Abstract
We evaluated the effect of photochemical alterations of chromophoric dissolved organic matter (CDOM) on bacterial abundance, activity and community composition in a coastal lagoon of the Atlantic Ocean with high dissolved organic carbon concentration. On two occasions during the austral summer, bacteria-free water of the lagoon was exposed to different regions of the solar spectrum (full solar radiation, UV-A + PAR, PAR) or kept in the dark. Subsequently, dilution cultures were established with bacterioplankton from the lagoon that were incubated in the pre-exposed water for 5 h in the dark. Cell abundance, activity, and community composition of bacterioplankton were assessed before and after incubation in the different treatments. Changes in absorption, fluorescence, and DOC concentration were used as proxies for CDOM photoalteration. We found a significant CDOM photobleaching signal, DOC loss, as well as a stimulation of bacterial activity in the treatments pre-exposed to UV radiation, suggesting increased bioavailability of DOM. Bacterial community analysis by fluorescence in situ hybridization revealed that this stimulation was mainly accompanied by the specific enrichment of Alpha- and Betaproteobacteria. Thus, our results suggest that CDOM photoalteration not only stimulates bacterioplankton growth, but also induces rapid changes in bacterioplankton composition, which can be of relevance for ecosystem functioning, particularly considering present and future changes in the input of terrestrial CDOM to aquatic systems.
Introduction
Dissolved organic matter (DOM) is composed of different classes of carbon moieties, some of which strongly absorb solar radiation (i.e., chromophoric DOM or CDOM) and influence the transmission of ultraviolet radiation (UVR, 280–400 nm) and photosynthetically active radiation (PAR, 400–700 nm) into the water column. The absorption of UVR by CDOM produces structural changes to this pool that reduce its ability to further absorb, a process known as photobleaching (see ref. 1 for a review). Furthermore, the sunlight-mediated conversion of CDOM into inorganic carbon, organic substrates (low-molecular-weight carbonyl compounds), and inorganic nutrients increases the turnover rates of CDOM in surface waters.2–5
In coastal ecosystems, a large fraction of CDOM is derived from the decomposition of terrestrial vegetation and microbial degradation is accelerated when this pool is exposed to UVR.6 In these ecosystems, the coupled photochemical and microbial degradation of CDOM is thought to be an important sink for organic carbon carried by rivers.7,8 Several studies have shown that bacterial activity usually increases significantly after exposure of CDOM to solar radiation.4,9,10,11–13 This increase in bacterial activity is driven by the formation of readily available low molecular weight organic photoproducts.1,9,14
In shallow coastal lagoons, the zone exposed to solar UVR can constitute an important fraction of the water column and negative effects of UVR on planktonic and benthic communities have been reported.11,15 Estuarine systems are characterized by the intense exchange of waters with different concentrations of CDOM.16 The change in dominance of freshwater and marine water masses with high and low CDOM concentration, respectively, is the main process governing changes in underwater UV attenuation in those systems.17,18
Several studies have addressed the effect of DOM photodegradation on bacterial activity and growth efficiency,14,19,20 however very little is known about whether the composition of the bacterial community is also affected by this process. For example, Abboudi et al.21 using 16S rDNA and rRNA-based capillary electrophoresis single strand conformational polymorphism (CE-SSCP) assessed changes in bacterial diversity due to phototransformation of DOM and found differences in the pattern and number of ribotypes. However, they did not identify the affiliation of the bacteria in the different light treatments.21
In the present study, we tested the idea that CDOM photoalteration favors specific bacterial taxa that are able to respond rapidly to changes in the bioavailability of organic substrates. Thus, we assessed how CDOM exposure to different regions of the solar spectrum affects bacterioplankton activity and community composition in the freshwater zone of a very productive coastal lagoon with high dissolved organic carbon (DOC) concentration. Our strategy was to assess those potential changes in bacterial community composition in the short term (hours) by a quantitative approach (fluorescent in situ hybridization with specific probes that target the most common bacterial groups found in aquatic ecosystems) that allows for establishing how significant those changes are.
Materials and methods
Study site
Laguna de Rocha is a highly productive shallow coastal lagoon (mean depth = 0.6 m, area = 72 km2) influenced by freshwater and marine intrusions. It is located on the south-eastern coast of South America (34° 33′ S, 54° 22′ W). The system communicates with the Atlantic Ocean through a single-mouth inlet on a sand barrier that opens naturally (occasionally by human action) when the water depth is higher than ~1.4 m depth. When the sand bar is opened, the marine intrusion progresses gradually and segregates a zone with brackish water and high PAR UVR penetration, and a zone that usually remains limnic and is characterized by its low transparency and high nutrient concentration.18
Sampling and water analyses
The northern zone of the lagoon was sampled in January and February 2004. Water samples from the upper 50 cm were collected with a 5 L sampler and basic parameters such as conductivity and temperature were measured in situ. For the analysis of DOC concentration and optical properties of CDOM, samples were filtered on the same day (within 6 h) through 0.2 μm polycarbonate filters (Millipore filters), previously soaked overnight in 10% HCl and rinsed with 20 ml of Milli-Q water. Filtered water was stored in precombusted (500 °C, 1 h) glass bottles (Teflon-capped) and kept in the dark at −20 °C for DOC analysis or at 4 °C for the optical characterization of CDOM (absorption and fluorescence).
The concentration of DOC was measured using a high temperature catalytic oxidation method (Shimadzu TOC analyzer model 5000). Previously to the analysis, the samples were thawed at room temperature and sonicated for 1 min at 13 W. The instrument was equipped with a Shimadzu platinized quartz catalyst for high sensitivity analysis. Vials for DOC estimation were also precombusted as described above for other glass material. Three to five injections were analyzed for each sample and blank (Milli-Q water). Blank values (0.03–0.05 mg DOC L−1) were subtracted from the average DOC values of the samples. Calibration of the instrument was done with potassium hydrogen phthalate (four points calibration curve). A consensus reference material (CRM) for DOC (batch 5 FS-2005: 0.57 mg C L−1 provided by RSMAS/MAC, University of Miami) was run in parallel. Results differed from the CRM given value by 5% and the coefficient of variation was better than 2%.
