Abstract
Myogenesis is a process whereby myoblasts differentiate and fuse into multinucleated myotubes, the precursors of myofibers. Various signals and factors modulate this process, and glucocorticoids (GCs) are important regulators of skeletal muscle metabolism. We show that glucocorticoid-induced leucine zipper (GILZ), a GC-induced gene, and the newly identified isoform long GILZ (L-GILZ) are expressed in skeletal muscle tissue and in C2C12 myoblasts where GILZ/L-GILZ maximum expression occurs during the first few days in differentiation medium. Moreover, we observed that GC treatment of myoblasts, which increased GILZ/L-GILZ expression, resulted in reduced myotube formation, whereas GILZ and L-GILZ silencing dampened GC effects. Inhibition of differentiation caused by GILZ/L-GILZ overexpression correlated with inhibition of MyoD function and reduced expression of myogenin. Notably, results indicate that GILZ and L-GILZ bind and regulate MyoD/HDAC1 transcriptional activity, thus mediating the anti-myogenic effect of GCs.
Keywords: Development Differentiation/Muscle, Hormones/Steroid, Cell Differentiation, Chromatin Histone Modification, Protein-Protein Interactions, GILZ, Glucocorticoids, HDAC1, MyoD, Myogenesis
Introduction
Glucocorticoids (GCs)3 are important agents widely employed in the therapy of inflammatory, autoimmune, and neoplastic diseases (1). They regulate cell survival, proliferation, and differentiation by modulating the expression of a variety of molecules and signaling cascades, in many cells and tissues. In particular, GCs are potent modulators of skeletal muscle metabolism, regulating the expression of contractile proteins and promoting muscle atrophy in vivo and in vitro (2, 3). Moreover, GC receptor activation takes part in angiotensin II-related muscle wasting (4). Recent reports have shown that activation of FoxO proteins and the consequent activation of the ubiquitin-proteasome pathway represent the molecular mechanisms responsible for GC-mediated muscle atrophy (5, 6). Nevertheless, GC effects on differentiating myoblasts have not been extensively investigated. In fact, despite the evidence that dexamethasone (DEX) treatment results in reduced myogenesis and inhibition of the activation of the adult stem cells in skeletal muscle tissue known as satellite cells (7, 8), the molecular determinants of these biological effects are still poorly understood.
We have previously identified a GC-induced, 15-kDa protein that we named glucocorticoid-induced leucine zipper (GILZ), which mediates some of the effects of GCs, such as regulation of thymocyte survival (9, 10), inhibition of NF-κB transcriptional activity (11–14), counteraction of extracellular signal-regulated kinases (ERKs) 1/2 activation (15, 16), and inhibition of Ras-driven cell proliferation and oncogenic Ras-dependent transformation (17). GILZ expression is not restricted to lymphoid cells, and GILZ has been shown to play regulatory roles in adipocytes, osteoblasts, and tubular renal cells (18–21). Moreover, this factor is expressed in a variety of tissues, including skeletal muscle tissue (22).
Myogenesis is a multistep process by which undifferentiated mononucleated precursors, the myoblasts, differentiate and fuse into multinucleated myotubes. This process takes place during skeletal muscle tissue development and during regeneration of damaged skeletal muscle tissue; in this latter case, skeletal muscle adult stem cells, the satellite cells, become activated, proliferate, and differentiate into fusion-competent myoblasts (23, 24). The myogenic development program is tightly regulated, in which myoblasts exit the cell cycle and express the muscle-related factors, including MyoD, which is the best characterized. MyoD activation represents the convergence of several signals from the plasma membrane to the nucleus, such as the activation of the pro-myogenic kinases p38 and Akt by the receptor for insulin and insulin-like growth factors 1 and 2 (25), the receptor of advanced glycation end products (26), and the cell-to-cell contact signaling mediators CDO (cell adhesion molecule-related/down-regulated by oncogenes) and N-Cadherin (27, 28). MyoD, which belongs to the basic helix-loop-helix protein superfamily, is expressed in proliferating, undifferentiated myoblasts, exhibiting a nuclear localization. In the nucleus MyoD is bound to Id1 and HDAC1, which render MyoD inactive (24, 29, 30). Sustained p38 and Akt kinase activities promote MyoD effects by enhancing its activation and binding to co-factors such as chromatin regulators (31–33). Activation of MyoD leads to the induction of myogenin, an early differentiation marker that is a muscle-related factor itself and is required for cell-to-cell fusion (24). Intriguingly, during the differentiation process, not all myoblasts undergo fusion; a fraction of them enter quiescence after cell cycle withdrawal and constitute a pool of reserve cells that reactivate, proliferate, and fuse after muscle damage to repair damaged myofibers. The events regulating the preservation of this pool of undifferentiated cells are still poorly understood. It is known that members of the TGF-β superfamily (i.e. TGF-β and myostatin), which are expressed and released by differentiating cells, act in an autocrine manner to counteract MyoD transcriptional activity and promote quiescence (34, 35).
In this study, we analyzed the expression and role of GILZ in GC-mediated inhibition of myogenic differentiation. During the course of these studies, we observed that, in addition to the 15-kDa GILZ, differentiating myoblasts expressed a 28-kDa GILZ isoform that we named long GILZ (L-GILZ). We found that both GILZ and L-GILZ were induced by DEX and contributed to DEX anti-myogenic effects. Moreover, GILZ and L-GILZ expression spontaneously rose during myoblast differentiation. Notably, both GILZ isoforms affected HDAC1 activity on myogenin promoter and thus inhibited MyoD transcriptional activity. Collectively, our data show that GILZ and L-GILZ mediate the DEX anti-myogenic effect during myoblast differentiation.
EXPERIMENTAL PROCEDURES
Cloning of L-GILZ
We designed primers according to the sequence uc009ulb.1 (University of California Santa Cruz (UCSC) Genome Browser mouse data base), which represents the longest transcript of GILZ gene. Total RNA isolated from C2C12 cells was extracted with TRIzol reagent (Invitrogen) and retro-transcribed with SuperScript III (Invitrogen). The primers used were 5′-CACTCCCCTTCTCACTCTGC-3′ (sense) and 5′-GAACTTTATAAGCAGTCATCCC-3′ (antisense).
Cell Cultures and Reagents
Primary myoblasts were isolated from 7-day-old C57Bl6/J mice using a modification of the procedure described by Rando and Blau (36). In brief, the cells were isolated from dissected muscle by trypsin (Invitrogen) treatment and were preplated onto tissue culture plastic twice for 1 h. The cells were cultured in DMEM with 20% fetal bovine serum. C2C12 myoblasts were obtained by Dr. Pier Lorenzo Puri (The Burnham Institute, La Jolla, CA) and were cultivated in DMEM, 20% fetal calf serum (growth medium (GM)) or differentiation medium (DM) (DMEM, 2% horse serum) at 37 °C with 5% CO2. TGF-β, trichostatin A (TSA), and DEX were purchased from Sigma-Aldrich.
Cell Transfections and Plasmids
C2C12 were transfected with Lipofectamine 2000 (Invitrogen). 24 h after transfection, the cells were switched to DM to induce myogenic differentiation. We transfected C2C12 with an enhanced green fluorescent protein vector to evaluate transfection efficiency (ranging from 40 to 45%) by fluorescence-activated cell sorter analysis. GILZ-Myc and L-GILZ-FLAG were cloned in pcDNA3.1 (Invitrogen), pcDNA3-MyoD-HA was a gift from Dr. Milena Grossi (University of Rome, “La Sapienza,” Rome); pGL3-myogenin-luc (MyoG-luc) was a gift from Dr. Pier Lorenzo Puri (The Burnham Institute, La Jolla, CA).
Retroviral Infections
shRNA for GILZ and L-GILZ knockdown were inserted into pSUPER.retro.puro (Oligoengine). The target sequences were: GILZ, 5′-CAAUUUCUCCAUCUCCUUC-3′; L-GILZ, 5′-CACUGACAAGCUGAACAAC-3′; GILZ/L-GILZ, 5′-ACAGCUUCACCUGACAAUG-3′; and scramble, 5′-GUACCGGACGAGUUAGAAC-3′. Retroviruses were generated with Phoenix packaging cells following the Nolan Lab protocol. 48 h after transfection, the medium was collected, filtered, and added with polybrene to a suspension of 105 C2C12 cells.
