Abstract
The anionic phospholipid cardiolipin and its precursor phosphatidylglycerol are synthesized and localized in the mitochondrial inner membrane of eukaryotes. They are required for structural integrity and optimal activities of a large number of mitochondrial proteins and complexes. Previous studies showed that loss of anionic phospholipids leads to cell inviability in the absence of mitochondrial DNA. However, the mechanism linking loss of anionic phospholipids to petite lethality was unclear. To elucidate the mechanism, we constructed a crd1Δrho° mutant, which is viable and mimics phenotypes of pgs1Δ in the petite background. We found that loss of cardiolipin in rho° cells leads to elevated expression of Swe1p, a morphogenesis checkpoint protein. Moreover, the retrograde pathway is activated in crd1Δrho° cells, most likely due to the exacerbation of mitochondrial dysfunction. Interestingly, the expression of SWE1 is dependent on retrograde regulation as elevated expression of SWE1 is suppressed by deletion of RTG2 or RTG3. Taken together, these findings indicate that activation of the retrograde pathway leads to up-regulation of SWE1 in crd1Δrho° cells. These results suggest that anionic phospholipids are required for processes that are essential for normal cell division in rho° cells.
Keywords: Cardiolipin, Cell Division, Mitochondrial DNA, Phospholipid, Yeast
Introduction
The phospholipid cardiolipin (CL)3 is ubiquitous in eukaryotes. It is predominantly present in the mitochondrial inner membrane, where it interacts with a large number of mitochondrial proteins (1, 2). The interactions of CL with mitochondrial membrane proteins are required for optimal protein function as dissociation of CL from these proteins results in inactivation of the complexes or decreases in protein activities (3–9).
In the yeast Saccharomyces cerevisiae, the absence of CL leads to reduced mitochondrial membrane potential, perturbation of coupling, instability of electron transport chain supercomplexes, and impaired protein import (10–15). The identification of the genes encoding the CL biosynthetic enzymes phosphatidylglycerolphosphate synthase (PGS1) (16, 17) and CL synthase (CRD1) (18–20) in yeast has enabled analyses of the functional role of CL in vivo. Mutants in CRD1, which are blocked in the final step of CL synthesis, can synthesize the precursor lipid phosphatidylglycerol (PG), the levels of which are significantly increased in the crd1Δ mutant (18, 19). Analysis of the crd1Δ mutant indicates that CL is not essential for yeast viability at optimal growth temperatures. In contrast, the pgs1Δ mutant cannot synthesize PG and CL (16). It appears that PG can substitute for CL for some mitochondrial and cellular functions as the pgs1Δ mutant exhibits a more severe growth defect than that of the crd1Δ mutant. Previous studies showed that the pgs1Δ mutant fails to maintain mtDNA without 1 m sorbitol and exhibits defective cell wall biogenesis and defective growth at 37 °C, even on non-fermentable carbon sources (21, 22). These defects suggest that CL biosynthesis plays an important role in numerous cellular processes apart from mitochondrial bioenergetics.
S. cerevisiae is a petite positive yeast that can grow without mtDNA (23). However, mutations in PGS1 lead to the petite lethal phenotype, as evidenced by the finding that the pgs1Δ mutant cannot survive ethidium bromide mutagenesis (17, 24, 25). The pgs1Δ mutant grown on glucose exhibits a spontaneous loss of mtDNA at 30 °C, which may thus contribute to the deleterious phenotypes of the mutant (21). Interestingly, disruption of CRD1 does not lead to loss of mtDNA or to petite lethality, most likely because the PG that accumulates in crd1Δ substitutes for some essential functions of CL.
Loss of mtDNA and mitochondrial dysfunction lead to a variety of cellular responses (26–28). One of the most dramatic of these is induction of mitochondrial retrograde regulation, a pathway of communication from mitochondria to the nucleus (29, 30). In this pathway, mitochondrial dysfunction is signaled to the nucleus, which responds with a wide range of changes in nuclear gene expression that may compensate for the mitochondrial defects (31). The key signaling proteins in the retrograde pathway are Rtg2p and the transcription factors Rtg1p and Rtg3p (32, 33). Rtg2p senses mitochondrial dysfunction and transmits this signal to the heterodimer Rtg1p/Rtg3p, which translocates from the cytoplasm to the nucleus and activates transcription of target genes, such as CIT2 and DLD3 (34, 35). The promoter regions of CIT2 and DLD3 contain an inverted repeat of an R box (GTCAC) that provides the binding sites for the Rtg1p/Rtg3p heterodimer (34, 36). The expression of CIT2 and DLD3 is dramatically increased in a strain- and carbon source-dependent manner when the retrograde response is activated (34, 35).
In addition to the mitochondrial retrograde response, loss of mitochondrial DNA in yeast is also associated with cell division defects. In a wide scale screen for mutants that produce rho° petite cells (lacking mtDNA), Newlon et al. (37, 38) found that mutations in CDC8 and CDC21, which encode thymidylate kinase and thymidylate synthase, respectively, arrested mtDNA replication and caused a high frequency of petite formation. In contrast, a cdc28 mutant exhibited increased mitochondrial genome stability and decreased rates of spontaneous and ethidium bromide-induced petite formation (39, 40). How these genes affect mitochondrial genome stability is unknown. During cell division, mtDNA replication and mitochondrial inheritance must be precisely controlled so that mitochondria are properly transmitted to daughter cells. In this light, it would be expected that some mutations affecting mitochondria might result in a cdc (cell division cycle) mutant phenotype. Previous studies have shown that ERV1, a flavin-linked sulfhydryl oxidase of the mitochondrial intermembrane space, is essential for cell viability and for the biogenesis of functional mitochondria (41). Elimination of ERV1 leads to loss of mtDNA and cell division delay with a cdc phenotype (42). Interestingly, some genes for maintenance of mitochondrial inheritance and morphology, including MMM1, MDM1, and OLE1, showed a significant association with cell division as mutants in these genes exhibit defects in mitochondrial distribution and morphology and are unable to complete cell division (43–45).
Because mitochondrial dysfunction and loss of mtDNA are associated with cdc defects, we hypothesized that the deleterious phenotypes seen in CL mutants could be attributed to perturbation of cell division. Although the pgs1Δ mutant is petite lethal unless supplemented with sorbitol, a crd1Δrho° mutant is viable and could be used to address the hypothesis. In this study, we demonstrated that loss of mtDNA in crd1Δ leads to cell division delay triggered by elevated expression of Swe1p, a morphogenesis checkpoint protein. The induction of Swe1p was dependent on the retrograde pathway, which was activated in the crd1Δrho° mutant. This is the first demonstration that mitochondrial dysfunction leads to cell division defects that are mediated by retrograde regulation.
