, 54, and 39 kDa ranges (Fig. S1) migrating along the same mass. Each individual spot was not of high enough abundance to identify the site of phosphorylation. Furthermore, phosposerine and phosphothreonine are labile (e.g. beta-elimination) so different mass spectrometric strategies need to be employed and require higher abundance of protein.
CRMP-2 was identified as a component of the post-synaptic density by mass spectrometry (Peng et al. 2004) and is of interest as a potential mediator of structural plasticity (see Discussion). Goα2 is another interesting candidate because it transduces signals from DA receptors (Neve et al. 2004). To determine if their localization is consistent with participation in AMPAR plasticity in NAc neurons, we performed immunocytochemical experiments in primary cultures prepared from postnatal rat NAc neurons. Both CRMP-2 and Goα2 are expressed in the processes and cell bodies of NAc medium spiny neurons (Fig. S2).
Discussion
AMPAR redistribution during cocaine withdrawal
The purpose of this study was to identify mechanisms that may contribute to AMPAR up-regulation after withdrawal from a sensitizing regimen of cocaine. Our present findings, along with prior studies (Boudreau and Wolf 2005; Boudreau et al. 2007; Kourrich et al. 2007; Ghasemzadeh et al. 2009), demonstrate increased surface and synaptic expression of GluR1/2-containing AMPARs in the NAc of cocaine-sensitized rats, developing between WD1 and WD7 and persisting at least through WD21. However, the relationship between AMPAR up-regulation and locomotor sensitization is complex. While intra-NAc injection of an AMPAR antagonist blocked expression of sensitization in two studies (Pierce et al. 1996; Bell et al. 2000), we and others have found that AMPAR up-regulation and locomotor sensitization can be dissociated (Boudreau and Wolf 2005; Bachtell and Self 2008; Wolf and Ferrario 2008). For example, rats express locomotor sensitization on WD1 before AMPARs are up-regulated (Boudreau and Wolf 2005). On the other hand, it is well accepted that neuroadaptations associated with locomotor sensitization can increase motivation to self-administer drugs (Vezina 2004). We propose that AMPAR up-regulation in the NAc is one of the links between locomotor sensitization and enhanced drug seeking, based on the evidence that drug seeking requires AMPAR transmission in the NAc (see Introduction) and that increased AMPAR transmission is associated with enhanced drug seeking (Suto et al. 2004; Conrad et al. 2008; Anderson et al. 2008; but see Famous et al. 2008). It should be noted that the results summarized above are based on studies of endogenous AMPAR function. Different conclusions have been reached based on viral-mediated over-expression of GluR1 (Bachtell et al. 2008).
Increased AMPAR surface expression in the NAc of sensitized rats was demonstrated by increased GluR1 S and S/I values; we also observed trends towards decreased I and increased S+I. These results suggest three possible mechanisms that may contribute to increased AMPAR surface expression: redistribution from intracellular compartments, stabilization of GluR1 on the surface (reducing turnover and degradation), and synthesis of additional GluR1. Distinguishing between these possibilities is beyond the scope of this study.
In the remainder of the Discussion, we describe changes in ERK2, CaMKII and PKA activation observed in the NAc after withdrawal from repeated cocaine exposure. Interestingly, in the NAc of amphetamine-sensitized rats, we observed neither AMPAR up-regulation nor the changes in signaling pathway activation reported here for cocaine-sensitized rats (Nelson et al. 2009). Another interesting comparison is between sensitized and non-sensitized rats in the present study. The latter exhibited a higher initial response to cocaine, as observed previously (Sabeti et al. 2003; Boudreau and Wolf 2005), raising the possibility that their pre-drug state resembles sensitization. Arguing against this, they were similar to saline controls with respect to AMPAR distribution, PKA activation, and ERK activation on WD1, WD7 and WD21 (although they had in common with sensitized rats an increase in pCaMKII and total CaMKII on WD7). Previous studies have found that non-sensitized rats are more similar to saline controls than cocaine-sensitized rats with respect to mitogen-activated protein kinase signaling (Boudreau et al. 2007) and cocaine-induced changes in DA clearance (Sabeti et al. 2003). Finally, it is important to note that the present results were obtained from analysis of total tissue homogenates because we wanted to measure signaling pathway activation in the same rats used to measure AMPAR surface expression with protein cross-linking. Future studies should examine synaptic fractions, as changes may be evident in these fractions that are not observed in total tissue homogenates (see Ghasemzadeh et al. 2009).
