Abstract
Adipocyte fatty acid-binding protein (A-FABP) has emerged as an important mediator of inflammation in macrophages. Macrophage-selective ablation of A-FABP alone is sufficient to prevent the development of high cholesterol diet-induced atherosclerosis in apoE-deficient mice. However, the precise mechanisms whereby A-FABP modulates inflammation remain elusive. Here, we report that A-FABP forms a finely tuned positive loop between JNK and activator protein-1 (AP-1) to exacerbate lipopolysaccharide (LPS)-induced inflammatory responses in macrophages. Real time PCR and luciferase reporter analysis showed that LPS induced A-FABP expression through transcriptional activation. This effect was mediated by JNK, which promoted the recruitment of c-Jun to a highly conserved AP-1 consensus binding motif located within the proximal region of the A-FABP promoter. LPS-induced transactivation of the A-FABP gene was diminished by either pharmacological inhibition of JNK or knocking down c-Jun or by mutating the AP-1 recognition site within the proximal region (−122 to −116 bp) of the A-FABP promoter. Conversely, the LPS-evoked phosphorylation of JNK, activation of AP-1, and production of pro-inflammatory cytokines were markedly attenuated by pharmacological or genetic suppression of A-FABP in macrophages. Furthermore, the LPS-induced elevation in A-FABP expression could also be prevented by the selective A-FABP inhibitor BMS309403. These findings support the notion that pharmacological inhibition of A-FABP represents a valid strategy for treating inflammation-related disorders such as atherosclerosis.
Keywords: Adipose Tissue, AP-1 Transcription Factor, Fatty Acid-binding Protein, Gene Expression, Inflammation, JNK, Jun Transcription Factor, Macrophage, Obesity
Introduction
Fatty acid-binding proteins are a family of small intracellular lipid chaperones that reversibly bind hydrophobic ligands, such as long chain fatty acids and eicosanoids, facilitating the intracellular diffusion of fatty acids between cellular compartments and enzymes (1, 2). Adipocyte fatty acid-binding protein (A-FABP,3 also known as aP2 and FABP4) is a member of the fatty acid-binding protein family that is abundantly expressed in adipose tissue (1, 2). In adipocytes, A-FABP regulates fatty acid storage and lipolysis. The latter effect is mediated by a direct interaction with hormone-sensitive lipase (3, 4). Mice deficient in A-FABP are partially protected against insulin resistance, dyslipidemia, and fatty liver disease in both genetic and dietary obesity (5–7).
In addition to its role in regulating lipid metabolism and insulin sensitivity, mounting evidence suggests that A-FABP is also a key player in inflammation. A-FABP is highly expressed in macrophages, where it regulates cholesterol ester accumulation and inflammatory responses (8). Macrophage-specific A-FABP deficiency protects against both early and advanced atherosclerosis in apolipoprotein E-deficient (apoE−/−) mice (8, 9). This protection is comparable with that observed in apoE−/− mice with total A-FABP deficiency, suggesting that the macrophage-specific actions of A-FABP are a predominant contributor to atherosclerotic lesion formation. At the molecular level, A-FABP deficiency in macrophages results in decreased production of a cluster of pro-inflammatory cytokines, such as tumor necrosis factor-α, interleukin-6, monocyte chemoattractant protein (MCP)-1, and interleukin-1β. These findings are corroborated by the observation that pharmacological inhibition of A-FABP by its selective inhibitors protects mice against atherosclerosis and compromises inflammatory responses in macrophage cells (10, 11). In addition, the pivotal role of A-FABP in inflammation is highlighted by the findings that A-FABP-deficient mice are resistant to develop several other inflammatory disorders, including allergic airway inflammation (12) and experimental autoimmune encephalomyelitis/multiple sclerosis (13).
Although initially thought to be an intracellular protein, recent studies showed that A-FABP is also secreted into the bloodstream in rodents and human (14, 15). The circulating concentration of A-FABP significantly correlates with several features of the metabolic syndrome, including central adiposity, dyslipidemia, fasting glucose, the indices of insulin resistance, and markers of inflammation (16, 17). Furthermore, an elevated level of serum A-FABP has been observed in a number of obesity-related inflammatory diseases, including type 2 diabetes, atherosclerosis, coronary heart disease, and nonalcoholic fatty liver disease (15, 18). A-FABP released from adipocytes suppresses cardiomyocyte contractile activity, implicating that it also functions as an endocrine factor (19). Taken in conjunction, these clinical and experimental data suggest that A-FABP might serve as an important mediator linking obesity with metabolic and cardiovascular diseases, partly through its pro-inflammatory actions in macrophages.
In macrophages, several pro-inflammatory stimuli have been shown to induce A-FABP expression, including phorbol 12-myristate 13-acetate, oxidized low density lipoproteins, and toll-like receptor agonists (8, 20–22). In particular, lipopolysaccharide (a major agonist of TLR4, is a potent inducer of A-FABP production in macrophages (21). TLR4, a key player in the innate immune system, is also actively involved in the etiology of obesity-related disorders such as insulin resistance and atherosclerosis (23). Interestingly, an earlier report demonstrated that LPS-induced pro-inflammatory responses are decreased in macrophages derived from A-FABP knock-out mice (8). However, the cellular mechanisms whereby LPS increases A-FABP expression and the precise role of A-FABP in LPS-induced pro-inflammatory pathways remain to be established.
To address these questions, we investigated the detailed signaling events underlying LPS-induced A-FABP expression in murine macrophages. Our results demonstrated that LPS induces the transactivation of the A-FABP gene through JNK, which in turn promotes the recruitment of c-Jun to a highly conserved activator protein-1 (AP-1) recognition site within the proximal region (−122 to −116 bp) of the A-FABP promoter. Interestingly, we found that A-FABP potentiates LPS-induced activation of the JNK/AP-1 signaling pathway, suggesting that it controls an autoregulatory loop aggravating the pro-inflammatory responses in macrophages.