EXPERIMENTAL PROCEDURES
Yeast Strains, Growth Media, and Growth Conditions
The S. cerevisiae strains and plasmids used in this work are listed in Table 1. Synthetic complete medium contained adenine (20.25 mg/liter), arginine (20 mg/liter), histidine (20 mg/liter), leucine (60 mg/liter), lysine (200 mg/liter), methionine (20 mg/liter), threonine (300 mg/liter), tryptophan (20 mg/liter), uracil (20 mg/liter), yeast nitrogen base without amino acids (Difco), and glucose (2%). Synthetic drop-out medium contained all of the above ingredients except the amino acid used as a selectable marker. Complex medium (YPD) contained yeast extract (1%), peptone (2%), and glucose (2%). YPDS medium was YPD supplemented with 1 m sorbitol. Solid medium contained agar (2%) in addition to the above mentioned ingredients.
TABLE 1.
Plasmids and yeast strains used in this study
| Plasmid/strains | Characteristics or genotype | Source or reference |
|---|---|---|
| pYPGK18 | 2μm, LEU2 | (93) |
| pYPGK18CRD1 | Derivative of pYPGK18, expresses CRD1 from PGK1 promoter | (94) |
| pYPGK18SWE1 | Derivative of pYPGK18, expresses SWE1 from PGK1 promoter | This study |
| pHS12-DsRed.T4 | Centromere, LEU2, express COX4-Discosoma red fluorescent protein from ADH1 promoter | (49) |
| pMS76 | Centromere, HIS3, derivative of pRS313, express CDC12-GFP | (95) |
| pAC115 | Centromere, TRP1, derivative of pRS414, express CDC11-GFP | (96) |
| pRS315(GFP-CDC3) | Centromere, LEU2, derivative of pRS315, express GFP-CDC3 | (97) |
| FGY3 | MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1 | (19) |
| FGY3 ρ° | rho° mutant derived from FGY3 | (21) |
| FGY2 | MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1, crd1Δ::URA3 | (19) |
| FGY2 ρ° | rho° mutant derived from FGY2 | This study |
| FGY3 swe1Δ | MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1, swe1Δ::KanMX4 | This study |
| FGY3 swe1Δ ρ° | rho° mutant derived from FGY3 swe1Δ | This study |
| FGY2 swe1Δ | MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1, crd1Δ::URA3, swe1Δ::KanMX4 | This study |
| FGY2 swe1Δ ρ° | rho° mutant, MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1, crd1Δ::URA3, swe1Δ::KanMX4 | This study |
| FGY3 rtg2Δ ρ° | rho° mutant, MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1, rtg2Δ::KanMX4 | This study |
| FGY2 rtg2Δ ρ° | rho° mutant, MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1, crd1Δ::URA3, rtg2Δ::KanMX4 | This study |
| FGY3 rtg3Δ ρ° | rho° mutant, MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1, rtg3Δ::KanMX4 | This study |
| FGY2 rtg3Δ ρ° | rho° mutant, MATα, ura3-52, lys2-801, ade2-101, trp1Δ1, his3Δ200, leu2Δ1, crd1Δ::URA3, rtg3Δ::KanMX4 | This study |
To construct deletion mutants, the entire open reading frame of the target gene was replaced by KanMX4 using PCR-mediated homologous recombination in the wild type strain. The KanMX4 cassette was amplified from pUG6 using primers consisting of 50 nucleotides identical to the target gene flanking regions at the 5′ end and 21 nucleotides for the amplification of the KanMX4 gene at the 3′ end. The PCR product was transformed into wild type cells, and transformants were selected on YPD medium containing G418 (200 μg/ml). Disruption of the target gene was confirmed by the absence of the target PCR product using primers against the target gene coding sequences.
To generate rho° derivatives, parental rho+ cells were cultured in YPD medium containing 20 μg/ml ethidium bromide to the early stationary phase. Single cells were then plated on solid YPD and replicated to YPGE. Colonies that were inviable on YPGE medium were tested for mitochondrial DNA deletion by staining with DAPI. Loss of mtDNA was confirmed by failure to complement rho− tester strains for growth on YPGE medium.
Plasmid Construction
To construct a SWE1-overexpressing plasmid, a 2533-bp sequence containing the entire open reading frame of SWE1 was amplified from yeast genomic DNA using SacI-tagged primer 18SWE1-f (5′-GCACACGAGCTCAGATGAGTTCTTTGGACGAGG-3′) and BamHI-tagged primer 18SWE1-r (5′-TATTGGGGATCCTATACAATGCGGCCCATAAG-3′). The PCR products were ligated to pYPGK18 cut with SacI and BamHI, downstream of the PGK1 promoter.
Phospholipid Determination
Yeast cells were grown in the presence of [32P]Pi (10 μCi/ml) at 30 °C to the early stationary phase. Cells were then washed and digested by Zmyolyase to yield spheroplasts. Total lipids were extracted from spheroplasts with chloroform:methanol (2:1) (v/v). The extracted lipids were applied to a boric acid-treated TLC plate, which was developed in the one-dimension solvent system chloroform/triethylamine/ethanol/water (30:35:35:7). Developed chromatograms were quantified by phosphorimaging (46).
Fluorescence and Microscopic Analysis
All microscopy was performed using an Olympus BX41 epifluorescence microscope. Images were acquired using an Olympus Q-Color3 digital CCD camera operated by QCapture2 software. Pictures in the same pattern were taken at the same magnification (×1000). To stain nuclear and mitochondrial DNA, yeast cells were cultured to the mid-log phase, fixed in 70% ethanol at room temperature for 30 min, washed two times with distilled water, and stained with 1 μg/ml DAPI (Sigma) for 5 min.
Visualization of chitin distribution was performed by Oregon Green 488-tagged wheat germ agglutinin (WGA) staining. Yeast cells were grown to the early stationary phase and washed once with PEM buffer containing 0.1 mm PIPES, 5 mm EGTA, 5 mm MgCl2 (pH 6.9). To 100 μl of cell suspension, 1 μl of WGA-Oregon Green 488 (Molecular Probes) was added, and cells were incubated at room temperature for 5 min (47).
The actin cytoskeleton was visualized using rhodamine-phalloidin (Molecular Probes), which binds specifically to polymerized actin. Yeast cells grown to the mid-log phase were harvested by centrifugation at 2000 × g and fixed in the medium with 4% formaldehyde for 10 min. The cells were resuspended in phosphate-buffered saline containing 4% formaldehyde and incubated for 60 min. Rhodamine-phalloidin (2 μl) was added to a 100-μl cell suspension according to the manufacturer's instructions followed by incubation in the dark for 60 min. Stained cells were washed three times with phosphate-buffered saline before visualization with fluorescence microscopy (48).
To stain mitochondria, cells grown to the mid-log phase were incubated with DiOC6(3) (Molecular Probes), a membrane potential-dependent probe, or MitoTracker Green FM (Molecular Probes), a membrane potential independent probe, in 10 mm HEPES buffer containing 5% glucose at room temperature for 15 min. Mitochondria were observed and photographed following the procedures of the manufacturer. In living cells, mitochondria were visualized using a fusion protein consisting of the mitochondrial signal sequence of Cox4p fused to Discosoma red fluorescent protein (COX4-DsRed), which was expressed by a centromere-based plasmid (49).
