Abstract
The molecular mechanism responsible for the regulation of the mitochondrial membrane proton conductance (G) is not clearly understood. This study investigates the role of the transmembrane potential (ΔΨm) using planar membranes, reconstituted with purified uncoupling proteins (UCP1 and UCP2) and/or unsaturated FA. We show that high ΔΨm (similar to ΔΨm in mitochondrial State IV) significantly activates the protonophoric function of UCPs in the presence of FA. The proton conductance increases nonlinearly with ΔΨm. The application of ΔΨm up to 220 mV leads to the overriding of the protein inhibition at a constant ATP concentration. Both, the exposure of FA-containing bilayers to high ΔΨm and the increase of FA membrane concentration bring about the significant exponential Gm increase, implying the contribution of FA in proton leak. Quantitative analysis of the energy barrier for the transport of FA anions in the presence and absence of protein suggests that FA− remain exposed to membrane lipids while crossing the UCP-containing membrane. We believe this study shows that UCPs and FA decrease ΔΨm more effectively if it is sufficiently high. Thus, the tight regulation of proton conductance and/or FA concentration by ΔΨm may be key in mitochondrial respiration and metabolism.
Abbreviations used: AA, arachidonic acid; BSA, albumin from bovine serum; DTT, dithiothreitol; EDTA, ethylenediamine-tetraacetic acid; EGTA, ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid; FA, free fatty acid; hUCP2, human uncoupling protein 2; LA, linoleic acid; Gm, membrane conductance; MES, 2-(N-morpholino)ethanesulfonic acid; mUCP1, murine uncoupling protein 1; OA, oleic acid; PMSF, phenylmethanesulfonyl fluoride; SLS, sodium lauryl sulfate; UCP, uncoupling proteins
Introduction
Proton leak represents downhill flux of H+ from mitochondrial intermembrane space to the matrix noncoupled to utilization of the released energy to carry out a useful work. Although such a proton leak lowers the efficiency of ATP synthesis, it may be physiologically important, e.g., for thermogenesis, as documented for the uncoupling protein 1 (UCP1) in brown adipose tissue (1,2). Another hypothesis, so-called mild uncoupling (3), claims that the regulated H+-leak leads to the attenuation of reactive oxygen species formation (4). The latter has been proposed as a main function of the recently discovered uncoupling proteins UCP2-UCP5 and is controversially debated (5–9).
Both phospholipids and proteins in the mitochondrial inner membrane are in discussion as important determinants of proton conductance (10,11). At least two mitochondrial carriers, UCP and adenine nucleotide translocase, are able to mediate a significant inhibitor-sensitive proton conductance when activated by small molecules. Adenine nucleotide translocase induces a carboxyatractylate-sensitive proton leak in the presence of FA (12–14), AMP (15), or alkenyls (16). The requirement for fatty acids and inhibition by purine nucleotides have been shown for UCP1, UCP2, and UCP3 in cells and isolated mitochondria (9,17), as well as in liposomes (18,19) and lipid bilayers (20–22), reconstituted with purified UCP1-UCP3. However, the obvious discrepancy exists between purine nucleotide concentrations that inhibit the mitochondrial proton conductance and the much higher concentration present in the brown adipocyte cytosol (23,24). Because the overriding effect of FA in presence of nucleotides is rather weak, the search for additional cofactors has been started. The proposed coenzyme Q, hydroxynonenal and superoxide (16,25,26) seem to activate not all UCPs and their activating function is highly controversial (27–29).
The inhibitor-nonsensitive (basal) proton conductance is present in mitochondria of all studied tissues, may essentially contribute to the metabolic rate, and is insensitive to all known activators or inhibitors (11,30,31). Differences in phospholipid composition and amount/composition of FAs have been hypothesized as possible causes (32). However, although patterns of fatty acid saturation degree correlate with proton leak in whole mitochondria (33), the contribution of the lipid bilayer to the total conductance of biological membranes seems to be rather negligible (34,35). As noted by Garlid et al. (34), leak pathways will be found near integral membrane proteins, where resistance is much lower.
The mitochondrial transmembrane electric potential (ΔΨm) seems to be very important to the proton leak. There are indices that some members of the mitochondrial anion carrier family can catalyze a FA-dependent proton leak only at high ΔΨm, e.g., in State 4 (36–40). The evaluation of the transmembrane potential role is nontrivial in living cells and in isolated mitochondria, because the absolute value of ΔΨm is not known and a variety of endogenous factors has to be taken into account. Also, in artificial systems, no consensus about the character of proton leak dependency on ΔΨm in the presence of uncouplers is achieved so far. Whereas experiments using liposomes show the linear dependence of proton leak on ΔΨm both with reconstituted UCP1 (41) and in the presence of the proton ionophores CCCP (42), a nonlinear dependence of proton leak on ΔΨm was found in experiments using bilayer membranes (43). Studies carried out in the proteoliposome system have been criticized because the electric potential was generated by a potassium concentration gradient in the presence of valinomycin.
We applied the transmembrane potential up to 210 mV directly to the solvent-free planar bilayer membranes made from Escherichia coli lipid extract. The aims of this study were: i), to compare the contribution of fatty acids or/and highly purified uncoupling proteins (UCP1, UCP2) to the protein-mediated and basal conductances at low and high transmembrane potentials; ii), to analyze the nonlinearity in the proton conductance at high potentials, which have been observed in experiments with isolated mitochondria and cells; and iii), to evaluate current hypotheses regarding the UCP-mediated proton transport.
Materials and Methods
mUCP1 and hUCP2 expression, extraction, purification, and reconstitution into liposomes
mUCP1
A fragment containing the coding region of murine UCP1 (mUCP1) was amplified by PCR from a mouse EST clone (IRAV p968C1023D) and cloned into the NdeI and EcoRI sites of the expression vector pET-24a (Novagen, Germany) under the control of an inducible T7 promoter. An internal NdeI site of the UCP1 coding frame was altered by generating a silent mutation from CATATG to CGTATG using the Quik Change II Site-Directed Mutagenesis Kit (Stratagene, The Netherlands) with the primer combination 5′-TGTACAGAGCTGGTAACGTATGACCTCATGAAGGGG-3′ and 5′CCCCTTCATGAGGTCATACGTTACCAGCTCTGTACA-3′. To avoid additional amino acids at the N-term of the expressed protein after cloning into pET-24a, a NdeI site was generated at the UCP1 start codon using the primer 5′GGCCATATGGTGAACCCGACAAC3′. The reverse primer 5′GCGAATTCTTATGTGGTACAATCCACTGTC3′ was used to introduce an EcoRI site downstream of the stop codon. Sequence analysis was carried out to control the sequence of the modified UCP1 coding frame after cloning the resulting PCR product into pET-24a.
The E. coli strain Rosetta (DE3) (Novagen, Germany) was transformed with the resulting plasmid pET-24a/UCP1 and grown overnight in the DYT medium containing 25 μg/mL kanamycin and 34 μg/mL chloramphenicol. Two milliliters of this culture were used to inoculate 1l DYT medium. Protein expression was induced by adding 1 M isopropyl-b-D-thiogalactopyranoside when the cultures reached an OD 600 of 0.3–0.5. Bacteria were harvested by centrifugation at 4°C (4500 × g, 10 min) 2.5 h later.
