Abstract
Caveolae are specialized lipid rafts that form flask-shaped invaginations of the plasma membrane. They are involved in cell signalling and transport and have been shown critically regulate vascular reactivity and blood pressure. The organization and functions of caveolae are mediated by coat proteins (caveolins) and support or adapter proteins (cavins). The caveolins, caveolin-1, -2, and -3, form the structural backbone of caveolae. These proteins are also highly integrated into caveolae function and have their own activity independent of caveolae. The cavins, cavins 1–4, are involved in regulation of caveolae and modulate the function of caveolins by promoting the membrane remodelling and trafficking of caveolin-derived structures. The relationships between these different proteins are complex and intersect with many aspects of cell function. Caveolae have also been implicated in chronic inflammatory conditions and other pathologies including atherosclerosis, inflammatory bowel disease, muscular dystrophy, and generalized dyslipidaemia. The pathogenic role of the caveolins is an emerging area, however, the roles of cavins in disease is just beginning to be explored. This review will examine the relationship between caveolins and cavins and explore the role of caveolae in inflammatory signalling mechanisms.
Keywords: Caveolin, Caveolae, Cardiovascular, Cell biology, Nitric oxide
1. Introduction
Caveolae are flask-shaped invaginations of the plasma membrane which participate in and are the site of regulation for many cellular functions. They were first discovered by Palade1 and Yamada,2 who noted non-clathrin coated, plasmalemmal vesicles of 50–100 nm. Caveolae occur at different densities in different cell types, being most prominent in vascular endothelial cells (ECs), adipocytes, fibroblasts, and epithelial cells. These specialized lipid rafts can function as cell signalling platforms and regulate the kinetics of vesicle transport, making them both versatile and highly integrated into cellular physiology. Enhanced cellular signalling within caveolae is due to the target rich environment formed through clustering of receptors and signalling molecules, thereby permitting efficient signal transduction. For purposes of macromolecule transport, multiple caveolae can form trans-endothelial channels and vesiculo-vacuolar organelles and/or cavicles.3 In terms of EC function, caveolae are important regulators of vascular tone through modulation of endothelial nitric oxide synthase (eNOS) activity. Specifically, regulation of eNOS within caveolae by the coat protein, caveolin-1 (Cav-1), is an important physiological mechanism for control of vascular reactivity, and this pathway is intimately involved with the progression of pathologies likely through suppression of pro-inflammatory signalling pathways.
Caveolae are specialized forms of lipid rafts. Contained within the plasma membrane, lipid rafts are dynamic assemblies of sphingolipids and cholesterol, and individual lipid raft domains can have differing affinities for proteins resulting in different functions. These rafts are highly important to cellular signal transduction as they can concentrate or segregate receptors and signalling intermediates and form a micro-environment where local kinases and phosphatases can modify downstream signalling events.4 In the case of caveolae, organelle size and specificity have been shown for several protein pairs, and clearly demonstrated for the Cav-1/eNOS interaction.5,6 Importantly, Cav-1 contained within non-caveolar, lipid rafts fails to exert its inhibitory effect on eNOS, whereas Cav-1 within caveolae is able to inhibit NO release due to its closer proximity to eNOS.7 Caveolae-mediated endocytosis differs mechanistically from clathrin-dependent endocytosis and other clathrin-independent (rhoA-dependent and cdc2 regulated) endocytosis; these differences involve cargo, biochemical sensitivities and specific adaptor and signalling proteins.8 A unique mechanism by which exogenous cholesterol and glycosphingolipids selectively stimulate caveolar endocytosis has also been suggested.8
The caveolae coat proteins, caveolins, have specific functional roles which can vary in different cell types. These coat proteins, Cav-1, caveolin-2 (Cav-2), and caveolin-3 (Cav-3), are the major structural components of caveolae.9,10 Cav-1 is present in most cell types of the cardiovascular system as is Cav-2, whereas Cav-3 is expressed primarily in vascular smooth muscle, cardiac and skeletal muscle. Evidence shows that Cav-1 or Cav-3 expression is necessary for the formation of caveolae, whereas Cav-2 expression is not required and its role less obvious.11–13 Endothelial Cav-1 is responsible for caveolae formation throughout the endothelium lining the entire cardiovascular system. Cav-1 regulates endothelial NO production,14 microvascular permeability, cellular Ca2+ entry, vascular remodelling, and angiogenesis.15–18 Cav-1 localizes at the plasma membrane and in the Golgi.19,20 Although a clear picture of Cav-1 trafficking to the plasma membrane is yet to emerge, several proteins involved in this process have been identified. Sato et al.21 show that ARF-1 is necessary for Cav-1 targeting to embryonic Cav-1 bodies in Caenorhabditis elegans. It has also recently been shown that amyloid beta protein stimulates the trafficking of Cav-1 from the Golgi to the plasma membrane, and insulin can acutely regulate Cav-1 trafficking.22,23 Furthermore, the endocytotic trafficking of Cav-1 has been shown to be regulated by Na/K-ATPase activity.24 Smart et al.25 suggested that Cav-1 targeting to the membrane is likely stabilized by its C-terminal palmitoylation.
The role of Cav-2 in the formation of caveolae is less clear, it is not necessary for lung endothelium or adipose tissue caveolae formation, but it is suggested to have a support role in caveolae assembly via its hetero-oligomerization with Cav-1.26,27 Additionally, Cav-2 is almost always co-expressed with Cav-1. Cav-2 can regulate the number of plasma membrane attached caveolae28 and is a regulator of mitosis through serine 36 phosphorylation, a process regulated by Cav-1.11 Cav-3 is expressed highly in striated muscle, localized to the sarcolemma, and is critical to caveolae formation and eNOS regulation in muscle cells, in the same manner as Cav-1 elsewhere.29–31 The caveolin support proteins, cavin-1 (polymerase transcript release factor, PTRF), cavin-2 (serum deprivation protein response, SDPR), cavin-3 (srd-related gene product that binds to c-kinase, SRBC), and cavin-4 (muscle-restricted coiled-coil protein, MURC), are newly documented and play important roles in the regulation of caveolin expression and caveolae morphology. The remainder of this review will focus on new information regarding caveolae regulatory proteins, and the involvement of caveolae and these proteins in vascular function and inflammation.