Real Time PCR Analysis
Total RNA was extracted using TRIzol (Invitrogen). Reverse transcription-PCR was done using QuantiTect reverse transcription (Qiagen). For real time PCR, the primers were: for GILZ, sense 5′-GGTGGCCCTAGACAACAAGA-3′ and antisense 5′-TCTTCTCAAGCAGCTCACGA-3′; for L-GILZ, sense 5′-ACCGCAACATAGACCAGACC-3′ and antisense 5′-TCTTCTCAAGCAGCTCACGA-3′; and for glyceraldehyde-3-phosphate dehydrogenase, sense 5′-GCCTTCCGTGTTCCTACCC-3′ and antisense 5′-CAGTGGGCCCTCAGAUGC-3′. PCR was done in CHROMO 4 (MJ Research Bio Rad, Milan, Italy) using a DyNAmo HS SYBR GREEN qPCR kit (Finnzymes; Celbio). Relative amounts of GILZ, L-GILZ, and glyceraldehyde-3-phosphate dehydrogenase mRNA were calculated by the Comparative ΔΔC(t) method. The C(t) values were determined using Opticon Monitor 2 software (MJ Research Bio Rad).
May-Grunwald-Giemsa Staining
The differentiation rate was determined by calculating the myogenic index as a percentage of nuclei in myotubes relative to total nuclei. 48 h after DM, the cells were fixed with paraformaldehyde (4% in PBS) for 10 min at room temperature and stained with May-Grunwald-Giemsa (Carlo Erba, Milan) as previously described (26). The cells were viewed in a phase contrast microscope (Olympus IX51) equipped with a digital camera (Olympus C-5050 ZOOM), and the images were acquired at 20× magnification. The images were analyzed using UTHSCSA ImageTool software for calculation of the myogenic index. We considered myotubes only multinucleated cells containing at least three nuclei.
Immunofluorescence
Cells seeded directly on glass coverslips were subjected to transfection after adherence. The cells were fixed with 4% paraformaldehyde for 10 min at room temperature and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 30 min at room temperature. After three washes with PBS, the cells were treated with sodium borohydride (Sigma-Aldrich) 1 mg/ml for 5 min at room temperature, washed again with PBS, and blocked overnight (3% bovine serum albumin and 1% glycine in PBS). Primary and secondary Abs were incubated for 1 h at room temperature in PBS, 3% bovine serum albumin, and 0.1% Triton X-100. Primary Abs were: mouse mAb anti-Myc (Invitrogen); mouse mAb anti-FLAG (Sigma-Aldrich). The anti-mouse secondary Ab was Alexa-Fluor 568-conjugated (Molecular Probes). Image acquisition was performed using a Leica (Milano, Italy) microscope equipped with Diagnostic Instruments Spot RT Color camera under oil immersion (Sigma) at 100× magnification; image merging was obtained using Diagnostic Instruments Spot Advance software.
For GILZ protein endogenous detection, the myoblasts were stained with GILZ monoclonal antibody (eBioscience) and mounted with Prolong antifade (Invitrogen). Confocal photomicrographs of immunostained tubules were acquired with a Leica TCS SP2 (Leica) equipped with three laser lines (argon 488, 543, and 633 nm). Each channel was acquired separately using specific laser lines to avoid bleed-through of the fluorochromes. The photomicrographs were acquired with LAS AF Software (Leica) at 1024 × 1024 pixels.
Immunohistochemistry
To detect myosin heavy chain (MyHC) by immunocytochemistry, C2C12 myoblasts cultivated in DM were fixed in cold methanol at −20 °C for 7 min and subjected to immunocytochemistry with a monoclonal anti-developmental MyHC antibody (Biogenesis) at a 1:1,000 dilution. The immune reaction product was visualized using the Vectastain Elite ABC kit (Vector Laboratories Inc.). The images were acquired at 20× magnification.
Immunoprecipitation
For endogenous co-immunoprecipitation (co-IP) assays, whole cell extracts were prepared with nondenaturing lysis buffer (10 mm Tris, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 5 mm EGTA). Immunoprecipitations were performed in co-IP buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 5 mm EDTA); antigen-antibody complexes were precipitated with protein A bound to agarose beads (Sigma-Aldrich) prior to SDS-PAGE. For IP rabbit anti-MyoD Ab (Santa Cruz) and control isotype Ab (Sigma-Aldrich) were used. For exogenous co-IP assays, HEK293 cells were transfected with different combinations of expression vectors for GILZ-Myc, MyoD-HA, HDAC1-FLAG, and HDAC2-FLAG. Total proteins were immunoprecipitated with an anti-Myc Ab (Invitrogen), and co-IP was revealed with anti-HA (Santa Cruz) or anti-FLAG (Sigma-Aldrich) Abs.
Western Blot
The proteins were separated on a SDS-PAGE and subjected to Western blotting as previously described (37). The following primary Abs were used: anti-GILZ (Santa Cruz), mAb anti-MyoD (Novocastra), mAb anti-myogenin (BD Pharmingen), mAb anti-MyHC (Novocastra), mAb anti-HDAC1 (Upstate Biotech), mAb anti-β-tubulin (Sigma-Aldrich), mAb anti-Myc and mAb anti-HA (Invitrogen), and mAb anti-FLAG (Sigma-Aldrich). Horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary Abs were from Thermo Scientific. Polyvinylidene difluoride membrane (Hybond plus) was from Amersham Biosciences. Western blot films were scanned, and band signal intensities were determined using Scion Image software (Scion Corporation).
Luciferase Assay
MyoD activity on myogenin promoter was performed using a kit from Roche Applied Science, following the manufacturer's instructions. Luciferase activity was measured 6 h after switching transfected C2C12 cells to DM. Western blot analysis with anti-HA, anti-FLAG, and anti-Myc Abs was assessed as described in the previous paragraph to check the protein levels of all ectopically expressed proteins (data not shown).
Chromatin Immunoprecipitation (ChIP)
The anti-HDAC1 and anti-acetyl-histone H3 (Upstate Biotech) ChIP was performed according to the protocol of ChIP assay kit from Upstate Biotech. The experiments were performed using 5 × 106 cells for sample. 2 μg of anti-HDAC1 or anti-acetyl-histone H3 or nonimmune rabbit IGG was added to the cell lysates for immunoprecipitations. After elution, the samples were deproteinated, and DNA was resuspended after precipitation with 20 μl of double distilled H2O. The samples were quantified by real time PCR using an Mx3000P real time PCR system (Stratagene). The sequences of the primers against the mouse myogenin promoter region used for CHIP were as follows: forward, 5′-GAATCACATGTAATCCACTGGA-3′, and reverse, 5′- ACGCCAACTGCTGGGTGCCA-3′. As negative control for CHIP PCR, a nonpromoter genomic region was amplified with the following primers: forward, 5′-TCAGAACCCAACTCCTTTGG-3′, and reverse, 5′-GCCTTCACAAGAGCAGGAAC-3′. The relative amount of immunoprecipitated DNA fragments were determined based on the threshold cycle (Ct) for each PCR product (38). The data were quantitatively analyzed according to the formula 2−Δ[Ct(IP)−Ct(input)] − 2−Δ[Ct(control IgG)−Ct(input)].
Enzyme-linked Immunosorbent Assay
Supernatants from differentiating C2C12 were collected, and TGF-β content was evaluated by sandwich enzyme-linked immunosorbent assay, following the manufacturer's recommendation. Anti-TGF-β antibody was purchased from Pharmingen.
Statistical Analysis
All of the experiments were repeated at least three times. Student's t test was used with the STATPAC computerized program for data analysis, and p < 0.05 was considered significant.
