Abstract
Aims
Phosphatase and tensin homolog (PTEN) is implicated as a negative regulator of vascular smooth muscle cell (SMC) proliferation and injury-induced vascular remodelling. We tested if selective depletion of PTEN only in SMC is sufficient to promote SMC phenotypic modulation, cytokine production, and enhanced neointima formation.
Methods and results
Smooth muscle marker expression and induction of pro-inflammatory cytokines were compared in cultured SMC expressing control or PTEN-specific shRNA. Compared with controls, PTEN-deficient SMC exhibited increased phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signalling and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activity, reduced expression of SM markers (SM-α-actin and calponin), and increased production of stromal cell-derived factor-1α (SDF-1α), monocyte chemotactic protein-1 (MCP-1), interleukin-6 (IL-6), and chemokine (C-X-C motif) ligand 1 (KC/CXCL1) under basal conditions. PI3K/Akt or mTOR inhibition reversed repression of SM marker expression, whereas PI3K/Akt or NF-κB inhibition blocked cytokine induction mediated by PTEN depletion. Carotid ligation in mice with genetic reduction of PTEN specifically in SMC (SMC-specific PTEN heterozygotes) resulted in enhanced neointima formation, increased SMC hyperplasia, reduced SM-α-actin and calponin expression, and increased NF-κB and cytokine expression compared with wild-types. Lesion formation in SMC-specific heterozygotes was similar to lesion formation in global PTEN heterozygotes, indicating that inactivation of PTEN exclusively in SMC is sufficient to induce considerable increases in neointima formation.
Conclusion
PTEN activation specifically in SMC is a common upstream regulator of multiple downstream events involved in pathological vascular remodelling, including proliferation, de-differentiation, and production of multiple cytokines.
Keywords: Vascular biology, NF-κB, Vascular injury, Vascular smooth muscle, Cytokines/chemokines
1. Introduction
Vascular smooth muscle cells (SMCs) play important roles in normal blood pressure homeostasis and in many pathologic conditions. In normal adult arteries, SMCs contract to maintain vascular tone, are quiescent and express high levels of SMC-specific proteins, such as smooth muscle myosin heavy chain, smooth muscle-α-actin, and calponin. However, in response to various insults, such as atherosclerosis and angioplasty, SMCs can undergo phenotypic modulation characterized by increased proliferation and migration, and decreased expression of smooth muscle markers.1 As a result of this phenotypic switch, SMCs contribute to neointima formation in these conditions.2,3
Proinflammatory cytokines and chemokines, induced in response to injury by several cell types including SMCs, have been shown to participate in pathological vascular remodelling.4 MCP-1 regulates inflammatory cell recruitment and vascular cell proliferation in several different experimental models of vascular injury.4–7 MCP-1 levels are increased in developing atherosclerotic plaques of experimental animal models and after carotid artery wire injury. Inhibition of MCP-1 ameliorates lesion formation, confirming a functional role for this chemokine. SDF-1α/CXCL12 causes mobilization and vascular recruitment of bone marrow-derived smooth muscle progenitor cells.8,9 Additional cytokines/chemokines, including CXCL1/KC and IL-6, have also been implicated in promoting atherosclerosis and neointima formation.10–13
PTEN, a lipid and protein phosphatase and an important tumour suppressor gene, acts as a key negative regulator of the PI3K pathway by dephosphorylating membrane PtdIns(3,4,5)P3.14 PTEN is essential for regulation of both basal and growth factor-stimulated PI3K-mediated signalling. Elevations in cellular PtdIns(3,4,5)P3 in response to dysregulated PTEN activity even in the absence of stimuli are sufficient to activate numerous downstream effectors, most notably Akt and mTOR, which participate in regulation of cell proliferation and survival.15 A growing body of information suggests that regulation of PTEN signalling plays a critical role in vascular injury. Huang and Kontos16 first demonstrated that PTEN overexpression blocks PDGF-induced SMC proliferation and migration, but promotes SMC apoptosis. Consistent with their findings, we showed that highly proliferative and relatively de-differentiated embryonic and neointimal SMCs exhibit low levels of PTEN compared with adult or medial SMC.17 Additionally, we demonstrated that PTEN is inactivated in replicating SMC of balloon-injured rat carotid arteries,18 consistent with a role in proliferation control. Exogenously administered PTEN has been shown to attenuate neointima size in two rodent injury models,19,20 while pharmacological upregulation of PTEN attenuated atherosclerotic lesion formation in high fat-fed rabbits.21 Recently, investigators demonstrated that miR-21 was increased in response to injury and that PTEN was a specific target of miR-21.22 Finally, data from ex vivo human specimens showed that reduced PTEN activity in saphenous vein grafts contributes to the poor long-term outcome seen with such grafts following coronary artery bypass surgery.23 Collectively, these data support the concept that an alteration in SMC PTEN signalling serves as a key initiating determinant driving pathological vascular remodelling.
We previously described the phenotype of smooth muscle-specific PTEN null mice generated by crossing SM22α-Cre transgenic mice with mice containing loxP sites flanking exons 4 and 5 of PTEN.24 Although early lethality of these mice precluded their use in vascular injury studies, we showed that homozygous knockout mice (SM22α-Cre/+; PTENflox/flox) exhibited medial and intimal SMC hyperplasia, vascular recruitment of progenitor/proinflammatory cells, and increased SMC expression of SDF-1α. PTEN-deficient SMC in vitro exhibited higher rates of proliferation under basal conditions that was mediated, in part, through increased SDF-1α production. Thus, our data suggested that loss of SMC PTEN signalling mediates key events in pathological vascular remodelling, including an alteration in SMC function and increased production of a pro-inflammatory chemokine. Our goal here was to further characterize the molecular consequences of PTEN depletion in SMCs. We hypothesized that, in addition to enhanced proliferation, PTEN deficiency would promote SMC de-differentiation and production of multiple inflammatory cytokines that contribute to enhanced neointima formation.
