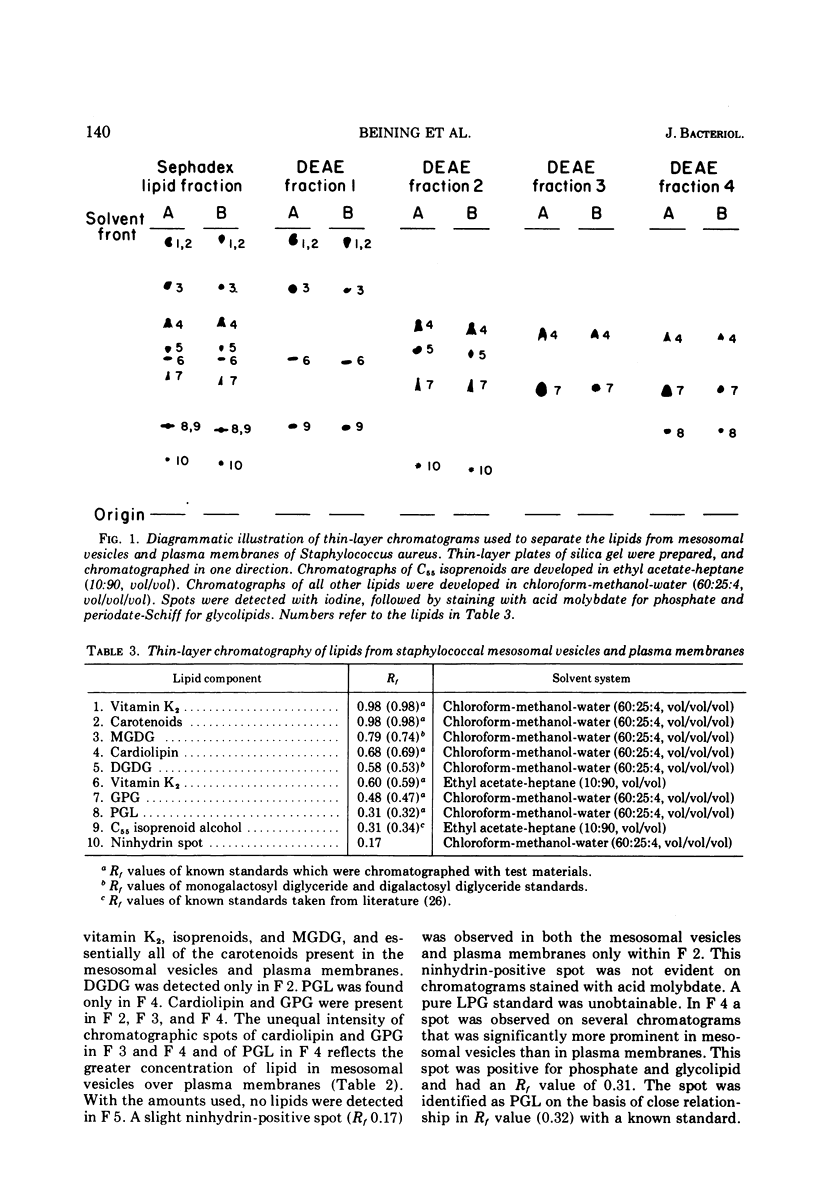
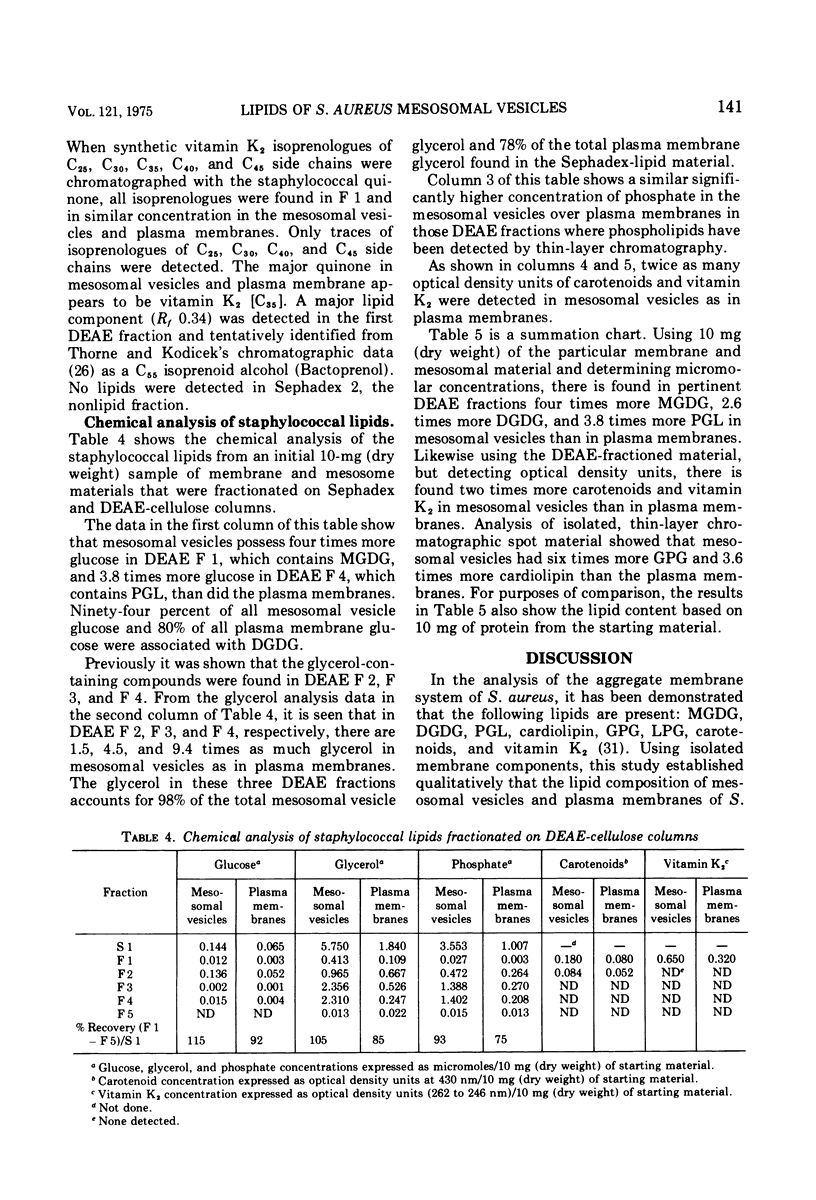
Abstract
Mesosomal vesicles and plasma membranes were isolated from Staphylococcus aureus ATCC 6538P by protoplasting and differential centrifugation. The lipids of each of the two membrane fractions were extracted with pyridine-acetic acid-N-butanol, and the nonlipid contaminants were removed by Sephadex treatment. The lipids were then separated by passage through diethylaminoethyl-cellulose columns and characterized by thin-layer chromatographic, chemical, and spectral analyses. The lipids were separated into four discrete diethylaminoethyl fractions: (i) vitamin K2, carotenoids, C55 isoprenoid alcohol, and monoglucosyl diglyceride; (ii) cardiolipin, carotenoids, phosphatidyl glycerol, diglucosyl diglyceride, and an unidentified ninhydrin-positive component; (iii) cardiolipid and phosphatidyl glyderol; (iv) cardiolipin, phosphatidyl glycerol, and phosphatidyl glucose. Qualitatively, no difference in lipid composition between mesosomal vesicles and plasma membranes was found. However, based on equal dry weights of membrane materials, a relative quantitative difference in the amount of specific lipids in mesosomal vesicles and plasma membranes was observed. There are 4 times more monoglucosyl diglyceride, 2.6 times more diglucosyl diglyceride, 3.8 times more phosphatidyl glucose, 2 times more carotenoids, and 2 times more vitamin K2 found in mesosomal vesicles than in plasma membranes. The concentration of cardiolipin and phosphatidyl glycerol is 3.6 and 6 times greater, respectively, in mesosomal vesicles.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Emdur L. I., Chiu T. H. Turnover of phosphatidylglycerol in Streptococcus sanguis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1137–1144. doi: 10.1016/s0006-291x(74)80097-5. [DOI] [PubMed] [Google Scholar]