CDOM absorbance was measured at room temperature in a double-beam spectrophotometer (Shimadzu UV1603) between 280 and 750 nm using acid-cleaned 5 cm fused silica cuvettes (Suprasil I) previously rinsed with Milli-Q water and twice with the filtered sample. Milli-Q water of low DOC concentration (0.05 mg L−1) was used as a reference. Absorption coefficients (a) were calculated as: a = 2.303D/l, where D is absorbance and l is the pathlength of the cuvette in metres. Correction for the scattering caused by particles was done by subtracting the absorbance value at 690 nm. For comparative purposes, we used the absorption value at 320 nm (a320), which is sensitive to changes caused by both UV-B and UV-A radiation. The CDOM absorption at 320 nm was also normalized to the DOC concentration (a*320).
Samples for fluorescence measurements were filtered as for DOC and the filtrates collected in 20 mL vials (acid washed, gently rinsed with Milli-Q, and pre-combusted as above). Fluorescence was measured at room temperature in an AMINCO Bowman Serie 2 spectrofluorometer (excitation at 355 nm, emission at 450, slit width of 5 nm) using a 1 cm quartz cuvette, pre-rinsed gently with Milli-Q water and with the filtered sample. Fluorescence standardization was done by normalizing the signal of the sample to the fluorescence of a solution of quinine sulfate 0.1 mg L−1 in 1 N H2SO4 defined as 1 quinine sulfate unit (QSU). Fluorescence was linear between 0.05 and 10 mg L−1 of quinine sulfate. To account for potential instrumental drift, separate quinine sulfate spectra were acquired on each day of measurement.
Experimental design
Two experiments were done during the austral summer of 2004 (January and February) to assess DOM photoalteration and its effects. First, the lagoon water was double-filtered as described above for DOC, but under sterile conditions (i.e. in a laminar flow) and kept in the dark at 4 °C until the experiment was set up (within 24 h). Then, the filtered water was distributed in spherical quartz bottles of 100 mL (diameter: 5 cm) that were previously acid-washed, rinsed with Milli-Q water, and muffled at 500 °C for 1 h. The bottles were placed in a circulating water bath at in situ temperature (24–25 °C) and were rotated regularly to assure homogeneous exposure to solar radiation. Triplicate bottles were exposed to natural solar radiation during 6 days using different filters to exclude different regions of the solar spectrum. Quartz bottles with no additional spectral filters were used for the ‘full spectrum’ treatment and those used as dark control were wrapped with three layers of aluminium foil. The bottles in the PAR treatment were wrapped with one layer of a vinyl chloride foil (CI Kasei Co., Tokyo, Japan, 50% transmittance at 405 nm) to exclude the UVR. To differentiate between UV-B (280–315 nm) and UV-A (315–400 nm) effects, quartz bottles were wrapped with one layer of Mylar-D (150 μm thickness, 50% transmission at 325 nm) to exclude the UV-B. Changes in the transmittance of the different foils were measured in a double-beam spectrophotometer, and foils were replaced, if necessary.
To prepare the bacterial inoculum, water from the same zone of the lagoon was collected on the sixth day of exposure and filtered through a glass-fiber filter with a ‘pore size’ of 1.2 μm (Whatman GF/C). Then, 10 ml of the filtrate were added to each bottle containing the water from the different treatments (1 : 10 dilution). Bacterial abundance, community composition, and activity were determined before the GF/C filtration and in the filtrate at time zero (i.e. before the inoculation into each treatment). Potential bacterial contamination in the pre-exposed filtered lagoon water proved negative as determined by inspection of samples from additional bottles (i.e. only used for this purpose) by epifluorescence microscopy (see below).
After 5 h incubation in the dark, bacterial abundance, activity, and community composition in each treatment were again assessed. This incubation time was chosen in order to minimize the effect of confinement on bacterial growth,22 and was based on previous data on bacterial activity for this lagoon, which is very high in summer.23
Radiation measurements
Irradiances in the UV-A and UV-B wavebands were measured in situ with an International Light radiometer (IL-1400A) consisting of the broadband SUL033/W (UV-A) and SUL240/W (UV-B) sensors. Incident radiation for UV-B and UV-A was integrated for the time of exposure.
Bacterial abundance
Water samples were fixed with paraformaldehyde (1% final concentration), kept in the dark at room temperature for 1 h, and then overnight at 4 °C. Total bacterial abundance was determined by epifluorescence microscopy after staining the samples with the fluorochrome 4′,6-diamino-2-phenylindole staining (DAPI, final concentration 1 μgmL−1) as previously described.24 After staining, samples were filtered onto polycarbonate filters (type GTTP; diameter, 25 mm; pore size, 0.2 μm; Millipore) using a cellulose nitrate filter (pore size, 0.45 μm; Sartorius) as support to optimize the distribution of cells on the filters. Bacterial enumeration was done by epifluorescence microscopy using a Zeiss Axioplan II Imaging epifluorescence microscope (Carl Zeiss, Jena, Germany).
Bacterial activity
Bacterial bulk activity was estimated following the incorporation of [3H]-L-leucine (Amersham, Little Chalfont, England) into bacterial biomass. Radiolabelled leucine (specific activity 173 Ci mmol−1) was added at saturating concentrations (20 nM) to triplicate sub-samples and to one formaldehyde-killed control (3% final concentration) from each water treatment. The treatments were incubated in a water bath in the dark at the in situ temperature (24–25 °C) for 1 h and fixed by the addition of formaldehyde (3% final concentration). Macromolecule extraction was done with cold TCA and cold ethanol.25 Measurements of [3H]-L-leucine incorporation were done in a Beckman LS5000TD liquid scintillation counter (Beckman, Fullerton, CA). Values were corrected for quenching (external standard method) and by subtraction of counts from the controls.