To view septin localization, CDC3-GFP, CDC11-GFP, or CDC12-GFP was expressed from centromeric plasmids. Yeast cells containing these septin-GFP constructs were grown to the mid-log phase, and live cells were examined directly under the fluorescence microscope using Olympus BX41 NIB filter.
Real-time PCR
Yeast cultures (10 ml) were grown to the early stationary phase, cells were harvested, and total RNA was isolated using the RNeasy mini kit (Qiagen). The RNA samples were treated with DNase from a DNA-free kit (Ambion) to remove contaminating genomic DNA. cDNAs were synthesized with a Reverse-iT first strand synthesis kit (ABgene) according to the manufacturer's protocol. Real-time PCR reactions were performed in a 50-μl volume using ABsolute quantitative PCR SYBR Green mix (ABgene) in a 96-well plate. Duplicates for each sample were included for each reaction. The real-time PCR primers used in this work are listed in Table 2. ACT1 was used as the internal control, and the RNA level of the gene of interest was normalized to ACT1 levels. PCR reactions were initiated at 95 °C for 10 min for denaturation followed by 40 cycles consisting of 30 s at 95 °C and 60 s at 57 °C.
TABLE 2.
The real-time PCR primers used in this study
| Gene | Primers | Sequence (5′ to 3′) | Product length |
|---|---|---|---|
| bp | |||
| ACT1 | Forward | TCGTGCTGTCTTCCCATCTATCG | 218 |
| Reverse | CGAATTGAGAGTTGCCCCAGAAG | ||
| SWE1 | Forward | GGTAATAGTAACAACGCTGGCACC | 309 |
| Reverse | CTGGATAATAGCACCTGCATTGCG | ||
| CIT2 | Forward | CGGAACTACCTAGTCATGTCGTTCA | 309 |
| Reverse | CATCCTTAGAACCAATCAAGTTGACCAG | ||
| DLD3 | Forward | ACGTCAGGGTCCAATAAGAGACAC | 258 |
| Reverse | CAAACCGGCTGCGTTTAATCTCTC |
Western Blot Analysis of Swe1p
Yeast cells grown to the mid-log phase were harvested and subjected to mechanical breakage with glass beads at 4 °C. After separation from glass beads and cell debris by centrifugation, protein extracts were boiled immediately with protein gel sample buffer. Total proteins (50 μg of protein) were separated on 8% SDS-PAGE and electrotransferred to a polyvinylidene difluoride membrane (Millipore). The membrane was incubated in 5% nonfat milk with anti-Swe1p rabbit IgG (y-311, Santa Cruz Biotechnology) followed by horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology). Swe1p was visualized using an ECL chemiluminescence detection system (GE Healthcare) with α-tubulin as the loading control.
Flow Cytometry
Yeast cells were cultured in YPD medium at 30 °C and harvested at the indicated growth phase. After cells were fixed with 75% ethanol and digested with RNase A at room temperature for 4 h, cellular DNA was stained with 50 μg/ml propidium iodide at 4 °C for 12 h. Stained cells were sonicated briefly before proceeding with flow cytometry. Cellular DNA quantification was performed using a FACSArray (BD Biosciences) 96-well plate flow cytometer.
RESULTS
Loss of mtDNA in crd1Δ Results in Slow Growth and Decreased PG Accumulation
As discussed above, pgs1Δ exhibits the petite lethal phenotype in the absence of sorbitol (21). To determine whether crd1Δ exhibits petite lethality, we generated a crd1Δrho° mutant. The crd1Δrho° cells were viable on YPD medium (Fig. 1A) but exhibited a very long lag in growth and decreased cell density in the stationary phase (Fig. 1B). One possible explanation for the severe growth defect in the crd1Δrho° cells is that the loss of mtDNA led to a decrease in synthesis of PG. Because the increase in PG in the crd1Δ mutant may partially compensate for loss of CL, the loss of mtDNA may diminish this excess PG to a level that can no longer compensate. This would be consistent with previous studies showing that PG and CL synthesis are decreased in the absence of a mitochondrial genome (50–54). Consistent with this, we found that loss of mtDNA led to a 42% decrease in CL levels in WT cells (Fig. 1C). In the crd1Δ mutant, which lacks CL but accumulates PG, the loss of mtDNA led to a 48% decrease in PG levels (Fig. 1C). Therefore, the decreased PG levels may contribute to the severe growth defect of crd1Δrho° cells.
FIGURE 1.
Loss of mtDNA in crd1Δ results in slow growth and decreased PG. A, yeast cells (except for pgs1Δ) were grown in YPD medium to the early stationary phase, and cells were seeded on solid YPD. The pgs1Δ cells were precultured in YPDS containing 1 m sorbitol and then switched to YPD medium for 18 h and plated on solid YPD. B, cells were grown in YPD at 30 °C. Cell growth was monitored by A550 at the indicated times. C, WT, WT rho°, crd1Δ, and crd1Δrho° cells were grown in YPD, and pgs1Δ was grown in YPDS, at 30 °C to the early stationary phase. Cells were harvested by centrifugation, and lipids were extracted and separated as described under “Experimental Procedures.” Phospholipids were separated by one-dimensional TLC and visualized by phosphorimaging. CL and PG were quantified using the ImageQuant software. The relative amount of 32P in CL and PG is presented as a percentage of the 32P incorporated into total phospholipids. Error bars represent the range of the three independent experiments. PA, phosphatidic acid; PE, phosphatidylethanolamine; PS, phosphatidylserine; PI, phosphatidylinositol; PC, phosphatidylcholine.
The crd1Δrho° Mutant Shows Cell Division Defects
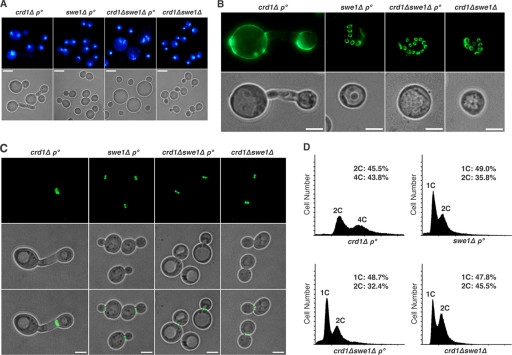
Previous studies demonstrated that the pgs1Δ mutant exhibits defects in cell wall biosynthesis and maintenance of cell integrity (21). Consequently, it is hypersensitive to cell wall-perturbing agents such as caffeine and calcofluor white and exhibits an enlarged cell morphology. Although PG accumulation is decreased in the crd1Δrho° mutant (Fig. 1C), crd1Δrho° cells were not sensitive to caffeine and calcofluor white (data not shown), suggesting that they contain sufficient PG to maintain cell wall integrity. However, crd1Δrho° cells did have an enlarged cell morphology phenotype, and 13.4% (±0.6% S.D.) of cells exhibited elongated buds in the mid-log phase, suggestive of a cell division defect (Fig. 2A). In the presence of a plasmid-borne copy of the CRD1 gene, wild type cell morphology was completely restored. This suggested that CL is essential for normal cell division in the absence of mtDNA. Consistent with this, DAPI staining of nuclear DNA revealed incoordination between nuclear division and cell division in crd1Δrho° cells. As seen in Fig. 2B, in contrast to rho° cells in the CRD1 background (WT rho°), crd1Δrho° cells exhibited multinucleate mother cells and anucleate daughter cells. Mother and daughter cells were characterized by failure to separate and were linked by long necks. DNA content analysis by flow cytometry also revealed a dramatic delay in cell division. Although the crd1Δ mutant showed a relatively normal distribution of cells in G1 and G2/M, the crd1Δrho° mutant accumulated cells with a G2/M DNA content and had essentially no cells in G1 (Fig. 2C). Interestingly, the mutant also accumulated cells with a DNA content greater than G2/M DNA content (Fig. 2C, indicated by 4C), suggesting additional DNA synthesis prior to cell division. As described further below, all of these cell division defects could be rescued by mutations in the cell cycle checkpoint gene, SWE1.