The protein purification was carried out according to the procedures, described previously (19) with some modifications. Pellet from 1 L culture was resuspended in 100 mL TE-buffer (100 mM Tris, 5 mM EDTA, pH 7.5) with addition of 5 mM DTT and 2 mM PMSF. Cells were decomposed by French press and centrifuged once more at 16,000 × g. The pellet was resuspended in PA-buffer (150 mM NaH2PO4 at pH 7.9, 25 mM EDTA, 5% ethylene glycol, 2% Triton X-100, 1 mM DTT, 2 mM PMSF) and again centrifuged at 1000 × g to eliminate cell debris. Inclusion bodies were obtained after supernatant centrifugation at 14,000 × g for 10 min. For the subsequent UCP1 purification, 10 mg protein of the inclusion bodies per 100 mg lipid were used. All experiments were carried out at 4°C, unless otherwise indicated. Inclusion bodies were washed in 20 mL TE/G-buffer (100 mM Tris at pH 7.5, 5 mM EDTA, 10% glycerin) first with additional 2% Triton X-100 and then with 0.1% SLS. For solubilization the inclusion bodies were incubated at room temperature in 20 mL TE/G-buffer with 2% SLS and 1 mM DTT. After centrifugation at 14,000 × g for 10 min the supernatant was gradually added to a mixture of 100 mg E. coli total or polar lipid (Avanti Polar Lipids, Alabaster, AL), 400 mg Triton X-114, 100 mg octyl-polyoxyethylene, 1 mM DTT, and 1 mM ATP. After incubation overnight, the mixture was concentrated with Amicon Ultra-15 filters (Millipore, Schwalbach, Germany) and dialyzed 2 h against TE/G-buffer supplemented with 1 mg/mL BSA and 1 mM DTT. Two further dialyses were carried out without DTT for a minimum of 12 h. For the buffer exchange, three additional dialyses were completed with an assay buffer (usually 50 mM KCl, 10 mM MES, 10 mM Tris, 0.6 mM EGTA; pH 7.0). To exclude chloride-mediated conductance, shown for UCP1 and UCP2 previously (44,45), a chloride-free buffer was used in all experiments.
To eliminate aggregated proteins, the dialysate was centrifuged at 14, 000 × g for 10 min. The supernatant was then filled in a column containing 1 g hydroxyapatite (Bio-Rad, Munich, Germany) to eliminate the decomposed protein (46,47) and equilibrated with an assay buffer (80 mM K2SO4, 2 mM EDTA, 30 mM K-TES). The sample was incubated with 10 ml Bio-Beads SM-2 (Bio-Rad, Germany) for 2 h and for additional 30 min after separation (48). The protein content of obtained proteoliposomes was measured by Micro BCA Protein Assay (Perbio Science, Bonn, Germany). The proteoliposomes were stored at −80°C until used.
hUCP2
Cloning and purification of human UCP2 (hUCP2) from inclusion bodies has been described previously (19,49–51). hUCP2 reconstitution in liposomes was similar to procedures described above for mUCP1.
Silver staining of recombinant mUCP1 (charge 11) and hUCP2 (charge 8) used in experiments were published previously (52). Charge numbers indicate the independent protein preparations. mUCP1 and hUCP2 are referred to as UCP1 and UCP2 in the following text.
Formation of planar membranes and measurements of membrane electrical parameters
Planar lipid bilayers were formed on the tip of one-way plastic pipettes as described previously (21). Polar or total lipid extracts from E. coli used for the formation of bilayer membrane were obtained from Avanti Polar Lipids and the hexane, hexadecane, K2SO4, TES, EGTA from Aldrich and Sigma GmbH (Munich, Germany). Long-chain FAs (oleic, linoleic, arachidonic) were purchased as chloroform solutions from Aldrich and Sigma GmbH. For FA reconstitution, UCP-containing proteoliposomes were mixed with FA-containing liposomes in required proportions.
Membrane formation was monitored by capacitance measurements (0.74 ± 0.06 μF/cm2). The capacitance was independent of protein and FA content. Current-voltage (I/V) characteristics of model membranes (Fig. 1) were measured by a patch-clamp amplifier (EPC 10, HEKA Elektronik, Dr. Schulze GmbH, Germany). Total membrane conductance (Gm) at zero voltage was determined from a linear fit of experimental data (I) on the interval between −50 and 50 mV. Membrane conductance at voltages in the range from −220 to 220 mV was calculated from a linear fit of voltage intervals with width of 10–20 mV.
Figure 1.
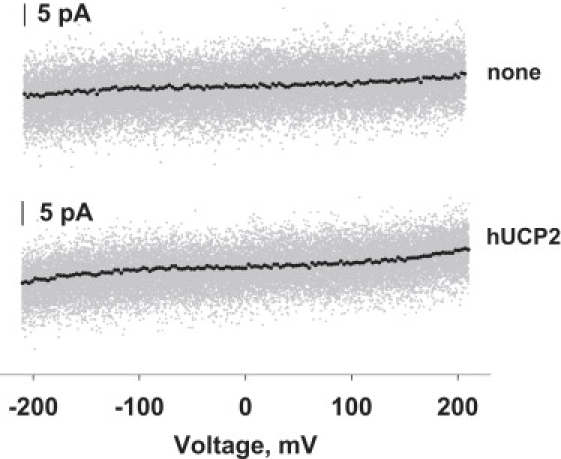
Representative volt-ampere characteristics of bilayer membranes from E. coli total lipid extract with and without hUCP2. hUCP2 concentration was ∼7 μg protein/mg of lipid respectively. Buffer solution contained 50 mM K2SO4, 25 mM TES, 0.6 mM EGTA at pH 7.35, and T = 37°C. Data were recorded using 10 kHz and 2.9 kHz filters. To obtain a spline line, the acquired 16,368 points were averaged (163 points per line point).
Results
The effect of the transmembrane potential on proton conductance was investigated in lipid membranes of different composition, e.g., unmodified membranes made from E. coli total lipid extract, membranes reconstituted with FA, UCP, or both. We compared two members of uncoupling protein subfamily, UCP1 and UCP2, because their functions are thought to be different: UCP1 is mainly involved in thermogenesis (53), whereas UCP2 is thought to attenuate reactive oxygen species production (54,55) or to regulate calcium homeostasis (56). Therefore, it can not be excluded that the proteins are regulated differently.
High membrane potential does not activate UCP1 and UCP2 in the absence of fatty acids
Fig. 1 shows the original recordings and averaged curves of volt-ampere characteristics of bilayer membranes made from E. coli total lipid extract only and membranes reconstituted with UCP2. The I/V behavior of a protein-free and UCP-containing membranes were similar (at 210 mV: I0 = 1.5 pA and Iucp2 = 2.9 pA, respectively), showing that in the absence of FA, UCP2 does not increase the membrane current, even at voltages above 120 mV. Similar results were obtained for mUCP1 (data not shown) that confirms our previous data obtained for hUCP1 (21).