RESULTS
GILZ Is Expressed in Muscle, and L-GILZ Is an Alternative Transcript Encoded by GILZ Gene
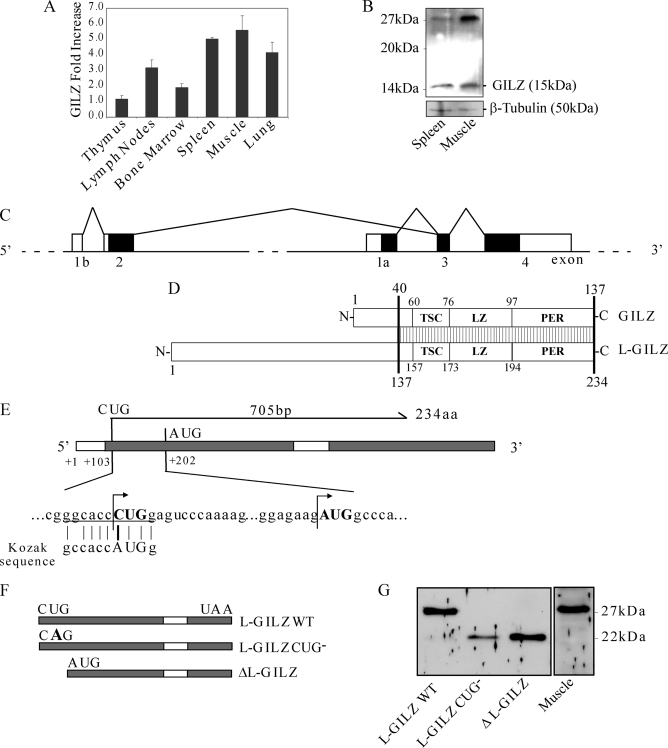
It has been previously reported that GILZ is expressed in a variety of human tissues (22). With real time PCR, we analyzed the expression of GILZ mRNA in different mouse tissues (Fig. 1A). We found that GILZ is expressed in various tissues, including adult skeletal muscle where its expression is comparable with that in lymphoid tissues, such as spleen (Fig. 1A). These results were confirmed by Western blot analysis (Fig. 1B). Interestingly, in addition to the expected 15-kDa GILZ band, a 28-kDa band was detected both in spleen and muscle extracts (Fig. 1B). Different GILZ splice variants, arising from alternative transcript of the first exon in the GILZ gene have been already described (16), but no one translates a protein of a putative molecular mass of ∼28 kDa. To investigate the nature of this protein and its possible relationship to GILZ, we initially performed a homology search in the UCSC Genome Browser BLAT search tool using GILZ cDNA sequence (GenBankTM accession number AF024519). Fig. 1C shows a schematic map of the analysis for GILZ genomic locus. Because a splice variant generated by exon 1b-2 that splices on GILZ exon 3 and 4 has been previously described (16), we investigated its expression in C2C12 murine myoblast cell line. Primers corresponding to the 5′- and 3′-untranslated regions were used for reverse transcriptase PCR of total RNA from C2C12 myoblasts. PCR product was cloned into pcDNA3.1 vector, and we named this sequence L-GILZ (GenBankTM accession number EU 818782). The longest open reading frame (ORF) predicted by software analysis starting from the first ATG, at position +202, is 22 kDa, but transient transfection of HEK293 cells with pcDNA3.1-L-GILZ resulted in the expression of a ∼28-kDa protein, similar to the band detected in muscle tissue (Fig. 1G, first lane). To explain this discrepancy, we postulated that this new variant starts from a noncanonical non-AUG translation start codon. Indeed, a noncanonical non-AUG (CUG) translation start codon is present at position +103, which is characterized by a high degree of homology with the Kozak consensus sequence (Fig. 1E). This hypothetical splice variant generated a transcript with an ORF of 705 bp and encodes a protein of 234 amino acids with a predictive molecular mass of 26-kDa that differed from GILZ protein only in the N-terminal part (National Center for Biotechnology Information (NCBI) accession number ACJ09091). This transcript shared an identical conserved TGF-β-stimulated clone (TSC) box, leucine zipper domain, and C-terminal region with GILZ (Fig. 1D). To confirm the involvement of the alternative translational start site, we generated a L-GILZ-CUG− mutant in which the thymidine of the starting codon of the cDNA was replaced by an adenosine (CUG to CAG). This mutation impaired L-GILZ transcription, and only a shorter protein product was detected following transfection (Fig. 1G, second lane). Thus, disruption of the starting CUG codon allowed the translation of a protein starting from a downstream in-frame AUG codon, characterized by a molecular mass of ∼22 kDa (Fig. 1, E and F). Next, we generated a GILZ mutant, named ΔL-GILZ, in which DNA started from position +202, deleting the part including the CUG start codon (Fig. 1F). Transfection of this mutant form in HEK293 cells induced the expression of a 22-kDa protein exhibiting an electrophoretic migration profile identical to that of the L-GILZ-CUG− mutant (Fig. 1G, third lane). Taken together, these results demonstrate that the new GILZ transcript variant L-GILZ generates a 28-kDa protein, which is characterized by a non-AUG translational start codon (NCBI accession number ACJ09091).
FIGURE 1.
A, GILZ mRNA expression in murine tissues. Total mRNA was isolated from both immune system-related and not related adult mouse tissue and underwent real time PCR analysis for GILZ mRNA semi-quantitative expression related to the housekeeping gene as described under “Experimental Procedures” (n = 3). GILZ mRNA from fresh thymus was used as unit. B, Western blot analysis of total protein extracted from adult mouse skeletal muscle and spleen. An anti-GILZ antibody recognizes the expected GILZ band and a higher molecular mass band that is expressed both in muscle and spleen. Western blot of β-tubulin is included to show total protein loading. C, schematic representation of GILZ locus. The conventional GILZ exons are named 1a, 3, and 4; exons 1b and 2 are alternative exons, and they represent the origin of a putative alternative GILZ isoform. D, schematic representation of GILZ alternative isoforms. The two predicted proteins share an amino acidic sequence that includes the TGF-β-stimulated clone box (TSC), leucine zipper domain (LZ), and proline- and glutamic acid-rich region (PER); the striped box represents region of identity. E, representation of L-GILZ ORF. Translation starts at a noncanonical CUG start codon, which is related to the most conserved Kozak sequence. F and G, mutagenic analysis of L-GILZ ORF. In F mutational strategy is schematically represented. Starting from L-GILZ ORF, a mutant carrying a point mutation on the CUG translation start site (L-GILZ-CUG−) and a mutant carrying a deletion upstream of the canonical AUG start codon (ΔL-GILZ) were generated. In G, WT L-GILZ and the mutants L-GILZ-CUG−and ΔL-GILZ were transfected in HEK293 cells. Western blot analyses with anti-GILZ antibody confirmed that the noncanonical CUG start codon is the translation start site and revealed that synthesis of a shorter protein (∼22 kDa) is allowed when the CUG codon is inactivated or when the ORF region upstream of the canonical AUG is deleted. WT, wild type.
Glucocorticoids Inhibit Myoblast Differentiation in a GILZ/L-GILZ-dependent Manner
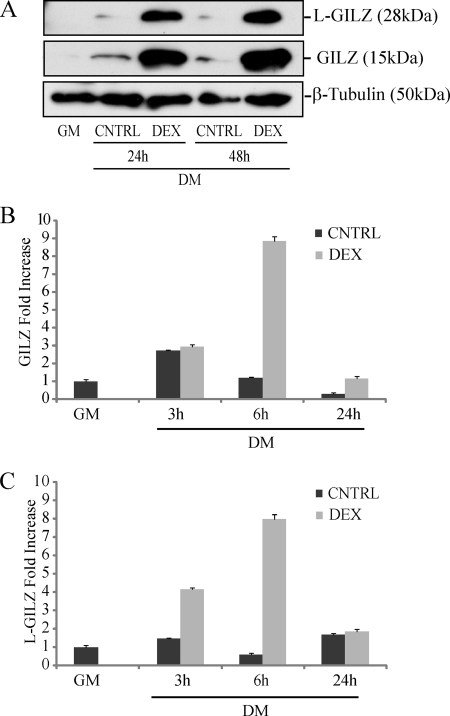
GILZ has been previously described as a GC-induced protein (9, 11). Because GCs are involved in skeletal muscle metabolism both at a physiological and pharmacological level, we treated them with DEX undifferentiated primary myoblasts derived from neonatal muscle of C57BL/6 mice. We performed Western blot analysis in myoblasts during differentation in vitro (DM) with or without DEX 10−6 m treatment. The results indicated that GILZ isoforms were not present in undifferentiated myoblasts (maintained in GM), whereas their expression was induced in differentiation conditions. Moreover, GILZ and L-GILZ were strongly up-regulated by DEX 10−6 m treatment as evidenced by protein expression (Fig. 2A). Real time analysis confirmed the up-regulation of GILZ and L-GILZ mRNA expression induced by DEX (Fig. 2, B and C). Together these results indicated that GILZ and L-GILZ are expressed in murine myoblast during differentiation and that GCs control L-GILZ as well as GILZ expression, as previously shown in other tissues (9, 21).
FIGURE 2.