AMPAR up-regulation is paralleled by ERK2 activation in sensitized rats
ERK is strongly implicated in addiction-related plasticity and learning (Lu et al. 2006; Girault et al. 2007). For example, both systemic (Valjent et al. 2005, 2006a; Ferguson et al. 2006) and intra-ventral tegmental area administration (Pierce et al. 1999) of drugs that inhibit ERK activation attenuate the development of behavioral sensitization. Our study focused on the relationship of ERK signaling to AMPAR up-regulation in the NAc. We found that ERK2 phosphorylation in the NAc of sensitized rats was increased on WD7 and WD21 but not WD1. AMPAR up-regulation followed the same time-course, suggesting that ERK2 may contribute to AMPAR up-regulation. This is consistent with evidence that ERK activation mediates activity-dependent AMPAR synaptic insertion in hippocampal neurons (Zhu et al. 2002), and studies showing that deletion of ERK1 (which promotes ERK2 activation) facilitates LTP in NAc neurons (Mazzucchelli et al. 2002). Further supporting a relationship between ERK2 and AMPAR surface expression in NAc neurons, a challenge injection of cocaine given to sensitized rats reversed both elevated AMPAR surface expression and ERK2 phosphorylation (Boudreau et al. 2007).
On the other hand, inhibition of ERK by systemic injection of the MEK inhibitor SL327 just before a cocaine challenge did not prevent expression of sensitization in mice (Valjent et al. 2005, 2006a). Given that ERK activation is closely linked to AMPAR up-regulation, this result provides indirect support for the idea that AMPAR up-regulation and the expression of locomotor sensitization can be dissociated (see previous section). However, in keeping with ERK's important role in maintaining drug-related memories (Miller and Marshall 2005; Valjent et al. 2006b) and evidence that enhanced AMPAR up-regulation is important for drug seeking (Suto et al. 2004; Anderson et al. 2008; Conrad et al. 2008), we suggest that the relationship between ERK and AMPAR transmission is relevant to drug craving despite its dissociation from the expression of locomotor sensitization.
Three prior studies examined basal ERK phosphorylation in the NAc after withdrawal from a sensitizing cocaine regimen, although they summed ERK1 and ERK2 measurements. Two found no change in ERK1/2 phosphorylation in early withdrawal (WD1, Berhow et al. 1996; WD7, Mattson et al. 2005). Kim and Kim (2008) observed that basal ERK1/2 phosphorylation was unchanged on WD1, elevated on WD7 and normalized by WD21. The shorter duration of activation in this latter study may reflect lower cocaine exposure compared with our own regimen.
While we have focused on ERK's role in AMPAR trafficking when interpreting our results, the ERK cascade has many cellular targets (Sweatt 2004). For example, ERK is best known as a regulator of transcription (Whitmarsh 2007). Withdrawal-dependent changes in ERK-mediated transcriptional regulation may contribute to the neuro-adaptations reported herein.
Adaptations involving CaMKII during cocaine withdrawal
Sensitized rats exhibited significantly decreased pCaMKII/CaMKII on WD1 compared with both saline controls and non-sensitized rats. On WD7, the earliest withdrawal time when sensitized rats showed AMPAR up-regulation, increased pCaMKII and total CaMKII were observed. However, these measures were also increased in non-sensitized rats, without a concomitant change in AMPARs. Thus, increased CaMKII activity may contribute to AMPAR up-regulation, but other changes, occurring selectively in sensitized rats, must also be required. Consistent with a possible role for CaMKII in AMPAR up-regulation, repeated DA treatment of cultured NAc neurons results in a withdrawal-dependent and CaMK-dependent increase in GluR1 surface expression (Sun et al. 2008).
The elevation in basal CaMKII measures observed on WD7 was no longer evident on WD21. However, on WD21 from the same regimen, CaMKII activation in the NAc is required for expression of behavioral sensitization in response to cocaine challenge (Pierce et al. 1998) and augmented stimulant-induced DA overflow in the NAc of cocaine-sensitized rats (Pierce and Kalivas 1997). Thus, even after the withdrawal-dependent increase in basal CaMKII measures subsides, acute CaMKII activation by cocaine contributes to behavioral and neurochemical responses. CaMKII activation in the NAc is also required for enhanced drug self-administration following sensitization (Loweth et al. 2008) and cocaine-primed reinstatement of drug-seeking (Anderson et al. 2008).
