Abstract
Deletion of two homologous genes, MRS3 and MRS4, that encode mitochondrial iron transporters affects the activity of the vacuolar iron importer Ccc1. Ccc1 levels are decreased in Δmrs3Δmrs4 cells, but the activity of the transporter is increased, resulting is reduced cytosolic iron. Overexpression of CCC1 in Δmrs3Δmrs4 cells results in a severe growth defect due to decreased cytosolic iron, referred to as the mitochondria-vacuole signaling (MVS) phenotype. Mutants were identified that suppress the MVS growth defect, and FRA1 was identified as a gene that suppresses the MVS phenotype. Overexpression of FRA1 suppresses altered transition metal metabolism in Δmrs3Δmrs4 cells, whereas deletion of FRA1 is synthetically lethal with Δmrs3Δmrs4. Fra1 binds to Tsa1, which encodes a thioredoxin-dependent peroxidase. Deletion of TSA1 or TRR1 is synthetically lethal in Δmrs3Δmrs4 cells, suggesting that Δmrs3Δmrs4 cells generate reactive oxygen metabolites. The generation of reactive oxygen metabolites in Δmrs3Δmrs4 cells was confirmed by use of the reporter molecule 2′,7′-dichlorodihydrofluorescein diacetate. These results suggest that mitochondria-induced oxidant damage is responsible for activating Ccc1 and that Fra1 and Tsa1 can reduce oxidant damage.
Keywords: Mitochondria, Oxidative Stress, Subcellular Organelles, Transport Metals, Yeast, Vacuole
Introduction
Mitochondria are well recognized as the site for energy production and biosynthetic pathways. Mitochondria are central for a variety of downstream signaling pathways initiated by perturbations in mitochondrial activities. Most notably, in many eukaryotes decreased mitochondrial function results in apoptosis. The budding yeast Saccharomyces cerevisiae has been useful in dissecting the genetics and biochemistry of mitochondrial signaling events. Loss of mitochondrial heme synthesis leads to changes in the transcription of a wide variety of genes mediated by the Hap family of transcription factors (1). Decreased mitochondrial respiration, independent of effects on heme synthesis, affects the transcription of nuclear genes that result in a reconfiguration of energy metabolism, referred to as the retrograde response (2). Finally, alteration in mitochondrial iron-sulfur cluster synthesis can induce expression of genes involved in iron metabolism (3).
We showed that deletion of two nuclear genes MRS3 and MRS4, which encode homologous mitochondrial iron transporters, had effects on cellular metal metabolism (4). Deletion of MRS3 or MRS4 individually had no phenotype, but a double deletion showed impaired mitochondrial iron metabolism when cells were grown on low iron medium (4–6). Mitochondrial iron-consuming processes such as heme and iron-sulfur cluster synthesis appeared normal when Δmrs3Δmrs4 cells were grown in iron-sufficient medium, suggesting that low affinity iron transport systems can supply iron to the mitochondria. In iron-sufficient medium, however, Δmrs3Δmrs4 cells showed abnormalities in iron homeostasis as well as alterations in response to other transition metals (4). We showed that many of the phenotypes in the double deletion could be suppressed by deleting CCC1. The vacuole in yeast is the iron storage organelle, and Ccc1 is the only known vacuolar iron importer (7). The observation that a deletion in CCC1 rescues the phenotype of a Δmrs3Δmrs4 cell suggests that loss of the mitochondrial iron transporters induces changes in vacuolar iron transport. Herein we provide genetic evidence for such a mitochondria-vacuole signaling system (MVS).2 We show that deletion of both MRS3 and MRS4 affects the activity of the vacuolar iron transporter Ccc1. Using a genetic screen for mutants defective in the MVS pathway, we identify FRA1 as a gene that affects MVS phenotypes. We provide genetic and biochemical data showing that Δmrs3Δmrs4 cells show increased levels of oxidants and that Fra1 interacts with the thioredoxins-dependent peroxiredoxin Tsa1 to reduce oxidant damage. Our data indicate that oxidant damage increases the activity of the vacuolar iron transporter.
EXPERIMENTAL PROCEDURES
Yeast Strains, Media, and Plasmids
All S. cerevisiae strains used in this work are derived from W303 or S288C background and are listed in Table 1. Most deletion strains were created by PCR from the diploid homozygous deletion collection (ATCC) using the PCR-mediated gene disruption technique of Amberg et al. (8). The primers used for deleting FRA1 were PR1122 (5′-ACC CGT CTT TAT AAC CCA CGT A-3′) and PR1129 (5′-TTG TGA CTC TTG CGG AAA GGT TCG-3′). The primers used for deleting FRA2 were PR1118 (5′-GTT TGT ATG TGG GCT TAA TCT GTT-3′) and PR1119 (5′-TTT TAC TTC ATA GGT TTG AAC TCG C-3′). The primers used for deleting TSA1 were PR1750 (5′-GCA ACC TCA TCT CTA CAT TC-3′) and PR1751 (5′-CGC CTA GAA GGG AAA TCA TA-3′). The primers used for deleting TRR1 were 5′-CGC TTC AGT ATA CAG TAC GG-3′ and 5′-GGG GAA GCG CAG CAA TAA TA-3′. Multiple deletion strains were generated by combining traditional mating, sporulation, and dissection or by direct chromosome recombination of PCR knock-out constructs.
TABLE 1.
| Strain | Genotype | Source |
|---|---|---|
| By4742 | MAT α his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0 | Ref. 4 |
| 25c (Δmrs3Δmrs4) | MAT α his3Δ1 leu2Δ0 ura3Δ0 Δmrs3Δmrs4::kanMX4 | Ref. 4 |
| 25CΔfra1 | MAT α his3Δ1 leu2Δ0 ura3Δ0 Δmrs3Δmrs4::kanMX4 Δfra1::HIS3 | This study |
| 25CΔfra2 | MAT α his3Δ1 leu2Δ0 ura3Δ0 Δmrs3Δmrs4::kanMX4 Δfra2::HIS3 | This study |
| 25CΔfra1Δccc1 | Δmrs3Δmrs4::kanMX4 Δfra1::HIS3 Δccc1::LEU2 | This study |
| Δfra1 | MAT α his3Δ1 leu2Δ0 ura3Δ0 Δfra1::HIS3 | This study |
| Δfra2 | MAT α his3Δ1 leu2Δ0 ura3Δ0 Δfra2::HIS3 | This study |
| mvs6-3 | MAT α his3Δ1 leu2Δ0 ura3Δ0 Δmrs3Δmrs4::kanMX4 | This study |
| mvs6-4 | MAT α his3Δ1 leu2Δ0 ura3Δ0 Δmrs3Δmrs4::kanMX4 | This study |
| mvs6-3Δfra1 | MAT α his3Δ1 leu2Δ0 ura3Δ0 Δmrs3Δmrs4::kanMX4 Δfra1::HIS3 | This study |
| DY150WT | MAT a ura3-52, leu2-3, 112, trp1-1, his3-11, ade2-1, can1-100(oc) | Ref. 4 |
| Δmrs3Δmrs4 | Δmrs3::kanMax, Δmrs4::kanMax | Ref. 4 |
| Δccc1 | Δccc1::HIS3 | Ref. 4 |
| Δmrs3Δmrs4Δaft1 | Δmrs3::kanMax, Δmrs4::kanMax Δaft1::HIS3 | Ref. 4 |
| Δtsa1 | Δtsa1::HIS3 | This study |
| Δmrs3Δmrs4Δtsa1 | (diploid)Δmrs3::kanMax, Δmrs4::kanMax Δtsa1::HIS3 | This study |
| Δmrs3Δmrs4Δtsa1Δccc1 | (diploid)Δmrs3::kanMax, Δmrs4::kanMax Δtsa1::HIS3 Δccc1::LEU2 | This study |
| Δtrr1 | Δtrr1::HIS3 | This study |
| Δmrs3Δmrs4Δtrr1 | Δmrs3::kanMax, Δmrs4::kanMax Δtrr1::HIS3 | This study |
| Δmrs3Δmrs4Δtrr1Δccc1 | Δmrs3::kanMax, Δmrs4::kanMax Δtrr1::HIS3 Δccc1::LEU2 | This study |
| DY150WT Rho° | MAT a ura3-52, leu2-3, 112, trp1-1, his3-11, ade2-1, can1-100(oc) | Ref. 4 |
| Δmrs3Δmrs4 Rho° | Δmrs3::kanMax, Δmrs4::kanMax | Ref. 4 |
Cells were grown in YPD (1% yeast extract, 2% peptone, 2% dextrose) or CM and its amino acid dropout selective media (0.67% yeast nitrogen base, 0.12% drop-out amino acid mix, 2% dextrose). Galactose medium was prepared by adding 2% galactose instead of 2% dextrose. Media was made iron-deficient by addition 80 μm bathophenanthroline sulfonate (BPS) and specific concentrations of ferrous ammonium sulfate. The concentration of added iron in μm is denoted as BPS(x). The BBLTM GasPakTM Plus anaerobic system (BD Biosciences) was used for anaerobic experiments.