FIGURE 2.
Cell division defects in crd1Δrho° cells. A, WT, rho°, crd1Δ, and crd1Δrho° cells were cultured in YPD medium. The crd1Δrho° cells transformed with pYPGK18 or pYPGK18CRD1 were cultured in synthetic drop-out medium (Leu−). Cell morphology was examined and photographed microscopically after growth to the mid-log phase at 30 °C. The scale bar represents 3 μm. B, cells were grown to the mid-log phase in YPD at 30 °C. The nucleus was visualized by staining with DAPI as described under “Experimental Procedures.” The scale bar represents 2 μm. C, yeast cells were grown to mid-log phase in YPD medium at 30 °C and processed for flow cytometry as described under “Experimental Procedures” (1C, G1 DNA content; 2C, G2/M DNA content; 4C, greater than G2/M DNA content). D, cells were grown to the early stationary phase in YPD at 30 °C. Chitin deposition was visualized by staining with WGA-Oregon Green 488 as described under “Experimental Procedures.” The scale bar represents 2 μm. E, cells transformed with the GFP-tagged Cdc3p were grown to the mid-log phase in synthetic drop-out medium at 30 °C, and living cells were examined directly under the fluorescence microscope. The scale bar represents 2 μm. All images in the same pattern were taken at the same magnification (×1000).
In addition to the incoordination between nuclear and cell division, chitin deposition was altered in crd1Δrho° cells (Fig. 2D). Chitin ring formation was absent, and bud scars were hardly distinguishable from the rest of the cell wall, both in morphologically abnormal cells and in cells with normal morphology, indicating that chitin deposition in the bud scars did not occur in these cells. Chitin plays an important role in cell division, and synthesis of chitin is regulated during the cell cycle. Chitin is distributed asymmetrically in the cell wall, largely at the presumptive bud site before bud emergence (55–57), and forms the primary septum at cytokinesis (58). The dynamic synthesis and restricted localization of chitin underscores its importance in cell division. Therefore, loss of normal chitin deposition was consistent with a cell division defect in crd1Δrho° cells.
During cell division, the formation of chitin rings is dependent on the presence of septins (59, 60). Yeast cells have five septins that are involved in vegetative growth, Cdc3p, Cdc10p, Cdc11p, Cdc12p, and Shs1 (61, 62). Early in the cell cycle, septins polymerize at the presumptive bud site, forming a ring that expands into an hourglass structure and acts as a scaffold to localize the chitin synthase III complex to the mother side of the neck, where it deposits chitin in the cell wall (58, 59, 62). Because chitin localization is dependent on the septins, the observation of defective chitin deposition in crd1Δrho° cells suggested that these cells have defects in septin localization. To test this possibility, strains containing Cdc3p-GFP, Cdc12-GFP, and Cdc11-GFP were used for visualization of septin rings. As seen in Fig. 2E and supplemental Fig. S9A, WT, WT rho°, crd1Δ, and crd1Δrho° cells with normal morphology had normally localized septin rings, whereas crd1Δrho° cells with abnormal morphology exhibited misplaced septin rings localized around the neck close to the side of the daughter cells, indicating that the misplaced septin ring may be a consequence of abnormal cell morphology. This result suggested that the chitin deposition is independent of septin in crd1Δrho° cells as crd1Δrho° cells did not exhibit ring-form chitin deposition.
Cell Division Defects in the crd1Δrho° Mutant Result from Elevated Expression of Swe1p
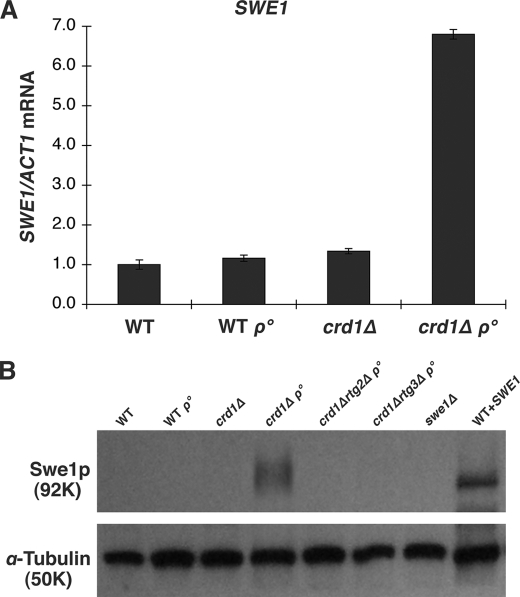
Coordination of cell cycle events during yeast cell division is maintained by the morphogenesis checkpoint control that monitors errors in critical cell cycle processes and acts to delay cell division until cells recover from errors (63–66). Cell division delay is mediated by Swe1p, which phosphorylates and inhibits the cyclin-dependent kinase Cdc28p (67, 68). Previous studies have shown that conditional overexpression of SWE1 can cause cell division delay and the formation of elongated buds, similar to the crd1Δrho° phenotype (67, 69). To explore a possible role for SWE1 in the crd1Δrho° phenotype, we examined the mRNA level of SWE1 in crd1Δrho° cells. The SWE1 mRNA was increased 6.8-fold in the mutant when compared with the WT (Fig. 3A). Consistent with the increased RNA levels, Swe1p was significantly accumulated in crd1Δrho° cells (Fig. 3B). These results raised the possibility that SWE1 up-regulation may contribute to the crd1Δrho° phenotype. Consistent with this possibility, we showed that constitutive overexpression of SWE1 in WT cells led to an elongated cell morphology and to the presence of binucleated cells, similar to the crd1Δrho° mutant (supplemental Fig. S1A). Cells constitutively overexpressing SWE1 also had a very similar DNA content profile to crd1Δrho° cells, with a loss of G1 cells, and an accumulation of cells with postreplicative (G2/M DNA content (2C) and greater than G2/M DNA content (4C)) DNA content (supplemental Fig. S1B).
FIGURE 3.