2. Caveolae regulatory proteins
Cavins act as regulators of caveolin function and organization, each of them has been assigned different roles based on caveolae morphologies and cell type. Importantly, these proteins function primarily as scaffolding proteins, though they also regulate availability of the caveolins.32,33 It should be noted that all cavin proteins share certain characteristics; namely leucine zipper-like domains for protein–protein interactions, PEST domains for protein turnover, and phosphorylation motifs.32 Additionally, all of the cavins can bind phosphatidylserine and are phosphorylated upon insulin stimulation.32 This section will focus on the individual contributions of the cavins to caveolae structure and function.
2.1. Cavin-1 (PTRF)
The first of the caveolin regulatory proteins to be identified was cavin-1, also known as PTRF.34 Upon its original identification, PTRF was thought to be a transcript release factor.35,36 The requirement of cavin-1 for caveolae formation and organization has been previously demonstrated.33,37,38 Early studies showed that cavin-1 co-localizes with Cav-1 in adipose tissue and co-distributes with Cav-1 in lipid rafts.33,39 Importantly, interactions between cavin-1 and Cav-1 are apparently not direct, as solubilization of caveolae disrupts the interactions and the interaction of cavin-1 with Cav-1 may be mediated by cytoskeletal interactions dependent on microtubules and actin filaments.33 Regulation of Cav-1 bioavailability by cavin-1 was demonstrated in vitro, as cavin-1 over-expression causes increased levels of Cav-1, and cavin-1 knockdown reduces Cav-1 levels.33 These data are similar to the well-appreciated stabilizing effect of Cav-2 with Cav-1 and visa versa.40 Liu et al.38 showed that genetic deletion of cavin-1 results in global loss of caveolae through decreased availability of all caveolin proteins, dyslipidaemia, reduced adipose tissue, and glucose intolerance, similar phenotypes to Cav-1/Cav-3 knockout mice. Hill et al.41 showed that cavin-1 associates with caveolae at the plasma membrane, where it is required for the formation of caveolae via sequestration of caveolins into caveolae. Additionally, these authors demonstrated that the loss of cavin-1 enhances the lateral mobility of Cav-1 and its accelerated lysosomal degradation.41 A recent study confirmed these results using live-cell imaging to show that Cav-1 scaffolds are trafficked to the plasma membrane independently from cavin-1, suggesting that cavin-1 interacts with Cav-1 once it is already in the membrane to direct the formation of caveolae.42 Importantly, in humans, mutations in cavin-1 result in a secondary deficiency of caveolins and muscular dystrophy; effects likely mediated via cavin-1/Cav-3 interactions.43
2.2. Cavin-2 (SPDR)
Cavin-2 or SDPR is required along with cavin-1 for caveolae formation.44,45 Gustincich and Schneider46 identified a gene they called serum deprivation response (called sdr) in 1993, and in 1998 Mineo et al.47 isolated the sdr protein and showed that it is a protein kinase C (PKC)α binding protein and localizes to caveolae. Hansen et al.45 showed that cavin-2 directly binds cavin-1 and recruits it to the plasma membrane, and that cavin-2 is required for the stable expression levels of both Cav-1 and cavin-1 proteins. Cavin-1/cavin-2 binding results in the formation of complexes containing Cav-1 contributing to stable caveolae structures.45 Interestingly, the over-expression of cavin-2 in cultured cells results in the formation of elongated tubular caveolae implying that it provides an organizational role to generate membrane curvature.45 Cavin-2 also exhibits functionality in caveolae signalling as a known substrate of PKC, and it is involved in the compartmentalization of PKC to caveolae.48 Additionally, pleckstrin, a platelet PKC substrate has been shown to directly bind cavin-2.49
2.3. Cavin-3 (SRBC)
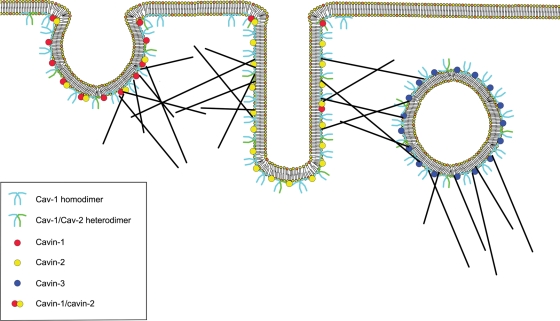
Cavin-3, or SRBC, was initially identified as a PKCδ binding protein and separately as a BRCA1-interacting protein.50,51 McMahon et al.52 were the first to show that cavin-3 is localized to caveolae and examine its role in caveolar function. Cavin-3 co-precipitates with Cav-1, has a similar distributions to Cav-1 and that either Cav-1 or Cav-3 must be present for cavin-3 localization to the plasma membrane.52 Furthermore, cavin-3 participates in the formation of caveolar vesicles (cavicles) based on two observations; cavin-3 remains associated with caveolae when budding occurs and the absence of cavin-3 impairs intracellular cavicle trafficking.52 This role for cavin-3, when compared with those of cavins-1 and -2, paints an interesting picture where cavin-1 in conjunction with normal levels of cavin-2 regulate the formation of traditional flask-shaped caveolae, excess of cavin-2 causes the formation of elongated tubular caveolae, and cavin-3 directs the budding and formation of cavicles (Figure 1).
Figure 1.
