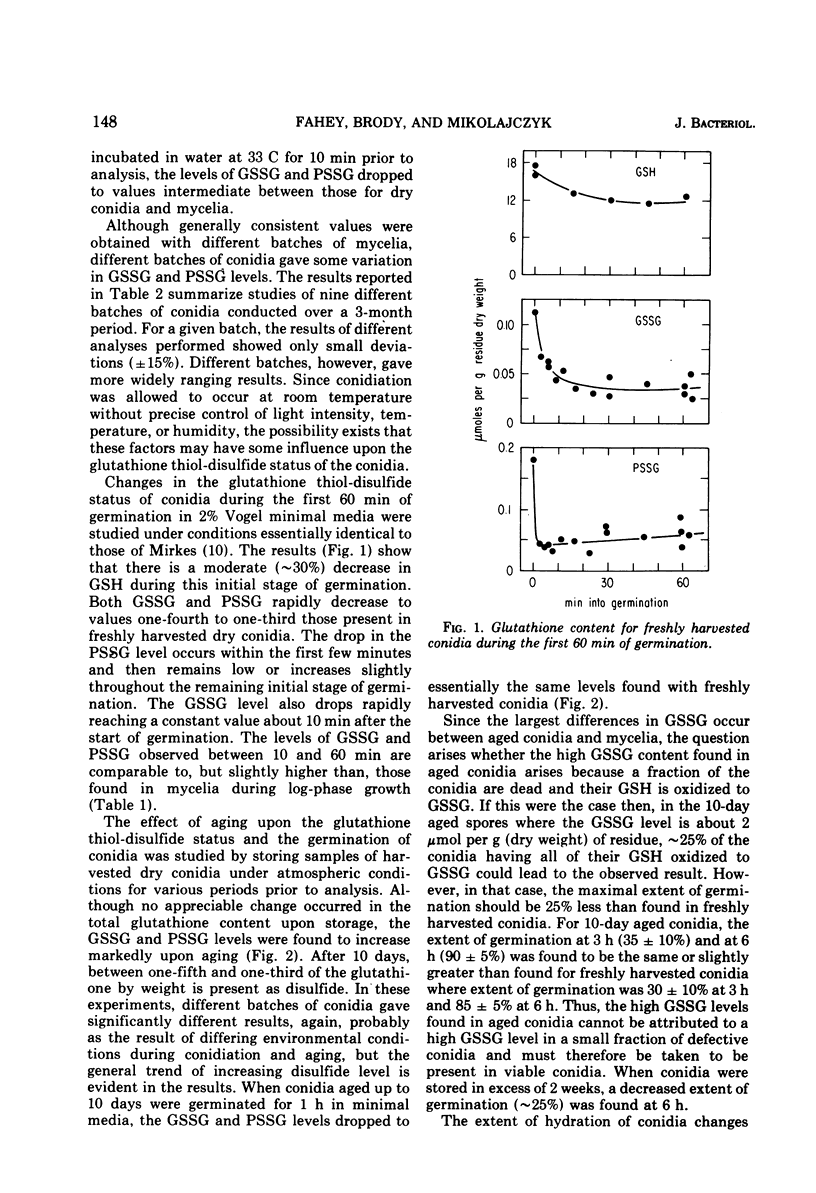
Abstract
Improved methods were developed for the determination of reduced glutathione (GSH), glutathione disulfide (GSSG), and protein-glutathione disulfide (PSSG) and applied to determine the glutathione status at various stages of the asexual life cycle for the band strain of Neurospora crassa. The GSH-GSSG ratio in freshly harvested dry conidia was found to be about 150 but decreased to around 6 when dryconidia were aged (stored) for 10 days after harvest. When conidia were germinated, this ratio increased to about 300 during the first 10 min of the 6-h germination process. In mycelia, during log-phase growth, the ratio was about 10-3. Changes in the ratio occurred primarily through changes in the GSSG content, which ranges from about 0.023 (mycelia) to 2(10-day aged conidia) mumol per g (dry weight) of residue, whereas GSH levels varied by a factor of about two. The PSSG content varied from 0.02 (mycelia) to 0.6 (10-day aged conidia) mumol per g (dry weight) of residue and generally paralleled the GSSG content. The results demonstrate the potential importance of thiol-disulfide reactions as a mechanism for the control of physiological properties associated with dormancy, and the observed changes in GSSG level are found to be compatible with the view that GSSG plays a role in the regulation of protein synthesis through control of polysome formation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOTT K. F., LUNDGREN D. G. THE RELATIONSHIP OF SULFHYDRYL AND DISULFIDE CONSTITUENTS OF BACILLUS CEREUS TO RADIORESISTANCE. Radiat Res. 1964 Feb;21:195–211. [PubMed] [Google Scholar]
- Barron E. S., Singer T. P. ENZYME SYSTEMS CONTAINING ACTIVE SULFHYDRYL GROUPS. THE ROLE OF GLUTATHIONE. Science. 1943 Apr 16;97(2520):356–358. doi: 10.1126/science.97.2520.356. [DOI] [PubMed] [Google Scholar]
- Cheng H. M., Aronson A. I., Holt S. C. Role of glutathione in the morphogenesis of the bacterial spore coat. J Bacteriol. 1973 Mar;113(3):1134–1143. doi: 10.1128/jb.113.3.1134-1143.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin G., Martic P. A., Doughty G. Kinetics of the reaction of N-ethylmaleimide with cysteine and some congeners. Arch Biochem Biophys. 1966 Sep 9;115(3):593–597. doi: 10.1016/0003-9861(66)90079-8. [DOI] [PubMed] [Google Scholar]
- Güntherberg H., Rost J. The true oxidized glutathione content of red blood cells obtained by new enzymic and paper chromatographic methods. Anal Biochem. 1966 May;15(2):205–210. doi: 10.1016/0003-2697(66)90025-x. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Vanderhoff G. A., Kosower E. M. Glutathione. 8. The effects of glutathione disulfide on initiation of protein synthesis. Biochim Biophys Acta. 1972 Jul 31;272(4):623–637. [PubMed] [Google Scholar]
- Mirkes P. E. Polysomes, ribonucleic acid, and protein synthesis during germination of Neurospora crassa conidia. J Bacteriol. 1974 Jan;117(1):196–202. doi: 10.1128/jb.117.1.196-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan R. D., Hoagland M. B. Cytoplasmic control of protein synthesis in rat liver. Biochim Biophys Acta. 1971 Nov 19;247(4):609–620. doi: 10.1016/0005-2787(71)90696-4. [DOI] [PubMed] [Google Scholar]
- SAKAI H., DAN K. Studies on sulfhydryl groups during cell division of sea urchin egg. I. Glutatione. Exp Cell Res. 1959 Jan;16(1):24–41. doi: 10.1016/0014-4827(59)90192-2. [DOI] [PubMed] [Google Scholar]
- Sargent M. L., Woodward D. O. Genetic determinants of circadian rhythmicity in Neurospora. J Bacteriol. 1969 Feb;97(2):861–866. doi: 10.1128/jb.97.2.861-866.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik O. A., Hoagland M. B., Pfefferkorn L. C., Bishop E. A. Inhibitors of protein synthesis in rat liver microsome fractions. J Biol Chem. 1967 Jan 10;242(1):131–139. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- VINTER V. Changes in radioresistance of sporulating cells of Bacillus cereus. Nature. 1961 Feb 18;189:589–590. doi: 10.1038/189589a0. [DOI] [PubMed] [Google Scholar]