Swe1p is up-regulated in crd1Δrho° cells. A, total RNA was extracted from cells grown to the mid-log phase in YPD at 30 °C. SWE1 mRNA levels were determined by real-time PCR as described under “Experimental Procedures.” Expression was normalized to the RNA levels of the internal control ACT1. Error bars represent the range of the three independent experiments. B, total protein was extracted from cells grown to the mid-log phase in YPD at 30 °C. Swe1p was identified by Western blot. Wild type cells transformed with pYPGK18SWE1 were used as a positive control, swe1Δ was used as a negative control, and α-tubulin was used as a loading control.
To determine whether Swe1p expression is required for the cell division defects of crd1Δrho° cells, we deleted SWE1 in the crd1Δrho° mutant. The phenotypes of elongated budding cells, aberrant chitin deposition, misplaced septin, and postreplicative DNA content were rescued in the crd1Δswe1Δ rho° mutant (Fig. 4 and supplemental Figs. S3 and S9B). However, disruption of SWE1 in crd1Δrho° was unable to alleviate the slow growth defects. The crd1Δswe1Δrho° mutant maintained a prolonged lag phase similar to that seen in crd1Δrho° cells (supplemental Fig. S4). A few crd1Δswe1Δrho° cells exhibited enlarged cell morphology with diffuse DNA fragmentation (Fig. 4A). To determine whether crd1Δswe1Δrho° cells are arrested in the cell cycle after DNA replication in lag phase, DNA content was monitored in the first 10 h after cells were inoculated in fresh medium. Cytometric analysis showed that crd1Δswe1Δrho° cells did not exhibit postreplicative DNA accumulation in lag phase (supplemental Fig. S5), indicating that the slow growth defect of crd1Δswe1Δrho° is not due to cell division delay after DNA replication. Taken together, these findings suggested that the crd1Δrho° mutant undergoes cell division defects controlled by Swe1p.
FIGURE 4.
Deletion of SWE1 in crd1Δrho° rescued cell morphology, chitin deposition, septin ring localization, and nuclear DNA content. A, the nucleus was visualized by staining with DAPI as described in the legend for Fig. 2. The scale bar represents 3 μm. B, chitin deposition was visualized by staining with WGA-Oregon Green 488 as described in the legend for Fig. 2. The scale bar represents 2 μm. C, Cdc3p-GFP was visualized as described in the legend for Fig. 2. The scale bar represents 2 μm. All images in the same pattern were taken at the same magnification (×1000). D, yeast cells were grown to mid-log phase in YPD medium at 30 °C and processed for flow cytometry as described under “Experimental Procedures” (1C, G1 DNA content; 2C, G2/M DNA content; 4C, greater than G2/M DNA content).
Stabilization and accumulation of Swe1p are triggered by actin depolarization (70, 71). We observed that actin polarization, as detected by phalloidin staining, was maintained in crd1Δrho° cells (supplemental Fig. S6). Similar to the WT, the actin cortical patches clustered at the bud tip. Because budding cells were elongated, the actin cable extended through the long neck to the bud tip. These results suggested that in crd1Δrho° cells, increased expression of the SWE1 gene leads to an accumulation of Swe1p (Fig. 3).
The Mitochondrial Retrograde Pathway Is Involved in Up-regulation of SWE1 in crd1Δrho° Cells
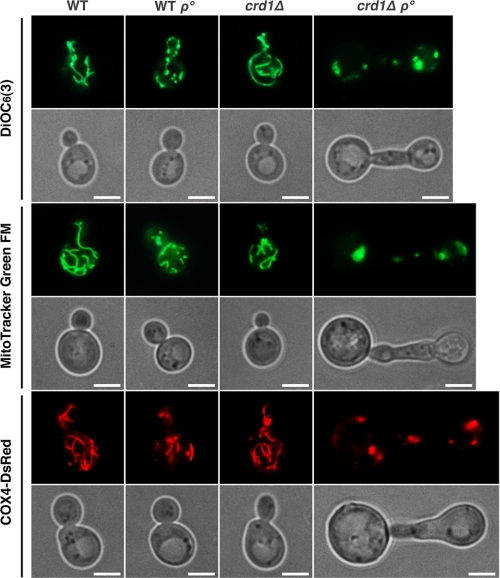
Although respiration is not essential for yeast cells, which can grow fermentatively on glucose, metabolic reactions performed in the mitochondria such as amino acid and lipid biosynthesis are essential (72–74). Yeast daughter cells cannot survive if they fail to inherit mitochondria from the mother cell (75–77). We predicted that loss of both CL and mtDNA drastically compromises mitochondrial function and contributes to severe growth defects and cell division delay in crd1Δrho° cells. To address this possibility, we observed mitochondrial morphology in dividing cells stained with DiOC6(3), which stains mitochondria in a potential-dependent manner, MitoTracker Green FM, which is potential-independent, and a plasmid-borne Cox4-DsRed fusion protein. As shown in Fig. 5, mitochondria in WT and crd1Δ cells exhibited an extended tubular morphology. The WT rho° cells displayed a reduced mitochondrial network. In contrast, normal tubule-like mitochondria were completely absent from crd1Δrho° cells, which contained large mitochondrial patches in mother and budding daughter cells. These results indicated that loss of both mtDNA and CL perturbed mitochondrial integrity.
FIGURE 5.
The crd1Δrho° mutant exhibited severe defects in mitochondrial integrity. Cells grown to the mid-log phase were incubated with DiOC6(3) or MitoTracker Green FM and examined under the fluorescence microscope. In living cells containing COX4-DsRed, mitochondria were visualized directly under the fluorescence microscope after cells were grown to the mid-log phase. All images were taken at the same magnification (×1000). The scale bar represents 2 μm.
As discussed above, the retrograde pathway is activated in yeast cells in response to mitochondrial dysfunction, resulting in increased expression of genes in anapleurotic pathways (29, 30). The degree to which the retrograde response is activated is dependent on the genetic background of cells and the carbon source used for growth (26, 27, 35, 78). For example, rho° cells in the PSY142 strain background exhibit a robust retrograde response in which CIT2 expression is up-regulated as much as 10-fold (26). In contrast, the expression of CIT2 is only slightly increased in rho° cells derived from the W303-1B strain background (27).