Bacterial community composition
Fixed bacteria were collected by filtration onto white 0.2 μm pore size polycarbonate filters, rinsed twice with 1× phosphatebuffered saline and once with deionized water, and then the filters were stored at −20 °C. FISH with horseradish-peroxidaselabelled probes and tyramide signal amplification (CARD-FISH) was performed as described previously.26 using a modified permeabilization protocol.27 The following bacterial group-specific oligonucleotide probes (ThermoHybrid, Germany) were assayed: ALF96828 (Alphaproteobacteria), BET42a29 (Betaproteobacteria), GAM42a29 (Gammaproteobacteria), CF319a30 (originally described to target the phylum Cytophaga-Flavobacter-Bacteroides, but now only Cytophaga-like within the Bacteroidetes), and HGC69a31 (Actinobacteria), as well as the probe EUB I-III for the Bacteria domain.32 To determine the relative abundance of each targeted group, bacterial counting was done by epifluorescence microscopy and a semi-automated counting system.33
Statistical analyses
One-way analysis of variance (ANOVA) was performed to test the significance of the differences observed in bacterial abundance, activity, and relative abundance of different phylogenetic groups among irradiation treatments after checking for homogeneity of variance and normal distribution. For all parameters, the average values were calculated from the three replicates in each treatment. Post-hoc comparisons were done with the Scheffé test. Differences were considered significant when p < 0.05. All statistical analyses were performed with Statistica 5.0 (StatSoft Inc.).
Results
Incident radiation and optical characteristics of CDOM
Values of incident integrated UV-B and UV-A radiation were 8.1 J cm−2 and 414.2 J cm−2, respectively, in January and 7.1 J cm−2 and 419.6 J cm−2 in February. Water temperature in both experiments was 24–25 °C. Conductivity increased from 0.54 mS cm−1 in January to 13.11 mS cm−1 in February.
In January, the in situ (i.e. before exposure) absorption coefficient (a320) for CDOM was approximately three times higher than in February (Table 1) coinciding with the lower water conductivity value. In January, a significant reduction in CDOM fluorescence (F) by 43 and 45% in the UVR + PAR (‘full spectrum’) and UV-A + PAR (Mylar) treatments, respectively, was observed compared to the control (Table 1). Absorption at 320 nm and DOC-specific absorption at 320 nm (a*320) decreased after exposure to UVR + PAR and UV-A + PAR (Table 1). In addition, the DOC content in the UVR + PAR (17.7 mg C L−1) and in the UV-A + PAR (17.9 mg C L−1) treatments was significantly different from that of the dark control (21.7 mg C L−1, p < 0.05). Further, the DOC concentration in the UVR + PAR treatment was significantly lower than in the PAR one (p < 0.05), suggesting that the observed decrease in a, a*320, F, and DOC in January was mostly due to the UVR, as values for PAR were not significantly different from those in the dark control. Within the UVR, the largest photoalteration (i.e. photobleaching and DOC loss) effect on CDOM was caused by UV-A radiation.
Table 1.
Optical characteristics of CDOM and DOC concentration in the initial sample and after exposure for 6 days to PAR, PAR + UV-A, PAR + UVR or kept in the dark. a320: absorption coefficients at 320 nm;F: fluorescence in quinine sulfate units (QSU); DOC: dissolved organic carbon; a*320: DOC-specific absorption at 320 nm. Values are the average of three replicates for each treatment ± 1 SD
| Experiment | Initial | UVR + PAR | UV-A + PAR | PAR | Dark | |
|---|---|---|---|---|---|---|
| January | a320/m−1 | 53.7 | 40.4 ± 4.5 | 43.2 ± 3.6 | 53.4 ± 3.7 | 53.8 ± 4.6 |
| F/QSU | 118 | 68 ± 4.6 | 66 ± 2.5 | 116 ± 3.8 | 120 ± 4.7 | |
| DOC/mg C L−1 | 21.3 | 17.7 ± 0.6 | 17.9 ± 1.4 | 20.4 ± 1.9 | 21.7 ± 0.1 | |
| a*320/m−1 [mg C L−1]−1 | 2.51 | 2.28 | 2.40 | 2.62 | 2.48 | |
| February | a320/m−1 | 16.5 | 12.9 ± 1.3 | 16.1 ± 2.6 | 16.4 ± 2.5 | 16.8 ± 3.0 |
| F/QSU | 64 | 28 ± 3.1 | 46 ± 2.2 | 60 ± 4.9 | 64 ± 3.8 | |
| DOC/mg C L−1 | 18.3 | 16.4 ± 2.5 | 16.7 ± 0.4 | 18.4 ± 0.9 | 18.0 ± 1.4 | |
| a*320/m−1 [mg C L−1]−1 | 0.90 | 0.74 | 0.96 | 1.00 | 0.93 |
In February, photobleaching was principally caused by UV-B radiation as suggested by the more pronounced decrease in a320 and in F in the UVR + PAR treatment (e.g. 56% compared to F in the control). Similarly, a*320 decreased in the UVR + PAR treatment by 20% related to the control (Table 1). However, similar to the experiment in January, DOC loss was mainly caused by UV-A radiation. In February, the initial a*320 was only 35.9% of that estimated in January (Table 1).