Gm in protein-free membranes at high membrane potentials depends on FA concentrations and FA saturation degree
Whereas the electrical current, Iucp2, and consequently membrane conductance, Gm, were similar in the presence and absence of protein (Fig. 1) at all applied potentials, the reconstitution of membranes with different concentrations of polyunsaturated arachidonic acid (5–40 mol %) led to ΔΨm-dependent Gm increase (Fig. 2 A). At 220 mV the Gm of the membranes reconstituted with AA at a concentration of 15 mol % was equal to 179.7 nS/cm2, which was comparable with Gm in the presence of UCP2 and AA at low voltages (22). The dependence of Gm on transmembrane potentials was strongly nonlinear. The reconstitution of more unsaturated arachidonic acid (Fig. 2 B, circles) led to the higher Gm in comparison to linoleic acid (Fig. 2 B, squares). These results confirmed observations made in planar lipid membranes (57,58). In our previous work, a slight augmentation of the membrane conductance at low potentials was only measured in the presence of AA, but not in the case of less unsaturated fatty acids (22).
Figure 2.
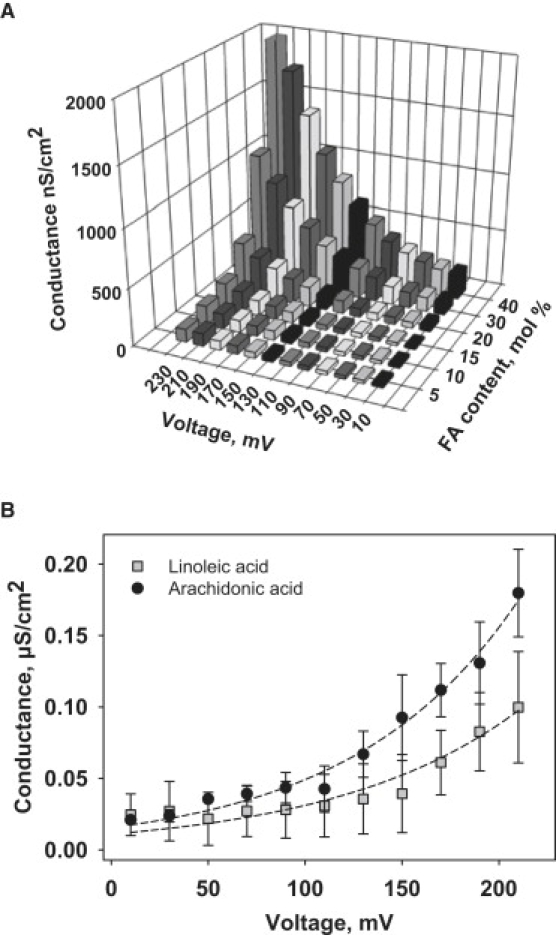
(A) Membrane conductance of bilayer lipid membranes reconstituted with various amounts of arachidonic acid at membrane potentials up to 230 mV. Bilayer lipid membranes were made from E. coli total lipid extract (1.5 mg/mL). Buffer contained 50 mM K2SO4, 25 mM TES, 0.6 mM EGTA at pH 7.35. (B) Conductance of bilayer lipid membranes reconstituted with linoleic (gray squares) and arachidonic (black circles) acids at membrane potentials ≤210 mV. The concentration of AA and OA was 15 mol % of lipid. Dashed lines represent the fit of the Eq. 1 to the experimental data.
Gm reconstituted with purified UCP1 and UCP2 in the presence of fatty acids increases nonlinearly with the increase of the membrane potential amplitude, ΔΨm
FAs were added directly to the lipid phase reconstituted with UCP before lipid membranes were formed, assuring the fast protein-FA interaction. The addition of the polyunsaturated AA to the UCP2-reconstituted membranes increased the current at 210 mV from Iucp2 = 2.9 pA to Iucp2,FA = 30.2 pA. Fig. 3 A shows Gm of membranes of different composition calculated at zero voltage (black) and after application of 210 mV (gray). In the absence of FAs, Gm of membranes reconstituted with UCP2 or UCP1 at 210 mV (Gucp2 = 120 ± 43.1 nS/cm2 and Gucp1 = 124 ± 30 nS/cm2, respectively) were similar to Gm of unmodified membranes (G0 = 127.7 ± 17.1 nS/cm2). Addition of AA to UCP2-containing membranes led to an ∼8-fold Gm increase at 0 mV and to an ∼16-fold at 210 mV (Fig. 3 A). We have shown previously that UCP1- and UCP2-dependent conductance was highest in the presence of polyunsaturated AA (22). Here we show that at 210 mV the membrane conductance was increased significantly for both oleic (OA, 0.95 ± 0.14 μS/cm2) and arachidonic (1.43 ± 0.19 μS/cm2) acids, thereby the UCP1-dependent Gm was higher in the presence of the more unsaturated AA (Fig. 3 B). Similar results in the presence of OA and AA were obtained also for membranes, reconstituted with UCP2 (data not shown). More pronounced effect of unsaturated FAs on UCPs at high potentials confirms our results obtained previously at low potentials in planar bilayer membranes (22), as well data obtained in mitochondria (59).
Figure 3.
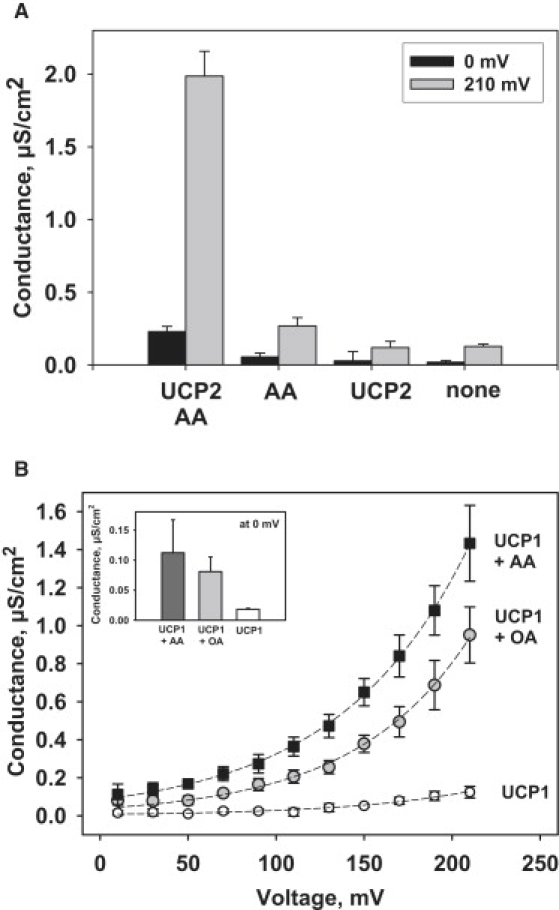
(A) Dependence of the total membrane conductance on membrane composition and applied voltage. hUCP2 concentration was ∼7 μg/mg of lipid. Buffer solution contained 50 mM K2SO4, 25 mM TES, 0.6 mM EGTA at pH 7.35, and T = 37°C. Each point with a vertical bar represents a mean value and a standard deviation from 9–18 experiments, carried out on three different days. (B) UCP1-mediated membrane conductance in the presence of arachidonic (AA, 20:4) and oleic (OA, 18:1) acids at different ΔΨm. Dashed lines represent the fit of the Eq. 1 to the experimental data. Inset: Comparison of G calculated at zero potential. Bilayer lipid membranes were made from E. coli total lipid extract. The concentration of AA and OA was 15 mol %, mUCP1 (charge 2) ∼2.6 μg/mg of lipid. Buffer solution contained 50 mM Na2SO4, 25 mM TES, 0.6 mM EGTA at pH 7.0.