Dynamic roles of the cavins in determining caveolae structure. Cav-1 and Cav-2 (light blue and green lines) dimerize to form the backbone of caveolae. Cavins as supports proteins then determine the final shape of this structure. Cavin-1 (red circle) directs formation of normal flask-shaped caveolae alone and through interactions with cavin-2 (combined red and yellow circles). Cavin-2 when expressed at higher levels than cavin-1 causes the formation of elongated caveolae which may be involved in channel formation. Finally, cavin-3 (blue circles) directs vesicle formation by caveolae. Dark lines show cytoskeletal components which are thought to mediate the interaction between cavins and caveolins.
2.4. Cavin-4 (MURC)
The most recent addition to the Cavin family is cavin-4, MURC. This cavin was discovered as a cardiac and skeletal muscle-specific cytosolic protein and sequence homology searches identified it as a cavin family member.53–55 Bastiani et al.55 characterized cavin-4 as a predominantly muscle expressed protein component associated with sarcolemmal caveolae complexes. On the basis of its expression in muscle and co-localization with Cav-3, a role is suggested for cavin-4 in caveolin-associated muscle disease due to altered cavin-4 distribution in patients with caveolinopathies.55 Although the cavin proteins have not yet been extensively studied their, as of now, known roles in caveolae regulation indicate their importance to the physiological roles of caveolae.
3. Caveolae and vascular function
3.1. Caveolae and eNOS
The only direct protein–protein interaction demonstrated in vivo between Cav-1 and a non-homologous protein is with eNOS. Other proteins have been shown to bind Cav-1 in vitro including dynamin-2 during Cav-1-mediated endocytosis.56 Evidence suggests that Src phosphorylates Cav-1 at tyrosine 14 suggesting a direct interaction in response to growth factor stimulation and cellular stress.57–59 Furthermore, the insulin receptor has been shown to phosphorylate Cav-1 at the same site.60 Interactions of phosphoCav-1 with Csk resulting in negative regulation of Src, and Cav-1 with fatty acid synthase have also been demonstrated.61,62 Lastly, Feng et al.63 showed that Cav-1 associates with TRAF2 enhancing TNFR signalling.
The relationship between caveolae and eNOS is one of the cornerstones of caveolae involvement in cardiovascular function. Loss of Cav-1 leads to persistent eNOS activation and high levels of NO in cells through the loss of Cav-1 inhibitory effect on eNOS. The Cav-1/eNOS interaction tonically inhibits eNOS activity resulting in sequestering of eNOS in caveolae and reduced NO production. Our group showed that eNOS dysregulation in global Cav-1 KO mice is normalized with EC-specific reconstitution of Cav-1.16,17,64 Previous studies from our laboratory using AP-Cav-1 peptides, the Cav-1 scaffolding domain fused to the cellular internalization peptide, antennopedia (AP), showed that the amino acids 82–101 of Cav-1 contain the binding site for eNOS5,6,30,65 and that F92 is the key residue for this inhibitory effect.14 It has also been shown that the genetic deletion of eNOS or pharmacological blockade, prevents many of the physiological changes observed in Cav-1 KO mice,66 highlighting the importance of Cav-1 regulation of eNOS function. The mechanism for reducing the inhibitory action of Cav-1 on eNOS under normal circumstances is regulated by the activator calcium-–calmodulin, hsp90 complex.30,67,68
3.2. Caveolae, vascular reactivity, and blood pressure
The genetic deletion of Cav-1, and concurrent loss of caveolae, results in dysregulation of NO synthesis, enhanced or reduced vascular permeability, impaired vascular reactivity and mechanotranduction, cell proliferation, and severe cardiovascular and pulmonary phenotypes.12,13,17,69–73 These phenotypes include cardiac hypertrophy, pulmonary hypertension, and pulmonary hyperplasia. Murata et al.16 examined vascular reactivity in Cav-1 KO and EC-specific Cav-1 reconstituted mice and found that reconstitution of Cav-1 restored vascular reactivity resulting in normal smooth muscle contractility and endothelium-dependent relaxation in response to phenylephrine and acetylcholine, respectively. Additionally, Cav-1 reconstitution partially rescues the cardiovascular and pulmonary phenotypes seen in Cav-1 KO mice, and corrected vascular leakage.16 The conditions seen in Cav-1 KO mice have been linked to hyperactivation of Akt and p42/p44 ERK signalling, and reduced signalling by these messengers in Cav-1 EC-specific reconstituted mice has been shown.16 Zhao et al.66 have recently shown that pulmonary hypertension, and seen in Cav-1 KO mice, is dependent on PKG nitration. Specifically, they showed that genetic deletion of eNOS in Cav-1 KO mice prevents pulmonary hypertension and that the activation of eNOS in Cav-1 KO mice causes nitrosative stress leading to the nitration of and the inactivation of PKG.66 EC-specific transgenic expression of Cav-1 reduces microvascular permeability when compared with that of Cav-1 KO, adding another aspect to caveolae regulation of vascular function.15
Caveolae are implicated in mechanosignalling74 and to sense changes in shear flow, indeed, genetic evidence shows that Cav-1 is required for short- and long-term mechanotransduction in the vasculature.17 Reduced systemic blood pressure (BP), as measured by conventional methods, with anaesthesia, in Cav-1 KO mice has been documented and we have shown that the restoration of Cav-1 to the endothelium returned systemic BP to normal, suggesting a role for caveolae in the regulation of BP.16 Recently, Desjardins et al.75 examined blood pressure in Cav-1 KO by implanted telemetry in awake mice and determined that Cav-1 regulation of eNOS NO production is important for control of central BP. They saw no differences in BP between Cav-1 KO and WT mice, and they showed increased levels of circulating Hb-NO and vessel relaxation in response to increased NO production by eNOS in Cav-1 KO mice, both of which were reversible with NOS inhibition.75 They suggest that Hb-NO acts as a ‘buffer’ for systemic BP, further indicating Cav-1 as an important target in vascular regulation.75
4. Caveolae and inflammation
4.1. Caveolae as a signalling platform for inflammation
The microdomain created by caveolae is ideal for promoting cell signalling both through localization of many different types of receptors and bioavailability of signalling molecules and through the direct actions of the caveolin proteins.76 Sequestered within caveolae, through interaction with Cav-1, are many G protein receptors, Gα subunits, tyrosine kinases and receptor tyrosine kinases (RTKs), GTPases, components of the MAPK pathway, and others.77,78 As far as specific actions of the caveolin proteins are concerned, Cav-1 modulation of eNOS regulates inflammatory signalling through local control of NO production.79,80 Cav-1 has also been shown to sequester p42/44 MAPK cascade members including EGFR, raf, MAP-1, and ERK-2, inhibiting the activity of this pathway.81 Another important inflammatory signalling mediator cyclooxygenase-2 (Cox-2) has recently been shown to be regulated by Cav-1 through Cav-1 binding of Cox-2 at the endoplasmic reticulum (ER) enhancing its degradation.82 Caveolae and Cav-1 are also involved in integrin signalling, a key component of mechanotransduction and the inflammatory response. Co-precipitation of Cav-1 and integrins in cell culture has been demonstrated.83,84 Additionally, Salani et al.85 have recently shown that Cav-1 is necessary for integrin-β1 localization to caveolae upon IGF signalling.