2. Methods
2.1. PTEN mutant mice and carotid artery ligation injury
Global PTEN null mice and PTENflox/flox mice were generously provided to us by Dr Tak Mak (Ontario Cancer Institute, University of Toronto, Toronto, Ontario).25 SM22α-Cre transgenic mice were generously provided to us by Dr J. Miano (U. Rochester, Rochester, NY).26 Global PTEN null mice were maintained as heterozygotes. PTENflox/flox mice were mated to SM22α-Cre transgenic mice to generate control mice (PTENflox/flox;+/+) and heterozygous mutant mice (PTENflox/+;Cre/+). To induce neointima formation, left carotid arteries were completely ligated just proximal to the carotid bifurcation.27 Right and left carotid arteries were harvested 14 days after ligation for morphometric analysis and for immunohistochemical analysis for BrdU incorporation and SM-α-actin, calponin, and NF-κB p65 expression. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the University of Colorado Institutional Animal Care and Use Committee, #36209(04)1E.
2.2. Cell culture and PTEN silencing
Rat aortic SMC clones stably expressing control or PTEN-specific shRNA were generated as previously described.24 Transient transductions using lentiviral particles expressing control or PTEN-specific shRNA were performed on primary rat aortic SMC (passage 3) according to protocols provided (OpenBiosystems, Huntsville, AL).
2.3. Additional methods
An expanded Methods section containing details regarding animals, vascular injuries, morphometric analyses, and immunohistochemistry, reagents, cell culture, qRT–PCR, western analyses and cytokine immunoassays, immunofluorescence and confocal microscopy, and plasmids and transient promoter assays is available in Supplementary material online.
2.4. Statistical analysis
Data are expressed as means ± SE and were determined using either two-tailed t-test analyses or one-way ANOVA followed by Fisher's exact test analyses. P-values <0.05 were considered statistically significant.
3. Results
3.1. PTEN depletion promotes enhanced PI3-kinase-Akt-mTOR signalling under basal conditions and induces SMC phenotypic modulation
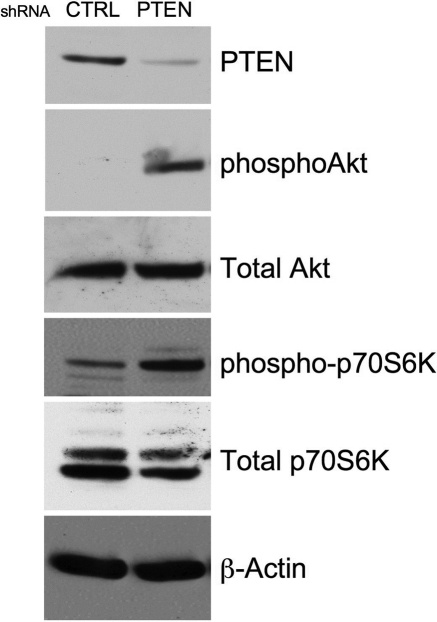
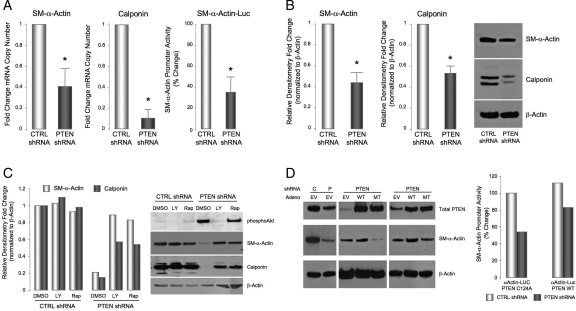
We previously reported that Akt activation promotes SMC proliferation17,24 and de-differentiation28,29 and that PTEN depletion, which is sufficient to activate Akt in the absence of other stimuli, stimulates SMC proliferation under basal conditions.24 To determine if PTEN depletion promotes SMC phenotypic modulation, aortic SMC stably expressing PTEN-specific or control shRNA were generated. PTEN-depleted SMC exhibited greater than an 80% decrease in PTEN expression and enhanced phosphorylation of Akt and p70S6-kinase, a direct downstream target of Akt/mTOR signalling, under basal, unstimulated conditions compared with controls (Figure 1). PTEN deficiency resulted in reduced SM-α-actin mRNA, protein, and promoter activity (Figure 2A and B). The reduction in SM-α-actin expression in PTEN-depleted SMC under basal conditions was similar to the decrease in expression following PDGF stimulation of wild-type SMC (data not shown).28 PTEN deficiency also decreased expression of calponin, a second SMC marker (Figure 2A and B). Inhibition of PI3K at least partially reversed the downregulation of both markers, confirming that the effects of PTEN depletion were mediated through activation of PI3-kinase/Akt (Figure 2C). Treatment with rapamycin, an mTOR inhibitor also reversed the effects mediated by PTEN depletion on SM marker expression (Figure 2C), indicating that SMC phenotypic modulation induced by PTEN depletion is dependent on increased Akt-mTOR signalling. To confirm that the decrease in SM markers was directly attributable to loss of PTEN, control or PTEN-deficient SMC were transduced with adenoviruses expressing wild-type or inactive PTEN. Re-expression of wild-type PTEN restored SM-α-actin expression, whereas mutant PTEN further reduced SM-α-actin expression likely through a dominant negative effect resulting in inhibition of any residual PTEN activity (Figure 2D). Re-expression of PTEN also reversed the repression of basal SM-α-actin promoter activity (Figure 2D). Finally, to confirm that effects on SMC differentiation markers are the result of PTEN depletion, and not an artefact of subculturing, early passage PTEN-depleted SMC pools were generated by transduction with lentiviral particles expressing control or PTEN-specific shRNA. Similar to above, low passage PTEN-deficient SMC expressed high levels of phosphorylated Akt under basal conditions and reduced levels of SM-α-actin mRNA and protein compared with cells transduced with a control virus; decreased SM-α-actin expression was reversed by LY294002 (see Supplementary material online, Figure S1).