FA-mediated Gm increase is inhibited at low pH
To evaluate UCP role in FA-mediated Gm increase at high potentials, we compared Gm dependence on pH in the presence of FA alone and in the presence of both, FA and protein (Fig. 4). Gm decreases at low pH (5.5) in both cases. However, in the presence of protein Gm seems to saturate on the alkaline side of the pK. In contrast, FA-mediated Gm in the absence of protein (Fig. 4, gray circles) has its maximum at pH between 7 and 7.5, which coincides with the pK of arachidonic acid. These results only partly support the data of Gutknecht (57), who showed that the proton transport mediated by long-chain FA is inhibited at low pH but saturates at alkaline pH even in the absence of protein. Moreover, the effect of pH on the membrane conductance was at least one order of magnitude larger than in our experiments. The reason for this discrepancy can be the presence of decane and chlordecane in the membranes formed by Gutknecht (57).
Figure 4.
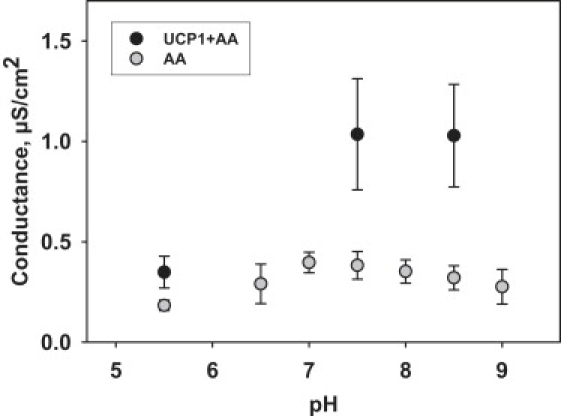
Dependence of the total membrane conductance on pH in membranes containing UCP and AA (black symbols) or only AA (gray symbols). The concentration of AA was 15 mol %, mUCP1(charge 12) ∼14.8 μg/mg of lipid. Buffer solution contained 50 mM Na2SO4, 10 mM MES, 10 mM TRIS, 0.6 mM EGTA, T = 32°C. Each point with a vertical bar represents a mean value and a standard deviation from 8–12 experiments carried out on three different days.
We believe the results in this study imply that the translocation of the fatty acid anion (FA−) is the rate limiting step in H+ transport and support the hypothesis that UCP accelerates this step by transport of FA anion.
Application of high ΔΨm leads to the overriding of the protein inhibition at a constant ATP concentration
One possibility to explain the discrepancy between millimolar PN concentrations present in mitochondria in vivo and the proton flux (see Introduction) is that under nonphosphorylating conditions (analogous to the State 4 in the isolated organelle preparation) the membrane potential, which is as high as ∼180–200 mV, releases the nucleotide-mediated inhibition and, hence, abolishes the respiratory control. To evaluate this hypothesis we compared the inhibitory effect of purine nucleotides at low and at higher voltages. Fig. 5 shows that AA-induced activity of protein at low voltage (Fig. 5, first bar chart) can be inhibited by 1 mM ATP to ∼50% (Fig. 5, second bar chart). At high potentials Gm was reduced to ∼40% (Fig. 5, 6th bar chart).
Figure 5.
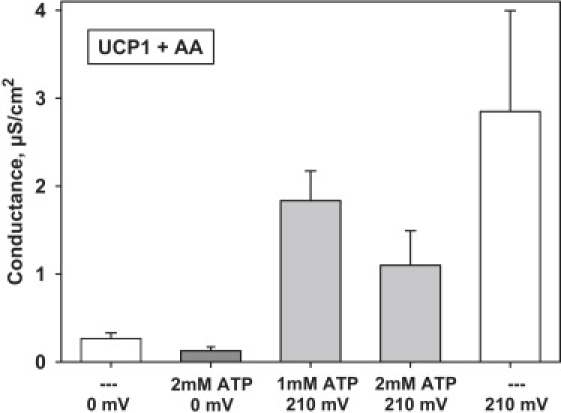
Comparison of the ATP inhibitory effect at low and at high voltages. Buffer solution contained 50 mM Na2SO4, 25 mM TES, 0.6 mM EGTA at pH = 7.4, and T = 32°C. Lipid membranes were made from E. coli polar lipid extract (1 mg/mL) and reconstituted with both UCP1 (∼10.4 μg/mg of lipid, charge 9) and 15 mol % arachidonic acid (AA). Each point with a vertical bar represents a mean value and a standard deviation from 8–15 experiments, carried out on five different days.
Although no relative reduction of ATP-inhibition could be observed, presented data showed that in the presence of ATP the absolute value of Gm is increased 10-fold at 210 mV, implying that the potential increase is more effective method in overriding of protein inhibition than FA addition. The dependency of nucleotide inhibition on the magnitude of the proton electrochemical potential was described previously by Rial et al. (59): at low potentials FA actions were blocked by inhibitory nucleotides, whereas under physiological conditions (high membrane potential?) FA overrode PN inhibition. Our data show that the modulation of the membrane potential can sufficiently explain these observations.
Comparison of relative conductances in the presence and absence of protein at high potentials
To get an insight to the mechanism of the proton transport mediated by UCP in the presence of FAs we have compared the relative membrane conductances (G/G0, where G is the conductance at voltage V and G0 is the conductance calculated at 0 mV) at various membrane compositions. Fig. 6 shows that the curve slopes are similar for protein-containing and protein-free membranes in the presence of FA (black and gray symbols) but differ than compared to the FA-free membranes (white symbols). Because this difference is likely to be quantitative and reflect the difference in energy barriers to protons and anions, we carry out a quantitative description of the barrier shape according to Garlid (34). The dependence of the proton leak on the applied potential is described by the simplified (i.e., neglecting part of equation for backflux) equation for ion leak (J) valid at high potentials, ΔΨ (34):
| (1) |
where J0 = PC is the exchange flux at Ψ = 0 and u is calculated as (−zFΔΨ)/RT, where F, z, R, and T have their usual meanings corresponding to the Faraday constant, ion charge, universal gas constant and temperature. β is equal to 1/2 N, where ion is crossing N uniformly high, sharp barriers at the membrane potential ΔΨ. For N = 2 one can calculate that β is 0.25; for N >2 the barrier can also be approached by trapezoid. For N > 10 β approaches to zero and the original Garlid's equation not neglecting backflux is simplified to the Goldman equation. Alternatively, a trapezoid barrier is introduced for which flux J can be expressed as follows:
| (2) |
where W is a fractional width of the trapezoid. At the limit W = 1, one gets the Goldman equation, whereas at the limit W = 0, the equation changes to Eq. 1 for a single sharp peak (β = 0.5).