EXPERIMENTAL PROCEDURES
Reagents
The antibody against c-Jun and siRNA against mouse c-Jun and scrambled siRNA were from Santa Cruz Biotechnology (Santa Cruz, CA), and antibodies against β-tubulin, phospho-JNK, and total JNK1 and ChIP grade protein G beads were from Cell Signaling Technology (Beverly, MA). Anti-mouse A-FABP was produced in-house by immunizing female New Zealand rabbits with recombinant mouse A-FABP expressed in Escherichia coli and was affinity-purified as described previously (24). TRIzol reagent, SYBR Green, and cell culture medium were purchased from Invitrogen. Superscript first-strand cDNA synthesis system was purchased from Promega (Madison, WI). Streptavidin-agarose beads and LPS (E. coli 026:B6) were from Sigma. The AP-1-luc reporter plasmid was obtained from Stratagene (La Jolla, CA). The A-FABP inhibitor BMS309403 was synthesized as described previously (25) and dissolved in dimethyl sulfoxide (DMSO).
Construction of Luciferase Reporter Vectors Driven by the Mouse A-FABP Promoters
The mouse A-FABP promoter/enhancer regions spanning from −5160 to +22 bp and −962 to +22 bp were subcloned into pGL3-Basic vector (Promega) to obtain the 5kb-luc reporter vector and 1kb-luc reporter vector, respectively. The putative AP-1-binding site was mutated by mutagenesis PCR using their wild type vectors as the templates. The constructs were confirmed by DNA sequencing. The sequences of all the primers used for the vector construction are listed in supplemental Table 1.
Transient Transfection and Luciferase Assay
The murine macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Cells were co-transfected with the luciferase reporters and siRNA (50 μm) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions, followed by treatment with various concentrations of LPS for different periods as specified in the figure legends. Afterward, cells were lysed in Reporter Lysis Buffer, and the luciferase activity was measured using Bright-GloTM luciferase assay system (Promega) as described previously (26). The siRNA sequences for A-FABP are listed in supplemental Table 2. A plasmid encoding a constitutively active JNK (27) was introduced into cells by electroporation using GenePulser Xcell (Bio-Rad).
Quantitative Real Time PCR
Total RNA was extracted from RAW 264.7 cells using TRIzol reagent. 1 μg of total RNA was reverse-transcribed into cDNA using Improm-II reverse. Each cDNA sample was analyzed for gene expression by quantitative real time PCR using the SYBR Green reagent on an Applied Biosystems Prism 7000 sequence detection system as described previously (24). Analysis was performed with ABI Prism 7000 SDS software. Sequences of the primers used for real time PCR are listed in supplemental Table 1.
Preparation of Nuclear Extracts
Nuclear extracts were prepared as described previously (28) with minor modifications. In brief, 80–90% confluent RAW 264.7 cells were harvested by scraping, washed in cold PBS, and incubated in three packed cell volumes of hypertonic buffer (10 mm HEPES, pH 8.0, 1.5 mm MgCl2, 200 mm sucrose, 0.5% Nonidet P-40, 10 mm KCl, 1 mm EDTA, 0.5 mm dithiothreitol plus protease and phosphatase inhibitors) for 5 min at 4 °C. The crude nuclei were collected by microcentrifugation, rinsed twice in hypertonic buffer, and resuspended in lysis buffer (PBS, pH 7.4, 1.0 mm EDTA, 2.5% glycerol, 1.0 mm dithiothreitol plus protease and phosphatase inhibitors). Nuclei were disrupted by sonication at 4 °C. The debris was removed by centrifugation at 4 °C and 13,500 × g for 10 min.
In Vitro DNA Protein Binding Assay
Binding of c-Jun to the A-FABP promoter DNA was assayed as described previously (29). Biotin-labeled oligonucleotide primers were synthesized by Invitrogen and used to amplify a 200-bp region in the mouse A-FABP promoter (−217 to −27) containing the putative AP-1 cis-element. The forward and reverse primer pairs used are listed in supplemental Table 1. The binding assay was performed by mixing 500 μg of nuclear extract proteins purified as above, 5 μg of biotin-labeled DNA, and 30 μl of streptavidin-agarose beads for 1 h at room temperature. Beads were then pelleted and washed four times with cold PBS. The binding proteins were eluted by boiling in 50 μl of SDS-PAGE loading sample buffer and separated on 10% SDS-PAGE, followed by Western blot analysis.
ChIP
The ChIP assay was performed as described previously (30) with minor modifications. 80–90% confluent RAW 264.7 cells were treated with or without LPS for various time periods and fixed with 1% formaldehyde for 15 min at room temperature. Cells were lysed, and chromatin was sheared by sonication at 4 °C. 25 μg of the lysates were incubated overnight at 4 °C with 2 μg of anti-c-Jun antibody or rabbit nonimmune IgG as negative control, followed by precipitation with ChIP grade protein G beads. The precipitates were washed extensively and eluted with the elution buffer (1% SDS, 0.1 m NaHCO3) at room temperature for 15 min. Input chromatin and immunoprecipitated chromatin were incubated at 65 °C overnight to reverse the cross-links. After digestion with protease K, DNA was extracted with phenol/chloroform and precipitated with ethanol. Purified DNA was analyzed by quantitative real time PCR using specific A-FABP promoter primers as listed in supplemental Table 1. All results were normalized to the respective input values.
Western Blot Analysis
60 μg of RAW 264.7 cell lysates were separated by SDS-PAGE and probed with various primary antibodies as indicated. The proteins were visualized by chemiluminescence detection, and the relative band densities were quantified using the MultiAnalyst software package (Bio-Rad) as described previously (24).
Statistical Analysis
Data are expressed as means ± S.D. Statistical significance was determined by one-way analysis of variance or Student's t test. In all statistical comparisons, a p value less than 0.05 was accepted to indicate a statistically significant difference.