DEX up-regulates GILZ and L-GILZ expression in primary myoblast cultures. A, Western blot analyses of GILZ/L-GILZ in primary myoblasts induced to differentiate for a 48-h absence (CNTRL) or in the presence of 10−6 m DEX. B and C, real time analysis of GILZ (B) and L-GILZ (C) expression in primary myoblasts induced to differentiate for 24 h absence (CNTRL) or in presence of 10−6 m DEX (n = 3).
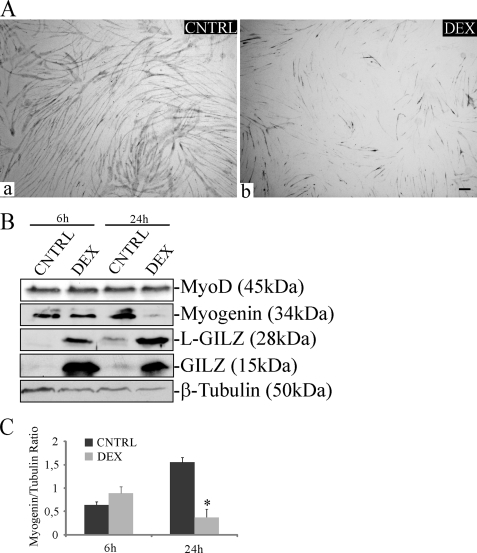
Next, to address the role of GILZ and L-GILZ in GC effects during myogenic differentiation, we employed C2C12 myoblasts, an established model of in vitro myogenesis (39). Morphological analyses, evaluated by staining of MyHC, a muscle differentiation marker, revealed a reduction of myotube formation in DEX-treated cells compared with the untreated controls (Fig. 3A). We also found that DEX treatment at 10−5 m, a concentration that is known to be pro-atrogenic in differentiated myotubes (5), caused a sustained induction of GILZ and L-GILZ expression (Fig. 3B) and reduced C2C12 myogenic potential at early differentiation stages. Indeed, in addition to a reduction of the fusion index, we observed a significant reduction of myogenin expression (Fig. 3B). On the contrary, MyoD protein did not undergo significant regulation (Fig. 3B).
FIGURE 3.
DEX reduces myogenic differentiation and up-regulates GILZ and L-GILZ expression in C2C12 myoblast cultures. A, C2C12 myoblasts were induced to differentiate for 48 h in absence (CNTRL, panel a) or in presence (panel b) of 10−5 m DEX. The cultures were subjected to MyHC staining. Bar, 50 μm. B, Western blot analyses of GILZ/L-GILZ induction by DEX and of expression of biochemical markers of differentiation in CNTRL and DEX-treated cells at the indicated time points. C, densitometric analysis of the Western blots from B are shown in C (myogenin/β-tubulin ratio).
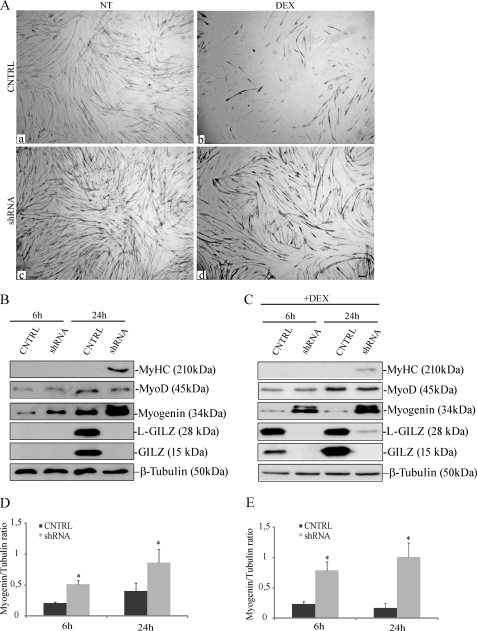
To test the contribution of GILZ/L-GILZ to DEX-induced regulation of myogenesis, we evaluated DEX effects on GILZ and/or L-GILZ silenced cells. We performed gene knockdown experiments by targeting GILZ and L-GILZ transcripts with specific shRNA sequences cloned into pSUPER.retro vector. Control cells were infected with a pSUPER.retro vector containing a scramble sequence. Gene knockdown efficiency was assessed in each experiment with real time PCR, with a degree of knockdown greater than 70% (not shown). Confluent control and L-GILZ/GILZ knockdown cells were switched to DM and subjected to MyHC staining 48 h later. We found that the simultaneous GILZ and L-GILZ knockdown resulted in a complete reversal of DEX-mediated anti-myogenic effects, as revealed by inhibition of myotube formation (Fig. 4A, compare panels b and d). Moreover, the individual contribution of GILZ and L-GILZ was assessed by shRNAs targeting specific for GILZ or L-GILZ mRNA sequences. The results showed that knocking down individual isoforms similarly dampened DEX activity (not shown). Furthermore, performing Western blot analysis to evaluate the expression of myogenic markers of differentiation, we found that GILZ and L-GILZ knockdown conferred resistance to DEX-induced inhibition of myogenin but not of MyoD expression, at 24 h upon DM switch (Fig. 4, B and C, compare second and third rows). To note, after 24 h in DM, the late myogenic marker of differentiation MyHC already appeared in GILZ/L-GILZ silenced myoblasts, either with or without DEX treatment (Fig. 4, B and C, first rows). Collectively, these results support the conclusion that GILZ and L-GILZ share anti-myogenic activity and mediate DEX-induced anti-myogenic effects.
FIGURE 4.
DEX effects on C2C12 differentiation are dependent on GILZ and L-GILZ expression, and knockdown of GILZ/L-GILZ results in enhanced myoblast differentiation. A, control (CNTRL) and or GILZ/L-GILZ silenced C2C12 myoblasts (shRNA) were induced to differentiate for 48 h in the presence or absence (NT) of 10−5 m DEX. The cultures were subjected to MyHC staining. Bar, 50 μm. B and C, Western blot of expression of biochemical markers of differentiation was taken over in CNTRL versus small hairpin GILZ + small hairpin L-GILZ (shRNA) C2C12 myoblasts with (C) or without (B) DEX treatment. Western blot of β-tubulin is included to show total protein loading. D, densitometric analysis of Western blots from B are shown in C (myogenin/β-tubulin ratio). *, p < 0.01 (n = 3). E, densitometric analysis of Western blots from C are shown in E (myogenin/β-tubulin ratio). *, p < 0.01 (n = 3).
GILZ and L-GILZ Expression Is Developmentally Regulated during Myogenesis, and Their Deregulation Affects Myoblast Differentiation
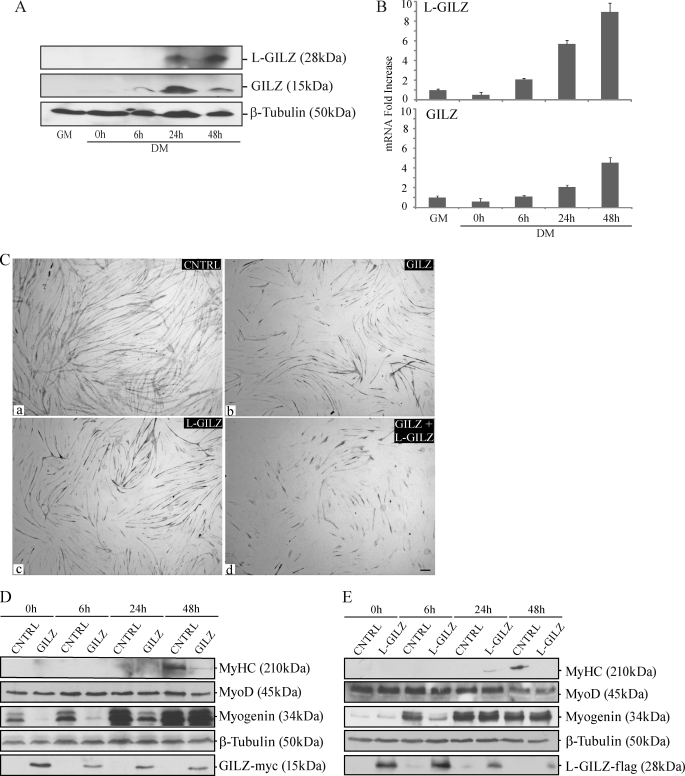
We found that GILZ and L-GILZ were undetectable in undifferentiated proliferating myoblasts, whereas both isoforms, either at the protein (Fig. 5A) or the mRNA (Fig. 5B) level, were expressed during myoblast differentiation, at a time when myotube formation became evident.