Bacterial response to sunlight-induced changes in DOM
In both experiments, bacterial abundance significantly increased in all treatments and in the control after 5 h of incubation in the dark (p < 0.05) (Fig. 1). However, no significant differences between the treatments and control were found in January. The highest bacterial abundance was observed in the UV-A + PAR treatment (Fig. 1A), but it was not significantly different from the dark control (p = 0.057). In February, bacterial numbers significantly increased in the UVR + PAR treatment compared to the control (p = 0.02, Fig. 1B).
Fig. 1.
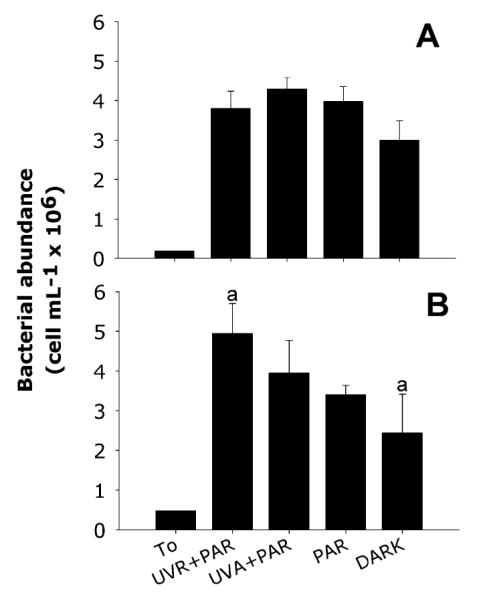
Bacterial abundance (cells mL−1 × 106; average ± 1 SD) measured at T0 and after 5 h incubation in each treatment for January (A) and February (B). Letters over the bars indicate those treatments showing significant differences.
After 5 h of incubation in the dark, bulk bacterial activity in the January experiment significantly increased in the treatment where CDOM was pre-exposed to UV-A + PAR (p = 0.01 compared to the dark control) (Fig. 2A). In February, bulk bacterial activity was clearly stimulated in the treatments where CDOM was pre-exposed to UVR, but it was significantly different from PAR (ANOVA, p = 0.028) and the dark control (ANOVA, p = 0.020) only in the UVR + PAR treatment. Nevertheless, the largest absolute increase in bacterial activity was observed when comparing the PAR and UV-A + PAR treatments (Fig. 2B).
Fig. 2.
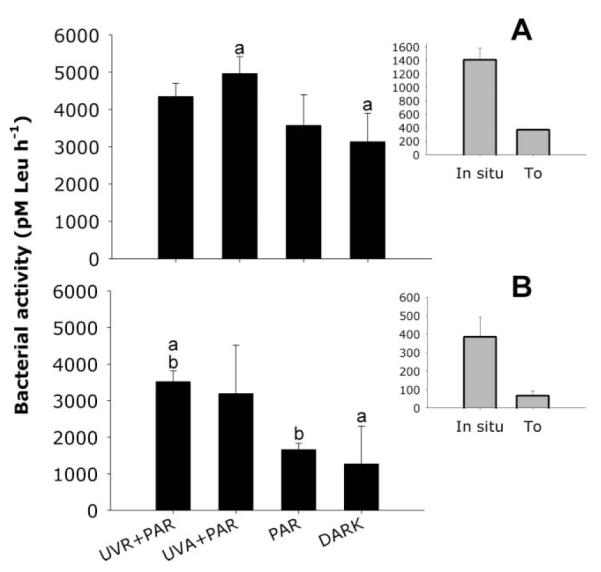
Bacterial activity (pM [3H]-L-leucine h−1; average ± 1 SD) in January (A) and February (B) for each water treatment. Bacterial activity in situ and after GF/C filtration (T0) are also shown (same unit as in the main graphs). Bars showing the same letters indicate those treatments where significant differences were found.
A marked increase in the detection level with the Eub I-III probe (domain Bacteria) was observed in both experiments. In January, this increase ranged from 50% at the beginning to 90.9 ± 0.74% of the DAPI-stained objects after 5 h of incubation (average ± 1 SD for all treatments), whereas in February ranged from 50% to 82.1 ± 5.2% (average ± 1 SD for all treatments).
In January, Beta- and Gammaproteobacteria were significantly enriched compared to the initial abundances (p < 0.05) (Fig. 3A). Betaproteobacteria was the only group showing significant differences among treatments (p = 0.017), specifically between UV-A + PAR and the dark control (p = 0.025), between UV-A + PAR and PAR (p = 0.035), and between both UV treatments (p = 0.02) (Fig. 3A). In the February experiment, the fractions of Beta- and Gammaproteobacteria again significantly increased in all treatments followed by Alphaproteobacteria that increased in the UV treatments (Fig. 3B). Significant differences among treatments were found for Alphaproteobacteria (p = 0.002), Betaproteobacteria (p = 0.03), and Gammaproteobacteria (p = 0.02) (Fig. 3B). The UVR + PAR treatment showed a significantly higher abundance of Alphaproteobacteria compared to PAR (p = 0.007) and the dark control (p = 0.008) (Fig. 3B). Significantly higher abundances of Betaproteobacteria were found in UVR + PAR than in the PAR (p = 0.015) and the dark control (p = 0.03) (Fig. 3B), and in UV-A + PAR compared to PAR (p = 0.03). For Gammaproteobacteria the trend was the opposite, so this group was extremely abundant in the PAR treatment and dark control reaching ~60% of the bacterial community and being significantly higher than in UVR + PAR (p = 0.02). The same was observed for the Cytophaga-like group, which showed a higher proportion of hybridized cells in the control than in the other treatments, being significantly different when compared to the PAR treatment (p < 0.05) (Fig. 3B). Abundances of Actinobacteria were under the detection limit of CARDFISH in both experiments.
Fig. 3.