CCC1 was cloned with either a MET3 promoter or a GAL1 promoter. FRA1 and FRA2 were cloned with their endogenous promoters or a GAL1 promoter. RIM2 was cloned with its own promoter. pHA-TSA1 and pHA-TSA1C4Ss were generous gifts from Dr. David Eide, University of Wisconsin.
UV Mutagenesis
We chose 50% killing for UV mutagenesis. The exposure time was determined by establishing a UV killing curve.
Library Construction
Genomic DNA was isolated from MVS mutant cells (mvs6-3 and mvs6-4), digested with Sau3AI, and applied to a 10–40% sucrose gradient to separate DNA fragments as described previously (9). The appropriate 10-kb-size DNA fractions were collected and ligated into pRS416 zero vector digested with BamHI.
Reverse Transcription-PCR, Western Blot, and Immunoprecipitation
mRNA was measured by reverse transcription-PCR. Briefly, first-strand cDNA was synthesized from total RNA using SuperScript III from Invitrogen. Reverse transcription-PCR used the following conditions: denaturation at 95 °C, annealing at 58 °C, elongation at 72 °C in a Roche Lightcycler 2.0 from Idaho Technology. Primers for FRA1 were 5′-ATG AGA GGA ACA TCG GCA TC-3′ and 5′-AAC AGA CCC ATT TCC AGC AC-3′. CMD1 was used as an internal control.
Cells were homogenized using glass beads and vacuoles isolated as described previously (7). Proteins were analyzed on a 12% SDS-PAGE, and Western blot transfer was performed. Antisera used for probing Westerns included: rabbit anti-CCC1 serum, used at a 1:500 dilution; rabbit anti-FRA1 serum and rabbit anti-FRA2 serum, used at a 1:3000 dilution; rabbit anti-HA (Invitrogen), used at a 1:5000 dilution; mouse anti-phosphoglycerate kinase (PGK) (Invitrogen), used at a 1:10,000 dilution; mouse anti-carboxypeptidase Y (Invitrogen), used at a 1:2000 dilution.
Affinity purification was performed using nickel-nitrilotriacetic acid-agarose beads (Qiagen) for HIS-tagged proteins or DYNAL magnetic beads with sheep anti-rabbit or sheep anti-mouse IgG (Invitrogen). Protein identification was provided by the Mass Spectrometry and Proteomics Core Facility, University of Utah.
LacZ Assay
The construction and measurement of FET3-lacZ and measurement of β-galactosidase activity was performed as described previously (10). Specific activity was calculated as nmol/min/mg of protein.
2′,7′-Dichlorodihydrofluorescein Diacetate (DCFDA) Assay
The production of reactive oxygen species was measured using DCFDA from Invitrogen. After the addition of 15 μm DCFDA in culture media for 1 h, 2 × 108 log-phase grown cells were harvested. Cells were washed once with water, twice with ice-cold phosphate-buffered saline, and homogenized by vortexing with glass beads at 4 °C for 10 min, and supernatant was collected by centrifugation at 14,000 × g for 10 min. Fluorescence was measured using an excitation wavelength of 504 nm and an emission wavelength of 524 nm in a PerkinElmer LS55 luminescence spectrometer. The results were normalized to protein concentration using the bicinchoninic acid assay (Pierce).
Measurement of Vacuolar 59Fe
Log phase-grown wild type or Δmrs3Δmrs4 cells (A600) were resuspended in iron-free Lim medium with 0.5 μm 59Fe, 1 mm ascorbate acid, and incubated at 30 °C for 10 min. Cells were washed with EDTA-containing buffers. The washed cells were resuspended in CM medium and incubated at 30 °C for another 2 h. After incubation, vacuoles were isolated as described previously (4). Radioactivity in vacuolar fractions was determined by gamma counting, and the data were for normalized to protein concentration.
RESULTS
Overexpression of CCC1 in Δmrs3Δmrs4 Cells Results in a Growth Defect Due to Cytosolic Iron Depletion
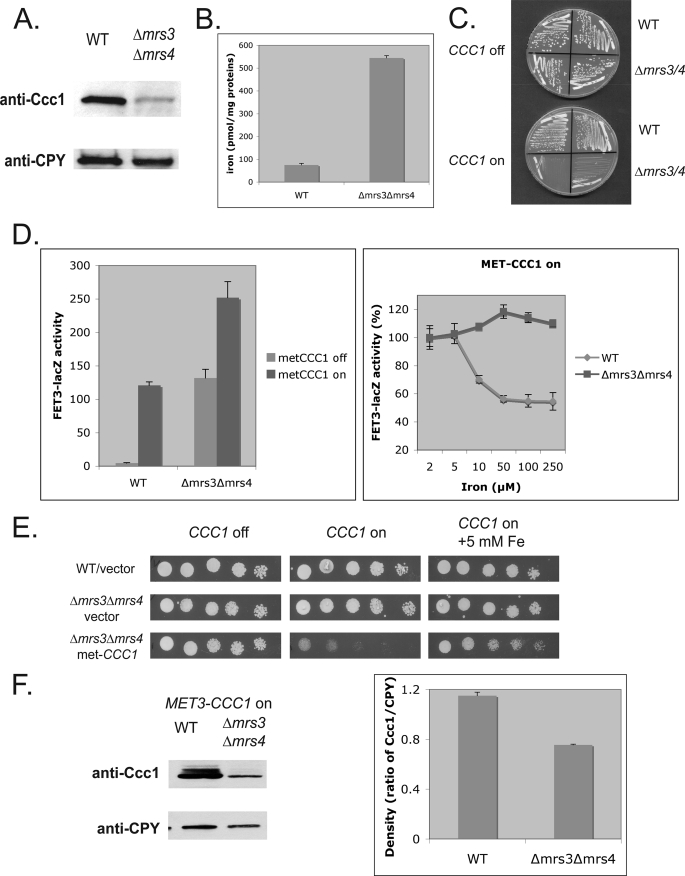
Deletion of MRS3 and MRS4 resulted in decreased cytosolic iron, which was mediated by the vacuolar iron transporter Ccc1 (4). To determine whether the amount or activity of Ccc1 was affected, we examined the level of Ccc1 in Δmrs3Δmrs4 cells. Western analysis showed that Ccc1 levels were much lower in Δmrs3Δmrs4 cells than in wild type cells (Fig. 1A). Studies have shown that CCC1 mRNA levels are increased by high iron through the transcription factor Yap5 (10) and the message destabilized under low iron by Cth1 and Cth2, which are expressed in low iron conditions through the low iron-sensing transcription factor Aft1 (11). The observation that cytosolic iron is decreased in the face of lower than normal amounts of Ccc1 suggests that deletion of MRS3/MRS4 increases the activity of Ccc1. Exposure of cells to radioactive iron showed that vacuoles from Δmrs3Δmrs4 cells contained higher levels of 59Fe than vacuoles from wild type cells (Fig. 1B). This result confirms our previous finding that Δmrs3Δmrs4 cells had increased vacuolar iron compared with wild type cells, as determined by inductively coupled plasma-optical emission spectroscopy (4). As the increased activity of endogenous Ccc1 led to decreased growth, we reasoned that increased expression of Ccc1 in Δmrs3Δmrs4 cells would lead to a profound growth defect. We transformed Δmrs3Δmrs4 cells with a plasmid containing a MET3-regulated CCC1. In the presence of methionine, Δmrs3Δmrs4 cells grew as well as wild type cells. In the absence of methionine, which leads to expression of CCC1, Δmrs3Δmrs4 cells showed a pronounced growth defect relative to control cells transformed with pMET3CCC1 (Fig. 1C). Similar results were observed using a GAL1CCC1 plasmid (data not shown).