Adaptations in PKA phosphorylation during cocaine withdrawal
We assessed PKA phosphorylation indirectly using an antibody recognizing PKA-phosphorylated substrates. In the NAc of sensitized rats, overall PKA substrate phosphorylation increased gradually during withdrawal, from levels significantly less than controls on WD1 to significantly greater than controls on WD21. Of the nine most prevalent PKA substrate bands, five showed greater phosphorylation on WD21. Because increased overall PKA phosphorylation temporally lagged behind AMPAR up-regulation (the latter was already evident on WD7), the two may be unrelated. However, our analyses likely did not detect all relevant PKA substrates. For example, studies in NAc and other regions suggest that PKA phosphorylation of GluR1 at Ser845 plays a permissive role in GluR1 synaptic targeting (Chao et al. 2002a; b; Esteban et al. 2003; Mangiavacchi and Wolf 2004; Sun et al. 2005, 2008; Gao et al. 2006; Oh et al. 2006; Man et al. 2007). In our NAc cross-linked samples, evaluation of Ser845 phosphorylation as a measure of PKA activity was not possible because cross-linking interfered with GluR1 phosho- Ser845 antibody binding (data not shown).
Several studies have measured PKA activity in the NAc of cocaine-sensitized rats. Using a regimen similar to our own, Crawford et al. (2004) found decreased PKA activity on WD1, while a study using a more aggressive regimen found increased PKA activity on WD1 (Terwilliger et al. 1991). Hope et al. (2005), using a regimen similar to our own, found increased PKA activity in the NAc of cocaine-sensitized rats on WD1 and WD7 but not WD21. Behavioral sensitization to cocaine challenge was expressed at all three withdrawal times, so Hope et al. (2005) concluded that PKA activation could not account for the persistence of behavioral sensitization. However, by using a different measure (phosphorylation of PKA substrates) that reflects the sum of all cocaine-induced adaptations that influence either PKA or phosphatase activity, we have found changes in PKA signaling that persist on WD21. Because PKA activity is not altered on WD21 (Hope et al. 2005), we suggest that a cocaine-induced decrease in phosphatase activity (e.g., Hu et al. 2005) accounts for increased PKA substrate phosphorylation in our study. Withdrawal-dependent changes in phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa may influence the balance between PKA and phosphatase activity (e.g., Bibb et al. 2001; Scheggi et al. 2004).
Interestingly, recent work has implicated Ca2+/calmodulin-stimulated adenylyl cyclases (types 1 and 8) in behavioral sensitization; double knockout of these isoforms enhanced the acute locomotor response to cocaine and prevented locomotor sensitization (DiRocco et al. 2009). Studies of acute cocaine action in the double knockout mice implicated ERK as a downstream target of Ca2+/calmodulin-stimulated adenylyl cyclases in NAc interneurons (DiRocco et al. 2009). These findings highlight the potential for important interactions between the signaling pathways examined in the present study.
Novel PKA substrates related to adaptations after long withdrawal
We used proteomic approaches to identify four putative PKA substrates showing elevated phosphorylation on WD21. A 39 kDa substrate was identified as Goα2. Its phosphorylation correlated with the magnitude of sensitization and GluR1 S/I on WD21, indicating a role for GPCR signaling in sensitization, which is not surprising. The 90 kDa substrate was identified as HSP-90, a contributor to diverse types of plasticity, including AMPAR cycling (Gerges et al. 2004). Interestingly, the other two substrates, α-tubulin and CRMP-2, are related to neuronal structure. CRMP-2 (also known as dihydropyrimidinase-related protein) is best known for its role in neurite outgrowth and remodeling (Arimura et al. 2004). However, it associates with NMDA receptors (Al-Hallaq et al. 2007) and its expression is regulated by cocaine (Tannu et al. 2008) and methamphetamine (Iwazaki et al. 2007; Kobeissy et al. 2008). In the cocaine study, CRMP-2 abundance was decreased in rhesus monkey NAc tissue obtained 18–24 h after the last self-administration session in a 18 month regimen (a trend towards decreased α-tubulin was also observed) (Tannu et al. 2008). Differences in species, duration of exposure, self-administration versus non-contingent cocaine administration, and withdrawal time may underlie the difference in direction of change in CRMP-2 abundance compared with our results, but it is very interesting that both studies identified CRMP-2 as a cocaine-modulated protein. Moreover, GluR1 and GluR2/3 levels were increased in NAc membrane fractions from the same rhesus monkeys, as well as in NAc membranes from human cocaine overdose victims (Hemby et al. 2005).