Figure 6.
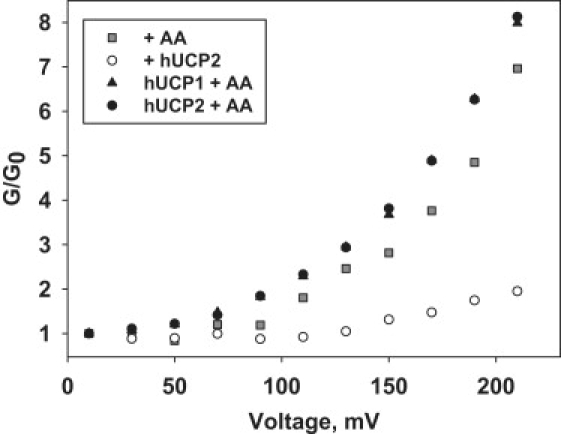
Comparison of relative conductances (G/G0) of the membranes containing UCP and AA (black symbols), AA alone (gray symbols) and pure membranes (white symbols). G is the conductance at the respective voltage and G0 is the conductance calculated at 0 mV. Lipid membranes were made from E. coli polar lipid extract (1.5 mg/mL) and reconstituted with ∼7 μg hUCP2 per mg of lipid. Arachidonic acid was added at a concentration of 15 mol %. Buffer solution contained 50 mM K2SO4, 25 mM TES, 0.6 mM EGTA at pH 7.35 and T = 37°C.
Fitting Eq. 1 to the experimental curves (Fig. 2 B and Fig. 3 B, dashed lines) resulted in β = (35 ± 0.3) × 10-2 and β = (30 ± 0.4) × 10-2 for membranes containing UCP + AA (Fig. 3 B) or AA alone (Fig. 2 B), respectively. It is reasonable to infer that the barrier in a protein-free membrane is trapezoidal (Eq. 2). The introduction of the protein raises additional possibilities (34). In our experiments, UCP lowered the energy barrier by a factor of 8. This could have occurred by a uniform perturbation of the protein-lipid interface, perhaps increasing the activation entropy of the FA diffusion, and thereby leaving a trapezoidal barrier. It could also have occurred by virtue of an energy well near the center of the membrane, created by a polar side chain of UCP. This would correspond to β = 0.25. The similarity of β values for membranes containing both UCP and AA or AA alone may indicate that the uncoupling protein recruits FAs from the lipid phase, meaning that the binding site for FA is localized in the membrane.
Discussion
The goal of this study was to get insight in the mechanism of UCP-mediated proton transfer. For this we compared the contributions of long-chain FA and uncoupling proteins (UCP1 and UCP2) to the lipid Gm at different transmembrane potentials.
A central point in the debate about UCP mechanism is the important question of whether or not FA are essential for UCP-mediated protonophoric function. Our group and other research groups led by Klingenberg, Garlid, and Jezek, respectively, supporting the first hypothesis used purified, reconstituted UCPs, whereas other groups led by Rial and Nicholls, respectively, which doubted the FA role in proton leak, used isolated mitochondria in the presence of BSA. The small leak in mitochondria in the absence of added FA observed in these experiments may be explained by the presence of endogenous FA produced in mitochondria through the action of phospholipases. In this study, we show that even at high potentials, no alterations in Gm are induced without FA. At the highest potentials described for mitochondria (∼210 mV), the Gm increase was most pronounced if protein and FA were reconstituted simultaneously. It confirms our observations made at low potentials (20–22).
In contrast to the membranes reconstituted with protein, the membrane conductance in the presence of FAs alone was significantly increased at high transmembrane potentials. Protonated (uncharged) FAs are known to diffuse quickly through the hydrophobic interior of the lipid bilayer, whereas FA− move slowly (t1/2 of minutes) (60–62). The molecular consequence of applying an electric field to the lipid membrane is poorly understood. It has been shown that an electric field creates a compressive stress in the membrane (63,64), however, the amplitude of such compression is rather small (65,66). ATR-FTIR, ESR, and 31P NMR studies with multilayers showed that the field influences the orientation of polar headgroups but without significant effects on the structure and dynamics of the hydrocarbon chains (67,68). The formation of membrane defects due to electric field application can lead to increased ion conductance with subsequent membrane rupture (69). Such defects were not observed in our experiments. In the case of charged FA anions, the supply of external energy would help to overcome the membrane energy barrier. For charged species, a trapezoidal energy barrier was described to be a good model for the force barrier (70,71).
Based on the experiments in the study, the catalyzing role of protein at high membrane potentials can be explained by protein-caused disturbance of the membrane barrier integrity (energy barrier?) leading to the acceleration of FA− flip-flop on the lipid-protein interface (34). The increase of the proton conductance in the presence of UCP and FA with increasing of FA unsaturation was explained recently by acceleration of FA flip-flop velocity due to increase of the membrane fluidity (22). The proposed nonspecific mechanism supports the idea that also other members of the mitochondrial carrier family, such as the adenine nucleotide and glutamate/aspartate transporters, dicarboxylate, oxoglutarate, and phosphate carriers may catalyze a fatty acid-dependent proton leak.
According to FA cycling hypothesis two steps in UCP-mediated proton transport are postulated: the flip-flop of protonated FAs along their interleaflet concentration gradient and the subsequent backward transport of the deprotonated (anionic) FAs by UCP (72,73). The similarity of the curve shapes for FA-containing membranes (Fig. 6) suggests that the protein acts to reduce the energy barrier of the existing pathway instead of providing a proteinaceous pathway for FA−. The size of the barrier is lowered by the protein, but its shape remains trapezoidal. In contrast, if the translocation of the anion would occur through the protein core, the barrier would adopt a sharp peak at the constriction zone (34), as suggested by the asymmetrical structure obtained for another member of the protein family (74). If the protein anion binding site would be additionally present, a barrier with two sharp peaks separated by an energy well would be appropriate, experimentally expecting β value of 0.25. A quantitative analysis of the I/V curves supports the observation that the FA− remains (at least partly) exposed to membrane lipids.
The nonlinear character of current-voltage curves in our experiments is in agreement with the strong nonlinear dependence of ion flux on the electrical membrane potential seen in mitochondria (75–78). In contrast, in liposomes reconstituted with UCP1 (41), it was shown that the H+ transport, measured in the presence of valinomycin, is linearly dependent on ΔΨm over a range from 0 to 150 mV. Several explanations for the divergence are possible. Because ΔΨm was created by a potassium gradient in these experiments, a potassium leak at high potentials may lower real ΔΨm. In addition, interactions between the positively charged valinomycin and the negatively charged fatty acid anions may hamper the analysis.
The nonlinear character of the curves attributes a modulating role to the membrane potential and may be an effective mechanism for the rapid regulation of proton leak and/or fatty acid concentration in the absence of proteins (72). Apart from the transmembrane potential, two factors were additionally able to amplify the FA-mediated leak: the FA saturation degree and the FA abundance in the membrane. The higher proton conductivity for unsaturated oleic acid in comparison with saturated palmitic acid in the absence of protein has been shown by Brunaldi et al. (58). Under similar conditions (ΔΨm = 0), we found a small but measurable increase in the membrane conductance after addition of polyunsaturated AA, but no effect in the presence of stearic, oleic, retinoic, and linoleic acids (22). Our data show that the differences in G, mediated by FAs of various saturation degrees and chain lengths become obvious at high membrane potentials.