Figure 1.
SMC PTEN depletion induces constitutive PI3K-Akt-mTOR signalling. Rat aortic SMCs stably expressing control (CTRL) or PTEN-specific shRNA were serum-restricted for 72 h. Whole cell lysates were analysed for total PTEN, phosphoAkt, total Akt, phospho-p70S6-kinase, and total p70S6-kinase. β-Actin was used as a loading control. Shown is a representative western of three independent experiments.
Figure 2.
PTEN depletion promotes SMC phenotypic modulation. (A) qRT–PCR for SM-α-actin and calponin mRNAs. β-Actin was used for normalization of cDNA. Shown are fold changes in mRNA copy number ± SE from CTRL SMC from three independent experiments; *P < 0.05. (A, right panel) CTRL and PTEN-deficient SMCs were transiently transfected with an SM-α-actin promoter-Luciferase reporter construct. Luciferase activity normalized to β-galactosidase was determined; shown are percent changes ± SE from control SMC. (B) Whole cell lysates were analysed for SM-α-actin and calponin protein levels. β-Actin was used as a loading control. Shown is a representative western and fold changes in densitometry measurements ± SE from CTRL SMC from three independent experiments; *P < 0.05. (C) CTRL and PTEN-deficient SMCs were serum-restricted in the presence or absence of the PI3-kinase inhibitor, LY294002 (10 µM), or the mTOR inhibitor, rapamycin (10 nM). Whole cell lysates were analysed for phosphoAkt, SM-α-actin, and calponin levels. β-Actin was used as a loading control. Shown is a representative western; fold changes in densitometry measurements from CTRL SMC are shown in the graph. (D, left) CTRL and PTEN-deficient SMCs were transiently transduced with empty vector adenovirus (EV) or adenoviruses encoding wild-type PTEN (WT) or phosphatase inactive PTEN (MT) (MOI = 100). Cells were maintained in 0.1% FCS and whole cell lysates analyszed for total PTEN and SM-α-actin levels. β-Actin was used as a loading control. (Right) CTRL and PTEN-deficient SMCs were transiently transfected with an expression plasmid encoding wild-type PTEN (PTEN WT) or phosphatase inactive PTEN (PTEN C124A), along with an SM-α-actin promoter-Luciferase reporter construct. Luciferase activity normalized to β-galactosidase was determined; shown are percent changes from control SMC.
3.2. SMC PTEN depletion induces NF-κB-dependent upregulation of a family of cytokines
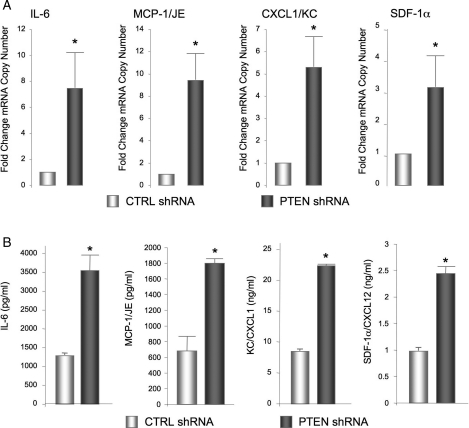
Increased production of pro-inflammatory cytokines/chemokines by SMC participates in pathological vascular remodelling. Our recent data demonstrate that PTEN-deficient SMC exhibit upregulation and secretion of the chemokine, CXCL12/SDF-1α.24 To assess the effects of PTEN depletion on the broader pattern of cytokine production, conditioned media from control and PTEN-deficient SMC were analysed using a commercial cytokine array (data not shown). Expression of candidate factors identified on the array was confirmed by quantitative RT–PCR. We found increased mRNA levels for IL-6, MCP-1/JE, CXCL1/KC, and CXCL12/SDF-1α (Figure 3A) in PTEN-depleted SMC compared with control SMC; levels of CX3CL1/fractalkine and CCL5/RANTES were not consistently changed (data not shown). Using ELISA assays with conditioned media, we verified the increase in mRNA resulted in increased protein release of all these cytokine/chemokines in the setting of PTEN depletion (Figure 3B). Similar results were obtained in low passage PTEN-deficient SMC pools (see Supplementary material online, Figure S2).
Figure 3.