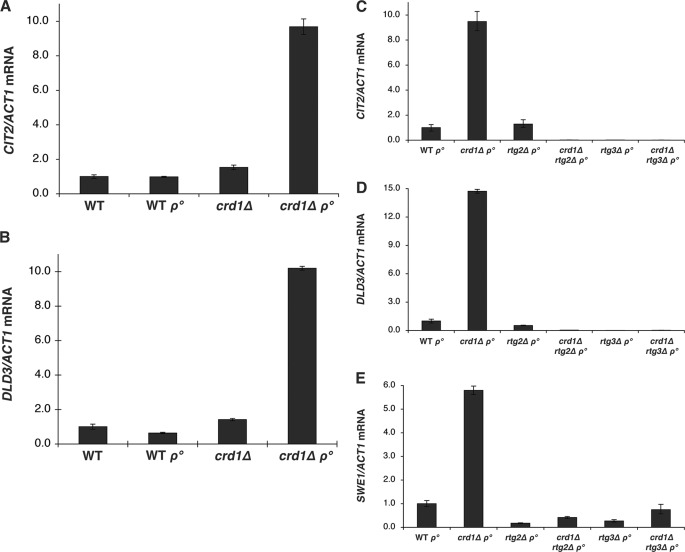
In the FGY3 strain background used in this study, neither crd1Δ nor rho° cells exhibited altered expression of CIT2 and DLD3 in glucose. Strikingly, however, the crd1Δrho° mutant exhibited a dramatic 10-fold increase in CIT2 and DLD3 expression (Fig. 6, A and B). To determine whether the increase in CIT2 and DLD3 resulted from activation of the retrograde pathway, RTG2 and RTG3 were deleted in crd1Δrho° cells. As expected, deletion of either RTG2 or RTG3 eliminated the increased expression of CIT2 and DLD3 (Fig. 6, C and D). Surprisingly, deletion of RTG2 or RTG3 from crd1Δrho° also reduced expression of SWE1 and Swe1p to wild type levels (Figs. 3B and 6E). These findings suggested that the mitochondrial retrograde pathway is involved in SWE1 expression. Moreover, the elongated budding cell morphology, defective chitin deposition, misplaced septin localization, and postreplicative DNA content were completely suppressed in crd1Δrtg2Δrho° and crd1Δrtg3Δrho° cells (supplemental Figs. S7–S10).
FIGURE 6.
Deletion of RTG2 or RTG3 suppressed elevated expression of SWE1 in crd1Δrho° cells. Total RNA was extracted from cells grown to the mid-log phase in YPD at 30 °C, and mRNA levels of CIT2, DLD3, and SWE1 were determined by real-time PCR as described under “Experimental Procedures.” Expression was normalized to the mRNA levels of the internal control ACT1. Error bars represent the range of the three independent experiments.
In conclusion, our results suggest a model in which loss of mtDNA in CL-deficient cells leads to severe mitochondrial dysfunction. This triggers activation of the retrograde pathway and up-regulation of SWE1, resulting in cell division delay.
DISCUSSION
The current study, which addressed the hypothesis that loss of CL from cells lacking mtDNA leads to cell division defects, has resulted in several novel findings. (i) The loss of CL from rho° cells led to cell division delay. (ii) Cell division delay was triggered by up-regulation of Swe1p. (iii) The induction of SWE1 stemmed from activation of the retrograde pathway as genetic elimination of the pathway decreased SWE1 expression. These findings indicate for the first time that mitochondrial anionic phospholipids are required for normal cell division in rho° cells and that cell division is controlled by the retrograde pathway in crd1Δrho° cells.
The finding of cell division defects in crd1Δrho° cells strongly supports the hypothesis that anionic phospholipids play an important role in maintenance of normal cell division in the absence of mtDNA. Unlike pgs1Δ, the crd1Δ mutant is not petite lethal and does not exhibit sensitivity to cell wall perturbing agents, suggesting that the phospholipid PG satisfies the anionic phospholipid requirement for cell wall biogenesis and cell viability (22). However, PG levels are significantly decreased in crd1Δrho° cells when compared with those of crd1Δ, and this deficiency may exacerbate mitochondrial dysfunction. Consistent with this, the normal tubular network of mitochondria is absent from crd1Δrho° cells (Fig. 5). Furthermore, crd1Δrho° cells fail to maintain mitochondrial integrity and contain fragmented mitochondrial patches in mother and budding daughter cells (Fig. 5). Restoration of CL by a plasmid borne CRD1 gene reverses the enlarged cell size and elongated budding cell phenotypes (Fig. 2A), indicating that the dysfunction is due to loss of CL from rho° cells. Other mutations that impair mitochondrial function and morphology have been linked to cell division defects, such as mmm1, mdm10, and mdm12 (43, 79, 80). Interestingly, deletion of any of these genes is synthetically lethal with crd1 (81). However, the mechanisms linking mitochondria to cell division have not been previously identified.
The presence of postreplicative DNA accumulation, multinucleate mother cells, and elongated anucleate budding daughter cells revealed the incoordination of nuclear division and cell division in crd1Δrho° cells (Fig. 2B). It appears that excess apical budding growth continues both in the presence and in the absence of nuclear division. This may result from elevated SWE1 expression because overexpression of SWE1 in WT cells can also cause the binucleated phenotype and cell division delay (supplemental Fig. S1). Swe1p negatively regulates the mitotic Clb-Cdc28p complexes by phosphorylating residue Tyr-19. As a consequence, Cdc28p cannot induce the switch from polar to isotropic bud growth, resulting in the formation of elongated buds (82, 83). Consistent with a previous study (67), we found that overexpression of SWE1 in WT cells resulted in the absence of a G1 DNA content peak and accumulation of G2/M and greater than G2/M DNA content (supplemental Fig. S1B). The increase in SWE1 expression and accumulation of Swe1p (Fig. 3) could thus easily account for the postreplicative DNA accumulation, multinucleate mother cells, elongated anucleate budding, misplaced septin, and defective chitin deposition in crd1Δrho° cells (Fig. 2, D and E). These findings are also consistent with reports that overexpression of SWE1 leads to elongated budding cells (67) and that Swe1p strongly influences septin localization by inhibiting Clb-Cdc28p (69). Defective chitin deposition may also be attributed to Swe1p-mediated inhibition of Cdc28p as the cdc28 mutant showed aberrant distribution of chitin in the cell wall (84).
Although disruption of SWE1 completely suppressed the postreplicative DNA accumulation, elongated budding cell phenotype, and restored localization of septin and chitin in crd1Δrho° cells (Fig. 4, A–C), it did not rescue the slow growth defect (supplemental Fig. S4). Although yeast can survive without mtDNA, which encodes respiratory chain components for oxidative phosphorylation, the tricarboxylic acid cycle and the biosynthesis of cellular metabolites, including some amino acids and lipids, are essential (72). These essential reactions require the import of nuclear-encoded proteins and the incorporation of lipids synthesized in other organelles (85). CL is localized in the mitochondrial inner membrane and affects the stability of various inner membrane protein complexes, including respiratory chain complexes and metabolite carriers (1). Moreover, CL is required for mitochondrial inner membrane fusion that facilitates content exchange and mtDNA maintenance (86). Surprisingly, we have recently shown that CL is involved in outer membrane protein biogenesis as the loss of CL impairs the assembly pathways of several mitochondrial outer membrane proteins (87). In addition, disruption of CRD1 is synthetically lethal with endoplasmic reticulum-mitochondria encounter structure (ERMES) components, which are indispensable for efficient interorganelle phospholipid exchange and mitochondrial morphology and inheritance (43, 79–81). As shown in Fig. 5, loss of mtDNA in crd1Δ led to severe mitochondrial morphology defects, which may affect the essential cellular functions of mitochondria, such as protein import, lipid exchange, and biosynthesis of metabolites. Therefore, severe mitochondrial defects are the main cause of growth defects in crd1Δrho°. Disruption of SWE1 in crd1Δrho° cells did not rescue mitochondrial morphology defects (supplemental Fig. S11), suggesting that the slow growth defect in swe1crd1Δrho° cells is due to exacerbated mitochondrial dysfunction. Similarly, despite suppression of postreplicative DNA content and Swe1p induction (Fig. 3 and supplemental Figs. S5 and S10), crd1Δrtg2Δrho° and crd1Δrtg3Δrho° cells exhibited slow growth and severe defects in mitochondrial integrity similar to that observed in crd1Δswe1Δrho° (supplemental Figs. S4 and S11). Although deletion of SWE1, RTG2, or RTG3 rescued cell division defects, it could not restore mitochondrial functions that require CL.