- Glaser L., Lindsay B. The synthesis of lipoteichoic acid carrier. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1131–1136. doi: 10.1016/s0006-291x(74)80096-3. [DOI] [PubMed] [Google Scholar]
- Houtsmuller U. M., van Deenen L. L. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim Biophys Acta. 1965 Dec 2;106(3):564–576. doi: 10.1016/0005-2760(65)90072-x. [DOI] [PubMed] [Google Scholar]
- Huff E., Cole R. M., Theodore T. S. Lipoteichoic acid localization in mesosomal vesicles of Staphylococcus aureus. J Bacteriol. 1974 Oct;120(1):273–281. doi: 10.1128/jb.120.1.273-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., WHITE D. C., SMITH S. L. THE 2-DESMETHYL VITAMIN K2'S. A NEW GROUP OF NAPHTHOQUINONES ISOLATED FROM HEMOPHILUS PARAINFLUENZAE. Biochemistry. 1964 Jul;3:949–954. doi: 10.1021/bi00895a018. [DOI] [PubMed] [Google Scholar]
- POLONOVSKI J., WALD R., PAYSANT-DIAMENT M. [The lipids of Staphylococcus aureus]. Ann Inst Pasteur (Paris) 1962 Jul;103:32–42. [PubMed] [Google Scholar]
- Patch C. T., Landman O. E. Comparison of the biochemistry and rates of synthesis of mesosomal and peripheral membranes in Bacillus subtilis. J Bacteriol. 1971 Jul;107(1):345–357. doi: 10.1128/jb.107.1.345-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett P., Marmion B. P., Shaw E. J., Lemcke R. M. Immunochemical analysis of Mycoplasma pneumoniae. 3. Separation and chemical identification of serologically active lipids. Aust J Exp Biol Med Sci. 1969 Apr;47(2):171–195. doi: 10.1038/icb.1969.19. [DOI] [PubMed] [Google Scholar]
- Shaw N. The detection of lipids on thin-layer chromatograms with the periodate-Schiff reagents. Biochim Biophys Acta. 1968 Oct 22;164(2):435–436. doi: 10.1016/0005-2760(68)90171-9. [DOI] [PubMed] [Google Scholar]