We speculate that phosphorylation of CRMP-2 and α-tubulin in sensitized rats may contribute to structural plasticity (increased dendritic branching and altered density or size of spines) observed in NAc medium spiny neurons after several weeks of withdrawal from cocaine (Robinson and Kolb 1999, 2004; Shen et al. 2009) and shown to be associated with behavioral sensitization (Li et al. 2004; but see Pulipparacharuvil et al. 2008). Some evidence suggests that AMPAR up-regulation can drive structural plasticity. For example, synaptic insertion of GluR1, through a structurally stabilizing effect mediated by its C-terminus, is required for spine enlargement during hippocampal LTP (Kopec et al. 2007).
Conclusions
As a first step towards understanding mechanisms that drive AMPAR up-regulation in the NAc of cocaine-sensitized rats, we measured signaling pathway activation and AMPAR surface expression in sensitized rats and controls at three withdrawal times. Our results demonstrate that behavioral sensitization to cocaine is associated with complex adaptations in ERK2, CaMKII and PKA signaling in the NAc that evolve gradually over 3 weeks of withdrawal. Most strikingly, ERK2 activation paralleled AMPAR surface expression throughout withdrawal. More work is required to determine how signaling pathway adaptations are linked to AMPAR and behavioral plasticity, but our results identify temporal windows in which to probe the role of candidate signaling pathways.
Supplementary Material
Acknowledgments
The authors would like to thank Mr Keith D. Philibert for his expertise in the proteomics aspects of this science. This work was supported by USPHS grants DA09621, DA015835 and DA00453 to MEW and S10RR19325 to the Midwest Proteome Center (MJG). ACB was supported by predoctoral National Research Service Award DA019762. CRF was supported by postdoctoral National Research Service Award DA024502.
Abbreviations used
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionate
- BS3
bis(sulfosuccinimidyl)suberate
- CaMKII
Ca2+-calmodulin-dependent protein kinase II
- DA
dopamine
- ERK
extracellular signal-regulated protein kinase
- GluR
glutamate receptor
- LTP
long-term potentiation
- NAc
nucleus accumbens
- PKA
protein kinase A
Footnotes
Supporting Information
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J. Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat. Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Arimura N, Menager C, Fukata Y, Kaibuchi K. Role of CRMP-2 in neuronal polarity. J. Neurobiol. 2004;58:34–47. doi: 10.1002/neu.10269. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J. Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur. J. Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacology. 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (Extracellular Signal Regulated Kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J. Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize upon cocaine challenge in association with altered activation of mitogen-activated protein kinases. J. Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:1976–248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee H-K, Huganir RL, Wolf ME. D1 dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J. Neurochem. 2002a;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J. Neurochem. 2002b;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J. Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J. Neurosci. 2000;20:89RC. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Choi FY, Kohutek JL, Yoshida ST, McDougall SA. Changes in PKA activity and Gs alpha and Golf alpha levels after amphetamine- and cocaine-induced behavioral sensitization. Synapse. 2004;51:241–248. doi: 10.1002/syn.10301. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanism of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- DiRocco DP, Scheiner ZS, Sindreu CB, Chan GC-K, Storm DR. A role for calmodulin-stimulated adenylyl cyclases in cocaine sensitization. J. Neurosci. 2009;29:2393–2403. doi: 10.1523/JNEUROSCI.4356-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha J-HJ, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J. Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J. Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- Gerges NA, Tran IC, Backos DS, Harrell JM, Chinkers M, Pratt WB, Esteban JA. Independent functions of hsp90 in neurotransmitter release and in the continuous synaptic cycling of AMPA receptors. J. Neurosci. 2004;24:4758–4766. doi: 10.1523/JNEUROSCI.0594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr. Opin. Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC. Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates. J. Neurochem. 2005;95:1785–1793. doi: 10.1111/j.1471-4159.2005.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Crombag HS, Jedynak JP, Wise RA. Neuroadaptations of total levels of adenylate cyclase, protein kinase A, tyrosine hydroxylase, cdk5 and neurofilaments in the nucleus accumbens and ventral tegmental area do not correlate with expression of sensitized or tolerant locomotor responses to cocaine. J. Neurochem. 2005;92:536–545. doi: 10.1111/j.1471-4159.2004.02891.x. [DOI] [PubMed] [Google Scholar]