RESULTS
LPS Induces A-FABP Expression through Transcriptional Activation in Macrophages
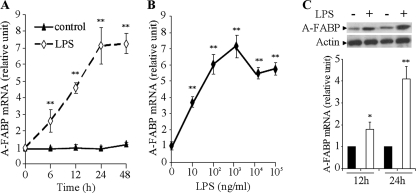
Quantitative real time PCR analysis showed that treatment of RAW 264.7 murine macrophage cells with LPS significantly increased the mRNA abundance of the A-FABP gene in a time- and concentration-dependent manner (Fig. 1, A and B). The mRNA abundance of A-FABP was increased approximately 3- and 7-fold, respectively, following stimulation with LPS at 1 and 100 ng/ml for 24 h. The stimulatory effect of LPS on A-FABP mRNA expression was slightly attenuated when its concentration was further increased to above 1000 ng/ml. Consistent with the changes in A-FABP mRNA abundance, the protein level of A-FABP in RAW 264.7 murine macrophages was also elevated following LPS stimulation (Fig. 1C). Induction of A-FABP mRNA and protein expression by LPS was also observed in human THP1 macrophages (data not shown).
FIGURE 1.
LPS potently induces A-FABP expression in RAW 264.7 macrophages. A, cells were stimulated with 100 ng/ml LPS or vehicle control, and the mRNA level of A-FABP was determined by quantitative real time PCR at the specified time points. B, RAW 264.7 cells were treated with various concentrations of LPS, and the mRNA level of A-FABP was determined 24 h after the treatment. C, protein level of intracellular A-FABP was analyzed by Western blotting at 12 and 24 h after stimulation with 100 ng/ml LPS. *, p < 0.05; **, p < 0.01 versus vehicle control at each time point (n = 5–6).
In the absence of LPS, treatment of RAW 264.7 macrophages with the transcription inhibitor actinomycin D for a period of 12 h did not cause a significant change in A-FABP mRNA abundance, suggesting that A-FABP mRNA is highly stable in this cell line (supplemental Fig. 1). However, the LPS-induced elevation of A-FABP mRNA was abolished when cells were incubated with actinomycin D, suggesting that the stimulatory effect of LPS on A-FABP mRNA expression in macrophages is due to the increased transcriptional activation but is not due to the enhanced mRNA stability.
LPS-evoked Transactivation of the A-FABP Gene Is Mediated by a Putative AP-1 Recognition Site Located between −122 and −116 bp of the Promoter
To further elucidate the molecular events by which LPS induces the transactivation of the A-FABP gene, we constructed two luciferase reporter vectors driven by 1 and 5 kb of the mouse A-FABP promoter, namely 1kb-luc and 5kb-luc. These reporter vectors were transfected into RAW 264.7 cells to test their response to LPS stimulation. This analysis revealed that LPS increased the luciferase activity driven by both the 1- and 5-kb A-FABP gene promoter (supplemental Fig. 2). Noticeably, time- and concentration-dependent studies showed that the kinetic changes of the luciferase activity driven by both 1- and 5-kb A-FABP promoters mirrored the changes in A-FABP mRNA abundance in response to LPS stimulation (Fig. 1). The maximal promoter activity was observed at 24 h after stimulation with 100 ng/ml LPS. The magnitude of the LPS-mediated increase of the luciferase activity was comparable between the 1- and 5-kb A-FABP promoters, suggesting that the LPS-responsive DNA element is located within the 1-kb promoter region of the A-FABP gene.
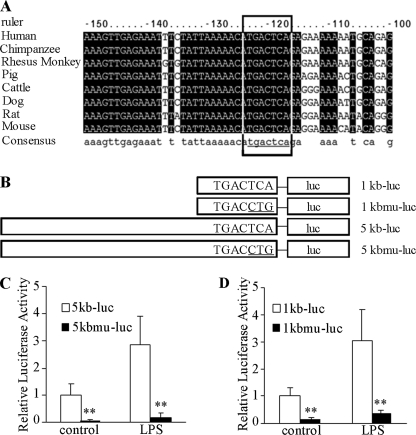
To map precisely the minimal cis-DNA element that mediates LPS-induced A-FABP expression, we analyzed potential transcription factor-binding sites within the 1-kb promoter region of the mouse A-FABP gene using Promoter 2.0 Prediction Server. This analysis identified a 7-bp DNA motif located between −122 and −116 bp of the mouse A-FABP gene promoter (Fig. 2A) that matches the consensus binding sequence for AP-1, a transcription factor that plays a key role in mediating the inflammatory responses and production of pro-inflammatory cytokines (31). Noticeably, this putative AP-1-binding site is highly conserved among eight mammalian species.
FIGURE 2.
Identification of a key promoter element responsible for LPS-induced transactivation of the A-FABP gene. A, multiple sequence alignment analysis of −150 to −100 bp of the A-FABP promoter region adjacent to the transcription initiation site from eight species. The 7-bp DNA motif inside the box is evolutionarily conserved and is identical to the AP-1 consensus recognition motif (TGACTCA). B, schematic presentation of luciferase reporter vectors driven by the 1-kb A-FABP gene promoter (1kb-luc), mutated 1-kb A-FABP gene promoter (1kbmu-luc), 5-kb A-FABP gene promoter (5kb-luc), and 5-kb mutated A-FABP gene promoter (5kb-luc). The three underlined nucleotides within the AP-1 recognition motif were mutated. C and D, comparison of the luciferase activity driven by the wild type and mutant A-FABP promoters as in B. RAW 264.7 macrophage cells were transfected with the luciferase reporter vector described in B, stimulated with 100 ng/ml LPS for 24 h, and then harvested for the luciferase assay. **, p < 0.01 versus wild type promoters (n = 4).
To investigate whether or not the putative AP-1 recognition site is indeed a critical cis-element mediating LPS-induced transactivation of the A-FABP gene, we generated two mutant luciferase reporter vectors, 1kbmu-luc and 5kbmu-luc, in which three nucleotides within the motif were mutated from 5′-TGACTCA-3′ to 5′-TGACCTG-3′ (Fig. 2A). In RAW 264.7 macrophage cells, the luciferase activity driven by either the 1- or 5-kb mutated promoter was decreased by ∼90% compared with their wild type form under basal conditions (Fig. 2, B and C). Furthermore, the mutation of these three nucleotides within the putative AP-1 cis-element led to an almost complete abolition of LPS-induced increase in luciferase activity, suggesting that the putative AP-1 recognition motif between −122 and −116 bp of the A-FABP promoter is indispensable for both basal and LPS-induced A-FABP expression in macrophage cells.