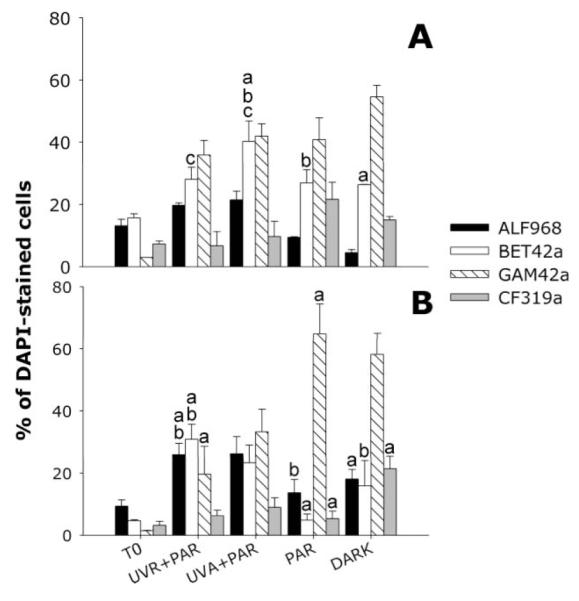
Bacterial community composition (CARD-FISH) in the different treatments of both experiments (% of DAPI-stained objects; average ± 1 SD). Assayed probes: BET42a (Betaproteobacteria), Alf968 (Alphaproteobacteria), Gam42a (Gammaproteobacteria), CF319a (Cytophaga-like). Panel A, January; B, February. Bars belonging to each probe showing the same letters indicate those treatments where significant differences were found for those bacterial groups.
Discussion
In this study, we found a clear photoalteration effect on CDOM when water from this coastal lagoon was exposed to solar radiation. CDOM photoalteration (i.e. DOC loss and photobleaching) was accompanied by a significant stimulation of bacterial activity. Stimulation of bacterial activity after CDOM exposure to solar UVR has been described for different aquatic ecosystems and seems to be related to the increased availability of organic substrates.9,34 For example, plant-derived DOM exposed to natural solar radiation stimulates bacterial growth through the progressive release of several short-chain fatty acids.10 One novel finding of our study, however, was the very rapid change in bacterial community composition observed in the experiments where CDOM underwent UVR-induced alteration.
DOM photoalteration
In January, the CDOM content of the lagoon water was higher than in February (Table 1) and the reduction in DOC concentration after UVR + PAR and UV-A + PAR exposure indicated direct photodegradation. The fact that the change in absorption and fluorescence between the UVR + PAR and UV-A + PAR treatments (Table 1) was not significantly different indicates a higher contribution of UV-A radiation. This is in agreement with a photobleaching model35 reported for lacustrine systems with DOC concentrations >3 mg L−1, as found in Laguna de Rocha.18
However, in February, UV-B radiation had a stronger photobleaching effect than UV-A as indicated by the strong decrease in a320 in the UVR + PAR treatment, though this effect was less pronounced when considering fluorescence (Table 1). Interestingly, DOC loss was again mainly caused by UV-A radiation and it was less pronounced than in January. The discrepancy between the wavebands causing the reduction in DOC concentration and the loss of chromophores and fluorophores in February may reflect a change in the CDOM pool associated to the intrusion of brackish water (conductivity increased by 24-fold). This change was clearly visible when the values of a*320 are compared (Table 1) and suggests that the CDOM in January (i.e. higher CDOM a320 and a*320) was mainly derived from terrestrial sources. It is arguable that the CDOM pool in February had different types of chromophores to that in January, but the methods used here do not allow for this comparison. Nevertheless, during marine intrusions as occurred in February in this lagoon, changes in underwater optical characteristics and in composition of CDOM are typically observed.15,18 In fact, the brackish area of Laguna de Rocha is characterized by low DOC concentrations and high UV penetration.15,18
Effect on bacterial abundance and activity
The observed increase in bacterial abundance and activity in all treatments and in the dark control during both experiments (Fig. 1 and 2) suggest that bacterial growth was stimulated by the dilution of bacteria into an environment enriched in bioavailable DOM, but also free of grazers. Dilution cultures have been used to study bacterial growth rates in situ,36,37 though there is evidence that dilution of the inoculum rapidly activates bacterial growth.22 In addition, in both experiments, it was clear that pre-filtration of the lagoon water to prepare the inoculum (GF/C filtration) caused a strong reduction in bacterial activity suggesting a relevant role of particle-attached bacteria in this system.
The significantly higher stimulation in the incorporation of leucine observed in January in the UV-A + PAR treatment (Fig. 2A) suggests that photodegradation of CDOM by UV-A radiation caused a positive feedback on bacterial activity. Similarly, in February the largest difference in bacterial activity was observed between the treatment where CDOM was pre-exposed to UV-A + PAR and only PAR. This pattern is coincident with the significant reduction in DOC concentration observed in the UV-A + PAR treatment in both experiments (Table 1). However, only in the UVR + PAR treatment it was significantly different from the control (Fig. 2B).