SMC PTEN depletion induces the upregulation of a family of cytokines. (A) qRT–PCR analysis for the indicated mRNAs in serum-restricted CTRL and PTEN-deficient SMCs. β-Actin was used for normalization of cDNA. Shown are fold changes in mRNA copy number ± SE from CTRL SMC from three independent experiments; *P < 0.05. (B) Conditioned media from SMCs described in (A) were analysed by ELISA for SDF-1α, IL-6, MCP-1, or CXCL1/KC protein levels. Shown are the means ± SE from three independent experiments; *P < 0.05.
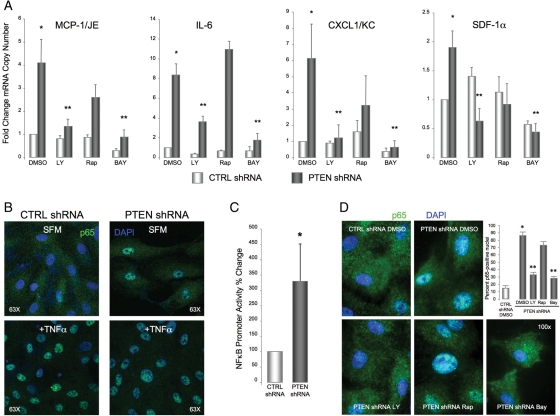
Activation of the transcription factor NF-κB has been implicated as critical for cytokine/chemokine production30–32 and previous studies in other systems have demonstrated increased NF-κB activity in the setting of PTEN loss and/or Akt activation.33,34 We used a pharmacological approach to determine if NF-κB activity promoted cytokine/chemokine production in PTEN-deficient SMC. Inhibition of PI3-kinase/Akt or inhibition of IKK, an upstream activator of NF-κB transcriptional activity, reversed the induction of all four cytokines mediated by PTEN deficiency (Figure 4A). In contrast to effects on SMC differentiation, inhibition of mTOR by rapamycin had varying effects on cytokine/chemokine production, either not affecting production (IL-6) or inducing a non-significant trend towards reduction (MCP-1/JE, CXCL1/KC, CXCL12/SDF-1α) thus supporting the concept that parallel, but independent pathways downstream of PTEN-Akt signalling regulate distinct SMC functions.
Figure 4.
Cytokine induction mediated by PTEN-depletion is dependent on NF-κB activity. (A) qRT–PCR analysis of serum-restricted CTRL and PTEN-deficient SMCs maintained in the presence or absence of the PI3K inhibitor, LY294002 (10 µM), the mTOR inhibitor, rapamycin (10 nM), or the IKK inhibitor, BAY11-7082 (5 µM). β-Actin was used for normalization of cDNA. Shown are fold changes in mRNA copy number ± SE from CTRL SMC from a minimum of four independent experiments; *P < 0.05 from CTRL DMSO; **P < 0.05 from PTEN DMSO. (B) SMCs were maintained under basal conditions (SFM) for 72 h or growth-arrested followed by stimulation with 100 ng/mL TNFα for 1 h then immunofluorescently stained for NF-κB p65 (green). Blue, DAPI; nuclei. (C) SMCs were transiently transfected with an NF-κB promoter-Luciferase reporter construct. Luciferase activity normalized to β-galactosidase was determined; shown are percent changes ± SE from CTRL SMC from three independent experiments; *P < 0.05. (D) SMCs were serum-restricted in the presence or absence of LY294002, rapamycin, or BAY11-7082 as in (A) and then immunofluorescently stained for NF-κB p65 (green). Blue, DAPI; nuclei. Shown in the graph are percent cells expressing nuclear p65 ± SE; *P < 0.05 from CTRL DMSO; **P < 0.05 from PTEN DMSO.
To confirm activation of NF-κB in the setting of PTEN depletion, subcellular localization of the NF-κB subunit, p65/RelA, was examined. Expression of p65/RelA was localized to the cytoplasm in control SMC under basal conditions (Figure 4B). In contrast, strong, nuclear localization of p65/RelA was observed in PTEN-deficient SMC maintained under basal conditions, consistent with NF-κB activation. The extent of nuclear localization was similar to that observed with TNFα stimulation, a known activator of NF-κB, which induced nuclear localization of p65/RelA in both control and PTEN-deficient SMC (Figure 4B). Using a reporter construct containing three consensus NF-κB elements upstream of a luciferase reporter, we also directly measured NF-κB activity. PTEN-deficient SMC exhibited increased basal NF-κB promoter activity (Figure 4C), which was blocked with re-expression of wild-type PTEN (see Supplementary material online, Figure S3). Finally, to confirm that Akt and NF-κB activities were coupled, we found that inhibition of PI3-kinase/Akt or IKK, but not mTOR, blocked nuclear localization of p65/RelA (Figure 4D).
