Abstract
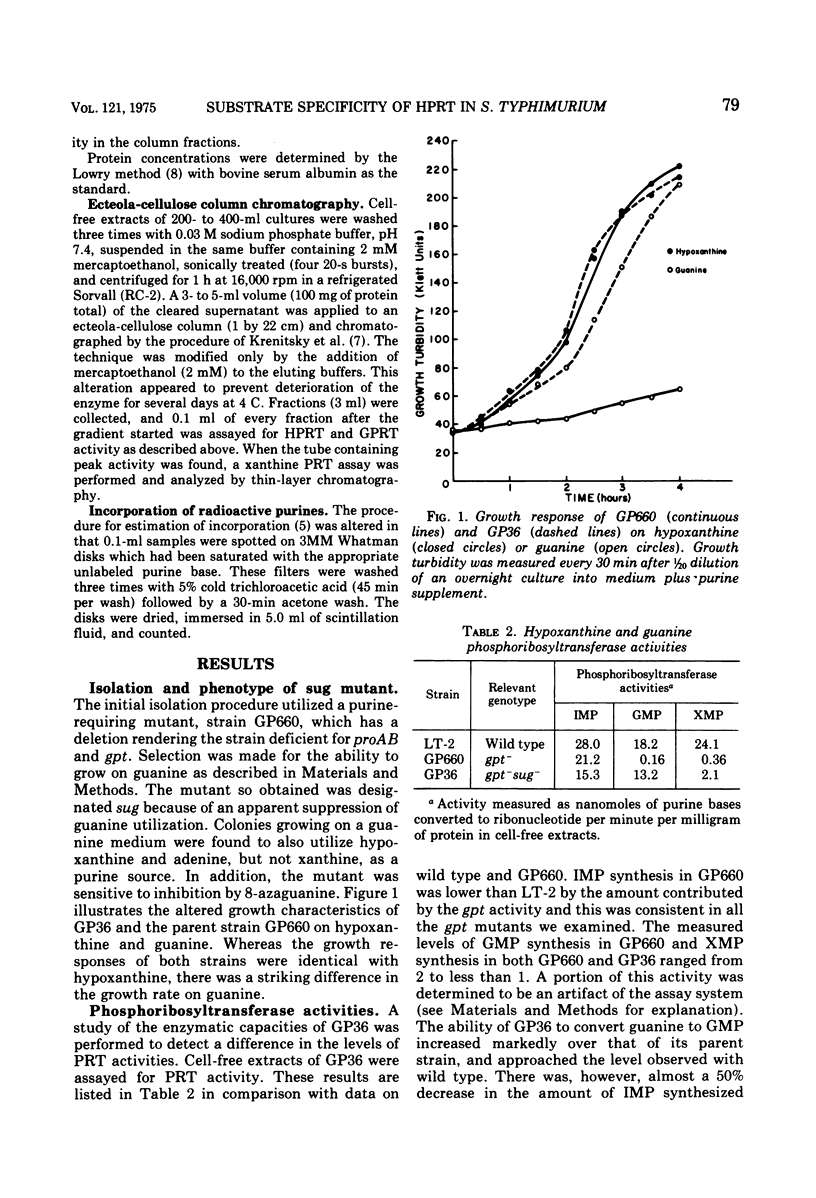
Salmonella typhimurium strain GP660 (proAB-gpt deletion, purE) lacks guanine phosphoribosyltransferase and hence cannot utilize guanine as a purine source and is resistant to inhibition by 8-azaguanine. Strain GP660 was mutagenized and a derivative strain (GP36) was isolated for utilization of guanine and hypoxanthine, but not xanthine, as purine sources. This alteration was designated sug. The strain was then sensitive to inhibition by 8-azaguanine. Column chromatographic analysis revealed the altered phosphoribosyltransferase peaks for both hypoxanthine and guanine to be located together, in the same position as hypoxanthine phosphoribosyltransferase (hpt gene product) of the wild-type strain. Genetic analysis showed the sug mutation to be allelic with hpt. Therefore sug represented a modification of the substrate specificity of the hpt gene product.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON M. R., MURRAY A. W. INHIBITION OF PRUINE PHOSPHORIBOSYLTRANSFERASES OF EHRLICH ASCITES-TUMOUR CELLS BY 6-MERCAPTOPURINE. Biochem J. 1965 Jan;94:64–70. doi: 10.1042/bj0940064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Purine phosphoribosyltransferases of Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):1010–1013. doi: 10.1128/jb.112.2.1010-1013.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E. Genetic control of bacterial purine phosphoribosyltransferases and an approach to gene enrichment. Adv Exp Med Biol. 1973;41:33–39. doi: 10.1007/978-1-4684-3294-7_5. [DOI] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Shumas S. R. Genetic separation of hypoxanthine and guanine-xanthine phosphoribosyltransferase activities by deletion mutations in Salmonella typhimurium. J Bacteriol. 1972 Nov;112(2):910–916. doi: 10.1128/jb.112.2.910-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Miller R. L. Guanine and xanthine phosphoribosyltransfer activities of Lactobacillus casei and Escherichia coli. Their relationship to hypoxanthine and adenine phosphoribosyltransfer activities. J Biol Chem. 1970 May 25;245(10):2605–2611. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