The mechanism whereby SWE1 expression is up-regulated in the crd1Δrho° mutant is not understood. Critical to understanding this mechanism is the induction of the retrograde response in crd1Δrho° cells. Neither WT rho° nor crd1Δ cells exhibited an increased retrograde response in the strain background used in this study (FGY3). In fact, the retrograde regulation appears to be triggered by altered mitochondrial metabolism, as opposed to an altered mitochondrial genome (26, 88). Deletion of RTG2 or RTG3 eliminated the elevated expression of SWE1, elongated budding cell morphology, misplaced septin ring, defective chitin deposition, and postreplicative DNA content in crd1Δrho° cells (Fig. 3 and supplemental Figs. S7–S10). One possible explanation for elevated SWE1 expression is that it is a target of the effectors of the retrograde pathway, Rtg1p/Rtg3p, that bind as a heterodimer to the R box (GTCAC) region in promoters of RTG target genes, including CIT2, DLD3, CIT1, ACO1, IDH1, and IDH2 (31, 33, 34, 36, 89). The R box is localized at −100 bp to −400 bp upstream from the open reading frame and is necessary for maximal gene expression. Inspection of the 5′-flanking region of the SWE1 gene revealed the presence of an R box localized at −356 bp, which could serve as a putative Rtg1p/Rtg3p binding site. Further work will be required to address this question.
Collectively, our results suggest that in the absence of both mtDNA and CL, cell division is compromised due to exacerbation of mitochondrial dysfunction, which triggers activation of the retrograde pathway. Swe1p is up-regulated by mitochondrial retrograde response and delays the cell division, possibly as a mechanism to avoid the generation of abnormal daughter cells. A model depicting this regulation is shown in Fig. 7.
FIGURE 7.
Model for retrograde pathway-mediated cell division delay in crd1Δrho° cells. The loss of mtDNA in crd1Δ cells leads to exacerbation of mitochondrial dysfunction, which is sensed by Rtg2p. The Rtg2p transmits this signal to the heterodimer Rtg1p/Rtg3p, resulting in translocation of Rtg1p/Rtg3p from the cytoplasm to the nucleus where it activates transcription of SWE1. The up-regulation of Swe1p serves as a compensatory mechanism to delay the cell division, preventing the generation of abnormal daughter cells.
The pgs1Δ mutant, lacking both PG and CL, loses mtDNA spontaneously in the absence of 1 m sorbitol and exhibits more severe growth defects than crd1Δrho° cells (Fig. 1A). It is tempting to speculate that the petite lethal phenotype of pgs1Δ is attributed to cell division defects due to the absence of both mtDNA and anionic phospholipids. Similar to crd1Δrho° cells, pgs1Δ cells display defective deposition of chitin (21), which indicates a possible cell division defect. The yeast cell wall consists of three major structural polysaccharides, mannans, glucans, and chitin (90, 91). Mannans and glucan are synthesized continuously during the cell cycle and are distributed uniformly in the cell wall, whereas chitin displays periodic synthesis and restricted localization (90). The importance of chitin distribution in cell division was underscored by the observation that some cell cycle mutations cause uniform distribution of chitin, such as cdc3 and cdc28 (84). In addition to the potential cell division defect due to the absence of both mtDNA and anionic phospholipids, the pgs1Δ cells may also be subject to defective cell division stemming from perturbation of cell wall biogenesis (21). Fks1p, a catalytic subunit of 1,3-β-d-glucan synthase, was barely detectable in pgs1Δ cells, which contained markedly reduced β-1,3-glucan (22). As the cell wall morphology checkpoint monitors cell wall biosynthesis during the cell division, the fks1 mutant, which has a significant defect in glucan synthesis, exhibits G2 arrest (92). Therefore, the defective cell wall might contribute to a cell division delay in pgs1Δ.
In summary, we have shown that CL is required for normal cell division in the absence of mtDNA. These studies show for the first time that mitochondrial dysfunction leads to cell division defects that are mediated by retrograde regulation. Our findings provide novel insights into the essential cellular functions of CL and shed light on the mechanism underlying petite lethality of pgs1Δ.
Acknowledgments
We thank Dr. Enrico Cabib (NIDDK, National Institutes of Health), Dr. Michael Snyder (Yale University), and Dr. Douglas I. Johnson (University of Vermont) for providing septin-GFP plasmids and Dr. Benjamin S. Glick (University of Chicago) for the plasmid pHS12-DsRed.T4. We also thank previous members of Greenberg laboratory Dr. Quan Zhong, Dr. Shulin Ju, Dr. Jingming Zhou, and Morgan Thompson for helpful advice.
This work was supported, in whole or in part, by National Institutes of Health Grant HL62263. This work was also supported by a grant from the Barth Syndrome Foundation.
This article was selected as a Paper of the Week.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S11.
- CL
- cardiolipin
- PG
- phosphatidylglycerol
- RTG
- retrograde regulation
- YPD
- yeast extract, peptone and dextrose
- YPDS
- yeast extract, peptone, dextrose and sorbitol
- YPGE
- yeast extract, peptone, glycerol, and ethanol
- WT
- wild type
- WGA
- wheat germ agglutinin
- GFP
- green fluorescent protein
- DsRed
- Discosoma red fluorescent protein
- DAPI
- 4′,6-diamidino-2-phenylindole
- PIPES
- 1,4-piperazinediethanesulfonic acid.