3.3. PTEN reduction enhances neointima formation in vivo
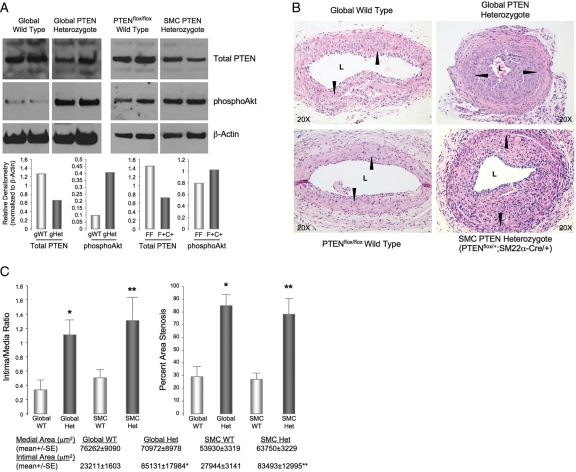
To study the effect of PTEN depletion on neointima formation, a carotid artery ligation model was used with two distinct genetic mouse models in which PTEN levels are either reduced in all tissues (global PTEN KO) or selectively in smooth muscle, using the Cre/Lox system in which Cre recombinase is driven by the SM22α promoter to determine the specific contribution of SMC-derived PTEN. Global homozygous deletion of PTEN is embryo lethal and smooth muscle-specific PTEN homozygous knockout mice (PTENfl/fl;SM22α-Cre+/−) die perinatally. Therefore injuries were performed on global PTEN heterozygotes (PTEN+/−) or SMC-specific PTEN heterozygotes (PTENfl/+;SM22α-Cre+/−) and compared with their respective littermate wild-type controls (WT or PTENfl/fl). Expression of PTEN was reduced and phosphorylation of Akt increased in lysates of whole aorta (Figure 5A) and whole lung (see Supplementary material online, Figure S4) from both PTEN+/− and PTENfl/+;SM22α-Cre+/− mice compared with controls. Four-month-old male mice were subjected to arterial injury and arteries examined 2 weeks post-injury. No arterial changes were observed in unligated arteries of all mice (data not shown). Injury to carotid arteries of control WT and PTENfl/fl mice resulted in formation of relatively small neointimal lesions by 2 weeks (Figure 5B), consistent with previous studies which showed that C57BL/6 mice are fairly resistant to neointima formation after carotid ligation.35,36 In contrast, ligation of carotids of PTEN+/− or PTENfl/+;SM22α-Cre+/− mutant mice resulted in the formation of large neointimal lesions (Figure 5B), indicating that reduced PTEN signalling leads to an exaggerated response to injury. Morphometric analysis confirmed a greater than a three-fold increase in intimal area in mutant mice compared with controls (Figure 5C). Medial area was not significantly different among the four genotypes; therefore resultant intima/media ratio and percent area stenosis were increased greater than a 2.5-fold in mutant mice compared with controls (Figure 5C). Interestingly, no differences were observed in intima/media ratio between global PTEN+/− and smooth muscle-specific PTENfl/+;SM22α-Cre+/− mutant mice thus demonstrating that restricting inactivation of PTEN specifically to SMC is sufficient to induce neointima formation.
Figure 5.
PTEN-deficient mutant mice exhibit enhanced neointima formation in response to vascular injury. (A) Western analysis of whole aorta from wild-type, global PTEN heterozygote (PTEN+/−), floxed wild-type (PTENfl/fl; +/+), and SMC-specific heterozygote (PTENfl/+; SM22α-Cre+/−) mice showing reduced total PTEN levels and increased phosphoAkt in tissues from PTEN heterozygous mice. Two mice per genotype were analysed; graphs show means of densitometry measurements. (B) Carotid artery ligation-induced vascular injuries were performed on mice described in (A) and arteries harvested 14-days post-injury. H&E staining on cross-sections from representative injured left carotid arteries. Arrowheads, internal elastic laminae. (C) Medial and intimal areas were measured using SPOT software. Intima-to-media ratios (left) and percent stenotic areas (right) are presented in the graphs. Percent area stenosis = 100 × [1.00 − (stenotic area/native vessel area)] = 100 × {1.00 − [(π × stenotic major axis × stenotic minor axis/4)/(π × native major axis × native minor axis/4)]}.45 *Different from global wild-type; **different from floxed wild-type; P < 0.05; n = 6.
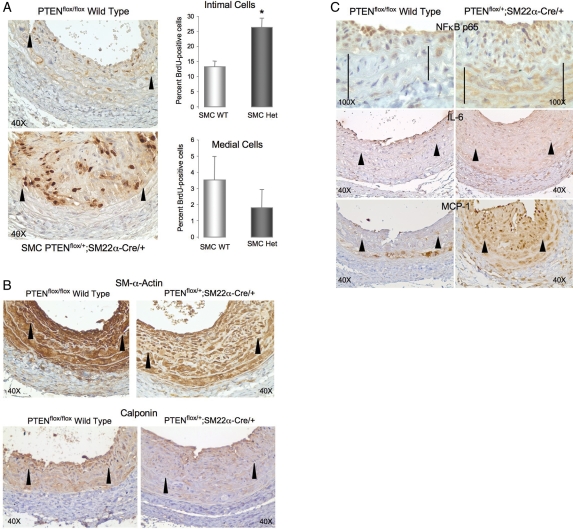
To determine if the exaggerated response to injury observed in smooth muscle-specific PTENfl/+;SM22α-Cre+/− mutant mice compared with control PTENfl/fl mice was due to increased proliferation, BrdU immunohistochemistry was performed. An increase in replicating neointimal cells was detected at 14 days post-injury in PTENfl/+;SM22α-Cre+/− mutant mice compared with controls (Figure 6A). TUNEL staining revealed very few TUNEL-positive cells and no differences in the degree of cell apoptosis between the two populations of mice (data not shown), suggesting that increased cell proliferation, rather than decreased cell death, contributes to the enhanced neointima formation observed in PTEN mutant mice. Consistent with our in vitro data, expression of SM-α-actin and calponin was reduced in injured vessels from PTENfl/+;SM22α-Cre+/− mutant mice compared with controls (Figure 6B). In addition, enhanced expression of NF-κB p65, IL-6, and MCP-1 was detected in injured vessels from PTENfl/+;SM22α-Cre+/− mutant mice compared with controls (Figure 6C).