LPS Induces the Recruitment of c-Jun to the AP-1-binding Site within the A-FABP Promoter
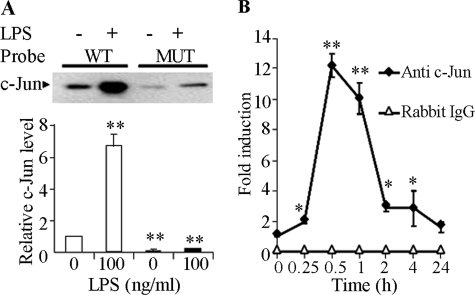
The transcription factor AP-1 is a heterodimeric protein consisting of the Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra1, and Fra2) families (32). We next investigated whether c-Jun mediates LPS-induced A-FABP expression through its interaction with the AP-1 recognition motif using a streptavidin pulldown assay (29). This analysis showed that the double-stranded DNA fragment spanning the sequence from −217 to −27 in the A-FABP promoter was bound to c-Jun derived from nuclear extracts of the macrophage cells, and that the interaction was further increased by more than 6-fold upon stimulation with LPS (Fig. 3A). By contrast, this interaction was abrogated by mutation of the three nucleotides within the AP-1 recognition motif, suggesting that c-Jun is indeed physically associated with the 7-bp cis-DNA element within the A-FABP promoter.
FIGURE 3.
LPS induces the recruitment of c-Jun to the A-FABP gene promoter through the AP-1 recognition site. A, streptavidin pulldown assay to detect the complex formation between c-Jun and the A-FABP promoter in vitro. Nuclear extract proteins from RAW 264.7 cells treated without or with 100 ng/ml LPS were incubated with a biotinylated wild type (WT) mouse A-FABP promoter spanning from −217 to −27 bp or a mutant (MUT) A-FABP promoter in which the three nucleotides within the AP-1 recognition motif were mutated as in Fig. 2. Following precipitation using streptavidin-coupled agarose beads, the proteins in the complexes were separated by SDS-PAGE and probed with anti c-Jun. The bar chart in the lower panel shows the relative amount of c-Jun that binds to the A-FABP promoter probes. Open bars, wild type probes; black bars, mutant probes. Data are expressed as fold of the wild type promoter DNA probe in untreated cells. **, p < 0.01 versus untreated wild type control group (n = 3). B, ChIP assay to quantify the interaction between c-Jun and the endogenous A-FABP promoter. Chromatin isolated from RAW 264.7 cells treated with 100 ng/ml LPS for various periods was subjected to immunoprecipitation using anti-c-Jun antibody as described under “Experimental Procedures.” The relative abundance of the 200-bp A-FABP promoter enriched by ChIP was quantified by real time PCR and expressed as fold of untreated control. *, p < 0.05; **, p < 0.01 versus the untreated control group (n = 4).
To further confirm the binding of c-Jun to the endogenous A-FABP gene promoter, RAW 264.7 cells were treated with LPS for various periods and subjected to ChIP assay using rabbit anti-c-Jun antibody or nonimmune rabbit IgG as a negative control (Fig. 3B). Quantitative real time PCR analysis showed that the soluble chromatin samples obtained from each time point (input) had equal amounts of chromatin fragments containing the A-FABP promoter. The association between c-Jun and the endogenous A-FABP promoter was detectable in untreated cells, started to increase at 15 min, and peaked at 30 min after LPS stimulation, after which the association was gradually diminished. This result suggests that LPS induces the recruitment of c-Jun to the endogenous A-FABP promoter in a temporal manner.
LPS-induced A-FABP Expression Is Dependent on the JNK/AP-1 Signaling Cascade
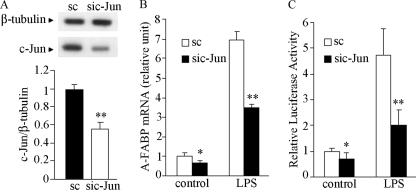
We next investigated whether c-Jun is directly involved in LPS-induced A-FABP expression by knocking down its expression in RAW 264.7 cells. Transfection of c-Jun siRNA for 24 h led to over a 50% decrease in the protein level of c-Jun compared with that transfected with the scrambled control (Fig. 4A). Knockdown of c-Jun expression by siRNA inhibited A-FABP mRNA expression by 36% in untreated cells and 48% in LPS-treated cells respectively (Fig. 4, B and C). Accordingly, both basal and LPS-stimulated promoter activity of the A-FABP gene was significantly reduced in cells transfected with c-Jun siRNA.
FIGURE 4.
Knockdown of c-Jun expression attenuates LPS-induced transcriptional activation of the A-FABP gene in murine macrophages. A, Western blot analysis for c-Jun protein levels from RAW 264.7 cells transfected with siRNA specific to c-Jun (sic-Jun) or scrambled control (sc) for 24 h. The bar chart below is the densitometric quantification of the blot. B, real time PCR analysis for A-FABP mRNA abundance in cells transfected with c-Jun siRNA or scrambled control in response to stimulation with 100 ng/ml LPS. C, effect of knocking down c-Jun on the 1-kb A-FABP promoter activity in the absence or presence of 100 ng/ml LPS for 24 h. *, p < 0.05; **, p < 0.01 versus the scrambled control group (n = 5–6).