- Hu X-T, White FJ. Glutamate receptor regulation of rat nucleus accumbens neurons in vivo. Synapse. 1996;23:208–218. doi: 10.1002/(SICI)1098-2396(199607)23:3<208::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Hu X-T, Ford K, White FJ. Repeated cocaine administration decreases calcineurin (PP2B) but enhances DARPP-32 modulation of sodium currents in rat nucleus accumbens neurons. Neuropsychopharmacology. 2005;30:916–926. doi: 10.1038/sj.npp.1300654. [DOI] [PubMed] [Google Scholar]
- Iwazaki T, McGregor IS, Matsumoto I. Protein expression profile in the striatum of rats with methamphetamine-induced behavioral sensitization. Proteomics. 2007;7:1131–1139. doi: 10.1002/pmic.200600595. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- Kim S, Kim JH. Time-dependent change of ERK phosphorylation levels in the nucleus accumbens during withdrawals from repeated cocaine. Neurosci. Lett. 2008;436:107–110. doi: 10.1016/j.neulet.2008.02.068. [DOI] [PubMed] [Google Scholar]
- Kobeissy FH, Warren MW, Ottens AK, Sadasivan S, Zhang Z, Gold MS, Wang KK. Psychoproteomic analysis of rat cortex following acute methamphetamine exposure. J. Proteome Res. 2008;7:1971–1983. doi: 10.1021/pr800029h. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J. Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioral sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur. J. Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Baker LK, Guptaa T, Guillory AM, Vezina P. Inhibition of CaMKII in the nucleus accumbens shell decreases enhanced amphetamine intake in sensitized rats. Neurosci. Lett. 2008;444:157–160. doi: 10.1016/j.neulet.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Man H-Y, Sekine-Aizawa Y, Huganir RL. Regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl Acad. Sci. USA. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J. Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J. Neurochemistry. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire E, Soderling ER. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for LTP. J. Biol. Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain post-synaptic density fractions by mass spectrometry. J. Biol. Chem. 2004;279:21003–21011. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Boeijinga PH, Lopes da Silva FH. Locally evoked potentials in slices of the rat nucleus accumbens: NMDA and non-NMDA receptor mediated components and modulation by GABA. Brain Res. 1990;529:30–41. doi: 10.1016/0006-8993(90)90808-o. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J. Neurosci. 1997;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J. Pharmacol. Exp. Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Pierce RC, Pierce-Bancroft AF, Prasad BM. Neutrophin-3 contributes to the initiation of behavioral sensitization to cocaine by activating the Ras/Mitogen-activated protein kinase signal transduction cascade. J. Neurosci. 1999;19:8685–8695. doi: 10.1523/JNEUROSCI.19-19-08685.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping A, Xi J, Prasad BM, Wang M-H, Kruzich PJ. Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res. 2008;1215:173–182. doi: 10.1016/j.brainres.2008.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Phil. Trans. R. Soc. B. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spiens in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur. J. Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl–1) doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J. Pharmacol. Exp. Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Scheggi S, Rauggi R, Gambarana C, Tagliamonte A, De Montis MG. Dopamine and cyclic AMP-regulated phosphoprotein-32 phosphorylation pattern in cocaine and morphine-sensitized rats. J. Neurochem. 2004;90:792–799. doi: 10.1111/j.1471-4159.2004.02510.x. [DOI] [PubMed] [Google Scholar]
- Shen H-W, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J. Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J. Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J. Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tannu NS, Howell LL, Hemby SE. Integrative proteomic analysis of the nucleus accumbens in rhesus monkeys following cocaine self-administration. Mol. Psychiatry. 2008;27 doi: 10.1038/mp.2008.53. [May Epub] ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Tullai JW, Cummins PM, Pabon A, Roberts JL, Lopingco MC, Shrimpton CN, Smith AI, Martignetti JA, Ferro ES, Glucksman MJ. The neuropeptide processing enzyme EC 3.4.24.15 is modulated by protein kinase A phosphorylation. J. Biol. Chem. 2000;275:36514–36522. doi: 10.1074/jbc.M001843200. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl Acad. Sci. USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol J-C, Trzaskos JM, Girault J-A, Hervé D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006a;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc. Natl Acad. Sci. USA. 2006b;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochem. Biophys. Acta. 2007;1773:1285–1298. doi: 10.1016/j.bbamcr.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. Alterations in AMPA receptor expression in the nucleus accumbens of cocaine-sensitized rats after re-exposure to cocaine. Soc. Neurosci. Abstr. 2008;34:662. [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychostimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl–1) doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, van Aiest L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.