Ca2+ regulation and signalling can also be involved in inflammatory responses. ECs sequester and store Ca2+ in the ER and it is used in receptor-induced signalling involving rapid release of these Ca2+ stores and then sustained entry of extracellular Ca2+. There are three phases to this process, initial ER Ca2+ release, sustained entry of Ca2+ into the cell, and tonic Ca2+ entry. The first can be stimulated by G protein-coupled receptors and RTKs. The major signalling pathway involved in calcium release is phospholipase C activation of inositoltriphosphate (IP3) and diacylgylcerol, where IP3 then activates receptors on the ER causing Ca2+ release. The sustained entry of Ca2+ is mediated by Ca2+ release activated Ca2+ channels and receptor-operated Ca2+ channels to promote signalling, and the tonic phase allows long-term Ca2+ signal propagation and replenishes intracellular Ca2+ stores. Importantly, Murata et al.16 have shown that Cav-1 is necessary for Ca2+ entry into ECs. In intact endothelium lining blood vessels, the loss of Cav-1 impairs Ca2+ entry and reconstitution of endothelial Cav-1 selectively rescues this phenotype. This evidence further indicates the importance of caveolae in cell signal transduction involved in maintaining vascular homeostasis. An impairment of Ca2+ signalling may be critical to pathological angiogenic processes which occur during chronic inflammation.86 Interestingly, recent data show that Cav-1 and Ca2+ antagonistically regulate eNOS in intact microvessels, agreeing with the above data, and they further show that the level of NO production directly determines the degree of vascular permeability increases which occur during inflammation.87
There are additional lines of evidence that indicate caveolae participate in inflammatory signalling. Garrean et al.88 show that Cav-1 can regulate NF-κB activation via its effects on eNOS resulting in reduced lipopolysaccharide (LPS)-driven lung inflammation in Cav-1−/− mice. Whereas its interaction with eNOS is the only direct protein–protein interaction that can be demonstrated in vivo at this time, implications from other studies suggest several other direct and indirect Cav-1 interactions that may have bearing on the evolution of the inflammatory response. Wang et al.89 have shown that the regulation of Cav-1/TLR4 interaction due to haemeoxygenase-1/carbon monoxide activity reduces the production of tumour necrosis factor-α and interleukin-6 in response to LPS-induced inflammation in macrophages suggesting an anti-inflammatory role here. Additionally, caveolae have also been shown to play a role in EC barrier dysfunction in the brain resulting in increased permeability through caveolae-mediated claudin-5 and occludin internalization.90
4.2. Caveolae and disease
The loss of caveolae through Cav-1 deletion is protective against atherosclerosis and inflammatory bowel disease (IBD). Frank et al.91 initially have shown that Cav-1 protects against atherosclerosis and LDL transcytosis in ECs implicating it as a regulator of plasma LDL level and composition suggesting a role in dislipidaemia. Our group has shown that genetic loss of caveolae results in reduced LDL uptake by the endothelium in vivo preventing development of atherosclerosis in Cav-1 KO mice, an effect that is reversed with endothelial-specific restoration of Cav-1.64 Additionally, Catalan et al.92 have indicated that Cav-1 is involved in inflammation associated with obesity, and Cav-1 regulates aspects of PMN activation, adhesion, and transmigration during lung inflammation.93
During IBD the loss of Cav-1, and thus caveolae, results in reduced tissue pathology likely due to decreased inflammatory and angiogenic signalling; this protection is also lost with EC-specific restoration of Cav-1.86 The reduction in angiogenesis signalling is also manifested in limb ischaemia studies73 perhaps via mislocalization of the vascular endothelial growth factor (VEGF)-R2 receptor. During chronic inflammation, as occurs during atherosclerosis, IBD, rheumatoid arthritis, age-related macular degeneration, and other diseases, angiogenic vascular responses are not properly regulated and contribute to the inflammatory conditions.94,95 In fact, our studies of Cav-l in experimental colitis models indicate that the protection seen in Cav-1 KO mice is conferred through a reduction in angiogenesis resulting secondarily in reduced inflammatory pathology.86 Important to this concept, Cav-1 is known to enhance tube formation in vitro by regulating EC differentiation, and to be important for the maturation of newly formed blood vessels.96,97 VEGF-R2, the major ligand for VEGF164, the pathological isoform of VEGF-A, has been shown to be sequestered in caveolae and is known to be involved in the pathological progression of IBD, rheumatoid arthritis, and others.94,98 Yu et al.17 have shown that Cav-1 over-expression reduces VEGF164-stimulated angiogenic responses in vivo demonstrating direct Cav-1 regulation of angiogenesis. Cav-1 at physiological levels is a positive regulator of angiogenic responses by coordinating the fidelity of signalling, however the loss or over-expression of Cav-1 in cases mentioned in this review results in impaired angiogenic responses, which may be due to the lack of the organelle, impaired signalling or excess levels of the scaffolding function of Cav-1.