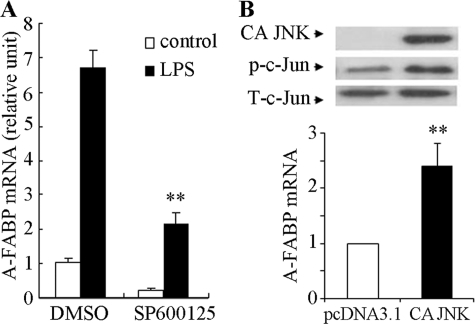
The c-Jun NH2-terminal kinase (JNK), also known as stress-activated MAPK, enhance AP-1-dependent transcriptional activity of c-Jun by phosphorylating c-Jun at Ser-63 and Ser-73 (33, 34). We next tested the role of JNK in regulating A-FABP expression. Inhibition of JNK by its chemical inhibitor SP600125 markedly suppressed both basal and LPS-stimulated A-FABP expression (Fig. 5A). Furthermore, the ectopic expression of a constitutively active form of JNK alone was sufficient to increase A-FABP mRNA expression even in the absence of LPS stimulation (Fig. 5B). Taken together, these data suggest JNK as a key mediator in LPS-induced A-FABP expression in macrophages.
FIGURE 5.
JNK is a key regulator of A-FABP expression in murine macrophages. A, pharmacological inhibition of JNK suppresses LPS-induced A-FABP expression. RAW 264.7 cells were treated with the JNK inhibitor SP600125 (5 μm) for 2 h prior to stimulation with 100 ng/ml LPS for 24 h. The A-FABP mRNA level was determined by real time PCR. B, ectopic expression of constitutively active JNK induces A-FABP expression. RAW 264.7 cells were transfected with an empty plasmid (pcDNA3.1) or a plasmid encoding hemagglutinin-tagged constitutively active JNK for 48 h. The expression of constitutively active JNK (CA JNK) and phosphorylation of c-Jun were analyzed by Western blotting (upper panel). The relative A-FABP mRNA abundance was quantified by real time PCR (lower panel). **, p < 0.01 versus control (n = 5).
A-FABP Mediates LPS-induced Activation of JNK/AP-1 Signaling Cascade and Production of Pro-inflammatory Cytokines
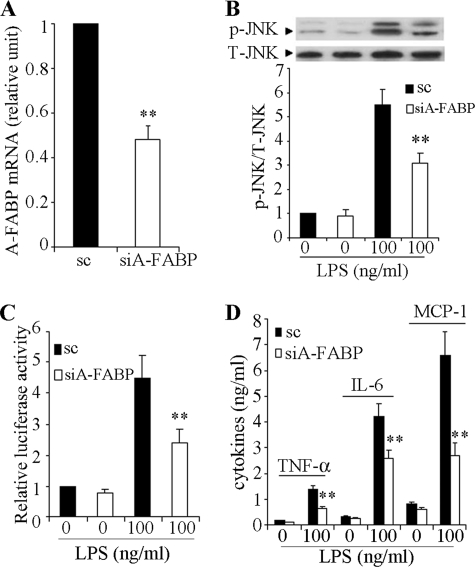
A number of recent studies demonstrate that A-FABP is an important player in inflammation, actively participating in the pro-inflammatory responses evoked by various stimuli (21, 22, 35). We therefore speculate that there might be a positive feedback loop between A-FABP and JNK/AP-1, which exacerbates LPS-induced inflammation in macrophages. We tested this hypothesis by knocking down A-FABP expression in RAW 264.7 cells. Transfection of A-FABP-specific siRNA for 24 h resulted in an ∼50% reduction in A-FABP expression compared with scrambled control (Fig. 6A). This decrease in A-FABP expression was accompanied by a significant reduction in LPS-induced phosphorylation of JNK, activation of the AP-1 transcriptional activity, and secretion of a panel of pro-inflammatory cytokines, including tumor necrosis factor α, interleukin-6, and MCP-1 (Fig. 6, B–D).
FIGURE 6.
LPS-induced activation of the JNK/AP-1 signaling cascade and production of pro-inflammatory cytokines are compromised by knocking down A-FABP expression in macrophages. RAW 264.7 cells were transfected with A-FABP-specific siRNA (siA-FABP) or scrambled control (sc) for 48 h. A, A-FABP expression as determined by real time PCR. B, cells were treated without or with 100 ng/ml LPS for 10 min, and phosphorylation of JNK was determined by semi-quantitative Western blot. C, luciferase activity in cells transfected with an AP-1 reporter vector. The cells were co-transfected with the reporter vector and siRNA for 24 h, followed by stimulation with 100 ng/ml LPS for another 24 h. D, levels of pro-inflammatory cytokines (tumor necrosis factor α (TNFα), interleukin-6 (IL6), and MCP-1) in the conditioned medium. **, p < 0.01 versus scrambled control (n = 5–7).
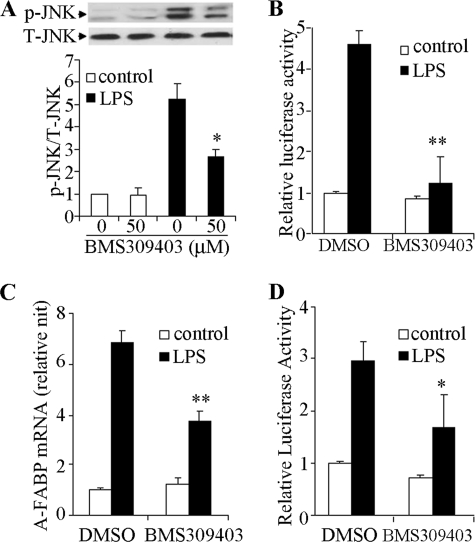
Treatment of RAW 264.7 cells with BMS309403, a selective inhibitor of A-FABP (10), also attenuated phosphorylation of JNK (Fig. 7A) and reduced the transactivation activity of AP-1 (Fig. 7B) in response to LPS stimulation. Interestingly, the LPS-induced elevation of endogenous A-FABP expression and the A-FABP promoter activity were also significantly suppressed by the A-FABP-selective inhibitor BMS309403 (Fig. 7, C and D), further highlighting the existence of a positive feedback loop that modulates A-FABP expression and inflammation. Real time PCR and Western blot analysis demonstrated that either pharmacological inhibition of A-FABP by BMS309403 or siRNA-mediated knockdown of A-FABP expression had no obvious effect on either mRNA or protein levels of c-Jun (data not shown) or JNK (Fig. 6B and Fig. 7A) under basal or LPS-stimulated conditions.
FIGURE 7.