FIGURE 5.
A and B, GILZ and L-GILZ are expressed in differentiating C2C12 myoblasts. The cells underwent lysis for protein extraction or RNA extraction after cultivation in GM or at the indicated time points in DM. Western blot (A) and real time PCR (B) are shown (n = 3). C–E, overexpression of GILZ and L-GILZ results in reduced C2C12 myoblast differentiation. C, morphological evaluation of GILZ and L-GILZ overexpression effects on myogenesis. C2C12 myoblasts were transfected with GILZ-Myc vector (GILZ, panel b) and/or L-GILZ-FLAG vector (L-GILZ, panel c; GILZ+L-GILZ, panel d) and then induced to differentiate. The cells transfected with empty vector were used as control (CNTRL, panel a). MyHC staining of myoblast cultures after 48 h in DM. Bar, 50 μm. D and E, Western blot analyses of myogenic markers (MyoD, myogenin, and MyHC) in control (CNTRL) and GILZ-Myc (D) or L-GILZ-FLAG-transfected cells (E) during cultivation in DM. Anti-Myc and anti-FLAG antibodies were employed to detect GILZ-Myc and L-GILZ-FLAG, respectively.
To elucidate the possible participation of GILZ and L-GILZ in the myogenic differentiation program, we transfected preconfluent C2C12 cells with GILZ-Myc- or L-GILZ-FLAG-expressing vectors, and 24 h later the cells were induced to differentiate by transfer to DM, and the extent of myogenic differentiation was evaluated by morphologic and biochemical analyses. C2C12 myoblasts transiently transfected with a pcDNA3.1 empty vector served as a control. We found a significant inhibition of myotube formation 48 h after the switch of GILZ-Myc and/or L-GILZ-FLAG transfected myoblast cultures to DM compared with control cells, as investigated by MyHC staining (Fig. 5C, panels b and c versus panel a). The expression levels of ectopic GILZ-Myc and L-GILZ-FLAG at different time points (at 0, 6, 24, and 48 h in DM) are shown in Fig. 5 (D and E, bottom rows). In parallel, we checked by Western blot the expression levels of the early myogenic marker myogenin and the late marker MyHC. We found that the expression of both markers was impaired in GILZ-Myc or L-GILZ-FLAG transfected cells, compared with controls (Fig. 5, D and E). Remarkably, the expression of MyoD was not significantly reduced (Fig. 5, D and 5E, second rows). Notably, at late differentiation stages, the effects of overexpressed GILZ or L-GILZ on myogenin expression were lost (Fig. 5, D and E, third rows, 24 and 48 h); this event can be regarded as a consequence of the time-dependent reduction of the expression of the ectopic proteins (Fig. 5, D and E, bottom rows, 24 and 48 h). This notwithstanding, the GILZ/L-GILZ-induced delay in myogenin accumulation resulted in a significant inhibition of myoblast fusion and MyHC expression. Thus, GILZ and L-GILZ overexpression appeared to inhibit myogenic differentiation by countering pro-myogenic events that induce cell-to-cell fusion.
These results are in agreement with a simultaneous decrease of GILZ and L-GILZ expression levels, induced by RNA interference (RNAi) previously shown in Fig. 4A, resulting in a significant increase in myotube formation (Fig. 4A, compare panels c and a). Moreover, biochemical analyses in GILZ silenced myoblasts showed a significant increase of MyHC and myogenin expression, whereas MyoD expression was not affected (Fig. 4B). These experiments revealed that the physiological up-regulation of GILZ and L-GILZ, which takes place after the differentiation onset, results in anti-myogenic effects that occur downstream of MyoD activation. Taken together, these results indicate that GILZ and L-GILZ behave as modulators of myogenic differentiation, being able to inhibit myogenesis and to dampen the expression of differentiation markers such as MyHC and myogenin.
GILZ and L-GILZ Target MyoD Transcriptional Activity
The results shown in Fig. 5 suggested that GILZ and L-GILZ inhibit myogenic differentiation at early stages and that their effects might not be related to repression of MyoD protein expression levels. Given that MyoD is indirectly activated by the sustained kinase activity of p38 and Akt (32), we looked for changes in the phosphorylation status of the two kinases; no detectable differences were detected when control and GILZ-Myc or L-GILZ-FLAG overexpressing cells were induced to differentiate (not shown). Moreover, flow cytometric assays did not reveal any significant effects on cell cycle and viability that could counteract myogenesis (not shown).
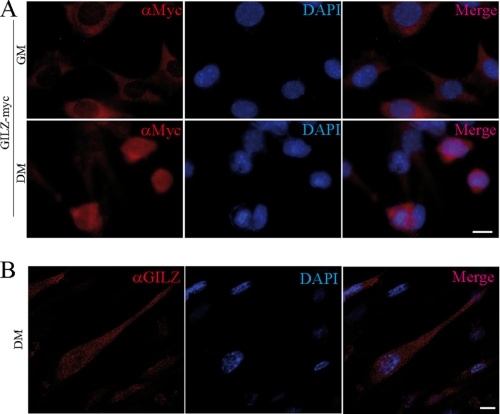
During myogenic differentiation, MyoD is stably expressed in the nucleus (24). We thus analyzed the subcellular localization of GILZ and L-GILZ. We analyzed the localization of ectopic GILZ and L-GILZ in C2C12 cells transiently transfected with GILZ-Myc- and L-GILZ-FLAG-expressing vectors. Immunofluorescence studies revealed a differential subcellular localization of GILZ-Myc and L-GILZ-FLAG (not shown) depending on culture medium conditions; in GM both GILZ and L-GILZ mainly localized to the cytoplasm (Fig. 6A, top row), whereas at 6 h after the switch to DM, they were localized to the nuclei (Fig. 6A, bottom row). Moreover, confocal images of C2C12 myoblasts at 24 h after the switch revealed a clear nuclear localization of GILZ protein (Fig. 6B).
FIGURE 6.
Subcellular localization analysis of overexpressed and endogenous GILZ in myoblasts. GILZ-Myc (A) was transfected in C2C12 myoblasts, which were kept in GM (upper row) or switched to DM (lower row). Anti-Myc antibody was employed for GILZ-Myc ectopic protein localization (red color). 4′,6′-Diamino-2-phenylindole (DAPI) has been employed for nuclear staining (blue color). Right panel of each row shows the image merge. Bar, 20 μm. B, confocal images of C2C12 myoblasts at 24 h after switch. Anti-GILZ antibody was employed for GILZ protein localization (red color). 4′,6′-Diamino-2-phenylindole has been employed for nuclear staining (blue color). The right lane shows the image merge. Bar, 10 μm.
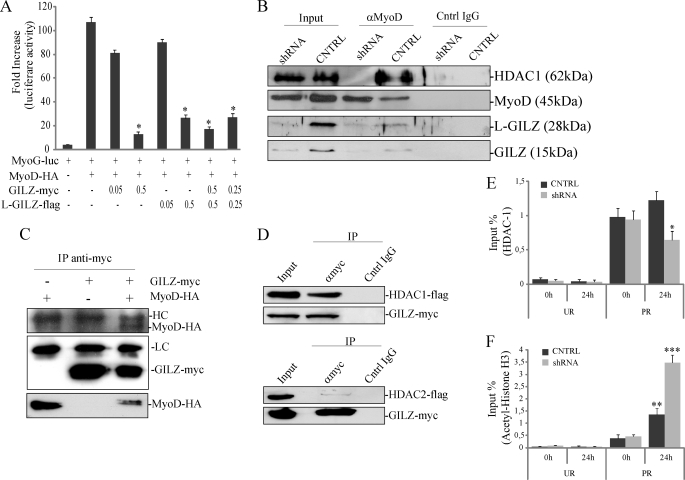
Because immunofluorescence results suggested that differentiation stimuli promoted a nuclear localization of GILZ and L-GILZ, we asked whether GILZ isoforms could directly target MyoD transcriptional activity. As shown in Fig. 7A, when a MyoD-HA-expressing vector was co-transfected with a luciferase reporter-gene containing the myogenin promoter (MyoG-luc) in C2C12 cells, a marked increase in luciferase activity was measured compared with base-line luminescence after the switch to DM. When GILZ and/or L-GILZ were co-transfected with MyoD-HA, we observed a dose-dependent reduction of luciferase activity, suggesting that overexpression of GILZ and L-GILZ resulted in a reduction of MyoD transcriptional activity on the myogenin promoter (Fig. 7A).