FIGURE 1.
Overexpression of Ccc1 in Δmrs3mrs4 cells decreases cytosolic iron leading to a poor growth phenotype. A, wild type (WT) and Δmrs3Δmrs4 cells were grown in CM medium with 500 μm iron overnight. Cells were homogenized, vacuoles were isolated, and samples were applied to a SDS-PAGE and examined by Western blot analysis. The blots were probed with a rabbit anti-Ccc1 or mouse anti-carboxypeptidase Y (CPY) followed by a peroxidase-conjugated goat anti-rabbit IgG or peroxidase-conjugated goat anti-mouse IgG. Carboxypeptidase Y was used as a loading control. B, wild type and Δmrs3Δmrs4 cells were grown in CM medium and then incubated with 59Fe, as described under “Experimental Procedures.” Vacuoles were isolated, and the amount of radioactivity in isolated vacuoles was determined. The data were normalized to vacuolar protein. C, wild type and Δmrs3Δmrs4 cells transformed with MET3CCC1 plasmid were grown for 2 days on plates with (CCC1 off) or without 10× methionine (CCC1 on). D, wild type and Δmrs3Δmrs4 cells were transformed with a MET3CCC1 plasmid together with a FET3-lacZ reporter plasmid. Cells were grown overnight at 30 °C with or without 10× methionine, and FET3-lacZ activity was measured (left panel). Cells were grown overnight at 30 °C with or without 10× methionine in the absence or presence of varying amounts of ferrous ammonium sulfate, and FET3-lacZ activity was measured (right panel). E, wild type cells or Δmrs3Δmrs4 transformed with a control vector or Δmrs3Δmrs4 cells transformed with a MET3CCC1 plasmid were serial-diluted and grown for 2 days on plates with (CCC1 off) or without 10× methionine (CCC1 on) and with or without five mm ferrous ammonium sulfate. F, wild type and Δmrs3Δmrs4 cells transformed with a MET3CCC1-FLAG plasmid were incubated at 30 °C in the absence of methionine for 6 h. Cells were homogenized with glass beads, cell lysates were applied to SDS-PAGE, and Western blot analysis with either anti-Ccc1 or anti- carboxypeptidase Y was performed as A (left panel). The data were quantified using densitometric analysis (right panel). All experiments were performed a minimum of three times, and error bars represent the S.E.
Increased expression of CCC1 in wild type cells led to decreased cytosolic iron as shown by increased activity of a FET3lacZ reporter construct, a surrogate assay for activation of the iron-regulon (Fig. 1D, left panel). In Δmrs3Δmrs4 cells, FET3lacZ activity is increased compared with wild type cells (4). Overexpression of CCC1 in Δmrs3Δmrs4 cells resulted in increased FET3lacZ activity, indicating a greater depletion of cytosolic iron. Iron supplementation reduced FET3lacZ activity in wild type cells overexpressing Ccc1 but did not suppress activity in Δmrs3Δmrs4 cells overexpressing Ccc1 (Fig. 1D, right panel). The addition of iron to the medium suppressed the poor growth resulting from overexpression of Ccc1 in Δmrs3Δmrs4 cells (Fig. 1E). Western blot analysis revealed that Ccc1 levels, expressed by the iron-independent MET3 promoter and lacking the normal 3′-untranslated region, were lower in Δmrs3Δmrs4 cells than in control cell incubated in the same medium (Fig. 1F). These results indicate that the Ccc1-mediated decrease in cell growth in Δmrs3Δmrs4 cells was not the result of an increase in Ccc1 level, indicating that the transport activity of Ccc1 must be affected.
Identification of MVS Mutants
We took advantage of the severe growth defect seen in Δmrs3Δmrs4 cells overexpressing CCC1 (referred to as the MVS phenotype) to identify mutants that could suppress the growth defect (Fig. 2A). Cells (Δmrs3Δmrs4 transformed with pMET3CCC1) were UV-mutagenized in the presence of methionine and then replica-plated to medium lacking methionine. Cells able to grow in the absence of methionine were selected and retested. We identified eight candidates that were able to grow in the absence of methionine. We focused our efforts on characterizing two mutants, mvs6-3 and mvs6-4. One of the phenotypes of Δmrs3Δmrs4 cells is an inability to grow on low iron plates, which reflects the absence of high affinity mitochondrial iron transport activity. When cured of the MET3CCC1 plasmid, the mutants showed no growth defect in the absence of methionine and a low iron growth defect, indicating that suppression of the MVS phenotype was not due to mutations that affected mitochondrial iron homeostasis (data not shown). These mutants, when retransformed with a MET3CCC1 or a GAL1-regulated CCC1 plasmid, did not show a growth defect in the absence of methionine or in galactose-containing medium, whereas the parent strain transformed with the same plasmid showed a growth defect (data not shown). These results show that the suppression of the MVS phenotype was not due to an alteration in MRS3/MRS4 or plasmid borne CCC1 but in genes that affected Ccc1 activity.
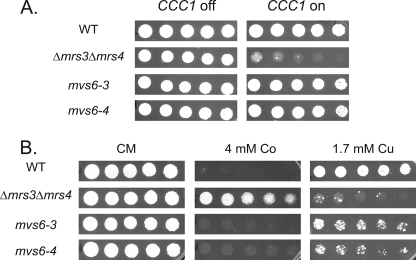
FIGURE 2.
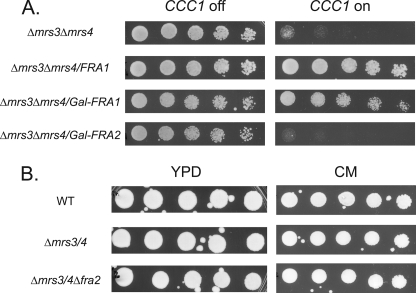
Identification of mvs mutants that suppress the poor growth phenotype of Δmrs3Δmrs4 cells overexpressing Ccc1. A, wild type (WT), Δmrs3Δmrs4, mvs6-3, and mvs6-4 cells were transformed with a MET3CCC1 plasmid, grown overnight in selection media, and washed, and serial dilutions were spotted on plates with (CCC1 off) or without 10× methionine (CCC1 on) and grown for 2 days at 30 °C. B, MVS mutant 6-3 and 6-4, cured of the MET3CCC1 plasmid, were spotted on plates containing the specified amounts of cobalt or copper and grown for 2 days at 30 °C. All experiments were performed a minimum of three times.
The Δmrs3Δmrs4 strain shows phenotypes related to transition metals, notably increased resistance to cobalt and decreased resistance to copper (4). These phenotypes were suppressed when CCC1 was deleted, showing that the phenotypes were due to Ccc1. MVS mutant cells show a metal phenotype more similar to wild type cells with increased cobalt sensitivity and decreased copper sensitivity (Fig. 2B).
We determined whether the MVS phenotype is recessive or dominant. MVS mutants were mated with Δmrs3Δmrs4 cells of the opposite mating type. The cells were transformed with pMET3CCC1 and then plated on medium either lacking or containing methionine. All diploids grew in the presence of methionine (data not shown). In the absence of methionine, homozygous Δmrs3Δmrs4 diploids showed a growth defect when CCC1 was expressed (see Fig. 4E). Diploids generated between mvs6-3 strains and parental Δmrs3Δmrs4 showed improved growth when CCC1 was expressed, indicating that the MVS phenotype was dominant.
FIGURE 4.