Cav-1 has also been in the pathological sequelae diabetes and fibrosis. Cav-1 has been shown to bind insulin receptor-β as a necessary component of receptor activation, and Cav-1 loss results in insulin resistance,99 and recently Fagerholm et al.100 have shown that tyrosine phosphorylation of Cav-1 causes endocytosis of activated insulin receptors. Although neither of these findings are directly related to caveolae-dependent-inflammatory signalling, this close relationship between Cav-1 and insulin regulation suggests that Cav-1 may be involved in diabetes-associated inflammation. In the context of fibrosis, the loss of Cav-1 promotes pulmonary and cardiac fibrosis, perhaps via regulation of angiotensin/TGF-β signalling pathways.101,102
Cav-3 defects have been closely tied to muscular dystrophy. Early studies showed that mutation of the Cav-3 gene results in autosomal dominant limb girdle muscular dystrophy characterized by calf hypertrophy and muscle weakness.103 Cav-3 has also been implicated in idiopathic hyperCKemia due to a sporadic Cav-3 gene mutation, in rippling muscle disease due to missense mutations of Cav-3, and in distal myopathy due to a heterozygous 80 G to A mutation of the Cav-3 gene.104–106 While not inflammatory conditions, these findings implicate the importance of caveolae in muscle homeostasis and show that Cav-3-dependent muscle caveolae are important to disease. Combined the data reviewed here shows significant evidence for caveolae and caveolin involvement in disease pathologies through inflammatory signalling, regulation of angiogenic responses and genetic control. While the role that cavins play in disease is as presently being explored is it likely that further study of their functions will shed mechanistic light on caveolae regulation of inflammation and disease.
5. Concluding remarks
Caveolae are clearly important to vascular function and homoestasis as are the caveolins and the cavin proteins. Caveolae support transcytosis in certain cell types and a plethora of signalling events, through sequestration of messengers and localization of receptors and their mere presence seems to be important during disease for inflammatory and angiogenic signalling making then critical regulators of vascular function from the single cell to the system wide level. As indicated herein the caveolins are directly responsible for many of the regulatory mechanisms attributed to caveolae. With the discovery of cavins, many caveolae functions can now begin to be mechanistically understood. Interestingly, cavins directly determine the shape of caveolae and the budding of cavicles making them key proteins for the regulation of all caveolae functions. The roles that cavins play in caveolae formation and signalling bear further investigation, research that will undoubtedly reveal new mechanisms of caveolae and caveolin function. Caveolae are clearly important during inflammation. Most studies of Cav-1 in inflammation indicate that the direct effects of Cav-1 regulation of other proteins is anti-inflammatory, however, genetic deletion of Cav-1 is protective against atherosclerosis and IBD. Although these findings seem to conflict, they underscore the overall importance of caveolae as a regulator of cell signalling. It could be that without caveolae inflammatory and angiogenic signalling are perturbed to the point where signalling becomes impossible, or conflicting signals override one another, resulting in protection more through inability to respond than differential regulation of signalling. Evidence for an extra-caveolar role (i.e. scaffolding functions) of caveolins is plentiful, and it is possible that Cav-1 itself can effect protein and vesicle trafficking, angiogenic and inflammatory signalling, and other cellular processes, however, more sophisticated approaches to delineate caveolae vs. caveolin/cavin functions are needed. Similarly, it is feasible that cavins may also function outside caveolae/cavicles, perhaps independent of caveolins. Regardless, the evidence for caveolae and Cav-1 regulation during inflammation is clear and additional studies will shed light on mechanisms of caveolae-dependent inflammatory responses and the new roles of cavins in these processes.
Funding
This work was supported by grants R01 HL64793, R01 HL61371, R01 HL57665, and P01 Hl70295 from the National Institutes of Health to W.C.S.
Acknowledgements
The authors wish to apologize for any omissions of citing primary research papers in lieu of reviews for the sake of space.
Conflict of interest: none declared.