The conductance increase mediated by fatty acids is strongly dependent on the FA mole fraction in the membrane-forming solution (Fig. 2). The studied concentrations of FA reflect the variation in normal levels of FA in biological membranes (1 mol % to ∼40 mol % of total lipids), which depend on the specific type of cell membrane and metabolic conditions (79). The dramatic concentration increase of free FAs under pathological conditions has been reported to be associated with ischemia, anoxia, hypothermia, stimulation of thermogenesis, ethanol abuse, hormone-induced lipolysis, and several hereditary disorders (80–82). Whereas under usual physiological conditions the uncoupling effect of FA is thought to be negligible (57), a rise in FA concentration combined with the alteration in transmembrane potential may contribute significantly to the FA-mediated proton leak under physiological or pathological conditions (83).
We believe experiments on lipid membranes show clearly that i), the electrical current, I, through lipid membranes reconstituted with FAs increases nonlinearly with increasing of FA concentration and/or ΔΨm, increasing the significance of FAs in proton leak under physiological conditions; and ii), FAs are absolutely necessary for the protonophoric function of UCPs (32) and this function is strongly activated by high ΔΨm. The latter finding indicates that UCPs should be regarded as mild uncouplers (84) lowering ΔΨm only if it is originally sufficiently high (e.g., in State 4 rather than in State 3). Due to the nonlinearity of the I/V curves, a slight drop in ΔΨm can lead to a significant reduction in proton leak rate and vice versa. Such regulation of proton conductance and/or FA concentration by ΔΨm may be key in mitochondrial respiration and metabolism.
Acknowledgments
The authors thank Dr. K. Garlid, Dr. P. Pohl, and Dr. Y. N. Antonenko for the helpful discussion, and J. A. Liebkowsky for the editorial assistance. Drs. S. S. Klishin and E. A. Sokolenko were supported by a DAAD Fellowship.
This work was supported by Deutsche Forschungsgemeinschaft (Po-524/2, Po-524/3, Po-524/5 and 436 TSE 113/44/0-1 to E.E.P.), Czech Academy of Sciences (AV0Z50110509), Czech Ministry of Education (ME09018), and Grant Agency of the Czech Republic (303/07/0105 to P.J. and M.J.).
Footnotes
Valeri Beck's present address is Bi-MS Beck, Berlin, Germany.
References
- 1.Nicholls D.G. A history of UCP1. Biochem. Soc. Trans. 2001;29:751–755. doi: 10.1042/bst0290751. [DOI] [PubMed] [Google Scholar]
- 2.Nedergaard J., Golozoubova V., Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 3.Skulachev V.P. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 4.Dlasková A., Hlavatá L., Jezek P. Oxidative stress caused by blocking of mitochondrial complex I H(+) pumping as a link in aging/disease vicious cycle. Int. J. Biochem. Cell Biol. 2008;40:1792–1805. doi: 10.1016/j.biocel.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Brand M.D., Affourtit C., Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Rousset S., Alves-Guerra M.C., Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53:130–135. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- 7.Horvath T.L., Diano S., Barnstable C. Mitochondrial uncoupling protein 2 in the central nervous system: neuromodulator and neuroprotector. Biochem. Pharmacol. 2003;65:1917–1921. doi: 10.1016/s0006-2952(03)00143-6. [DOI] [PubMed] [Google Scholar]
- 8.Nedergaard J., Cannon B. The ‘novel’ ‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp. Physiol. 2003;88:65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- 9.Krauss S., Zhang C.Y., Lowell B.B. The mitochondrial uncoupling-protein homologues. Nat. Rev. Mol. Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 10.Brown G.C., Brand M.D. On the nature of the mitochondrial proton leak. Biochim. Biophys. Acta. 1991;1059:55–62. doi: 10.1016/s0005-2728(05)80187-2. [DOI] [PubMed] [Google Scholar]
- 11.Porter R.K., Hulbert A.J., Brand M.D. Allometry of mitochondrial proton leak: influence of membrane surface area and fatty acid composition. Am. J. Physiol. 1996;271:R1550–R1560. doi: 10.1152/ajpregu.1996.271.6.R1550. [DOI] [PubMed] [Google Scholar]
- 12.Brand M.D., Pakay J.L., Cornwall E.J. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 2005;392:353–362. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreyev A.Yu., Bondareva T.O., Volkov N.I. Carboxyatractylate inhibits the uncoupling effect of free fatty acids. FEBS Lett. 1988;226:265–269. doi: 10.1016/0014-5793(88)81436-4. [DOI] [PubMed] [Google Scholar]
- 14.Andreyev A.Yu., Bondareva T.O., Vygodina T.V. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur. J. Biochem. 1989;182:585–592. doi: 10.1111/j.1432-1033.1989.tb14867.x. [DOI] [PubMed] [Google Scholar]
- 15.Cadenas S., Buckingham J.A., Brand M.D. AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem. J. 2000;351:307–311. [PMC free article] [PubMed] [Google Scholar]
- 16.Echtay K.S., Esteves T.C., Brand M.D. A signaling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabalina I.G., Backlund E.C., Nedergaard J. Within brown-fat cells, UCP1-mediated fatty acid-induced uncoupling is independent of fatty acid metabolism. Biochim. Biophys. Acta. 2008;1777:642–650. doi: 10.1016/j.bbabio.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Klingenberg M., Echtay K.S. Uncoupling proteins: the issues from a biochemist point of view. Biochim. Biophys. Acta. 2001;1504:128–143. doi: 10.1016/s0005-2728(00)00242-5. [DOI] [PubMed] [Google Scholar]
- 19.Jabůrek M., Varecha M., Garlid K.D. Transport function and regulation of mitochondrial uncoupling proteins 2 and 3. J. Biol. Chem. 1999;274:26003–26007. doi: 10.1074/jbc.274.37.26003. [DOI] [PubMed] [Google Scholar]
- 20.Urbánková E., Voltchenko A., Pohl E.E. Transport kinetics of uncoupling proteins. Analysis of UCP1 reconstituted in planar lipid bilayers. J. Biol. Chem. 2003;278:32497–32500. doi: 10.1074/jbc.M303721200. [DOI] [PubMed] [Google Scholar]
- 21.Beck V., Jabůrek M., Pohl E.E. A new automated technique for the reconstitution of hydrophobic proteins into planar bilayer membranes. Studies of human recombinant uncoupling protein 1. Biochim. Biophys. Acta. 2006;1757:474–479. doi: 10.1016/j.bbabio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Beck V., Jabůrek M., Pohl E.E. Polyunsaturated fatty acids activate human uncoupling proteins 1 and 2 in planar lipid bilayers. FASEB J. 2007;21:1137–1144. doi: 10.1096/fj.06-7489com. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls D.G., Locke R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Grav H.J., Pedersen J.I., Christiansen E.N. Conditions in vitro which affect respiratory control and capacity for respiration-linked phosphorylation in brown adipose tissue mitochondria. Eur. J. Biochem. 1970;12:11–23. doi: 10.1111/j.1432-1033.1970.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 25.Echtay K.S., Winkler E., Klingenberg M. Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature. 2000;408:609–613. doi: 10.1038/35046114. [DOI] [PubMed] [Google Scholar]