The stimulation of bacterial activity after irradiating CDOM has been described by several authors for different aquatic ecosystems.9,10,34 For example, CDOM photodegradation caused a significant increase in bacterial activity in the freshwater zone of the York River estuary, USA.38 This stimulation is attributed to the increase in the bioavailability of simple organic substrates after CDOM exposure to UVR.9,10 However, Abboudi et al.21 observed a decrease by 20 to 40% in bacterial activity and respiration when they incubated a bacterial community from a eutrophic lagoon in pre-irradiated CDOM from the same lagoon. These contrasting results suggest that the change in bacterial activity observed after CDOM is photodegraded depends on the quality and origin of this pool and it may be different for different ecosystems.14,21 In fact, other authors have found that seawater DOM that was initially bioreactive turned biologically more recalcitrant following exposure to solar radiation, while DOM of initially low bioavailability was rendered more bioavailable by photochemical processes.19
The fast generation times of the bacterial community observed in our study, i.e. ~67 min in the UV-A + PAR treatment in January and ~89 min in the UVR + PAR treatment in February, were similar to those observed for marine isolates grown in artificial media with low organic carbon content (<5 mg L−1).39 This suggests that the community growing in our experiments was composed by the so-called “eutrophic” bacterial species.40 Pinhassi and Hagströml41 reported that this bacterial strategy might be dominant in some habitats such as brackish waters. Laguna de Rocha supports bacterial production rates similar to those found for eutrophic water systems,23 which together with the high DOC content measured in this study could account for the high bacterial growth rates observed. When we calculated the amount of carbon (C) incorporated per cell during 5 h, we found an average of 17.4 (±1.9) and 11.2 (±2.5) fg C per cell for the first and second experiment, respectively. According to Simon and Azam,42 bacteria with cell volumes between 0.026 and 0.4 μm3 have a cell-specific carbon content from 10.4 to 53.3 fg, respectively. Hence, the carbon incorporated by the bacterial community during our experiments agreed with that expected on theoretical grounds.42
Effect on bacterial community composition
In both experiments, CARD-FISH detection levels after 5 h of incubation were higher in all treatments than in the inocula, which coincided with the observed increase in bacterial activity (Fig. 2 and 3). The proportions of cultivable cells43 and of cells with high metabolic activity typically increase during confinement experiments.14,44,45 Prefiltration and confinement of bacterioplankton during enrichments,46 dilutions,22 and incubation in mesocosms47,48 can result in changes of taxonomical composition of bacterial communities.49,50 This was also observed in our experiments, where the relative abundance of Gammaproteobacteria increased particularly in all treatments including the control (Fig. 3A and B). Enrichment of members of Gammaproteobacteria are usually observed during incubations of marine waters,22,48,49,51 and in our study it was probably a consequence of the exclusion of predators by filtration. However, the fact that the increase was less pronounced under UVR, particularly during the experiment in February (Fig. 3B), suggests that members of this group were negatively affected by the photochemical alteration of CDOM or, most probably, were outcompeted by the other bacterial groups. In fact, similar experiments in an alpine lake have indicated that members of Betaproteobacteria rapidly outcompete other bacterial groups.14 Thus, it seems arguable that members of Gammaproteobacteria are at disadvantage when CDOM is photoaltered and changes in availability and substrate quality take place. However, the broad target of the probe used in our study does not allow for making generalizations.
Abboudi et al.21 also found that CDOM exposed to sunlight caused changes in bacterial community structure in three contrasting coastal sites in the Northwestern Mediterranean Sea. However, they did not identify which bacterial groups or populations were enriched. Our experiments in Laguna de Rocha showed that the most significant change occurring within the bacterial community in relation to the pre-exposure of CDOM to UVR was that of Beta- and Alphaproteobacteria (Fig. 3). Similarly, in a study assessing the enrichment of bacterial populations in DOC-enriched water from a bog lake, stimulation of Betaproteobacteria growth was observed.52 As suggested by several authors,14,52 it is possible that members of the Betaproteobacteria are well adapted to abrupt changes in substrate availability responding rapidly to conditions such as those we had in our experiments. In particular, this change was pronounced in February, when Betaproteobacteria represented a low percentage of the DAPI-stained cells at T0 (Fig. 3B). By contrast, at T0 in the January experiment, Betaproteobacteria were dominant together with Alphaproteobacteria (Fig. 3A). In a previous study about the microbial community composition of Laguna de Rocha,23 the dominant bacterial group in both regions (i.e. freshwater and brackish) of the lagoon during an annual study was the Alphaproteobacteria. Our results extend these findings in that also Betaproteobacteria can occur in high relative abundance in this lagoon.
In summary, our results illustrate that UVR promotes the alteration of CDOM, which in turn stimulates growth of particular groups within the bacterial community such as Betaproteobacteria and Alphaproteobacteria. This suggests that phototransformation of CDOM is another factor to take into account when assessing temporal changes in bacterial community composition. The relevance of this process will depend on the hydrological regime, which also strongly influences bacterial dynamics in this type of coastal ecosystem.23 Finally, our results are important considering present and future changes in the input of terrestrial CDOM to aquatic ecosystems. In connection to this, a major task is still the identification of the bacterial ‘species’ responsible for the rapid shift observed in community composition and the assessment of what consequences those changes have for carbon cycling.
Acknowledgements
We thank R. Amann from the Max Planck Institute for Marine Microbiology for hosting the first author in his lab. This work was supported by the International Foundation for Science (grant IFS A-2917/2), the Max Planck Society and the Austrian Science Fund (FWF 19245-B03) to R. Sommaruga.
Footnotes
This article was published as part of the themed issue on “Environmental effects of UV radiation”.