References
- 1.Palade GE. Fine structure of blood capillaries. J Appl Phys. 1953;24:1424. [Google Scholar]
- 2.Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746:334–348. doi: 10.1016/j.bbamcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. doi:10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 5.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. doi:10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J Biolo Chem. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. doi:10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 7.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci USA. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. doi:10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng ZJ, Singh RD, Marks DL, Pagano RE. Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids. Mol Membr Biol. 2006;23:101–110. doi: 10.1080/09687860500460041. doi:10.1080/09687860500460041. [DOI] [PubMed] [Google Scholar]
- 9.Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett. 2004;9:195–220. [PubMed] [Google Scholar]
- 10.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–595. doi: 10.1080/07853890410018899. doi:10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 11.Sowa G, Xie L, Xu L, Sessa WC. Serine 23 and 36 phosphorylation of caveolin-2 is differentially regulated by targeting to lipid raft/caveolae and in mitotic endothelial cells. Biochemistry. 2008;47:101–111. doi: 10.1021/bi701709s. doi:10.1021/bi701709s. [DOI] [PubMed] [Google Scholar]
- 12.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. doi:10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 13.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 14.Bernatchez PN, Bauer PM, Yu J, Prendergast JS, He P, Sessa WC. Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proc Natl Acad Sci USA. 2005;102:761–766. doi: 10.1073/pnas.0407224102. doi:10.1073/pnas.0407224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer PM, Yu J, Chen Y, Hickey R, Bernatchez PN, Looft-Wilson R, et al. Endothelial-specific expression of caveolin-1 impairs microvascular permeability and angiogenesis. Proc Natl Acad Sci USA. 2005;102:204–209. doi: 10.1073/pnas.0406092102. doi:10.1073/pnas.0406092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem. 2007;282:16631–16643. doi: 10.1074/jbc.M607948200. doi:10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, et al. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest. 2006;116:1284–1291. doi: 10.1172/JCI27100. doi:10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007;67:2849–2856. doi: 10.1158/0008-5472.CAN-06-4082. doi:10.1158/0008-5472.CAN-06-4082. [DOI] [PubMed] [Google Scholar]
- 19.Glenney JR, Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA. 1992;89:10517–10521. doi: 10.1073/pnas.89.21.10517. doi:10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000;275:21605–21617. doi: 10.1074/jbc.M002558200. doi:10.1074/jbc.M002558200. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Sato M, Audhya A, Oegema K, Schweinsberg P, Grant BD. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol Biol Cell. 2006;17:3085–3094. doi: 10.1091/mbc.E06-03-0211. doi:10.1091/mbc.E06-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igbavboa U, Sun GY, Weisman GA, He Y, Wood WG. Amyloid beta-protein stimulates trafficking of cholesterol and caveolin-1 from the plasma membrane to the Golgi complex in mouse primary astrocytes. Neuroscience. 2009;162:328–338. doi: 10.1016/j.neuroscience.2009.04.049. doi:10.1016/j.neuroscience.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Wang AX, Liu Z, Chai W, Barrett EJ. The trafficking/interaction of eNOS and caveolin-1 induced by insulin modulates endothelial nitric oxide production. Mol Endocrinol. 2009;23:1613–1623. doi: 10.1210/me.2009-0115. doi:10.1210/me.2009-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, et al. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol. 2008;182:1153–1169. doi: 10.1083/jcb.200712022. doi:10.1083/jcb.200712022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razani B, Wang XB, Engelman JA, Battista M, Lagaud G, Zhang XL, et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. doi:10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheiffele P, Verkade P, Fra AM, Virta H, Simons K, Ikonen E. Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J Cell Biol. 1998;140:795–806. doi: 10.1083/jcb.140.4.795. doi:10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sowa G, Pypaert M, Fulton D, Sessa WC. The phosphorylation of caveolin-2 on serines 23 and 36 modulates caveolin-1-dependent caveolae formation. Proc Natl Acad Sci USA. 2003;100:6511–6516. doi: 10.1073/pnas.1031672100. doi:10.1073/pnas.1031672100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. doi:10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, et al. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. doi:10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 31.Venema VJ, Ju H, Zou R, Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem. 1997;272:28187–28190. doi: 10.1074/jbc.272.45.28187. doi:10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 32.Rahman A, Sward K. The role of caveolin-1 in cardiovascular regulation. Acta Physiol (Oxf) 2009;195:231–245. doi: 10.1111/j.1748-1716.2008.01907.x. doi:10.1111/j.1748-1716.2008.01907.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;283:4314–4322. doi: 10.1074/jbc.M707890200. doi:10.1074/jbc.M707890200. [DOI] [PubMed] [Google Scholar]
- 34.Vinten J, Johnsen AH, Roepstorff P, Harpoth J, Tranum-Jensen J. Identification of a major protein on the cytosolic face of caveolae. Biochim Biophys Acta. 2005;1717:34–40. doi: 10.1016/j.bbamem.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Jansa P, Mason SW, Hoffmann-Rohrer U, Grummt I. Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. EMBO J. 1998;17:2855–2864. doi: 10.1093/emboj/17.10.2855. doi:10.1093/emboj/17.10.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansa P, Grummt I. Mechanism of transcription termination: PTRF interacts with the largest subunit of RNA polymerase I and dissociates paused transcription complexes from yeast and mouse. Mol Gen Genet. 1999;262:508–514. doi: 10.1007/s004380051112. doi:10.1007/s004380051112. [DOI] [PubMed] [Google Scholar]