Figure 6.
SMC-specific PTEN-deficient mutant mice exhibit increased intimal cell proliferation, reduced SM marker expression, and overexpression of NF-κB p65 and cytokines. (A) BrdU immunohistochemistry on injured left carotid arteries from wild-type (PTENfl/fl;+/+) and SMC-specific heterozygote (PTENfl/+;SM22α-Cre+/−) mice (brown nuclei). Percent replicating cells was determined independently for the arterial media and intima and data presented in the graphs as means ± SE; *different from wild-type; P < 0.05. (B and C) Immunohistochemistry for SM-α-actin (B, upper), calponin (B, lower), NF-κB p65 (C, upper), IL-6 (C, middle), or MCP-1 (C, lower) on injured left carotid arteries from wild-type (PTENfl/fl;+/+) and SMC-specific heterozygote (PTENfl/+;SM22α-Cre+/−) mice (brown reaction colour). Arrowheads, internal elastic lamina; lines in C, the arterial media; n = 4.
4. Discussion
Restenosis is one of the major limitations of percutaneous angioplasty procedures. SMC accumulation in the arterial intima is a key event in the pathogenesis of post-angioplasty/in-stent restenosis. Injury to the arterial wall induces dedifferentiation, migration, and proliferation of medial-derived SMC and initiates an inflammatory response, all of which contribute to restenosis.3 Increased production of multiple cytokines and chemokines by several cell types, including SMC, occurs following injury.4,37,38 These factors participate in the remodelling process through direct effects on SMC and through the recruitment of inflammatory cells, therefore placing SMC as both mediators and effectors of the injury response. However, despite important previous studies, the underlying molecular programs activated in SMC in response to injury are not clearly defined. Under physiological conditions, SMC are highly quiescent suggesting that active growth inhibitory pathways repress SMC activation. Previous work from our group demonstrated that decreased PTEN activity is associated with highly proliferative SMC phenotypes17,18 and that molecular depletion of PTEN induces autonomous SMC proliferation and secretion of the chemokine CXCL12/SDF1α, which establishes an autocrine growth loop.24 In this study, we used in vitro and in vivo molecular approaches to further define the consequences of PTEN depletion on SMC function and neointima formation. The results of this study show that PTEN inactivation promotes SMC de-differentiation by decreasing expression of smooth muscle-specific markers, increases production of a family of pro-inflammatory cytokines and chemokines in an NF-κB-dependent manner, and enhances neointima formation.
PTEN depletion in cultured SMC was sufficient to suppress expression of SM markers and induce cytokine expression, even in the absence of any additional stimuli. The ability of both PI3K and mTOR inhibitors to reverse suppression of SM markers mediated by PTEN depletion indicates the involvement of a PI3K/Akt/mTOR axis in control of SMC gene expression. Restoration of PTEN to PTEN-deficient SMC also reversed SM-α-actin expression although it is interesting that exogenously added PTEN did not fully restore SM-α-actin expression. At this time, we are unclear of the reason; however this could be due to exogenously added PTEN being targeted by the PTEN shRNA and therefore subject to continual degradation and/or alterations in sub-cellular compartmentalization. Alternatively, this could be due to off-target effects of the PTEN shRNA. Nevertheless, these data are consistent with our previous work implicating Akt activation in mediating the suppressive effects of PDGF on SMC gene expression.28 Our data are also consistent with previous studies showing that rapamycin induces contractile protein expression in cultured synthetic state SMC.39 In contrast, cytokine production induced by PTEN depletion appeared to be mediated in large part by an mTOR-independent pathway that involves PI3K-Akt-mediated activation of NF-κB, consistent with findings in other systems.40 Thus our data demonstrate that de-differentiation and cytokine production are regulated by distinct parallel, but independent downstream pathways activated in SMC in response to PTEN inactivation. These data support a model in which PTEN signalling specifically in SMC controls multiple downstream events involved in pathological vascular remodelling, including proliferation, de-differentiation, and production of multiple cytokines.
The present data verify our previous report, which showed induction of SDF-1α by PTEN depletion, and expand on these findings by showing that PTEN depletion also induces the secretion of multiple cytokines/chemokines, including MCP-1, IL-6, and KC/CXCL1. Increased production of each of these factors has been demonstrated in the early stages of restenosis and others have reported distinct pathophysiological effects induced by specific chemokines on injury-induced vascular remodelling.4,11–13,37 Libby et al.41 proposed a cascade model suggesting that restenosis is triggered by vascular injury-induced growth factor/cytokine production by SMC that establishes autocrine and paracrine signalling loops resulting in sustained neointima formation. Our studies support this model and provide a molecular switch underlying cytokine production by SMC. We have already shown SDF-1α promotes SMC growth in the absence of other stimuli. The specific autocrine and paracrine effects of PTEN-regulated SMC-derived MCP-1, IL-6, and CXCL1/KC remain to be determined. From a therapeutic perspective, our data indicate that targeting the PTEN/Akt pathway will impact production of multiple cytokines, and therefore would likely to be more effective than blockade of individual cytokines in preventing restenosis.