Selective A-FABP inhibitor BMS309403 attenuates LPS-induced activation of the JNK/AP-1 signaling pathway and A-FABP expression in macrophages. RAW 264.7 cells were pretreated with BMS309403 (50 μm) for 2 h followed by stimulation with 100 ng/ml LPS. A, Western blot analysis to detect JNK phosphorylation at 10 min after stimulation with or without LPS. B, luciferase activity in cells transfected with an AP-1 reporter vector for 24 h in the absence or presence of LPS. C, real time PCR analysis for A-FABP mRNA abundance in the absence or presence of LPS and/or BMS309403 as indicated for 24 h. D, luciferase activity driven by 1-kb A-FABP promoter at 24 h after LPS stimulation. *, p < 0.05; **, p < 0.01 versus the control group without BMS309403 (n = 5–6).
DISCUSSION
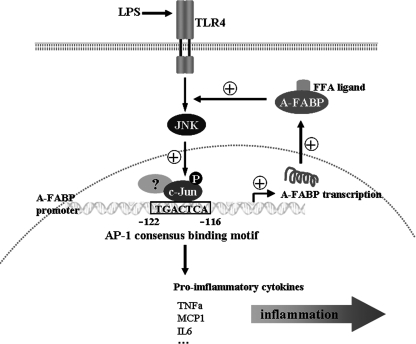
This study provides evidence showing that A-FABP potentiates LPS-induced inflammation by forming a positive feedback loop with the JNK signaling cascade (Fig. 8). In response to LPS stimulation, activated JNK increases A-FABP expression by inducing the phosphorylation of c-Jun, which in turn binds to a highly conserved AP-1 cis-element within the A-FABP gene promoter and enhances the gene transcription. Conversely, elevated A-FABP potentiates LPS-elicited JNK phosphorylation and subsequent activation of the AP-1 complex. Interestingly, pharmacological inhibition of A-FABP not only reduces LPS-induced JNK phosphorylation and AP-1 activity but also decreases A-FABP expression by suppressing the transcriptional activation of the gene promoter, suggesting the existence of an autoregulatory mechanism that tightly controls the feedback loop between A-FABP, JNK, and its downstream target c-Jun.
FIGURE 8.
Schematic representation of the molecular pathway whereby A-FABP mediates the inflammatory responses by forming a positive feedback loop with JNK and AP-1 in macrophages. FFA, free fatty acids; TNFα, tumor necrosis factor α; IL6, interleukin-6.
Consistent with our findings, an intimate connection between A-FABP and JNK has been implicated in a number of previous animal studies demonstrating that JNK and A-FABP null mice exhibit similar protection against obesity-induced insulin resistance, metabolic syndrome, and fatty liver disease (5, 8, 36–39). Furthermore, genetic ablation of A-FABP and JNK2 (one of the three JNK isoforms) renders apoE-deficient mice a comparable degree of resistance to high cholesterol diet-induced atherosclerosis (38). In apoE-deficient mice, JNK is selectively activated in the atherosclerotic lesion area (38), and this change is accompanied by elevated A-FABP expression.4
A growing body of evidence suggests JNK as a central mediator of obesity-related pathologies, including insulin resistance, type 2 diabetes, and vascular dysfunction (37, 39). In both dietary and genetic obesity, the activity of JNK is markedly elevated in several metabolically active organs, especially in adipose tissue (37). Activated JNK can directly induce insulin resistance by increasing serine phosphorylation of insulin receptor substrates 1 and 2 (IRS1 and IRS2), thereby preventing them from being recruited to activated insulin receptors. In addition, JNK in macrophages can cause insulin resistance indirectly, by mediating stress-induced production of pro-inflammatory cytokines, which in turn trigger local and systemic inflammation (36). Selective ablation of JNK1 in either adipose tissue or macrophages is sufficient to alleviate high fat diet-induced systemic insulin resistance and glucose intolerance (36, 39). Likewise, obesity-induced macrophage infiltration in adipose tissue and production of pro-inflammatory cytokines are markedly attenuated by selective ablation of JNK1 in hematopoietic cells (mainly macrophages), suggesting a central role of JNK in obesity-related inflammation (36). Because both adipocytes and macrophages are the major sites of A-FABP expression, it is conceivable that A-FABP and JNK act in a synergistic manner to perpetuate macrophage infiltration and inflammation in adipose tissue, consequently leading to systemic insulin resistance and metabolic syndrome.
Another notable finding of this study is that both siRNA-mediated knockdown of A-FABP and pharmacological inhibition of this protein attenuate LPS-induced activation of JNK and its downstream AP-1 complex in macrophages, suggesting that A-FABP is required for the maximal activation of the JNK signaling. In support of this notion, the oral administration of the A-FABP inhibitor BMS309403 in mice decreases obesity-induced JNK activation in adipose tissue (10). However, the mechanism by which A-FABP potentiates JNK activation is unknown at this stage. Two members of the MAPK kinase family, including MKK4 and MKK7, are directly involved in JNK activation by inducing dual phosphorylation on threonine and tyrosine residues (40). In addition, JNK activity can be modulated by its interacting proteins (41). Beyond its role as a lipid chaperone, A-FABP also modulates signal transduction and gene expression by fatty acid-dependent interaction with a number of intracellular proteins, including HSL(3), Jak2 (42), and peroxisome proliferator-activated receptor γ (43). The peroxisome proliferator-activated receptor agonists induce the expression of c-Jun in HT-29 colon cancer cells (44). However, this study found that neither pharmacological nor genetic inhibition of A-FABP affects the expression of c-Jun or JNK. Noticeably, a recent study showed that A-FABP mediates lipids-induced endoplasmic reticulum stress in macrophages (45). The endoplasmic reticulum stress has been shown to be a potent activator of JNK (46). Whether or not A-FABP potentiates LPS-induced activation of the JNK/c-Jun signaling pathway through endoplasmic reticulum stress warrants further investigation.