- 37.Aboulaich N, Vainonen JP, Stralfors P, Vener AV. Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem J. 2004;383:237–248. doi: 10.1042/BJ20040647. doi:10.1042/BJ20040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, et al. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8:310–317. doi: 10.1016/j.cmet.2008.07.008. doi:10.1016/j.cmet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. doi:10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. doi:10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 41.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. doi:10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayer A, Stoeber M, Bissig C, Helenius A. Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic. 2009 doi: 10.1111/j.1600-0854.2009.01023.x. doi:10.1111/j.1600-0854.2009.01023.x. Published online ahead of print 3 December 2009. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119:2623–2633. doi: 10.1172/JCI38660. doi:10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nabi IR. Cavin fever: regulating caveolae. Nat Cell Biol. 2009;11:789–791. doi: 10.1038/ncb0709-789. doi:10.1038/ncb0709-789. [DOI] [PubMed] [Google Scholar]
- 45.Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11:807–814. doi: 10.1038/ncb1887. doi:10.1038/ncb1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustincich S, Schneider C. Serum deprivation response gene is induced by serum starvation but not by contact inhibition. Cell Growth Differ. 1993;4:753–760. [PubMed] [Google Scholar]
- 47.Mineo C, Ying YS, Chapline C, Jaken S, Anderson RG. Targeting of protein kinase Calpha to caveolae. J Cell Biol. 1998;141:601–610. doi: 10.1083/jcb.141.3.601. doi:10.1083/jcb.141.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustincich S, Vatta P, Goruppi S, Wolf M, Saccone S, Della Valle G, et al. The human serum deprivation response gene (SDPR) maps to 2q32-q33 and codes for a phosphatidylserine-binding protein. Genomics. 1999;57:120–129. doi: 10.1006/geno.1998.5733. doi:10.1006/geno.1998.5733. [DOI] [PubMed] [Google Scholar]
- 49.Baig A, Bao X, Wolf M, Haslam RJ. The platelet protein kinase C substrate pleckstrin binds directly to SDPR protein. Platelets. 2009;20:446–457. doi: 10.3109/09537100903137314. doi:10.1080/09537100903137314. [DOI] [PubMed] [Google Scholar]
- 50.Izumi Y, Hirai S, Tamai Y, Fujise-Matsuoka A, Nishimura Y, Ohno S. A protein kinase Cdelta-binding protein SRBC whose expression is induced by serum starvation. J Biol Chem. 1997;272:7381–7389. doi: 10.1074/jbc.272.11.7381. doi:10.1074/jbc.272.11.7381. [DOI] [PubMed] [Google Scholar]
- 51.Xu XL, Wu LC, Du F, Davis A, Peyton M, Tomizawa Y, et al. Inactivation of human SRBC, located within the 11p15.5-p15.4 tumor suppressor region, in breast and lung cancers. Cancer Res. 2001;61:7943–7949. [PubMed] [Google Scholar]
- 52.McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28:1001–1015. doi: 10.1038/emboj.2009.46. doi:10.1038/emboj.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogata T, Ueyama T, Isodono K, Tagawa M, Takehara N, Kawashima T, et al. MURC, a muscle-restricted coiled-coil protein that modulates the Rho/ROCK pathway, induces cardiac dysfunction and conduction disturbance. Mol Cell Biol. 2008;28:3424–3436. doi: 10.1128/MCB.02186-07. doi:10.1128/MCB.02186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tagawa M, Ueyama T, Ogata T, Takehara N, Nakajima N, Isodono K, et al. MURC, a muscle-restricted coiled-coil protein, is involved in the regulation of skeletal myogenesis. Am J Physiol. 2008;295:C490–C498. doi: 10.1152/ajpcell.00188.2008. doi:10.1152/ajpcell.00188.2008. [DOI] [PubMed] [Google Scholar]
- 55.Bastiani M, Liu L, Hill MM, Jedrychowski MP, Nixon SJ, Lo HP, et al. MURC/cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol. 2009;185:1259–1273. doi: 10.1083/jcb.200903053. doi:10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao Q, Chen J, Cao H, Orth JD, McCaffery JM, Stan RV, et al. Caveolin-1 interacts directly with dynamin-2. J Mol Biol. 2005;348:491–501. doi: 10.1016/j.jmb.2005.02.003. doi:10.1016/j.jmb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- 58.Lee H, Volonte D, Galbiati F, Iyengar P, Lublin DM, Bregman DB, et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. doi:10.1210/me.14.11.1750. [DOI] [PubMed] [Google Scholar]
- 59.Volonte D, Galbiati F, Pestell RG, Lisanti MP. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr(14)) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J Biol Chem. 2001;276:8094–8103. doi: 10.1074/jbc.M009245200. doi:10.1074/jbc.M009245200. [DOI] [PubMed] [Google Scholar]
- 60.Kimura A, Mora S, Shigematsu S, Pessin JE, Saltiel AR. The insulin receptor catalyzes the tyrosine phosphorylation of caveolin-1. J Biol Chem. 2002;277:30153–30158. doi: 10.1074/jbc.M203375200. doi:10.1074/jbc.M203375200. [DOI] [PubMed] [Google Scholar]
- 61.Lu TL, Kuo FT, Lu TJ, Hsu CY, Fu HW. Negative regulation of protease-activated receptor 1-induced Src kinase activity by the association of phosphocaveolin-1 with Csk. Cell Signal. 2006;18:1977–1987. doi: 10.1016/j.cellsig.2006.03.002. doi:10.1016/j.cellsig.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Di Vizio D, Adam RM, Kim J, Kim R, Sotgia F, Williams T, et al. Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle. 2008;7:2257–2267. doi: 10.4161/cc.7.14.6475. [DOI] [PubMed] [Google Scholar]
- 63.Feng X, Gaeta ML, Madge LA, Yang JH, Bradley JR, Pober JS. Caveolin-1 associates with TRAF2 to form a complex that is recruited to tumor necrosis factor receptors. J Biol Chem. 2001;276:8341–8349. doi: 10.1074/jbc.M007116200. doi:10.1074/jbc.M007116200. [DOI] [PubMed] [Google Scholar]
- 64.Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. doi:10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. doi:10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 66.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. doi:10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. doi:10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 68.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. doi:10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 69.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. doi:10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 70.Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, et al. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. doi:10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 71.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. doi:10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wunderlich C, Schober K, Lange SA, Drab M, Braun-Dullaeus RC, Kasper M, et al. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340:702–708. doi: 10.1016/j.bbrc.2005.12.058. doi:10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 73.Sonveaux P, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, et al. Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ Res. 2004;95:154–161. doi: 10.1161/01.RES.0000136344.27825.72. doi:10.1161/01.RES.0000136344.27825.72. [DOI] [PubMed] [Google Scholar]
- 74.Rizzo V, Sung A, Oh P, Schnitzer JE. Rapid mechanotransduction in situ at the luminal cell surface of vascular endothelium and its caveolae. J Biol Chem. 1998;273:26323–26329. doi: 10.1074/jbc.273.41.26323. doi:10.1074/jbc.273.41.26323. [DOI] [PubMed] [Google Scholar]