We reported previously that injury-induced inactivation of PTEN in SMC precedes neointima formation supporting the concept that loss of SMC PTEN is a key initiating determinant in driving lesion formation. An unanticipated, but key finding in this study is that genetic reductions of PTEN selectively in SMC are sufficient to induce the development of large neointimal lesions by 14 days post-injury which are not significantly different from those formed in global PTEN heterozygotes, where other vascular and circulating cells (i.e. endothelial cells, fibroblasts, inflammatory cells) are also deficient in PTEN. Thus inactivation of PTEN in those cells appears not to be a major contributor to neointima formation. It should be noted, however, that PTEN reduction in other cell types (e.g. endothelial cells) might be protective against injury-induced neointimal lesion formation. In the present study, consistent with our in vitro data, in vivo reductions of PTEN in SMC promoted a switch to a highly proliferative, relatively dedifferentiated SMC phenotype. In addition, NF-κB was constitutively active in PTEN-depleted SMC in the absence of stimuli and injured vessels from SMC-specific PTEN heterozygotes exhibited enhanced staining for NF-κB. While increased NF-κB activity has been associated with the progression of atherosclerosis,42,43 less is known of its role during the progression of restenosis. However, Yamasaki et al.44 demonstrated that local delivery of an NF-κB decoy reduced neointima formation in a porcine coronary artery balloon injury model. NF-κB serves a pivotal role as a key regulator of inflammatory gene expression. Consistent with this, inhibition of NF-κB activity in vitro blocked the upregulation of several pro-inflammatory cytokines/chemokines induced by PTEN depletion placing NF-κB as a central regulator of inflammatory events associated with PTEN inactivation.
In summary, we show that a reduction of PTEN specifically in SMC is sufficient to promote the development of large neointimal lesions in a genetic strain of mice that are normally resistant to neointima formation in response to carotid ligation injury. These changes are associated with a de-differentiated, pro-inflammatory phenotype, consistent with clinical findings especially in select subpopulations of individuals who are susceptible to restenosis. Therefore our studies suggest that activation of PTEN in resident SMC normally promotes a differentiated, growth-inhibitory, and anti-inflammatory phenotype indicating a critical role for SMC PTEN in the control of multiple events that contribute to pathological vascular remodelling. Moreover, our data highly support the proposal that pathological remodelling reflects an underlying defect in anti-proliferative processes and, as shown here, SMC PTEN signalling events. An important potential implication of our findings is that individuals with dysfunctional PTEN expression or activity might be genetically predisposed to enhanced responses to arterial interventions. Therefore new generation therapeutics that specifically target PTEN to inhibit SMC phenotypic modulation, proliferation, and inflammatory mediator production could have important clinical implications in the treatment of restenosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by grants from the American Heart Association (GIA #0850231Z) to M.C.M.W.-E. and from the National Institutes of Health (1RO1 HL88643 and 2PO1 HL014985-36) to M.C.M.W.-E. and (2PO1 HL014985-36 and DK19928) to R.A.N.
Supplementary Material
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis, the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J Clin Pathol. 2006;59:232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 5.Taubman MB, Rollins BJ, Poon M, Marmur J, Green RS, Berk BC, et al. JE mRNA accumulates rapidly in aortic injury and in platelet-derived growth factor-stimulated vascular smooth muscle cells. Circ Res. 1992;70:314–325. doi: 10.1161/01.res.70.2.314. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, et al. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–314. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
- 7.Egashira K, Nakano K, Ohtani K, Funakoshi K, Zhao G, Ihara Y, et al. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Arterioscler Thromb Vasc Biol. 2007;27:2563–2568. doi: 10.1161/ATVBAHA.107.154609. [DOI] [PubMed] [Google Scholar]
- 8.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, et al. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 9.Schober A, Knarren S, Lietz M, Lin EA, Weber C. Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein E-deficient mice. Circulation. 2003;108:2491–2497. doi: 10.1161/01.CIR.0000099508.76665.9A. [DOI] [PubMed] [Google Scholar]
- 10.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Liu Z, Li Q, Karpurapu M, Kundumani-Sridharan V, Cao H, et al. An essential role for gp130 in neointima formation following arterial injury. Circ Res. 2007;100:807–816. doi: 10.1161/01.RES.0000261350.61711.9e. [DOI] [PubMed] [Google Scholar]
- 12.Marmur JD, Poon M, Rossikhina M, Taubman MB. Induction of PDGF-responsive genes in vascular smooth muscle. Implications for the early response to vessel injury. Circulation. 1992;86:III53–60. [PubMed] [Google Scholar]
- 13.Zeiffer U, Schober A, Lietz M, Liehn EA, Erl W, Emans N, et al. Neointimal smooth muscle cells display a proinflammatory phenotype resulting in increased leukocyte recruitment mediated by P-selectin and chemokines. Circ Res. 2004;94:776–784. doi: 10.1161/01.RES.0000121105.72718.5C. [DOI] [PubMed] [Google Scholar]
- 14.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 15.Dahia PL. PTEN, a unique tumor suppressor gene. Endocr Relat Cancer. 2000;7:115–129. doi: 10.1677/erc.0.0070115. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Kontos CD. Inhibition of vascular smooth muscle cell proliferation, migration, and survival by the tumor suppressor protein PTEN. Arterioscler Thromb Vasc Biol. 2002;22:745–751. doi: 10.1161/01.atv.0000016358.05294.8d. [DOI] [PubMed] [Google Scholar]