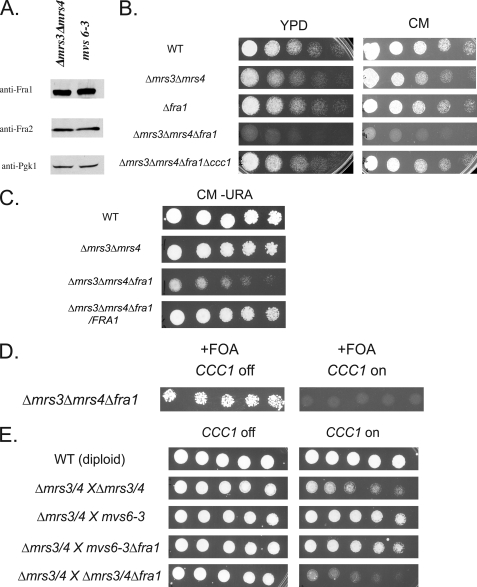
MVS mutants do not show increased amounts of Fra1, but deletion of FRA1 affects the MVS phenotype. A, Δmrs3Δmrs4 and mvs6-3 cells were grown to log phase. Cells were harvested and lysed with glass beads, lysates were run on SDS-PAGE, and Western blot analysis was performed using rabbit anti-Fra1, rabbit anti-Fra2, or mouse anti-phosphoglycerate kinase (PGK1) followed by peroxidase-conjugated goat anti-rabbit IgG or peroxidase-conjugated goat anti-mouse IgG. B, wild type (WT), Δmrs3Δmrs4, Δfra1, Δmrs3Δmrs4Δfra1, and Δmrs3Δmrs4Δfra1Δccc1 were serial-diluted onto YPD or CM plates and grown for 2 days at 30 °C. C, wild type, Δmrs3Δmrs4, Δmrs3Δmrs4Δfra1, and Δmrs3Δmrs4Δfra1 with FRA1 plasmid were serial-diluted onto CM-URA plates and grown for 2 days at 30 °C. D, Δmrs3Δmrs4Δfra1 cells were transformed with pFRA1 (URA3 marker) and pMET3CCC1 (LEU2 marker). Cells were grown without leucine overnight, washed, and spotted in serial dilution on fluoroorotic acid (FOA) plates with (CCC1 off) or without 10× methionine (CCC1 on) and grown for 2 days at 30 °C. E, diploid wild type cells, Δmrs3Δmrs4/Δmrs3Δmrs4, mvs6-3/Δmrs3Δmrs4, mvs6-3Δfra1/Δmrs3Δmrs4, and Δmrs3Δmrs4Δfra1/Δmrs3Δmrs4 cells were transformed with pMET3CCC1, serial diluted on plates with (CCC1 off) or without 10× methionine (CCC1 on), and grown for 2 days at 30 °C. All experiments were performed a minimum of three times.
Identification of FRA1 as an MVS Gene
To identify MVS genes, we generated a genomic library from mvs6-3 in a low copy vector and then transformed that library into Δmrs3mrs4 cells containing the MET3CCC1 plasmid. Transformed cells were plated in the absence of methionine, and resistant colonies were identified. We identified 12 colonies that were resistant to the growth inhibitory effects of increased CCC1 expression. Plasmids were isolated from those colonies, sequenced, and then subcloned to identify the gene responsible for suppressing the MVS phenotype. We determined that the plasmids contained either of two genes, RIM2 or FRA1, responsible for suppressing the MVS phenotype (Fig. 3A). RIM2 and FRA1 from a genomic library of mvs6-4 were also identified as suppressors of the MVS phenotype. RIM2 encodes a member of the mitochondrial carrier family, which is a homologue of MRS3 and MRS4. Expression of RIM2 partially suppressed the low iron growth defect of Δmrs3Δmrs4 cells (Fig. 3B). This result suggests RIM2 encodes a functional homologue of MRS3/MRS4. Because the MVS growth deficit requires deletion of both MRS3 and MRS4, increased expression of RIM2 complements the effects of MRS3/MRS4 deletions.
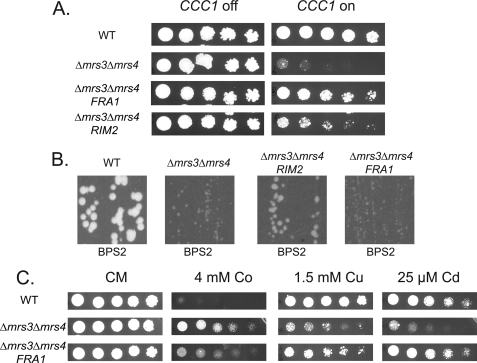
FIGURE 3.
Identification of RIM2 and FRA1 as suppressors of the MVS phenotype. A, wild type (WT) or Δmrs3Δmrs4 cells were transformed with a control plasmid pFRA1, or pRIM2 under the control of their endogenous promoters. The cells were also transformed with pMET3CCC1, serial diluted onto plates with (CCC1 off) or without 10× methionine (CCC1 on) and grown for 2 days at 30 °C. B, wild type or Δmrs3Δmrs4 cells transformed with a control plasmid, pRIM2, or pFRA1 under the control of their endogenous promoters were grown for 2 days at 30 °C on low iron (BPS2) plates. C, wild type, Δmrs3Δmrs4, and Δmrs3Δmrs4 with pFRA1 were grown for 2 days on CM plates supplemented with 4 mm Co2+, 1.5 mm Cu2+, and 25 μm Cd2+. All experiments were performed a minimum of three times.
Expression of FRA1 did not complement the growth phenotype of Δmrs3Δmrs4 on low iron (Fig. 3B). FRA1 was previously identified as part of a cytosolic protein complex involved in the signaling pathway leading to transcription of the iron regulon (12). Expression of FRA1 isolated from a wild type library, cloned in the same low copy vector as used to construct the genomic library, permitted a Δmrs3Δmrs4 (pMET3CCC1) strain to survive. To determine whether the expression of FRA1 affects other MVS phenotypes, a FRA1-containing plasmid was transformed into Δmrs3Δmrs4 cells, and the metal phenotypes of the transformed cells were examined. Δmrs3Δmrs4 cells are Cu+-and Cd2+-sensitive but are Co2+-resistant; transformation with FRA1 plasmids normalizes metal sensitivity in Δmrs3Δmrs4 cells (Fig. 3C).
Deletion of FRA1 Exacerbates the MVS Phenotype by Affecting Ccc1 Activity
Sequencing of FRA1 isolated from either mvs6-3 or mvs6-4 did not reveal any mutation in the coding sequence or in the promoter (500 bp upstream from the ATG). The above data suggest that even low copy expression of FRA1 may affect the MVS phenotype. We examined whether there was increased expression of FRA1 in MVS cells. Western blot analysis did not show any increase in Fra1 levels in mvs6-3. (Fig. 4A). Reverse transcription-PCR analysis also did not reveal any increase in FRA1 mRNA levels in mvs6-3 (1.15 ± 0.05) or mvs6-4 (1.26 ± 0.13) compared with parental Δmrs3Δmrs4 cells. These results do not reflect an inability to detect changes in mRNA, as we could detect increased mRNA levels in cells transformed with a low copy plasmid containing FRA1 expressed by its endogenous promoter (3.59 ± 0.62).
We used a genetic approach to determine the role of FRA1 in the MVS phenotype. Deletion of FRA1 in wild type cells did not show any growth defect (Fig. 4B). In contrast, deletion of FRA1 in Δmrs3Δmrs4 cells led to a severe growth defect. If the growth defect is due to an effect on Ccc1 activity, then deletion of CCC1 should suppress the defect. Indeed, deletion of CCC1 in a Δmrs3Δmrs4Δfra1 strain suppressed the poor growth. The poor growth of the Δmrs3Δmrs4Δfra1 strain was suppressed by transformation with a FRA1-containing plasmid (Fig. 4C). The Δmrs3Δmrs4Δfra1 strain grew poorly and could not be transformed with pMET3CCC1 even in the presence of high methionine. To examine the effect of increased expression of CCC1 in the triple deletion strain, we transformed the strain with a FRA1 plasmid and then with a MET3CCC1 plasmid. The FRA1 plasmid had the URA3 gene, permitting us to use fluoroorotic acid to select against the presence of the plasmid. In the presence of methionine, Δmrs3Δmrs4Δfra1 (pMET3CCC1) cells are viable (Fig. 4D). In the absence of methionine, Δmrs3Δmrs4Δfra1(pMET3CCC1) cells lost viability. These results show that the loss of FRA1 exacerbates the MVS phenotype.