FIGURE 7.
GILZ and L-GILZ target MyoD transcriptional activity. A, MyoD transcriptional activity was measured with a luciferase reporter gene containing the myogenin promoter (MyoG-luc). *, p < 0.01 (n = 3). B, MyoD interacts with GILZ, L-GILZ, and HDAC1 in differentiating myoblasts. C2C12 myoblasts were cultivated in DM for 24 h before lysis. The cell lysates were subjected to immunoprecipitation with an anti-MyoD polyclonal antibody, and MyoD, GILZ, L-GILZ, and HDAC1 were identified by Western blot. C, GILZ directly interacts with MyoD. HEK293 cells were transfected with different combinations of plasmids encoding GILZ-Myc and MyoD-HA. GILZ-Myc was immunoprecipitated with an anti-Myc antibody, and co-immunoprecipitation was revealed with an anti-HA antibody. The bottom row represents MyoD-HA input. D, GILZ is able to interact with HDAC1 but not with HDAC2. HEK293 cells were co-transfected with expression vectors encoding GILZ-Myc and HDAC1-FLAG (upper panels) or HDAC2-FLAG (lower panels); immunoprecipitation was carried out with an anti-Myc antibody, and co-immunoprecipitation was revealed with an anti-FLAG antibody. E and F, for ChIP analysis, equivalent amounts of chromatin from same lysate used in endogenous IP assay described in B, control (CNTRL), and GILZ/L-GILZ silenced C2C12 myoblasts (shRNA) were immunoprecipitated in parallel with normal rabbit IgG and antibodies specific for HDAC1 (E) or acetylated H3 histones (F). The purified myogenin promoter then were analyzed by real time PCR. Bar graphs show the relative levels of bound DNA in HDAC1 (E) or acetylated H3 histones (F) immunoprecipitates, and results from three independent ChIP experiments combined are expressed as percentages of input chromatin, normalized to control IgG samples. UR, unrelated genomic region; PR, specific promoter region. *, p < 0.05 fourth column versus third column; **, p < 0.05 third column versus first column; ***, p < 0.05, fourth column versus third column (n = 3).
Next, we asked whether GILZ and/or L-GILZ could interact with MyoD at the protein level in differentiating myoblasts. The results showed that GILZ and L-GILZ co-immunoprecipitated with MyoD (Fig. 7B, fourth lane). To address the molecular mechanism by which GILZ/L-GILZ inhibited MyoD activity, we searched for a possible role for HDAC1. In fact, it has been previously suggested that GILZ could act as a transcriptional repressor via interaction with and recruitment of HDAC1 (19). Moreover, HDAC1 is known to be a MyoD partner when myoblasts are cultivated in GM (30) and during early stages of differentiation (40). We found that HDAC1 co-immunoprecipitated with MyoD (Fig. 7B, fourth lane). Intriguingly, in the absence of GILZ/L-GILZ expression, when both isoforms were knocked down by RNAi, HDAC1 did not bind MyoD (Fig. 7B, third lane).
As further evidence of protein-to-protein interaction, we performed a co-immunoprecipitation experiment in MyoD-HA/GILZ-Myc transfected HEK293 cells; we confirmed that GILZ and MyoD interacted in a protein-to-protein manner (Fig. 7C). In the same way, co-immunoprecipitation assay performed in HEK293 cells co-transfected with GILZ-Myc and HDAC1-FLAG or HDAC2-FLAG revealed that GILZ was able to interact directly with HDAC1 but not HDAC2 (Fig. 7D).
To understand the significance of the GILZ/HDAC1 interaction, we investigated whether GILZ/L-GILZ could affect HDAC1 recruitment to myogenin promoter, at the same binding site where MyoD is present. In fact, it has been already shown that HDAC1, depending on differentiation status, co-localizes with and binds to MyoD on myogenin promoter and thus represses MyoD transcriptional activity (41). To this end, ChIP assay was performed in differentiated C2C12 using an anti-HDAC1 antibody. The results showed that HDAC1 bound to the region containing the MyoD-binding site in the myogenin promoter after 24 h in DM (Fig. 7E). When GILZ/L-GILZ isoforms were both silenced by RNAi, the degree of binding of HDAC1 in myogenin locus was noticeably lower (Fig. 7E), thus indicating that GILZ/L-GILZ contribute to HDAC1/MyoD interaction on the myogenin promoter region. Moreover, in the same experiment, we performed ChIP assay to assess the acetylation status of myogenin promoter. Acetylation of histones is an essential process in transcriptional activation (42), and it has been already shown that MyoD transcriptional activity mediates histone acetylation in myogenin promoter. Specific antibody for the acetylated form of H3 histones (AcH3) was employed in ChIP assay; the results showed that acetylation of H3 histones was specifically induced after 24 h in DM (Fig. 7F, third column). When GILZ and L-GILZ were both silenced by RNAi, acetylation of myogenin locus was significantly higher (Fig. 7F, fourth column). These results are consistent with previous data showing an increase in the myotube formation in GILZ/L-GILZ silenced C2C12 cells in DM conditions (Fig. 4A) and suggest that the observed increase in myogenin expression upon GILZ/L-GILZ silencing (Fig. 4, B–E) is in part dependent on the reduction of HDAC1 activity.
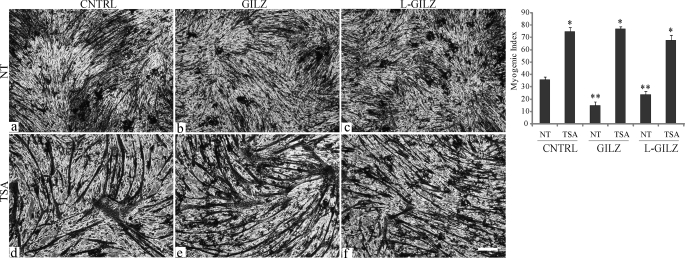
Finally, to confirm the possible involvement of HDAC1 in GILZ/L-GILZ-mediated inhibition of myogenesis, we treated GILZ-Myc- and L-GILZ-FLAG-transfected C2C12 cells with TSA (50 nm), a pharmacological inhibitor of HDACs (43, 44), prior to switching the cells from GM to DM. Morphological analysis revealed that TSA treatment reverted GILZ and L-GILZ anti-myogenic effects (Fig. 8), further suggesting that the GILZ/L-GILZ anti-myogenic effect was dependent on HDAC1 activity. Collectively, these data indicate that GILZ and L-GILZ are involved in the MyoD/HDAC1 interaction and the consequent inhibition of myogenin expression.
FIGURE 8.
TSA treatment reverts GILZ- and L-GILZ-induced inhibition of myogenic differentiation. C2C12 myoblasts were transfected with control empty vector (CNTRL, panels a and d), with a GILZ-Myc-expressing vector (GILZ, panels b and e), or with an L-GILZ-FLAG-expressing vector (L-GILZ, panels c and f). The upper row (panels a–c) shows the morphological analyses of untreated (NT) transfected cells, whereas the lower row (panels d–f) shows the morphological analyses of transfected cells that underwent treatment with 50 nm TSA prior to switching to DM. Bar, 100 mm. The myogenic index is shown in the right panel. *, p < 0.01; **, p < 0.01 (n = 3).
DISCUSSION
The results described here indicate that GILZ and L-GILZ are involved in the regulation of myogenesis and mediate GC-induced anti-myogenic activity. Skeletal muscle regeneration, after direct trauma or primary and secondary myopathies, is unsatisfactory. In fact, myofibers mainly die by necrosis, with consequent local inflammation, and this event contributes to create, in the regenerating muscle, a micro-environment that does not favor satellite cell survival and myoblast fusion. Intriguingly, inflammation-induced monocyte recruitment to the damaged skeletal muscle tissue contributes to satellite cell activation (45). However, in severe myopathic diseases, such as, for example, Duchenne dystrophy, this regenerative capacity is exhausted, because of altered satellite cell regeneration, prolonged inflammation, impaired vascular adaptation, and fibrosis (46, 47). Given the important role of inflammation in myopathies, GCs are employed in the pharmacological treatment of these diseases (48–50). However, this treatment is unsatisfactory in the long term because GCs are active on virtually all tissues including skeletal muscle tissue, promoting the conversion of proteins into glucose during stress or pharmacological treatments (51), and thus GC-related side effects on skeletal muscle tissue, such as wasting and interference with myogenic differentiation (2), probably contribute to the failure of anti-dystrophic therapy and to other characteristic GC-mediated side effects such as those typical of the iatrogenic Cushing syndrome (52). This notwithstanding, molecular determinants of myogenesis and antagonists of muscle inflammation are worthy of investigation.