- Short S. A., White D. C. Metabolism of the glycosyl diglycerides and phosphatidylglucose of Staphylococcus aureus. J Bacteriol. 1970 Oct;104(1):126–132. doi: 10.1128/jb.104.1.126-132.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Strominger J. L. Isolation of C 55 -isoprenylpyrophosphate from micrococcus lysodeikticus. J Biol Chem. 1972 Aug 25;247(16):5107–5112. [PubMed] [Google Scholar]
- TABER H. W., MORRISON M. ELECTRON TRANSPORT IN STAPHYLOCOCCI. PROPERTIES OF A PARTICLE PREPARATION FROM EXPONENTIAL PHASE STAPHYLOCOCCUS AUREUS. Arch Biochem Biophys. 1964 May;105:367–379. doi: 10.1016/0003-9861(64)90021-9. [DOI] [PubMed] [Google Scholar]
- Theodore T. S., Cole R. M., Huff E. Localization of glycerol phosphate in mesosomal vesicles of staphylococcus aureus. Biochem Biophys Res Commun. 1974 Jul 10;59(1):215–220. doi: 10.1016/s0006-291x(74)80195-6. [DOI] [PubMed] [Google Scholar]
- Theodore T. S., Panos C. Protein and fatty acid composition of mesosomal vesicles and plasma membranes of Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):571–576. doi: 10.1128/jb.116.2.571-576.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore T. S., Popkin T. J., Cole R. M. The separation and isolation of plasma membranes and mesosomal vesicles from Staphylococcus aureus. Prep Biochem. 1971;1(3):233–248. doi: 10.1080/00327487108081942. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Ellar D. J. Properties of plasma and mesosomal membranes isolated from Micrococcus lysodeikticus: rates of synthesis and characterisation of lipids. Biochim Biophys Acta. 1973 Aug 23;316(2):180–195. doi: 10.1016/0005-2760(73)90008-8. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Kodicek E. The structure of bactoprenol, a lipid formed by lactobacilli from mevalonic acid. Biochem J. 1966 Apr;99(1):123–127. doi: 10.1042/bj0990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