References
- 1.Osburn CL, Morris DP. In: Photochemistry of chromophoric dissolved organic matter in natural waters, in UV effects in aquatic organisms and ecosystems. Helbling EW, Zagarese H, editors. The Royal Society of Chemistry; Cambridge: 2003. pp. 185–217. [Google Scholar]
- 2.Bushaw KL, Zepp RG, Tarr MA, Schulz-Janders D, Bourbonniere RA, Hodson RE, Miller WL, Bronk DA, Moran MA. Photochemical release of biologically available nitrogen from aquatic dissolved organic matter. Nature. 1996;381:404–407. [Google Scholar]
- 3.Miller WL, Moran MA. Interaction of photochemical and microbial processes in the degradation of refractory dissolved organic matter from a coastal marine environment. Limnol. Oceanogr. 1997;42:1317–1324. [Google Scholar]
- 4.Moran MA, Zepp RG. Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol. Oceanogr. 1997;42:1307–1316. [Google Scholar]
- 5.Biddanda BA, Cotner JB. Enhancement of dissolved organic matter bioavailability by sunlight and its role in the carbon cycle of Lakes Superior and Michigan. J. Great Lakes Res. 2003;29:228–241. [Google Scholar]
- 6.Zepp RG, Callaghan TV, Erickson DJ. Interactive effects of ozone depletion and climate change on biogeochemical cycles. Photochem. Photobiol. Sci. 2003;2:51–61. doi: 10.1039/b211154n. [DOI] [PubMed] [Google Scholar]
- 7.Kieber RJ, Zhou X, Mopper K. Formation of carbonyl compounds from UV-induced photodegradation of humic substances in natural waters: Fate of riverine carbon to the sea. Limnol. Oceanogr. 1990;35:1503–1515. [Google Scholar]
- 8.Mopper K, Kieber DJ. Marine photochemistry and its impact on carbon cycling. In: Mora S, Demers S, Vernet M, editors. The effects of UV radiation in the marine environment. Cambridge University Press; Cambridge: 2000. pp. 101–129. [Google Scholar]
- 9.Lindell MJ, Granéli W, Tranvik LJ. Enhanced bacterial growth in response to photochemical transformation of dissolved organic matter. Limnol. Oceanogr. 1995;40:195–199. [Google Scholar]
- 10.Wetzel RG, Hatcher PG, Bianchi TS. Natural photolysis by ultraviolet irradiance of recalcitrant dissolved organic matter to simple substrates for rapid bacterial metabolism. Limnol. Oceanogr. 1995;40:1369–1380. [Google Scholar]
- 11.Lindell MJ, Granéli W, Tranvik LJ. Impact of solar (UV)-radiation on bacterial growth in lakes. Aquat. Microb. Ecol. 1996;11:135–141. [Google Scholar]
- 12.Jørgensen NOG, Tranvik L, Edling H, Granéli W, Lindell M. Effects of sunlight on occurrence and bacterial turnover of specific carbon and nitrogen compounds in lake water. FEMS Microb. Ecol. 1998;25:217–227. [Google Scholar]
- 13.Bertilsson S, Tranvik LJ. Photochemically produced carboxylic acids as substrates for freshwater bacterioplankton. Limnol. Oceanogr. 1998;43:885–895. [Google Scholar]
- 14.Pérez M, Sommaruga R. Interactive effects of solar radiation and dissolved organic matter on bacterial activity and community structure. Environ. Microbiol. 2007;9:2200–2210. doi: 10.1111/j.1462-2920.2007.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conde D, Aubriot L, Bonilla S, Sommaruga R. Marine intrusions in a coastal lagoon enhance the negative effect of solar UV radiation on phytoplankton photosynthetic rates. Mar. Ecol. Prog. Ser. 2002;240:57–70. [Google Scholar]
- 16.Stedmon CA, Markager S, Kaasb H. Optical properties and signatures of chromophoric dissolved organic matter (CDOM) in Danish coastal waters. Estuar. Coast. Shelf Sci. 2000;51:267–278. [Google Scholar]
- 17.Kuhn P, Brownman H, McArthur B, St-Pierre JF. Penetration of ultraviolet radiation in the waters of the estuary and Gulf of St. Lawrence. Limnol. Oceanogr. 1999;44:710–716. [Google Scholar]
- 18.Conde D, Aubriot L, Sommaruga R. Changes in UV penetration associated with marine intrusions and freshwater discharge in a shallow coastal lagoon of the Southern Atlantic Ocean. Mar. Ecol. Prog. Ser. 2000;207:19–31. [Google Scholar]
- 19.Obernosterer I, Sempéré R, Herndl GJ. Ultraviolet radiation induces reversal of the bioavailability of DOM to marine bacterioplankton. Aquat. Microb. Ecol. 2001;24:61–68. [Google Scholar]
- 20.Moran MA, Covert JS. Photochemically mediated linkages between dissolved organic matter and bacterioplankton. In: Findlay S, Sinsabaugh R, editors. Aquatic Ecosystems: Interactivity of Dissolved Organic Matter. 2003. pp. 244–262. (Academics Aquatic Ecology Series). [Google Scholar]
- 21.Abboudi M, Jeffrey WH, Ghiglione JF, Pujo-Pay M, Oriol L, Sempéré R, Charriere B, Joux F. Effects of photochemical transformations of dissolved organic matter on bacterial metabolism and diversity in three contrasting coastal sites in the Northwestern Mediterranean Sea during summer. Microb. Ecol. 2008;55:344–357. doi: 10.1007/s00248-007-9280-8. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs BM, Zubkov MV, Sahm K, Burkill PH, Amann R. Changes in community composition during dilution cultures of marine bacterioplankton as assessed by flow cytometric and molecular biological techniques. Environ. Microbiol. 2000;2:191–201. doi: 10.1046/j.1462-2920.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 23.Piccini C, Conde D, Alonso C, Sommaruga R, Pernthaler J. Blooms of single bacterial species in a coastal lagoon of the southwestern Atlantic Ocean. Appl. Environ. Microbiol. 2006;72:6560–6568. doi: 10.1128/AEM.01089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter KG, Feig YS. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980;25:943–948. [Google Scholar]
- 25.Kirchman DL, Ducklow HW. Estimating conversion factors for the thymidine and leucine methods for measuring bacterial production. In: Kemp PF, Sherr BF, Sherr EB, Cole J, editors. Handbook of methods in aquatic microbial ecology. Lewis Publishers; Boca Raton, Florida: 1993. pp. 513–517. [Google Scholar]