The data showing that deletion of FRA1 in a Δmrs3Δmrs4 strain affects the MVS phenotype does not answer the question of whether FRA1 is responsible for the suppression of the MVS phenotype in the MVS mutant cells. To examine if FRA1 is necessary for suppressing the MVS phenotype, we deleted FRA1 in mvs6-3 and examined the effect of that deletion. As mentioned above, mvs6-3 is dominant. A diploid strain between two Δmrs3Δmrs4 haploids shows the MVS phenotype of poor growth when CCC1 is overexpressed, whereas a diploid between Δmrs3Δmrs4 and mvs6-3 grew better (Fig. 4E). Deletion of one copy of FRA1 in a diploid Δmrs3Δmrs4 did not rescue the MVS phenotype. A diploid of Δmrs3Δmrs4 and mvs6-3Δfra1 still showed suppression of the MVS phenotype. We conclude from these results that FRA1 is required to suppress the MVS defect, but the mutation in mvs6-3 is not in FRA1 but, rather, affects FRA1 acting in trans.
FRA2 Is Not Involved in the MVS Phenotype
FRA1 was identified through its effect on induction of the iron-regulon, as a deletion in FRA1 increases transcription of Aft1-regulated genes such as FET3 (12) and siderophore transporters (13). A large scale genomic screen identified Fra2 as a binding partner of Fra1 (14), and deletion of FRA2 also resulted in induction of the iron-regulon (12, 13). Deletion of both FRA1 and FRA2 did not induce the iron regulon beyond that seen in single deletions (12). To determine whether FRA2 is involved in the MVS phenotype, we examined the effect of overexpressing FRA2 in Δmrs3Δmrs4 cells. Overexpression of FRA2 did not affect the pMET3CCC1-induced growth defect in Δmrs3Δmrs4 cells (Fig. 5A). Deletion of FRA2 in Δmrs3Δmrs4 cells did not affect cell growth (Fig. 5B). These results suggest that FRA2 does not affect the MVS phenotype.
FIGURE 5.
FRA2 is not involved in the MVS phenotype. A, Δmrs3Δmrs4 cells were transformed with empty vector, pFRA1 was transformed with endogenous promoter, pFRA1 was transformed with a GAL1 promoter, or pFRA2 was transformed with a GAL1 promoter followed by transformation with pMET3CCC1. Cells were washed and spotted in serial dilution on galactose plates with (CCC1 off) or without 10× methionine (CCC1 on) and grown for 3 days at 30 °C. Galactose was used as a carbon source to turn on GAL1-regulated genes. B, wild type (WT), Δmrs3Δmrs4, and Δmrs3Δmrs4Δfra2 cells were serial-diluted on YPD or CM plates and grown for 2 days at 30 °C. All experiments were performed a minimum of three times.
Examination of the Role of FRA1 in the MVS Phenotype
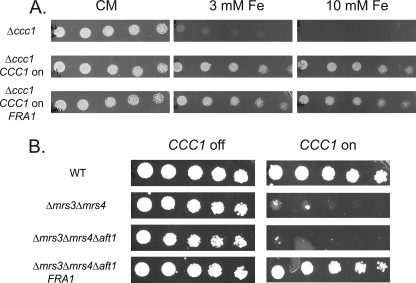
Because expression of FRA1 can affect the behavior of Ccc1 in Δmrs3Δmrs4 cells, we then asked whether expression of FRA1 affects the ability of Ccc1 to transport iron into the vacuole in cells containing MRS3/MRS4. Deletion of CCC1 results in sensitivity to high iron; thus, reduced expression of CCC1 might lead to a growth defect in high iron medium. Transformation of FRA1 into wild type cells (data not shown) or into Δccc1 cells transformed with MET3CCC1 plasmid did not affect growth on high iron plates (Fig. 6A). Based on these results we conclude that Fra1 does not affect Ccc1 directly but only in the absence of MRS3 and MRS4.
FIGURE 6.
Fra1 does not affect Ccc1 directly, and the MVS phenotype is independent of Aft1. A, Δccc1 cells containing pMET3CCC1 were transformed with empty vector or pFRA1, grown in selection media overnight, washed, serial-diluted onto plates with (CCC1 off) or without 10× methionine (CCC1 on) supplemented with 3 or 10 mm ferrous ammonium sulfate and grown for 2 days at 30 °C. B, wild type (WT), Δmrs3Δmrs4, Δmrs3Δmrs4Δaft1, and Δmrs3Δmrs4Δaft1 with pFRA1 were transformed with pMET3CCC1, grown in selective media overnight, washed, serial-diluted onto plates with (CCC1 off) or without 10× methionine (CCC1 on) and grown for 2 days at 30 °C. All experiments were performed a minimum of three times.
In wild type cells, deletion of FRA1 increases expression of the iron-regulon, as shown by increased expression of a FET3-lacZ reporter construct (12). We determined if the FRA1 effect on the MVS phenotype required Aft1, the transcriptional regulator of the iron-regulon. Deletion of AFT1 did not suppress the MVS phenotype, and transformation of FRA1 into Δmrs3Δmrs4Δaft1 cells suppressed the grown defect (Fig. 6B). These results show that the ability of FRA1 to suppress the growth defect of the MVS phenotype is independent of Aft1.
Deletion of MRS3 and MRS4 Leads to Chronic Oxidative Stress
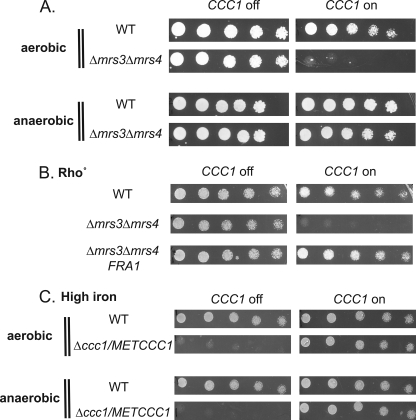
The MVS phenotype is oxygen-dependent, as cells grown anaerobically did not show MVS growth defect (Fig. 7A). Suppression of the MVS growth defect did not result from a loss of respiration, as shown by the fact that MVS Rho° cells generated by loss of the mitochondrial genome still showed the MVS phenotype (Fig. 7B). We considered the possibility that the loss of the MVS phenotype might reflect the inability of CCC1 to function anaerobically. To determine whether MET3CCC1 functions anaerobically, we took advantage of the observation that deletion of CCC1 results in toxicity to high iron. We determined that Δccc1 cells are sensitive to high iron under anaerobic conditions and that expression of pMET3CCC1 in Δccc1 cells can suppress high iron toxicity (Fig. 7C). These results show that Ccc1 can function under anaerobic conditions and suggest that activation of Ccc1 leading to the MVS phenotype may involve oxygen-derived metabolites.
FIGURE 7.
The MVS phenotype is oxygen-dependent. A, wild type (WT) and Δmrs3Δmrs4 cells transformed with pMET3CCC1 were grown in selective media overnight, washed, serial-diluted onto plates with (CCC1 off) or without 10× methionine (CCC1 on), and grown under aerobic or anaerobic conditions for 3 days at 30 °C. B, wild type Rho° cells with empty vector, Δmrs3Δmrs4 Rho° cells with empty vector, and Δmrs3Δmrs4 rho° cells with pFRA1 were transformed with pMET3CCC1 grown in selection media overnight, washed, serial-diluted onto plates with (CCC1 off) or without 10× methionine (CCC1 on), and grown for 2 days at 30 °C. C, wild type cells and Δccc1 cells were transformed with pMET3CCC1 grown in selection media overnight, washed, serial-diluted onto plates with (CCC1 off) or without 10× methionine (CCC1 on), and grown under aerobic or anaerobic conditions for 3 days at 30 °C. All experiments were performed a minimum of three times.