In the present study we focused our attention in GILZ, a rapidly GC-induced protein shown to be a relevant mediator of GC effects in immune-related cells (9, 11–13, 53) and to be expressed in mesenchyme-derived cells (19, 21, 54). We found that in skeletal muscle tissue GILZ is partnered by a longer alternative isoform, L-GILZ, which shares with GILZ a large portion of primary structure. Notably, all previously described GILZ functional domains (14, 17) are also present in L-GILZ. GCs were able to induce both GILZ and L-GILZ in primary myoblasts and C2C12 myoblasts, and their induction was long lasting and associated with inhibition of myogenic differentiation. Also, forced expression of GILZ and/or L-GILZ in C2C12 cells resulted in anti-myogenic effects, thus mimicking GC effects. Moreover, experiments with an RNAi approach revealed that both GILZ and L-GILZ contribute to GC anti-myogenic effects. Notably, experiments of selective RNAi directed toward GILZ or L-GILZ revealed that the specific knockdown of either GILZ isoform was able to dampen GC effects, although to a smaller extent compared with the simultaneous GILZ/L-GILZ gene silencing. In fact, when both proteins were knocked down, GCs completely lost their anti-myogenic effects, and formation of large caliber myotubes was allowed.
Moreover, we found that GILZ and L-GILZ were spontaneously regulated during C2C12 myogenic differentiation, and RNAi experiments indicated that inhibition of GILZ/L-GILZ expression was sufficient to enhance C2C12 myoblast differentiation. These results suggest that GILZ and L-GILZ might act as developmentally regulated modulators of myogenic differentiation. In this context, we investigated the possible function of GILZ and L-GILZ in regulating the TGF-β superfamily signaling, a well described system of myogenic terminal differentiation regulation (35). The results showed that no reciprocal regulation of expression was found between GILZ/L-GILZ and TGF-β (supplemental Figs. S1 and S2).
At a molecular level, we found that overexpressed GILZ and L-GILZ inhibited not only cell-to-cell fusion, but also the expression of the muscle transcription factor myogenin. Because the two GILZ isoforms did not affect MyoD protein expression levels, nor did they interfere with the activation of pro-myogenic kinases such as p38 and Akt, we looked for a direct action of GILZ/L-GILZ on MyoD activity. We found that GILZ and L-GILZ counteracted MyoD transcriptional activity. This effect was due to a protein-to-protein interaction between GILZ and L-GILZ with MyoD. Moreover, we found that GILZ was able to interact with HDAC1, thus suggesting that GILZ and L-GILZ act as transcriptional repressors by the recruitment of HDAC1. Considering that TSA, a known HDAC inhibitor, reverted the differentiation block induced by the forced expression of GILZ or L-GILZ, we speculate that those molecules act through regulation of HDAC1 activity. Collectively, our data support the idea that GILZ/L-GILZ could be targets for future therapies of myopathies and for control of GC-mediated anti-myogenic effects. Indeed, GC treatment has been reported to result in reduced myogenesis and inhibition of the activation of muscle satellite cells (7, 8). These observations might call for a reconsideration of anti-inflammatory GC therapy of myopathies as well for new therapeutic strategies aimed to counter GC side effects at the muscle tissue level. In fact, our present results show that GCs increase GILZ/L-GILZ in myoblasts and exert anti-myogenic effects via induction of GILZ/L-GILZ. Therefore, countering GILZ and L-GILZ expression and/or function in muscle tissue might promote satellite cell activation and contractile protein accumulation in myofibers.
Currently, efforts are dedicated to the synthesis and characterization of the so-called “safe GCs” (55). Given the tissue specificity of different GC receptor isoforms (51, 56) and the specificity of post-transductional modifications that differentially affect target gene expression (57), GCs endowed with the ability to selectively activate specific GC receptor in inflammatory cells could be made available, thus protecting skeletal muscle tissue against GC-induced GILZ/L-GILZ expression and wasting. In this respect, GILZ/L-GILZ null mice could provide an interesting tool in the upcoming future to verify whether absence of GILZ/L-GILZ in skeletal muscle tissue enhances muscle regeneration in vivo and whether GCs might be more effective in the symptomatic treatment of dystrophy when GILZ and L-GILZ cannot be pharmacologically induced in muscle tissue.
Supplementary Material
Acknowledgments
We are grateful to Dr. Pier Lorenzo Puri (The Burnham Institute, La Jolla, CA) and Dr. Milena Grossi (University of Rome “La Sapienza”, Rome, Italy) for C2C12 cells and plasmids.
This work was been supported by grants from Associazione Italiana per la Ricerca sul Cancro Milan (to C. R.) and by Ministero Istruzione Università della Ricerca, Fondo per gli Investimenti della Ricerca di Base Grant RBPR05NWWC CHEM-PROFARMA-NET.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- GC
- glucocorticoid
- GILZ
- glucocorticoid-induced leucine zipper
- L-GILZ
- long GILZ
- DEX
- dexamethasone
- TGF
- transforming growth factor
- DMEM
- Dulbecco's modified Eagle's medium
- GM
- growth medium
- DM
- differentiation medium
- TSA
- trichostatin A
- HA
- hemagglutinin
- shRNA
- small hairpin RNA
- PBS
- phosphate-buffered saline
- Ab
- antibody
- mAb
- monoclonal Ab
- MyHC
- myosin heavy chain
- IP
- immunoprecipitation
- ChIP
- chromatin immunoprecipitation
- ORF
- open reading frame
- RNAi
- RNA interference.
REFERENCES
- 1.Rhen T., Cidlowski J. A. (2005) N. Engl. J. Med. 353, 1711–1723 [DOI] [PubMed] [Google Scholar]
- 2.Jackman R. W., Kandarian S. C. (2004) Am. J. Physiol. Cell Physiol. 287, C834–C843 [DOI] [PubMed] [Google Scholar]
- 3.Menconi M., Fareed M., O'Neal P., Poylin V., Wei W., Hasselgren P. O. (2007) Crit. Care Med. 35, S602–608 [DOI] [PubMed] [Google Scholar]
- 4.Song Y. H., Li Y., Du J., Mitch W. E., Rosenthal N., Delafontaine P. (2005) J. Clin. Invest. 115, 451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S. H., Goldberg A. L. (2004) Cell 117, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stitt T. N., Drujan D., Clarke B. A., Panaro F., Timofeyva Y., Kline W. O., Gonzalez M., Yancopoulos G. D., Glass D. J. (2004) Mol. Cell 14, 395–403 [DOI] [PubMed] [Google Scholar]
- 7.Betters J. L., Long J. H., Howe K. S., Braith R. W., Soltow Q. A., Lira V. A., Criswell D. S. (2008) Muscle Nerve 37, 203–209 [DOI] [PubMed] [Google Scholar]
- 8.Dekelbab B. H., Witchel S. F., DeFranco D. B. (2007) Steroids 72, 705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Adamio F., Zollo O., Moraca R., Ayroldi E., Bruscoli S., Bartoli A., Cannarile L., Migliorati G., Riccardi C. (1997) Immunity 7, 803–812 [DOI] [PubMed] [Google Scholar]
- 10.Delfino D. V., Agostini M., Spinicelli S., Vito P., Riccardi C. (2004) Blood 104, 4134–4141 [DOI] [PubMed] [Google Scholar]