- 17.Mourani PM, Garl PJ, Wenzlau JM, Carpenter TC, Stenmark KR, Weiser-Evans MC. Unique, highly proliferative growth phenotype expressed by embryonic and neointimal smooth muscle cells is driven by constitutive Akt, mTOR, and p70S6K signaling and is actively repressed by PTEN. Circulation. 2004;109:1299–1306. doi: 10.1161/01.CIR.0000118462.22970.BE. [DOI] [PubMed] [Google Scholar]
- 18.Garl PJ, Wenzlau JM, Walker HA, Whitelock JM, Costell M, Weiser-Evans MC. Perlecan-induced suppression of smooth muscle cell proliferation is mediated through increased activity of the tumor suppressor PTEN. Circ Res. 2004;94:175–183. doi: 10.1161/01.RES.0000109791.69181.B6. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Niu XL, Pippen AM, Annex BH, Kontos CD. Adenovirus-mediated intraarterial delivery of PTEN inhibits neointimal hyperplasia. Arterioscler Thromb Vasc Biol. 2005;25:354–358. doi: 10.1161/01.ATV.0000151619.54108.a5. [DOI] [PubMed] [Google Scholar]
- 20.Koide S, Okazaki M, Tamura M, Ozumi K, Takatsu H, Kamezaki F, et al. PTEN reduces cuff-induced neointima formation and proinflammatory cytokines. Am J Physiol Heart Circ Physiol. 2007;292:H2824–H2831. doi: 10.1152/ajpheart.01221.2006. [DOI] [PubMed] [Google Scholar]
- 21.Chen WJ, Lin KH, Lai YJ, Yang SH, Pang JH. Protective effect of propylthiouracil independent of its hypothyroid effect on atherogenesis in cholesterol-fed rabbits: PTEN induction and inhibition of vascular smooth muscle cell proliferation and migration. Circulation. 2004;110:1313–1319. doi: 10.1161/01.CIR.0000140764.15398.F3. [DOI] [PubMed] [Google Scholar]
- 22.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 23.Mitra AK, Jia G, Gangahar DM, Agrawal DK. Temporal PTEN inactivation causes proliferation of saphenous vein smooth muscle cells of human CABG conduits. J Cell Mol Med. 2008;13:177–187. doi: 10.1111/j.1582-4934.2008.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, et al. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ Res. 2008;102:1036–1045. doi: 10.1161/CIRCRESAHA.107.169896. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 26.Miano JM, Ramanan N, Georger MA, de Mesy Bentley KL, Emerson RL, Balza RO, Jr, et al. Restricted inactivation of serum response factor to the cardiovascular system. Proc Natl Acad Sci USA. 2004;101:17132–17137. doi: 10.1073/pnas.0406041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan-Albuquerque N, Garat C, Desseva C, Jones PL, Nemenoff RA. Platelet-derived growth factor-BB-mediated activation of Akt suppresses smooth muscle-specific gene expression through inhibition of mitogen-activated protein kinase and redistribution of serum response factor. J Biol Chem. 2003;278:39830–39838. doi: 10.1074/jbc.M305991200. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan-Albuquerque N, Garat C, Van Putten V, Nemenoff RA. Regulation of SM22 alpha expression by arginine vasopressin and PDGF-BB in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;28:H1444–H1452. doi: 10.1152/ajpheart.00306.2003. [DOI] [PubMed] [Google Scholar]
- 30.Dwarakanath RS, Sahar S, Reddy MA, Castanotto D, Rossi JJ, Natarajan R. Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-kappa B (NF-kappa B) J Mol Cell Cardiol. 2004;3:585–595. doi: 10.1016/j.yjmcc.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 32.Monaco C, Paleolog E. Nuclear factor kappaB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc Res. 2004;61:671–682. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, et al. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005;24:1021–1031. doi: 10.1038/sj.onc.1208296. [DOI] [PubMed] [Google Scholar]
- 34.Koul D, Yao Y, Abbruzzese JL, Yung WK, Reddy SA. Tumor suppressor MMAC/PTEN inhibits cytokine-induced NFkappaB activation without interfering with the IkappaB degradation pathway. J Biol Chem. 2001;276:11402–11408. doi: 10.1074/jbc.M007806200. [DOI] [PubMed] [Google Scholar]
- 35.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol. 2000;156:1741–1748. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhel DG, Zhu B, Witte DP, Hui DY. Distinction in genetic determinants for injury-induced neointimal hyperplasia and diet-induced atherosclerosis in inbred mice. Arterioscler Thromb Vasc Biol. 2002;22:955–960. doi: 10.1161/01.atv.0000017994.77066.75. [DOI] [PubMed] [Google Scholar]
- 37.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 38.Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007;97:730–737. [PubMed] [Google Scholar]
- 39.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, et al. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 40.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby P, Schwartz D, Brogi E, Tanaka H, Clinton SK. A cascade model for restenosis. A special case of atherosclerosis progression. Circulation. 1992;86:III47–52. [PubMed] [Google Scholar]
- 42.Bourcier T, Sukhova G, Libby P. The nuclear factor kappa-B signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J Biol Chem. 1997;272:15817–15824. doi: 10.1074/jbc.272.25.15817. [DOI] [PubMed] [Google Scholar]
- 43.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki K, Asai T, Shimizu M, Aoki M, Hashiya N, Sakonjo H, et al. Inhibition of NFkappaB activation using cis-element 'decoy' of NFkappaB binding site reduces neointimal formation in porcine balloon-injured coronary artery model. Gene Ther. 2003;10:356–364. doi: 10.1038/sj.gt.3301875. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz RS, Murphy JG, Edwards WD, Camrud AR, Vliestra RE, Holmes DR. Restenosis after balloon angioplasty. A practical proliferative model in porcine coronary arteries. Circulation. 1990;82:2190–2200. doi: 10.1161/01.cir.82.6.2190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.