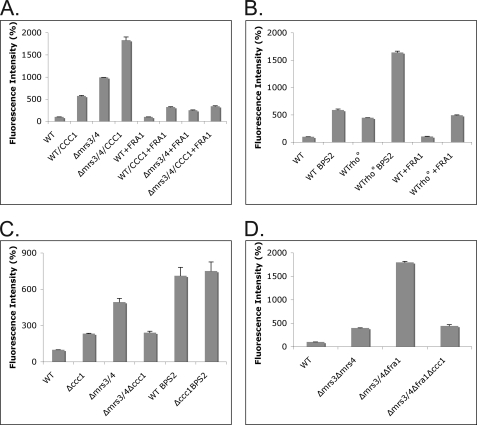
We utilized the oxidant-sensitive dye DCFDA to determine whetherΔmrs3Δmrs4 cells showed oxidant stress. DCFDA is not fluorescent but can react with oxygen radicals to produce the fluorescent derivative DCF. Expression of CCC1 in wild type cells resulted in an increase in DCF fluorescence relative to wild type cells (Fig. 8A). Δmrs3Δmrs4 cells showed an increase in DCF fluorescence compared with wild type cells. Overexpression of CCC1 in Δmrs3Δmrs4 cells resulted in a further increase in DCF. Overexpression of FRA1 in wild type cells had no effect on DCF fluorescence but reduced DCF fluorescence in wild type cells overexpressing CCC1 and in Δmrs3Δmrs4 cells and in Δmrs3Δmrs4 cells overexpressing CCC1. These results show a correlation between oxidant damage and the MVS phenotype.
FIGURE 8.
The MVS phenotype leads to oxidant damage that is reduced by Fra1. A, wild type (WT), Δmrs3Δmrs4, wild type with pFRA1, and Δmrs3Δmrs4 with pFRA1 cells were transformed with pMET3CCC1 plasmid, grown in selective media overnight, and incubated with DCFDA for 1 h, cells were washed and lysed using glass beads, and fluorescence intensity was measured on a luminescence spectrometry as described under “Experimental Procedures.” The results were normalized for protein and plotted as the percentage of intensity compared with wild type control cells. All strains contained pMET3CCC1; strains expressing the MET3-regulated CCC1 (incubated in the absence of methionine) are labeled CCC1. B, wild type Rho+ and wild type Rho° cells transformed with pFRA1 were grown overnight in either CM or CM BPS2 media, DCFDA was added for 1 h, and fluorescence intensity was measured as above. C, wild type, Δccc1, Δmrs3Δmrs4, and Δmrs3Δmrs4Δccc1 cells were grown in CM or BPS2 media overnight and incubated with DCFDA as above. D, wild type, Δmrs3Δmrs4, Δmrs3Δmrs4Δfra1, and Δmrs3Δmrs4Δfra1Δccc1 cells were grown as in A, and fluorescence intensity was measured as described. All experiments were performed a minimum of three times, and error bars represent the S.E.
Wu et al. (15) showed that incubation of cells in zinc-limited medium led to an increase in DCF fluorescence. Incubation of wild type cells in iron-limited medium also resulted in an increase in DCF fluorescence (Fig. 8B). As described above, the MVS phenotype is dependent on oxygen but independent of mitochondrial respiration. The loss of mitochondrial respiration did not prevent the generation of DCF fluorescence by wild type cells incubated in iron-limited medium, suggesting again that respiratory activity is not responsible for oxidant generation. Overexpression of FRA1 in wild type or Rho° cells had little effect on DCF fluorescence.
We then examined the role of Ccc1 in DCF activity. Deletion of CCC1 led to an increase in DCF fluorescence (Fig. 8C). There was a higher level of DCF fluorescence in Δmrs3Δmrs4 cells, which was reduced by deletion of CCC1. We note that the amount of fluorescence did not return to wild type levels. The level of DCF fluorescence in wild type cells incubated in iron-limited medium was not reduced upon deletion of CCC1. This result together with that in Fig. 8A shows that Ccc1 is involved in the generation of oxidants in Δmrs3Δmrs4 cells but is not solely responsible for generation of oxidants in that strain.
Deletion of FRA1 in Δmrs3Δmrs4 cells resulted in a synthetic growth defect, which was rescued upon deletion of CCC1. As shown above, overexpression of FRA1 reduces oxidant stress in Δmrs3Δmrs4 cells overexpressing CCC1, leading us to examine the level of oxidant stress in Δmrs3Δmrs4Δfra1 cells. Deletion of FRA1 in Δmrs3Δmrs4 cells resulted in a dramatic increase in DCF fluorescence (Fig. 8D). Deletion of CCC1 reduced DCF fluorescence to that seen in Δmrs3Δmrs4 cells. This result indicates that oxidants, by affecting Ccc1 activity, led to a further increase in oxidant stress.
Genetic and Physical Interaction between FRA1 and TSA1
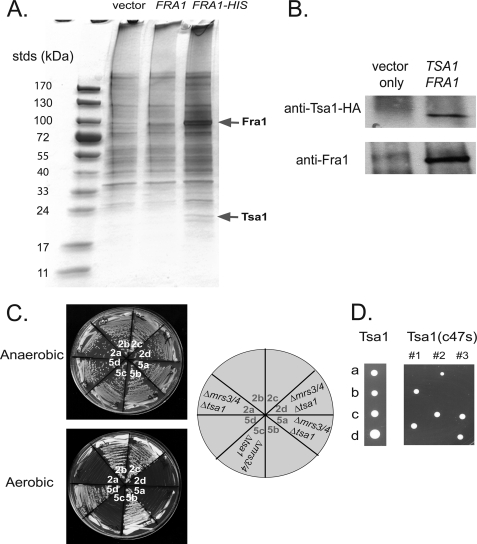
To determine how FRA1 might affect the MVS phenotype, we examined Fra1 for binding partners. Cells were transformed with a construct expressing His-tagged Fra1, which was functional. Affinity purification of His-tagged Fra1 revealed the presence of a protein band that was not present in cells transformed with Fra1 lacking the HIS epitope (Fig. 9A). Mass spectroscopy identified the band as TSA1, a member of the family of thiol-specific peroxiredoxins that is involved in intracellular signaling and in the breakdown of hydrogen peroxide and organic hydroperoxides. We confirmed the interaction of Fra1 and Tsa1 using cells transformed with a functional HA-tagged Tsa1 construct. Immunoprecipitation of HA-Tsa1 led to the co-immunoprecipitation of Fra1 (Fig. 9B).
FIGURE 9.
Fra1 binds to Tsa1. A, wild type cells with empty vector, GAL1-regulated pFRA1, or GAL1-regulated FRA1-6XHIS were grown overnight in selective media, washed, and resuspended in 1.0% Triton X-100 plus 25 mm Tris-HCl, pH 7.4, with glass beads. Cells were vortexed for 10 min, centrifuged at 14,000 × g, and Fra1-His6 affinity-purified using nickel-nitrilotriacetic acid agarose according to the manufacturer's protocol. Eluted samples were run on SDS-PAGE and silver-stained. Protein bands were excised from the gel and analyzed by mass spectrometry. B, Δfra1 cells were transformed with GAL1 promoter pFRA1 and empty vector or pHA-TSA1. Cells were grown in galactose medium to induce the expression of Fra1, lysed as described in A, and immunoprecipitated for HA-Tsa1 with anti-HA antibody and protein A/G-agarose. Beads were boiled, and samples were run on SDS-PAGE followed by Western analysis probing with rabbit anti-HA and rabbit anti-Fra1 followed by peroxidase-conjugated goat-anti rabbit IgG. C, Δmrs3Δmrs4 diploid cells were deleted for TSA1 by deletion PCR as described under “Experimental Procedures.” Triple deletion diploids were dissected, and tetrads were grown on YPD in an anaerobic chamber. 2:2 segregation of tetrads was verified by the deletion markers. Two sets of tetrads (2 and 5) were grown on YPD for 2 days under aerobic or anaerobic conditions. D, diploid cells of Δmrs3Δmrs4Δtsa1 were either transformed with pHA-TSA1 or pHA-TSA1(c47s). After sporulation and dissection, tetrads (a, b, c, d spores) were grown on CM-URA plates at 30 °C for 3 days.