- 26.Echtay K.S., Roussel D., Brand M.D. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 27.Shabalina I.G., Petrovic N., Nedergaard J. UCP1 and defense against oxidative stress. 4-Hydroxy-2-nonenal effects on brown fat mitochondria are uncoupling protein 1-independent. J. Biol. Chem. 2006;281:13882–13893. doi: 10.1074/jbc.M601387200. [DOI] [PubMed] [Google Scholar]
- 28.Silva J.P., Shabalina I.G., Larsson N.G. SOD2 overexpression: enhanced mitochondrial tolerance but absence of effect on UCP activity. EMBO J. 2005;24:4061–4070. doi: 10.1038/sj.emboj.7600866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombardi A., Grasso P., Goglia F. Interrelated influence of superoxides and free fatty acids over mitochondrial uncoupling in skeletal muscle. Biochim. Biophys. Acta. 2008;1777:826–833. doi: 10.1016/j.bbabio.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Brand M.D., Turner N., Hulbert A.J. Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem. J. 2003;376:741–748. doi: 10.1042/BJ20030984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brookes P.S., Buckingham J.A., Brand M.D. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standard metabolic rate and phospholipid fatty acid composition. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1998;119:325–334. doi: 10.1016/s0305-0491(97)00357-x. [DOI] [PubMed] [Google Scholar]
- 32.Stuart J.A., Cadenas S., Brand M.D. Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochim. Biophys. Acta. 2001;1504:144–158. doi: 10.1016/s0005-2728(00)00243-7. [DOI] [PubMed] [Google Scholar]
- 33.Brand M.D., Couture P., Hulbert A.J. Evolution of energy metabolism. Proton permeability of the inner membrane of liver mitochondria is greater in a mammal than in a reptile. Biochem. J. 1991;275:81–86. doi: 10.1042/bj2750081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garlid K.D., Beavis A.D., Ratkje S.K. On the nature of ion leaks in energy-transducing membranes. Biochim. Biophys. Acta. 1989;976:109–120. doi: 10.1016/s0005-2728(89)80219-1. [DOI] [PubMed] [Google Scholar]
- 35.Brookes P.S., Hulbert A.J., Brand M.D. The proton permeability of liposomes made from mitochondrial inner membrane phospholipids: no effect of fatty acid composition. Biochim. Biophys. Acta. 1997;1330:157–164. doi: 10.1016/s0005-2736(97)00160-0. [DOI] [PubMed] [Google Scholar]
- 36.Schönfeld P., Wieckowski M.R., Wojtczak L. Long-chain fatty acid-promoted swelling of mitochondria: further evidence for the protonophoric effect of fatty acids in the inner mitochondrial membrane. FEBS Lett. 2000;471:108–112. doi: 10.1016/s0014-5793(00)01376-4. [DOI] [PubMed] [Google Scholar]
- 37.Wojtczak L., Wieckowski M.R., Schönfeld P. Protonophoric activity of fatty acid analogs and derivatives in the inner mitochondrial membrane: a further argument for the fatty acid cycling model. Arch. Biochem. Biophys. 1998;357:76–84. doi: 10.1006/abbi.1998.0777. [DOI] [PubMed] [Google Scholar]
- 38.Skulachev V.P. Anion carriers in fatty acid-mediated physiological uncoupling. J. Bioenerg. Biomembr. 1999;31:431–445. doi: 10.1023/a:1005492205984. [DOI] [PubMed] [Google Scholar]
- 39.Heimpel S., Basset G., Klingenberg M. Expression of the mitochondrial ADP/ATP carrier in Escherichia coli. Renaturation, reconstitution, and the effect of mutations on 10 positive residues. J. Biol. Chem. 2001;276:11499–11506. doi: 10.1074/jbc.M010586200. [DOI] [PubMed] [Google Scholar]
- 40.Krämer R. Mitochondrial carrier proteins can reversibly change their transport mode: the cases of the aspartate/glutamate and the phosphate carrier. Exp. Physiol. 1998;83:259–265. doi: 10.1113/expphysiol.1998.sp004111. [DOI] [PubMed] [Google Scholar]
- 41.Klingenberg M., Winkler E. The reconstituted isolated uncoupling protein is a membrane potential driven H+ translocator. EMBO J. 1985;4:3087–3092. doi: 10.1002/j.1460-2075.1985.tb04049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnamoorthy G., Hinkle P.C. Non-ohmic proton conductance of mitochondria and liposomes. Biochemistry. 1984;23:1640–1645. doi: 10.1021/bi00303a009. [DOI] [PubMed] [Google Scholar]
- 43.Benz R., McLaughlin S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone) Biophys. J. 1983;41:381–398. doi: 10.1016/S0006-3495(83)84449-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang S.G., Klingenberg M. Chloride channel properties of the uncoupling protein from brown adipose tissue mitochondria: a patch-clamp study. Biochemistry. 1996;35:16806–16814. doi: 10.1021/bi960989v. [DOI] [PubMed] [Google Scholar]
- 45.Jezek P., Garlid K.D. New substrates and competitive inhibitors of the Cl- translocating pathway of the uncoupling protein of brown adipose tissue mitochondria. J. Biol. Chem. 1990;265:19303–19311. [PubMed] [Google Scholar]
- 46.Lin C.S., Klingenberg M. Isolation of the uncoupling protein from brown adipose tissue mitochondria. FEBS Lett. 1980;113:299–303. doi: 10.1016/0014-5793(80)80613-2. [DOI] [PubMed] [Google Scholar]
- 47.Lin C.S., Klingenberg M. Characteristics of the isolated purine nucleotide binding protein from brown fat mitochondria. Biochemistry. 1982;21:2950–2956. doi: 10.1021/bi00541a023. [DOI] [PubMed] [Google Scholar]
- 48.Rigaud J.L., Pitard B., Levy D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta. 1995;1231:223–246. doi: 10.1016/0005-2728(95)00091-v. [DOI] [PubMed] [Google Scholar]
- 49.Záckova M., Jezek P. Reconstitution of novel mitochondrial uncoupling proteins UCP2 and UCP3. Biosci. Rep. 2002;22:33–46. doi: 10.1023/a:1016009022186. [DOI] [PubMed] [Google Scholar]
- 50.Jaburek M., Garlid K.D. Reconstitution of recombinant uncoupling proteins: UCP1, -2, and -3 have similar affinities for ATP and are unaffected by coenzyme Q10. J. Biol. Chem. 2003;278:25825–25831. doi: 10.1074/jbc.M302126200. [DOI] [PubMed] [Google Scholar]
- 51.Jabůrek M., Varecha M., Garlid K.D. Alkylsulfonates as probes of uncoupling protein transport mechanism. Ion pair transport demonstrates that direct H(+) translocation by UCP1 is not necessary for uncoupling. J. Biol. Chem. 2001;276:31897–31905. doi: 10.1074/jbc.M103507200. [DOI] [PubMed] [Google Scholar]