- 26.Pernthaler A, Pernthaler J, Amann R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 2002;68:3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teira E, Reinthaler T, Pernthaler A, Pernthaler J, Herndl GJ. Combining catalyzed reporter deposition-fluorescence in situ hybridization and microautoradiography to detect substrate utilization by Bacteria and Archaea in the deep ocean. Appl. Environ. Microbiol. 2004;70:4411–4414. doi: 10.1128/AEM.70.7.4411-4414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neef A. Anwendung der in situ Einzelzell-Identifizierung von Bakterien zur Populationsanalyse in komplexen mikrobiellen Biozönosen. Technische Universität München; 1997. PhD thesis. [Google Scholar]
- 29.Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: Problems and solutions. Syst. Appl. Microbiol. 1992;15:593–600. [Google Scholar]
- 30.Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer KH. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology. 1996;142:1097–1106. doi: 10.1099/13500872-142-5-1097. [DOI] [PubMed] [Google Scholar]
- 31.Roller C, Wagner M, Amann R, Ludwig W, Schleifer KH. Probing of Gram-positive bacteria with high DNA G + C content using 23S rRNA-targeted oligonucleotides. Microbiology. 1994;140:2849–2858. doi: 10.1099/00221287-140-10-2849. [DOI] [PubMed] [Google Scholar]
- 32.Daims H, Bruhl A, Amann R, Schleifer KH, Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: Development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 1999;22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 33.Pernthaler J, Pernthaler A, Amann R. Automated enumeration of groups of marine picoplankton after fluorescence in situ hybridization. Appl. Environ. Microbiol. 2003;69:2631–2637. doi: 10.1128/AEM.69.5.2631-2637.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kieber DJ, McDaniel J, Mopper K. Photochemical source of biological substrates in seawater: implications for carbon cycling. Nature. 1989;341:637–639. [Google Scholar]
- 35.Reche I, Pace ML, Cole J. Modeled effects of dissolved organic carbon and solar spectra on photobleaching in lake ecosystems. Ecosystems. 2000;3:419–432. [Google Scholar]
- 36.Kirchman DL, Ducklow HW, Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl. Environ. Microbiol. 1982;44:1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ammermann JW, Fuhrman JA, Hagström A, Azam F. Bacterioplankton growth in seawater: I. Growth, kinetics and cellular characteristics in seawater cultures. Mar. Ecol. Prog. Ser. 1984;18:31–39. [Google Scholar]
- 38.McCallister SL, Bauer JE, Kelly J, Ducklow HW. Effects of sunlight on decomposition of estuarine dissolved organic C, N and P and bacterial metabolism. Aquat. Microb. Ecol. 2005;40:25–35. [Google Scholar]
- 39.Pernthaler A, Pernthaler J, Eilers H, Amann R. Growth patterns of two marine isolates: adaptations to substrate patchiness? Appl. Environ. Microbiol. 2001;67:4077–4083. doi: 10.1128/AEM.67.9.4077-4083.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schut F, Prins RA, Gottschal JC. Oligotrophy and pelagic marine bacteria: facts and fiction. Aquat. Microb. Ecol. 1997;12:177–202. [Google Scholar]
- 41.Pinhassi J, Hagström A. Seasonal succession in marine bacterioplankton. Aquat. Microb. Ecol. 2000;21:245–256. [Google Scholar]
- 42.Simon M, Azam F. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 1989;51:201–213. [Google Scholar]
- 43.Flõrdh K, Cohen PS, Kjelleberg S. Ribosomes exist in large excess over the apparent demand for protein synthesis during carbon starvation in marine Vibrio sp. strain CCUG 15956. J. Bacteriol. 1992;174:6780–6788. doi: 10.1128/jb.174.21.6780-6788.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasol JM, Morán XAG. Effects of filtration on bacterial activity and picoplankton community structure as assessed by flow cytometry. Aquat. Microb. Ecol. 1999;16:251–264. [Google Scholar]
- 45.Sherr EB, Sherr BF, Sigmon CT. Activity of marine bacteria under incubated and in situ conditions. Aquat. Microb. Ecol. 1999;20:213–223. [Google Scholar]
- 46.Suzuki MT. Effect of protistan bacterivory on coastal bacterioplankton diversity. Aquat. Microb. Ecol. 1999;20:261–272. [Google Scholar]
- 47.Schõfer H, Servais P, Muyzer G. Successional changes in the genetic diversity of a marine assemblage during confinement. Arch. Mikrobiol. 2000;173:138–145. doi: 10.1007/s002039900121. [DOI] [PubMed] [Google Scholar]
- 48.Schäfer H, Bernard L, Courties C, Lebaron P, Servais P, Pukall R, Stackebrandt E, Troussellier M, Guindulain T, Vives-Rego J, Muyzer G. Microbial community dynamics in Mediterranean nutrientenriched seawater mesocosms: changes in the genetic diversity of bacterial populations. FEMS Microbiol. Ecol. 2001;34:243–253. doi: 10.1111/j.1574-6941.2001.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 49.Eilers H, Pernthaler J, Glöckner FO, Amann R. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 2000;66:3044–3051. doi: 10.1128/aem.66.7.3044-3051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massana R, Pedrós-Alió C, Casamayor EO, Gasol JM. Changes in marine bacterioplankton phylogenetic composition during incubations designed to measure biogeochemically significant parameters. Limnol. Oceanogr. 2001;46:1181–1188. [Google Scholar]
- 51.Giuliano L, De Domenico E, Höfle MG, Yakimov MM. Identification of culturable oligotrophic bacteria within naturally occurring bacterioplankton communities of the Ligurian Sea by 16S rRNA sequencing and probing. Microb. Ecol. 1999;37:77–85. doi: 10.1007/s002489900132. [DOI] [PubMed] [Google Scholar]
- 52.Burkert U, Warnecke F, Babenzien D, Zwirnmann E, Pernthaler J. Members of a readily enriched β-Proteobacterial clade are common in the surface waters of a humic lake. Appl. Environ. Microbiol. 2003;69:6550–6559. doi: 10.1128/AEM.69.11.6550-6559.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