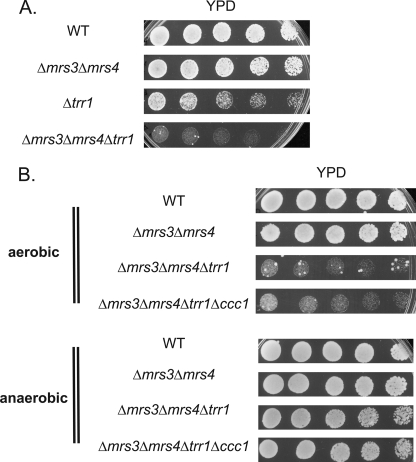
Overexpression of TSA1 in Δmrs3Δmrs4 (pMETCCC1) cells did not affect DCFDA fluorescence (data not shown); however, genetic experiments show an interaction between the MVS phenotype and TSA1. Deletion of TSA1 in Δmrs3Δmrs4 cells did not affect growth when cells were grown anaerobically, but the triple deletion did not grow under aerobic conditions (Fig. 9C). High copy expression of FRA1 did not suppress the lack of growth in Δmrs3Δmrs4Δtsa1 cells (data not shown). Deletion of CCC1 in Δmrs3Δmrs4Δtsa1 also did not suppress the growth defect. Tsa1 is reported to have two functions; as a peroxiredoxin it can reduce oxidant damage due to oxygen radicals such as the hydroxyl radical and as a chaperone affecting protein folding (16). The two functions are separable, as mutation in cysteine 47 affects the peroxiredoxin activity but not the chaperone activity. Transformation of Δmrs3Δmrs4Δtsa1 cells with TSA1C47S did not suppress the lethality of Δmrs3Δmrs4Δtsa1 cells, whereas transformation with wild type TSA1 did (Fig. 9D). This result indicates that the chaperone activity of Tsa1 is not involved in suppression of the MVS phenotype. The peroxiredoxin activity of Tsa1 is dependent on the thioredoxin reductase Trr1. Deletion of TRR1 had a small effect on cell growth, but deletion of TRR1 in Δmrs3Δmrs4 cells resulted in a severe growth defect under aerobic (Fig. 10A) but not anaerobic conditions (Fig. 10B). The severe aerobic defect was not suppressed by deletion of CCC1. This result shows that the peroxiredoxin activity is required to suppress oxidant generation in Δmrs3Δmrs4 cells.
FIGURE 10.
Deletion of TRR1 in Δmrs3Δmrs4 cells leads to synthetic poor growth. A, wild type (WT), Δmrs3Δmrs4, Δtrr1, and Δmrs3Δmrs4Δtrr1 cells were grown overnight, washed, spotted in serial dilutions on YPD plates, and grown for 2 days at 30 °C. B, wild type, Δmrs3Δmrs4, Δmrs3Δmrs4Δtrr1, and Δmrs3Δmrs4Δtrr1Δccc1 cells were grown overnight, washed, spotted in serial dilutions on YPD plates, and grown for 2 days at 30 °C under aerobic or anaerobic conditions. All experiments were performed a minimum of three times.
DISCUSSION
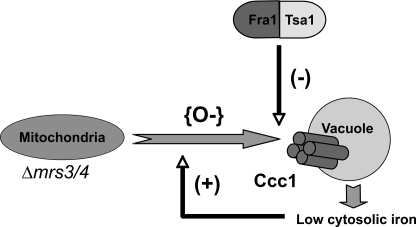
All organisms tightly regulate the concentration of iron in biological fluids, including cell cytosol. Iron transport activity is highly regulated, and most iron transporters are regulated at many levels. The high affinity cell surface iron transport system in yeast comprised of Fet3/Ftr1 is regulated transcriptionally (17) and post-translationally (18). The vacuolar iron importer Ccc1 is regulated at the transcriptional level by the high iron-sensing transcriptional activator Yap5 (10) and post-transcriptionally by message destabilization by Cth1 and Cth2, which are induced by iron deficit (11). In this manuscript we provide evidence to show that the activity of Ccc1 is affected by alteration in oxygen radicals; these radicals result from changes in mitochondrial function as a result of deletion of the iron transporters MRS3 and MRS4. Our results lead to a model that shows that the activity of Ccc1 can be altered in response to increased oxidative stress (Fig. 11).
FIGURE 11.
Model for the role of Fra1 and Tsa1 in the MVS pathway. Deletion of mitochondrial iron transporter MRS3 and MRS4 results in the generation of oxygen radicals (O−), which signals to increase vacuolar transporter Ccc1 activity. Ccc1 imports more iron into the vacuole, giving rise to less iron in the cytosol. Low cytosolic iron promotes more oxidative damage. Fra1 affects the Ccc1 response to mitochondrial-induced oxidant damage by binding to Tsa1 and reducing oxidants.
Deletion of MRS3 and MRS4 Leads to Chronic Oxidant Stress
Mrs3 and Mrs4 and their mammalian homologues mitoferrin 1 and mitoferrin 2 were identified as mitochondrial iron transporters (4–6, 19). They are members of the large family of mitochondrial carrier facilitators, most of which are antiporters that mediate metabolite transport across the mitochondrial inner membrane. Evidence that Mrs3/Mrs4 mediate iron transport comes from studies in which the absence of both MRS3 and MRS4 in yeast or vertebrates leads to a deficit in mitochondrial iron-consuming processes such as heme and iron-sulfur cluster synthesis. In yeast, the deficit in mitochondrial iron-consuming processes is only apparent when cells are placed in low iron medium, suggesting the presence of other mitochondrial iron transporters that have a lower affinity than Mrs3/Mrs4. Even in the presence of iron-replete medium, Δmrs3Δmrs4 cells show alterations in transition metal homeostasis (4). Data presented here confirm those observations and show that Δmrs3Δmrs4 cells have chronic oxidative stress, as shown by measurement of DCFDA fluorescence and the synthetic lethality of deletions in TRR1 and TSA1 in Δmrs3Δmrs4 cells. Trr1 and Tsa1 are linked components of an antioxidant system that resolves oxidant damage (16). The synthetic lethality of TRR1 or TSA1 deletions with MRS3 and MRS4 only occurs under aerobic conditions, supporting the view that Δmrs3Δmrs4 cells have chronic oxidant damage. Increased oxidants, as assessed by DCF fluorescence, occurs in Δmrs3Δmrs4 cells regardless of whether they were respiratory competent or not. This result indicates that an intact respiratory system and the increased oxygen consumption seen in respiring cells is not the source of reactive oxidants. A similar result was reported by Milgrom et al. (20), who demonstrated that cells lacking the vacuolar H+-ATPase showed chronic oxidative stress that was independent of mitochondrial respiration.
Oxidant Stress in Δmrs3Δmrs4 Cells Leads to Increased Vacuolar Iron Transport Activity
The chronic oxidative stress in Δmrs3Δmrs4 cells leads to an increase in the activity of Ccc1 resulting in increased vacuolar iron transport. The changes in cytosolic iron levels seen in Δmrs3Δmrs4 cells are dependent on the presence of Ccc1. Deletion of CCC1 in Δmrs3Δmrs4 cells restores altered transition metal metabolism, whereas expression of CCC1 in Δmrs3Δmrs4 cells results in a profound decrease in cytosolic iron. The level of Ccc1 in Δmrs3Δmrs4 cells is less than in wild type cells, suggesting that alterations in cytosolic iron result from an increase in Ccc1 transport activity. There is no in vitro assay for vacuolar iron transport, and our conclusion that the activity of Ccc1 is altered is based on the use of reporter constructs that assay high or low cytosolic iron and on growth phenotypes that are iron-dependent. The level of Ccc1 mRNA is highly regulated by high iron-dependent transcriptional activation and a post-transcriptional low iron-dependent destabilization of CCC1 mRNA levels. Given the multiplicity of regulatory mechanisms that govern Ccc1 levels, it is not surprising that environmental factors can regulate Ccc1 activity. The mechanism by which oxidative stress alters Ccc1 transport activity remains to be defined. We suggest that Ccc1 activity may increase upon exposure to high iron, as toxic levels of iron would increase oxidant damage. We are currently testing this hypothesis.