- 52.Smorodchenko A., Rupprecht A., Pohl E.E. Comparative analysis of uncoupling protein 4 distribution in various tissues under physiological conditions and during development. Biochim. Biophys. Acta. 2009;1788:2309–2319. doi: 10.1016/j.bbamem.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 53.Golozoubova V., Hohtola E., Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 54.Echtay K.S., Pakay J.L., Brand M.D. Hydroxynonenal and uncoupling proteins: a model for protection against oxidative damage. Biofactors. 2005;24:119–130. doi: 10.1002/biof.5520240114. [DOI] [PubMed] [Google Scholar]
- 55.Arsenijevic D., Onuma H., Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 56.Trenker M., Malli R., Graier W.F. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutknecht J. Proton conductance caused by long-chain fatty acids in phospholipid bilayer membranes. J. Membr. Biol. 1988;106:83–93. doi: 10.1007/BF01871769. [DOI] [PubMed] [Google Scholar]
- 58.Brunaldi K., Miranda M.A., Procopio J. Fatty acid flip-flop and proton transport determined by short-circuit current in planar bilayers. J. Lipid Res. 2005;46:245–251. doi: 10.1194/jlr.M400155-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Rial E., Poustie A., Nicholls D.G. Brown-adipose-tissue mitochondria: the regulation of the 32000-Mr uncoupling protein by fatty acids and purine nucleotides. Eur. J. Biochem. 1983;137:197–203. doi: 10.1111/j.1432-1033.1983.tb07815.x. [DOI] [PubMed] [Google Scholar]
- 60.Kamp F., Hamilton J.A. pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc. Natl. Acad. Sci. USA. 1992;89:11367–11370. doi: 10.1073/pnas.89.23.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pohl E.E., Peterson U., Pohl P. Changes of intrinsic membrane potentials induced by flip-flop of long-chain fatty acids. Biochemistry. 2000;39:1834–1839. doi: 10.1021/bi9919549. [DOI] [PubMed] [Google Scholar]
- 62.Pohl E.E., Voltchenko A.M., Rupprecht A. Flip-flop of hydroxy fatty acids across the membrane as monitored by proton-sensitive microelectrodes. Biochim. Biophys. Acta. 2008;1778:1292–1297. doi: 10.1016/j.bbamem.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 63.Coster H.G. Electromechanical stresses and the effect of pH on membrane structure. Biochim. Biophys. Acta. 1975;382:142–146. doi: 10.1016/0005-2736(75)90172-8. [DOI] [PubMed] [Google Scholar]
- 64.Bamberg E., Benz R. Voltage-induced thickness changes of lipid bilayer membranes and the effect of an electrin field on gramicidin A channel formation. Biochim. Biophys. Acta. 1976;426:570–580. doi: 10.1016/0005-2736(76)90400-4. [DOI] [PubMed] [Google Scholar]
- 65.Sugár I.P. The effects of external fields on the structure of lipid bilayers. J. Physiol. (Paris) 1981;77:1035–1042. [PubMed] [Google Scholar]
- 66.Pohl P., Rokitskaya T.I., Saparov S.M. Permeation of phloretin across bilayer lipid membranes monitored by dipole potential and microelectrode measurements. Biochim. Biophys. Acta. 1997;1323:163–172. doi: 10.1016/s0005-2736(96)00185-x. [DOI] [PubMed] [Google Scholar]
- 67.Stulen G. Electric field effects on lipid membrane structure. Biochim. Biophys. Acta. 1981;640:621–627. doi: 10.1016/0005-2736(81)90092-4. [DOI] [PubMed] [Google Scholar]
- 68.Le Saux A., Ruysschaert J.M., Goormaghtigh E. Membrane molecule reorientation in an electric field recorded by attenuated total reflection Fourier-transform infrared spectroscopy. Biophys. J. 2001;80:324–330. doi: 10.1016/S0006-3495(01)76017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glaser R.W., Leikin S.L., Sokirko A.I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim. Biophys. Acta. 1988;940:275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- 70.Hladky S.B. The energy barriers to ion transport by nonactin across thin lipid membranes. Biochim. Biophys. Acta. 1974;352:71–85. doi: 10.1016/0005-2736(74)90180-1. [DOI] [PubMed] [Google Scholar]
- 71.Hall J.E., Mead C.A., Szabo G. A barrier model for current flow in lipid bilayer membranes. J. Membr. Biol. 1973;11:75–97. [Google Scholar]
- 72.Skulachev V.P. Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 1991;294:158–162. doi: 10.1016/0014-5793(91)80658-p. [DOI] [PubMed] [Google Scholar]
- 73.Garlid K.D., Orosz D.E., Jezek P. On the mechanism of fatty acid-induced proton transport by mitochondrial uncoupling protein. J. Biol. Chem. 1996;271:2615–2620. doi: 10.1074/jbc.271.5.2615. [DOI] [PubMed] [Google Scholar]
- 74.Pebay-Peyroula E., Dahout-Gonzalez C., Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 75.Nicholls D.G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur. J. Biochem. 1974;50:305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- 76.Nicholls D.G. The effective proton conductance of the inner membrane of mitochondria from brown adipose tissue. Dependency on proton electrochemical potential gradient. Eur. J. Biochem. 1977;77:349–356. doi: 10.1111/j.1432-1033.1977.tb11674.x. [DOI] [PubMed] [Google Scholar]
- 77.Brown G.C., Brand M.D. Changes in permeability to protons and other cations at high proton motive force in rat liver mitochondria. Biochem. J. 1986;234:75–81. doi: 10.1042/bj2340075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorgato M.C., Ferguson S.J. Variable proton conductance of submitochondrial particles. Biochemistry. 1979;18:5737–5742. doi: 10.1021/bi00592a034. [DOI] [PubMed] [Google Scholar]
- 79.Hauser H., Howell K., Bowyer D.E. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim. Biophys. Acta. 1980;602:567–577. doi: 10.1016/0005-2736(80)90335-1. [DOI] [PubMed] [Google Scholar]
- 80.Boime I., Smith E.E., Hunter F.E. The role of fatty acids in mitochondrial changes during liver ischemia. Arch. Biochem. Biophys. 1970;139:425–443. doi: 10.1016/0003-9861(70)90496-0. [DOI] [PubMed] [Google Scholar]
- 81.Conner W.E., Lin D.S., Colvis C. Differential mobilization of fatty acids from adipose tissue. J. Lipid Res. 1996;37:290–298. [PubMed] [Google Scholar]
- 82.Chien K.R., Han A., Willerson J.T. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ. Res. 1984;54:313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- 83.Schönfeld P., Schild L., Kunz W. Long-chain fatty acids act as protonophoric uncouplers of oxidative phosphorylation in rat liver mitochondria. Biochim. Biophys. Acta. 1989;977:266–272. doi: 10.1016/s0005-2728(89)80080-5. [DOI] [PubMed] [Google Scholar]
- 84.Skulachev V.P. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]