This study shows that the potentiating effect of A-FABP on JNK activation is dependent on its fatty acid binding capacity, because the LPS-induced phosphorylation of JNK and activation of the AP-1 complex are largely abrogated by BMS309403, a selective inhibitor of A-FABP that competitively binds within the fatty acid-binding pocket and inhibits the binding of A-FABP to endogenous free fatty acids (25). These findings further support the notion that A-FABP is at the crossroads of lipid metabolism and inflammation and also raise the possibility that long chain fatty acids-induced JNK activation in macrophages may also depend on A-FABP.
In summary, this study shows that A-FABP, along with JNK and AP-1, forms a finely tuned positive feedback loop to perpetuate inflammatory responses in macrophages. Disruption of this feedback loop by molecular or pharmacological inhibition of either A-FABP or JNK causes a similar effect in attenuating JNK activation and production of pro-inflammatory cytokines. Indeed, both JNK and A-FABP have been proposed as promising therapeutic targets for obesity-related metabolic disorders and atherosclerosis (1, 47). The small molecule inhibitors of both JNK and A-FABP have been shown to be efficacious in alleviating insulin resistance, hyperglycemia, fatty liver, and atherosclerosis in rodent models (10, 47). However, the use of small molecule JNK inhibitors for treating obesity-related metabolic disease and atherosclerosis faces several formidable challenges. First, there exist three isoforms of JNK involved in a wide range of biological functions, such as cell growth, differentiation, and apoptosis (48). Second, JNK1 and JNK2 are expressed in almost all the cells and tissues, and it appears difficult to develop an isoform-specific inhibitor that would inhibit JNK specifically in adipose tissue or macrophages. In this connection, A-FABP inhibitors may have a more favorable selectivity than JNK inhibitors, because the expression of A-FABP is restricted mainly to adipocytes and macrophages. Therefore, pharmacological inhibition of A-FABP may represent a promising strategy to selectively block the inflammation associated with obesity and atherosclerosis.
Supplementary Material
This work was supported by Research Grants Council of Hong Kong, the National Natural Science Foundation of China Joint Research Scheme (Projects N_HKU 735/08 and 30831160518), and the Collaborative Research Fund (HKU 2/07C) from the Research Grants Council of Hong Kong.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1 and 2.
P. Vanhoutte and A. Xu, unpublished observations.
- A-FABP
- adipocyte fatty acid-binding protein
- LPS
- lipopolysaccharide
- ChIP
- chromatin immunoprecipitation
- JNK
- c-Jun NH2-terminal kinases
- TLR
- toll-like receptor
- SAPK
- stress-activated MAPK
- MAPK
- mitogen-activated protein kinase
- siRNA
- small interfering RNA.
REFERENCES
- 1.Furuhashi M., Hotamisligil G. S. (2008) Nat. Rev. Drug Discov. 7, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hertzel A. V., Bernlohr D. A. (2000) Trends Endocrinol. Metab. 11, 175–180 [DOI] [PubMed] [Google Scholar]
- 3.Jenkins-Kruchten A. E., Bennaars-Eiden A., Ross J. R., Shen W. J., Kraemer F. B., Bernlohr D. A. (2003) J. Biol. Chem. 278, 47636–47643 [DOI] [PubMed] [Google Scholar]
- 4.Smith A. J., Thompson B. R., Sanders M. A., Bernlohr D. A. (2007) J. Biol. Chem. 282, 32424–32432 [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil G. S., Johnson R. S., Distel R. J., Ellis R., Papaioannou V. E., Spiegelman B. M. (1996) Science 274, 1377–1379 [DOI] [PubMed] [Google Scholar]
- 6.Maeda K., Cao H., Kono K., Gorgun C. Z., Furuhashi M., Uysal K. T., Cao Q., Atsumi G., Malone H., Krishnan B., Minokoshi Y., Kahn B. B., Parker R. A., Hotamisligil G. S. (2005) Cell Metab. 1, 107–119 [DOI] [PubMed] [Google Scholar]
- 7.Uysal K. T., Scheja L., Wiesbrock S. M., Bonner-Weir S., Hotamisligil G. S. (2000) Endocrinology 141, 3388–3396 [DOI] [PubMed] [Google Scholar]
- 8.Makowski L., Boord J. B., Maeda K., Babaev V. R., Uysal K. T., Morgan M. A., Parker R. A., Suttles J., Fazio S., Hotamisligil G. S., Linton M. F. (2001) Nat. Med. 7, 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boord J. B., Maeda K., Makowski L., Babaev V. R., Fazio S., Linton M. F., Hotamisligil G. S. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 1686–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuhashi M., Tuncman G., Görgün C. Z., Makowski L., Atsumi G., Vaillancourt E., Kono K., Babaev V. R., Fazio S., Linton M. F., Sulsky R., Robl J. A., Parker R. A., Hotamisligil G. S. (2007) Nature 447, 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hertzel A. V., Hellberg K., Reynolds J. M., Kruse A. C., Juhlmann B. E., Smith A. J., Sanders M. A., Ohlendorf D. H., Suttles J., Bernlohr D. A. (2009) J. Med. Chem. 52, 6024–6031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shum B. O., Mackay C. R., Gorgun C. Z., Frost M. J., Kumar R. K., Hotamisligil G. S., Rolph M. S. (2006) J. Clin. Invest. 116, 2183–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds J. M., Liu Q., Brittingham K. C., Liu Y., Gruenthal M., Gorgun C. Z., Hotamisligil G. S., Stout R. D., Suttles J. (2007) J. Immunol. 179, 313–321 [DOI] [PubMed] [Google Scholar]