- 75.Desjardins F, Lobysheva I, Pelat M, Gallez B, Feron O, Dessy C, et al. Control of blood pressure variability in caveolin-1-deficient mice: role of nitric oxide identified in vivo through spectral analysis. Cardiovasc Res. 2008;79:527–536. doi: 10.1093/cvr/cvn080. doi:10.1093/cvr/cvn080. [DOI] [PubMed] [Google Scholar]
- 76.Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. doi: 10.1126/science.1310359. doi:10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 78.Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes. Science. 1996;274:239–242. doi: 10.1126/science.274.5285.239. doi:10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- 79.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–1367. doi: 10.1038/82176. doi:10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 80.Zhu L, Schwegler-Berry D, Castranova V, He P. Internalization of caveolin-1 scaffolding domain facilitated by Antennapedia homeodomain attenuates PAF-induced increase in microvessel permeability. Am J Physiol Heart Circ Physiol. 2004;286:H195–H201. doi: 10.1152/ajpheart.00667.2003. doi:10.1152/ajpheart.00667.2003. [DOI] [PubMed] [Google Scholar]
- 81.Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, et al. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. doi:10.1016/S0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 82.Chen SF, Liou JY, Huang TY, Lin YS, Yeh AL, Tam K, et al. Caveolin-1 facilitates cyclooxygenase-2 protein degradation. J Cell Biochem. 2010;109:356–362. doi: 10.1002/jcb.22407. [DOI] [PubMed] [Google Scholar]
- 83.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, et al. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. doi:10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 84.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. doi:10.1016/S0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 85.Salani B, Briatore L, Contini P, Passalacqua M, Melloni E, Paggi A, et al. IGF-I induced rapid recruitment of integrin beta1 to lipid rafts is Caveolin-1 dependent. Biochem Biophys Res Commun. 2009;380:489–492. doi: 10.1016/j.bbrc.2009.01.102. doi:10.1016/j.bbrc.2009.01.102. [DOI] [PubMed] [Google Scholar]
- 86.Chidlow JH, Jr, Greer JJ, Anthoni C, Bernatchez P, Fernandez-Hernando C, Bruce M, et al. Endothelial caveolin-1 regulates pathologic angiogenesis in a mouse model of colitis. Gastroenterology. 2009;136:575–584. doi: 10.1053/j.gastro.2008.10.085. e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou X, He P. Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq006. doi:10.1093/cvr/cvq006. Published online ahead of print 17 February 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, et al. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 89.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. doi:10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 90.Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem. 2009;284:19053–19066. doi: 10.1074/jbc.M109.000521. doi:10.1074/jbc.M109.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frank PG, Pavlides S, Cheung MW, Daumer K, Lisanti MP. Role of caveolin-1 in the regulation of lipoprotein metabolism. Am J Physiol. 2008;295:C242–C248. doi: 10.1152/ajpcell.00185.2008. doi:10.1152/ajpcell.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Catalan V, Gomez-Ambrosi J, Rodriguez A, Silva C, Rotellar F, Gil MJ, et al. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin Endocrinol (Oxf) 2008;68:213–219. doi: 10.1111/j.1365-2265.2007.03021.x. [DOI] [PubMed] [Google Scholar]
- 93.Hu G, Ye RD, Dinauer MC, Malik AB, Minshall RD. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L178–L186. doi: 10.1152/ajplung.00263.2007. doi:10.1152/ajplung.00263.2007. [DOI] [PubMed] [Google Scholar]
- 94.Chidlow JH, Jr, Langston W, Greer JJ, Ostanin D, Abdelbaqi M, Houghton J, et al. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014–2030. doi: 10.2353/ajpath.2006.051021. doi:10.2353/ajpath.2006.051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chidlow JH, Jr, Shukla D, Grisham MB, Kevil CG. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am J Physiol Gastrointest Liver Physiol. 2007;293:G5–G18. doi: 10.1152/ajpgi.00107.2007. doi:10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- 96.Liu J, Wang XB, Park DS, Lisanti MP. Caveolin-1 expression enhances endothelial capillary tubule formation. J Biol Chem. 2002;277:10661–10668. doi: 10.1074/jbc.M110354200. doi:10.1074/jbc.M110354200. [DOI] [PubMed] [Google Scholar]
- 97.Dewever J, Frerart F, Bouzin C, Baudelet C, Ansiaux R, Sonveaux P, et al. Caveolin-1 is critical for the maturation of tumor blood vessels through the regulation of both endothelial tube formation and mural cell recruitment. Am J Pathol. 2007;171:1619–1628. doi: 10.2353/ajpath.2007.060968. doi:10.2353/ajpath.2007.060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. doi:10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamamoto M, Toya Y, Schwencke C, Lisanti MP, Myers MG, Jr, Ishikawa Y. Caveolin is an activator of insulin receptor signaling. J Biol Chem. 1998;273:26962–26968. doi: 10.1074/jbc.273.41.26962. doi:10.1074/jbc.273.41.26962. [DOI] [PubMed] [Google Scholar]
- 100.Fagerholm S, Ortegren U, Karlsson M, Ruishalme I, Stralfors P. Rapid insulin-dependent endocytosis of the insulin receptor by caveolae in primary adipocytes. PLoS One. 2009;4:e5985. doi: 10.1371/journal.pone.0005985. doi:10.1371/journal.pone.0005985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–2906. doi: 10.1084/jem.20061536. doi:10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol. 2003;284:C457–C474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 103.Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, et al. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet. 1998;18:365–368. doi: 10.1038/ng0498-365. doi:10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- 104.Carbone I, Bruno C, Sotgia F, Bado M, Broda P, Masetti E, et al. Mutation in the CAV3 gene causes partial caveolin-3 deficiency and hyperCKemia. Neurology. 2000;54:1373–1376. doi: 10.1212/wnl.54.6.1373. [DOI] [PubMed] [Google Scholar]
- 105.Betz RC, Schoser BG, Kasper D, Ricker K, Ramirez A, Stein V, et al. Mutations in CAV3 cause mechanical hyperirritability of skeletal muscle in rippling muscle disease. Nat Genet. 2001;28:218–219. doi: 10.1038/90050. doi:10.1038/90050. [DOI] [PubMed] [Google Scholar]
- 106.Tateyama M, Aoki M, Nishino I, Hayashi YK, Sekiguchi S, Shiga Y, et al. Mutation in the caveolin-3 gene causes a peculiar form of distal myopathy. Neurology. 2002;58:323–325. doi: 10.1212/wnl.58.2.323. [DOI] [PubMed] [Google Scholar]