- 11.Ayroldi E., Migliorati G., Bruscoli S., Marchetti C., Zollo O., Cannarile L., D'Adamio F., Riccardi C. (2001) Blood 98, 743–753 [DOI] [PubMed] [Google Scholar]
- 12.Berrebi D., Bruscoli S., Cohen N., Foussat A., Migliorati G., Bouchet-Delbos L., Maillot M. C., Portier A., Couderc J., Galanaud P., Peuchmaur M., Riccardi C., Emilie D. (2003) Blood 101, 729–738 [DOI] [PubMed] [Google Scholar]
- 13.Cannarile L., Cuzzocrea S., Santucci L., Agostini M., Mazzon E., Esposito E., Muia C., Coppo M., Di Paola R., Riccardi C. (2008) Gastroenterology 136, 530–541 [DOI] [PubMed] [Google Scholar]
- 14.Di Marco B., Massetti M., Bruscoli S., Macchiarulo A., Di Virgilio R., Velardi E., Donato V., Migliorati G., Riccardi C. (2007) Nucleic Acids Res. 35, 517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayroldi E., Zollo O., Macchiarulo A., Di Marco B., Marchetti C., Riccardi C. (2002) Mol. Cell Biol. 22, 7929–7941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soundararajan R., Wang J., Melters D., Pearce D. (2007) J. Biol. Chem. 282, 36303–36313 [DOI] [PubMed] [Google Scholar]
- 17.Ayroldi E., Zollo O., Bastianelli A., Marchetti C., Agostini M., Di Virgilio R., Riccardi C. (2007) J. Clin. Invest. 117, 1605–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller O. G., Parnova R. G., Centeno G., Rossier B. C., Firsov D., Horisberger J. D. (2003) J. Am. Soc. Nephrol. 14, 1107–1115 [DOI] [PubMed] [Google Scholar]
- 19.Shi X., Shi W., Li Q., Song B., Wan M., Bai S., Cao X. (2003) EMBO Rep. 4, 374–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soundararajan R., Zhang T. T., Wang J., Vandewalle A., Pearce D. (2005) J. Biol. Chem. 280, 39970–39981 [DOI] [PubMed] [Google Scholar]
- 21.Zhang W., Yang N., Shi X. M. (2008) J. Biol. Chem. 283, 4723–4729 [DOI] [PubMed] [Google Scholar]
- 22.Cannarile L., Zollo O., D'Adamio F., Ayroldi E., Marchetti C., Tabilio A., Bruscoli S., Riccardi C. (2001) Cell Death Differ. 8, 201–203 [DOI] [PubMed] [Google Scholar]
- 23.Buckingham M., Relaix F. (2007) Annu. Rev. Cell Dev. Biol. 23, 645–673 [DOI] [PubMed] [Google Scholar]
- 24.Tapscott S. J. (2005) Development 132, 2685–2695 [DOI] [PubMed] [Google Scholar]
- 25.Musarò A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E. R., Sweeney H. L., Rosenthal N. (2001) Nat. Genet. 27, 195–200 [DOI] [PubMed] [Google Scholar]
- 26.Sorci G., Riuzzi F., Arcuri C., Giambanco I., Donato R. (2004) Mol. Cell Biol. 24, 4880–4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovett F. A., Gonzalez I., Salih D. A., Cobb L. J., Tripathi G., Cosgrove R. A., Murrell A., Kilshaw P. J., Pell J. M. (2006) J. Cell Sci. 119, 4828–4840 [DOI] [PubMed] [Google Scholar]
- 28.Takaesu G., Kang J. S., Bae G. U., Yi M. J., Lee C. M., Reddy E. P., Krauss R. S. (2006) J. Cell Biol. 175, 383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lluís F., Perdiguero E., Nebreda A. R., Muñoz-Cánoves P. (2006) Trends Cell Biol. 16, 36–44 [DOI] [PubMed] [Google Scholar]
- 30.Puri P. L., Iezzi S., Stiegler P., Chen T. T., Schiltz R. L., Muscat G. E., Giordano A., Kedes L., Wang J. Y., Sartorelli V. (2001) Mol. Cell 8, 885–897 [DOI] [PubMed] [Google Scholar]
- 31.Sartorelli V., Puri P. L., Hamamori Y., Ogryzko V., Chung G., Nakatani Y., Wang J. Y., Kedes L. (1999) Mol. Cell 4, 725–734 [DOI] [PubMed] [Google Scholar]
- 32.Serra C., Palacios D., Mozzetta C., Forcales S. V., Morantte I., Ripani M., Jones D. R., Du K., Jhala U. S., Simone C., Puri P. L. (2007) Mol. Cell 28, 200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simone C., Forcales S. V., Hill D. A., Imbalzano A. N., Latella L., Puri P. L. (2004) Nat. Genet. 36, 738–743 [DOI] [PubMed] [Google Scholar]
- 34.Carlson M. E., Hsu M., Conboy I. M. (2008) Nature 454, 528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S. J. (2004) Annu. Rev. Cell Dev. Biol. 20, 61–86 [DOI] [PubMed] [Google Scholar]
- 36.Rando T. A., Blau H. M. (1994) J. Cell Biol. 125, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruscoli S., Di Virgilio R., Donato V., Velardi E., Baldoni M., Marchetti C., Migliorati G., Riccardi C. (2006) Eur. J. Pharmacol. 529, 63–70 [DOI] [PubMed] [Google Scholar]
- 38.Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 39.Blais A., Tsikitis M., Acosta-Alvear D., Sharan R., Kluger Y., Dynlacht B. D. (2005) Genes Dev. 19, 553–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mal A., Sturniolo M., Schiltz R. L., Ghosh M. K., Harter M. L. (2001) EMBO J. 20, 1739–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mal A., Harter M. L. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1735–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grunstein M. (1997) Nature 389, 349–352 [DOI] [PubMed] [Google Scholar]
- 43.Iezzi S., Di Padova M., Serra C., Caretti G., Simone C., Maklan E., Minetti G., Zhao P., Hoffman E. P., Puri P. L., Sartorelli V. (2004) Dev. Cell 6, 673–684 [DOI] [PubMed] [Google Scholar]
- 44.Minetti G. C., Colussi C., Adami R., Serra C., Mozzetta C., Parente V., Fortuni S., Straino S., Sampaolesi M., Di Padova M., Illi B., Gallinari P., Steinkühler C., Capogrossi M. C., Sartorelli V., Bottinelli R., Gaetano C., Puri P. L. (2006) Nat. Med. 12, 1147–1150 [DOI] [PubMed] [Google Scholar]
- 45.Chazaud B., Sonnet C., Lafuste P., Bassez G., Rimaniol A. C., Poron F., Authier F. J., Dreyfus P. A., Gherardi R. K. (2003) J. Cell Biol. 163, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunelli S., Sciorati C., D'Antona G., Innocenzi A., Covarello D., Galvez B. G., Perrotta C., Monopoli A., Sanvito F., Bottinelli R., Ongini E., Cossu G., Clementi E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter J. D., Khanna S., Kaminski H. J., Rao J. S., Merriam A. P., Richmonds C. R., Leahy P., Li J., Guo W., Andrade F. H. (2002) Hum. Mol. Genet. 11, 263–272 [DOI] [PubMed] [Google Scholar]
- 48.Khan M. A. (1993) J. Neurol. Sci. 120, 8–14 [DOI] [PubMed] [Google Scholar]
- 49.Lundberg I., Kratz A. K., Alexanderson H., Patarroyo M. (2000) Arthritis Rheum. 43, 336–348 [DOI] [PubMed] [Google Scholar]
- 50.Mastaglia F. L., Garlepp M. J., Phillips B. A., Zilko P. J. (2003) Muscle Nerve 27, 407–425 [DOI] [PubMed] [Google Scholar]
- 51.Gross K. L., Cidlowski J. A. (2008) Trends Endocrinol. Metab. 19, 331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hopkins R. L., Leinung M. C. (2005) Endocrinol. Metab. Clin. North Am. 34, 371–384 [DOI] [PubMed] [Google Scholar]
- 53.Cannarile L., Fallarino F., Agostini M., Cuzzocrea S., Mazzon E., Vacca C., Genovese T., Migliorati G., Ayroldi E., Riccardi C. (2006) Blood 107, 1039–1047 [DOI] [PubMed] [Google Scholar]
- 54.Ingram W. J., Wicking C. A., Grimmond S. M., Forrest A. R., Wainwright B. J. (2002) Oncogene 21, 8196–8205 [DOI] [PubMed] [Google Scholar]
- 55.Lu N. Z., Collins J. B., Grissom S. F., Cidlowski J. A. (2007) Mol. Cell Biol. 27, 7143–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pujols L., Mullol J., Roca-Ferrer J., Torrego A., Xaubet A., Cidlowski J. A., Picado C. (2002) Am. J. Physiol. Cell Physiol. 283, C1324–C1331 [DOI] [PubMed] [Google Scholar]
- 57.Blind R. D., Garabedian M. J. (2008) J. Steroid Biochem. Mol. Biol. 109, 150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.