- 14.Xu A., Wang Y., Xu J. Y., Stejskal D., Tam S., Zhang J., Wat N. M., Wong W. K., Lam K. S. (2006) Clin. Chem. 52, 405–413 [DOI] [PubMed] [Google Scholar]
- 15.Xu A., Tso A. W., Cheung B. M., Wang Y., Wat N. M., Fong C. H., Yeung D. C., Janus E. D., Sham P. C., Lam K. S. (2007) Circulation 115, 1537–1543 [DOI] [PubMed] [Google Scholar]
- 16.Yeung D. C., Xu A., Tso A. W., Chow W. S., Wat N. M., Fong C. H., Tam S., Sham P. C., Lam K. S. (2009) Diabetes Care 32, 132–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabre A., Lazaro I., Cofan M., Jarauta E., Plana N., Garcia-Otin A. L., Ascaso J. F., Ferre R., Civeira F., Ros E., Masana L. (April11, 2009) J. Lipid Res., 10.1194/jlr.M800626-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milner K. L., van der Poorten D., Xu A., Bugianesi E., Kench J. G., Lam K. S., Chisholm D. J., George J. (2009) Hepatology 49, 1926–1934 [DOI] [PubMed] [Google Scholar]
- 19.Lamounier-Zepter V., Look C., Alvarez J., Christ T., Ravens U., Schunck W. H., Ehrhart-Bornstein M., Bornstein S. R., Morano I. (2009) Circ. Res. 105, 326–334 [DOI] [PubMed] [Google Scholar]
- 20.Fu Y., Luo N., Lopes-Virella M. F., Garvey W. T. (2002) Atherosclerosis 165, 259–269 [DOI] [PubMed] [Google Scholar]
- 21.Kazemi M. R., McDonald C. M., Shigenaga J. K., Grunfeld C., Feingold K. R. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1220–1224 [DOI] [PubMed] [Google Scholar]
- 22.Pelton P. D., Zhou L., Demarest K. T., Burris T. P. (1999) Biochem. Biophys. Res. Commun. 261, 456–458 [DOI] [PubMed] [Google Scholar]
- 23.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. (2006) J. Clin. Invest. 116, 3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui X., Zhu W., Wang Y., Lam K. S., Zhang J., Wu D., Kraegen E. W., Li Y., Xu A. (2009) J. Biol. Chem. 284, 14050–14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulsky R., Magnin D. R., Huang Y., Simpkins L., Taunk P., Patel M., Zhu Y., Stouch T. R., Bassolino-Klimas D., Parker R., Harrity T., Stoffel R., Taylor D. S., Lavoie T. B., Kish K., Jacobson B. L., Sheriff S., Adam L. P., Ewing W. R., Robl J. A. (2007) Bioorg. Med. Chem. Lett. 17, 3511–3515 [DOI] [PubMed] [Google Scholar]
- 26.Xu A., Wong L. C., Wang Y., Xu J. Y., Cooper G. J., Lam K. S. (2004) FEBS Lett. 572, 129–134 [DOI] [PubMed] [Google Scholar]
- 27.Zheng C., Xiang J., Hunter T., Lin A. (1999) J. Biol. Chem. 274, 28966–28971 [DOI] [PubMed] [Google Scholar]
- 28.Deng W. G., Wu K. K. (2003) J. Immunol. 171, 6581–6588 [DOI] [PubMed] [Google Scholar]
- 29.Wu K. K. (2006) Methods Mol. Biol. 338, 281–290 [DOI] [PubMed] [Google Scholar]
- 30.Wang Q. F., Lauring J., Schlissel M. S. (2000) Mol. Cell. Biol. 20, 9203–9211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vigouroux C., Maachi M., Nguyên T. H., Coussieu C., Gharakhanian S., Funahashi T., Matsuzawa Y., Shimomura I., Rozenbaum W., Capeau J., Bastard J. P. (2003) AIDS 17, 1503–1511 [DOI] [PubMed] [Google Scholar]
- 32.Ransone L. J., Verma I. M. (1990) Annu. Rev. Cell Biol. 6, 539–557 [DOI] [PubMed] [Google Scholar]
- 33.Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. (1991) Nature 353, 670–674 [DOI] [PubMed] [Google Scholar]
- 34.Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. (1991) Nature 354, 494–496 [DOI] [PubMed] [Google Scholar]
- 35.Fu Y., Luo N., Lopes-Virella M. F. (2000) J. Lipid Res. 41, 2017–2023 [PubMed] [Google Scholar]
- 36.Solinas G., Vilcu C., Neels J. G., Bandyopadhyay G. K., Luo J. L., Naugler W., Grivennikov S., Wynshaw-Boris A., Scadeng M., Olefsky J. M., Karin M. (2007) Cell Metab. 6, 386–397 [DOI] [PubMed] [Google Scholar]
- 37.Hirosumi J., Tuncman G., Chang L., Görgün C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002) Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- 38.Ricci R., Sumara G., Sumara I., Rozenberg I., Kurrer M., Akhmedov A., Hersberger M., Eriksson U., Eberli F. R., Becher B., Borén J., Chen M., Cybulsky M. I., Moore K. J., Freeman M. W., Wagner E. F., Matter C. M., Lüscher T. F. (2004) Science 306, 1558–1561 [DOI] [PubMed] [Google Scholar]
- 39.Sabio G., Das M., Mora A., Zhang Z., Jun J. Y., Ko H. J., Barrett T., Kim J. K., Davis R. J. (2008) Science 322, 1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis R. J. (2000) Cell 103, 239–252 [DOI] [PubMed] [Google Scholar]
- 41.Dickens M., Rogers J. S., Cavanagh J., Raitano A., Xia Z., Halpern J. R., Greenberg M. E., Sawyers C. L., Davis R. J. (1997) Science 277, 693–696 [DOI] [PubMed] [Google Scholar]
- 42.Thompson B. R., Mazurkiewicz-Muñoz A. M., Suttles J., Carter-Su C., Bernlohr D. A. (2009) J. Biol. Chem. 284, 13473–13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan N. S., Shaw N. S., Vinckenbosch N., Liu P., Yasmin R., Desvergne B., Wahli W., Noy N. (2002) Mol. Cell. Biol. 22, 5114–5127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimada T., Kojima K., Yoshiura K., Hiraishi H., Terano A. (2002) Gut 50, 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., Hotamisligil G. S. (2009) Nat. Med. 15, 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 47.Bennett B. L., Satoh Y., Lewis A. J. (2003) Curr. Opin. Pharmacol. 3, 420–425 [DOI] [PubMed] [Google Scholar]
- 48.Lin A. (2003) BioEssays 25, 17–24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.