Fra1 and Tsa1 Play a Role in Modulating the Oxidative Stress Activation of Ccc1
We took advantage of the MVS growth defect to identify FRA1 as a suppressor of the poor growth phenotype. FRA1 had been identified in two independent genetic screens as being involved in activation of the iron-regulon. Deletion of FRA1 led to increased activity of a FET3 reporter construct (12) and increased expression of SIT1 (ARN1), the siderophore transporter for ferroxamine B (13). Induction of these iron acquisition genes was mediated through the low iron-sensing transcription factor Aft1. Large scale interaction studies, confirmed by immunoprecipitation of tagged proteins, showed that Fra1 was found in a complex with Fra2, Grx3, and Grx4 (14). Deletion of FRA2 also led to an increase in Aft1-dependent gene expression as shown by increased expression of a FET3 reporter construct and induction of ferroxamine B transport (12, 13). A double deletion of GRX3/GRX4 was shown to also lead to induction of the iron-regulon (21, 22). These results led us to propose a model in which Fra1, Fra2, Grx3, and Grx4 participated in a signaling complex, which affected the activity of Aft1 (12).
Overexpression of Fra1 suppresses the MVS phenotype, whereas deletion of FRA1 in Δmrs3Δmrs4 cells leads to a synthetic growth defect. This synthetic growth defect can be suppressed by deletion of CCC1 but not by deletion of AFT1. These results suggest that changes in Aft1 activity are secondary to changes in cytosolic iron. Overexpression of FRA1 suppressed the MVS phenotype but did not fully suppress the increased DCF fluorescence seen in Δmrs3Δmrs4 cells or affect DCF fluorescence in wild type Rho° cells. This result suggests that oxidants initially generated by mitochondria are not affected by overexpression of Fra1. Deletion of FRA1 in Δmrs3Δmrs4 cells showed increased oxidants and a severe growth defect under aerobic conditions. The synthetic growth defect and the increased DCF fluorescence were suppressed by deleting CCC1. These results suggest that a major function of Fra1 is to prevent the effect of oxidant stress on Ccc1. Increased vacuolar iron uptake itself leads to increased oxidant stress, which is most notable when Ccc1 is overexpressed. The increase in oxidant stress due to Ccc1 activity may reflect a mitochondrial response to low iron, as iron deprivation increases DFCDA fluorescence even in Rho° cells.
FRA1 encodes an aminopeptidase P-like protein; family members are manganese-containing enzymes that can hydrolyze peptides with an N-terminal Xaa-Pro motif. The function of Fra1 in this regard, however, has not yet been tested. Here we show that Fra1 was bound to Tsa1. The large scale genomic screen (14), confirmed by our findings (12), showed that Fra1 also bound to Grx3 and Grx4. The finding that Fra1 can interact with two different antioxidant systems suggests that it may play a generic role in sensing or modulating oxidant damage.
Acknowledgments
We express our appreciation to members of the Kaplan laboratory for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK052380 and DK035034 (to J. K.). This work was also supported by Center of Excellence in Molecular Hematology Grant 5P30DK72437.
- MVS
- mitochondria-vacuole signaling
- BPS
- bathophenanthroline disulfonate
- CM
- complete medium
- DCFDA
- dichlorodihydrofluorescein (DCF) diacetate
- YPD
- yeast extract/peptone/dextrose.
REFERENCES
- 1.Mense S. M., Zhang L. (2006) Cell Res. 16, 681–692 [DOI] [PubMed] [Google Scholar]
- 2.Liu Z., Butow R. A. (2006) Annu. Rev. Genet 40, 159–185 [DOI] [PubMed] [Google Scholar]
- 3.Chen O. S., Crisp R. J., Valachovic M., Bard M., Winge D. R., Kaplan J. (2004) J. Biol. Chem. 279, 29513–29518 [DOI] [PubMed] [Google Scholar]
- 4.Li L., Kaplan J. (2004) J. Biol. Chem. 279, 33653–33661 [DOI] [PubMed] [Google Scholar]
- 5.Foury F., Roganti T. (2002) J. Biol. Chem. 277, 24475–24483 [DOI] [PubMed] [Google Scholar]
- 6.Mühlenhoff U., Stadler J. A., Richhardt N., Seubert A., Eickhorst T., Schweyen R. J., Lill R., Wiesenberger G. (2003) J. Biol. Chem. 278, 40612–40620 [DOI] [PubMed] [Google Scholar]
- 7.Li L., Chen O. S., McVey Ward D., Kaplan J. (2001) J. Biol. Chem. 276, 29515–29519 [DOI] [PubMed] [Google Scholar]
- 8.Amberg D. C., Botstein D., Beasley E. M. (1995) Yeast 11, 1275–1280 [DOI] [PubMed] [Google Scholar]
- 9.Tuttle M. S., Radisky D., Li L., Kaplan J. (2003) J. Biol. Chem. 278, 1273–1280 [DOI] [PubMed] [Google Scholar]
- 10.Li L., Bagley D., Ward D. M., Kaplan J. (2008) Mol. Cell Biol. 28, 1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puig S., Vergara S. V., Thiele D. J. (2008) Cell Metab. 7, 555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumánovics A., Chen O. S., Li L., Bagley D., Adkins E. M., Lin H., Dingra N. N., Outten C. E., Keller G., Winge D., Ward D. M., Kaplan J. (2008) J. Biol. Chem. 283, 10276–10286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesuisse E., Knight S. A., Courel M., Santos R., Camadro J. M., Dancis A. (2005) Genetics 169, 107–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho Y., Gruhler A., Heilbut A., Bader G. D., Moore L., Adams S. L., Millar A., Taylor P., Bennett K., Boutilier K., Yang L., Wolting C., Donaldson I., Schandorff S., Shewnarane J., Vo M., Taggart J., Goudreault M., Muskat B., Alfarano C., Dewar D., Lin Z., Michalickova K., Willems A. R., Sassi H., Nielsen P. A., Rasmussen K. J., Andersen J. R., Johansen L. E., Hansen L. H., Jespersen H., Podtelejnikov A., Nielsen E., Crawford J., Poulsen V., Sørensen B. D., Matthiesen J., Hendrickson R. C., Gleeson F., Pawson T., Moran M. F., Durocher D., Mann M., Hogue C. W., Figeys D., Tyers M. (2002) Nature 415, 180–183 [DOI] [PubMed] [Google Scholar]
- 15.Wu C. Y., Bird A. J., Winge D. R., Eide D. J. (2007) J. Biol. Chem. 282, 2184–2195 [DOI] [PubMed] [Google Scholar]
- 16.Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H., Lee J. R., Lee S. S., Moon J. C., Yun J. W., Choi Y. O., Kim W. Y., Kang J. S., Cheong G. W., Yun D. J., Rhee S. G., Cho M. J., Lee S. Y. (2004) Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi-Iwai Y., Dancis A., Klausner R. D. (1995) EMBO J. 14, 1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felice M. R., De Domenico I., Li L., Ward D. M., Bartok B., Musci G., Kaplan J. (2005) J. Biol. Chem. 280, 22181–22190 [DOI] [PubMed] [Google Scholar]
- 19.Shaw G. C., Cope J. J., Li L., Corson K., Hersey C., Ackermann G. E., Gwynn B., Lambert A. J., Wingert R. A., Traver D., Trede N. S., Barut B. A., Zhou Y., Minet E., Donovan A., Brownlie A., Balzan R., Weiss M. J., Peters L. L., Kaplan J., Zon L. I., Paw B. H. (2006) Nature 440, 96–100 [DOI] [PubMed] [Google Scholar]
- 20.Milgrom E., Diab H., Middleton F., Kane P. M. (2007) J. Biol. Chem. 282, 7125–7136 [DOI] [PubMed] [Google Scholar]
- 21.Ojeda L., Keller G., Muhlenhoff U., Rutherford J. C., Lill R., Winge D. R. (2006) J. Biol. Chem. 281, 17661–17669 [DOI] [PubMed] [Google Scholar]
- 22.Pujol-Carrion N., Belli G., Herrero E., Nogues A., de la Torre-Ruiz M. A. (2006) J. Cell Sci. 119, 4554–4564 [DOI] [PubMed] [Google Scholar]