REFERENCES
- 1.Schlame M., Rua D., Greenberg M. L. (2000) Prog. Lipid Res. 39, 257–288 [DOI] [PubMed] [Google Scholar]
- 2.Hoch F. L. (1992) Biochim. Biophys. Acta 1113, 71–133 [DOI] [PubMed] [Google Scholar]
- 3.Yu C. A., Yu L. (1980) Biochemistry 19, 5715–5720 [DOI] [PubMed] [Google Scholar]
- 4.Drees M., Beyer K. (1988) Biochemistry 27, 8584–8591 [DOI] [PubMed] [Google Scholar]
- 5.Beyer K., Nuscher B. (1996) Biochemistry 35, 15784–15790 [DOI] [PubMed] [Google Scholar]
- 6.Hayer-Hartl M., Schägger H., von Jagow G., Beyer K. (1992) Eur. J. Biochem. 209, 423–430 [DOI] [PubMed] [Google Scholar]
- 7.Gomez B., Jr., Robinson N. C. (1999) Biochemistry 38, 9031–9038 [DOI] [PubMed] [Google Scholar]
- 8.Robinson N. C., Zborowski J., Talbert L. H. (1990) Biochemistry 29, 8962–8969 [DOI] [PubMed] [Google Scholar]
- 9.Sedlák E., Robinson N. C. (1999) Biochemistry 38, 14966–14972 [DOI] [PubMed] [Google Scholar]
- 10.Jiang F., Ryan M. T., Schlame M., Zhao M., Gu Z., Klingenberg M., Pfanner N., Greenberg M. L. (2000) J. Biol. Chem. 275, 22387–22394 [DOI] [PubMed] [Google Scholar]
- 11.Koshkin V., Greenberg M. L. (2000) Biochem. J. 347, 687–691 [PMC free article] [PubMed] [Google Scholar]
- 12.Koshkin V., Greenberg M. L. (2002) Biochem. J. 364, 317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeiffer K., Gohil V., Stuart R. A., Hunte C., Brandt U., Greenberg M. L., Schägger H. (2003) J. Biol. Chem. 278, 52873–52880 [DOI] [PubMed] [Google Scholar]
- 14.Zhang M., Mileykovskaya E., Dowhan W. (2002) J. Biol. Chem. 277, 43553–43556 [DOI] [PubMed] [Google Scholar]
- 15.Zhang M., Mileykovskaya E., Dowhan W. (2005) J. Biol. Chem. 280, 29403–29408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang S. C., Heacock P. N., Clancey C. J., Dowhan W. (1998) J. Biol. Chem. 273, 9829–9836 [DOI] [PubMed] [Google Scholar]
- 17.Dzugasová V., Obernauerová M., Horváthová K., Vachová M., Záková M., Subík J. (1998) Curr. Genet. 34, 297–302 [DOI] [PubMed] [Google Scholar]
- 18.Chang S. C., Heacock P. N., Mileykovskaya E., Voelker D. R., Dowhan W. (1998) J. Biol. Chem. 273, 14933–14941 [DOI] [PubMed] [Google Scholar]
- 19.Jiang F., Rizavi H. S., Greenberg M. L. (1997) Mol. Microbiol. 26, 481–491 [DOI] [PubMed] [Google Scholar]
- 20.Tuller G., Hrastnik C., Achleitner G., Schiefthaler U., Klein F., Daum G. (1998) FEBS Lett. 421, 15–18 [DOI] [PubMed] [Google Scholar]
- 21.Zhong Q., Gvozdenovic-Jeremic J., Webster P., Zhou J., Greenberg M. L. (2005) Mol. Biol. Cell 16, 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong Q., Li G., Gvozdenovic-Jeremic J., Greenberg M. L. (2007) J. Biol. Chem. 282, 15946–15953 [DOI] [PubMed] [Google Scholar]
- 23.Contamine V., Picard M. (2000) Microbiol. Mol. Biol. Rev. 64, 281–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subı̆k J. (1974) FEBS Lett. 42, 309–313 [DOI] [PubMed] [Google Scholar]
- 25.Janitor M., Subík J. (1993) Curr. Genet. 24, 307–312 [DOI] [PubMed] [Google Scholar]
- 26.Epstein C. B., Waddle J. A., Hale W., 4th, Davé V., Thornton J., Macatee T. L., Garner H. R., Butow R. A. (2001) Mol. Biol. Cell 12, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traven A., Wong J. M., Xu D., Sopta M., Ingles C. J. (2001) J. Biol. Chem. 276, 4020–4027 [DOI] [PubMed] [Google Scholar]
- 28.Parikh V. S., Morgan M. M., Scott R., Clements L. S., Butow R. A. (1987) Science 235, 576–580 [DOI] [PubMed] [Google Scholar]
- 29.Liu Z., Butow R. A. (2006) Annu. Rev. Genet. 40, 159–185 [DOI] [PubMed] [Google Scholar]
- 30.Butow R. A., Avadhani N. G. (2004) Mol. Cell 14, 1–15 [DOI] [PubMed] [Google Scholar]
- 31.Liu Z., Butow R. A. (1999) Mol. Cell. Biol. 19, 6720–6728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z., Sekito T., Spírek M., Thornton J., Butow R. A. (2003) Mol. Cell 12, 401–411 [DOI] [PubMed] [Google Scholar]
- 33.Sekito T., Thornton J., Butow R. A. (2000) Mol. Biol. Cell 11, 2103–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chelstowska A., Liu Z., Jia Y., Amberg D., Butow R. A. (1999) Yeast 15, 1377–1391 [DOI] [PubMed] [Google Scholar]
- 35.Liao X. S., Small W. C., Srere P. A., Butow R. A. (1991) Mol. Cell. Biol. 11, 38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia Y., Rothermel B., Thornton J., Butow R. A. (1997) Mol. Cell. Biol. 17, 1110–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newlon C. S., Fangman W. L. (1975) Cell 5, 423–428 [DOI] [PubMed] [Google Scholar]
- 38.Newlon C. S., Ludescher R. D., Walter S. K. (1979) Mol. Gen. Genet. 169, 189–194 [DOI] [PubMed] [Google Scholar]
- 39.Devin A. B., Prosvirova T. Yu., Peshekhonov V. T., Chepurnaya O. V., Smirnova M. E., Koltovaya N. A., Troitskaya E. N., Arman I. P. (1990) Yeast 6, 231–243 [DOI] [PubMed] [Google Scholar]
- 40.Koltovaya N. A., Arman I. P., Devin A. B. (1998) Yeast 14, 133–146 [DOI] [PubMed] [Google Scholar]
- 41.Lisowsky T. (1992) Mol. Gen. Genet. 232, 58–64 [DOI] [PubMed] [Google Scholar]
- 42.Lisowsky T. (1994) Curr. Genet. 26, 15–20 [DOI] [PubMed] [Google Scholar]
- 43.Burgess S. M., Delannoy M., Jensen R. E. (1994) J. Cell Biol. 126, 1375–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McConnell S. J., Yaffe M. P. (1992) J. Cell Biol. 118, 385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart L. C., Yaffe M. P. (1991) J. Cell Biol. 115, 1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaden D. L., Gohil V. M., Gu Z., Greenberg M. L. (2005) Anal. Biochem. 338, 162–164 [DOI] [PubMed] [Google Scholar]
- 47.Hasek J. (2006) Methods Mol. Biol. 313, 85–96 [DOI] [PubMed] [Google Scholar]
- 48.Amberg D. C. (1998) Mol. Biol. Cell 9, 3259–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bevis B. J., Glick B. S. (2002) Nat. Biotechnol. 20, 83–87 [DOI] [PubMed] [Google Scholar]
- 50.Gaynor P. M., Hubbell S., Schmidt A. J., Lina R. A., Minskoff S. A., Greenberg M. L. (1991) J. Bacteriol. 173, 6124–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang F., Gu Z., Granger J. M., Greenberg M. L. (1999) Mol. Microbiol. 31, 373–379 [DOI] [PubMed] [Google Scholar]
- 52.Zhao M., Schlame M., Rua D., Greenberg M. L. (1998) J. Biol. Chem. 273, 2402–2408 [DOI] [PubMed] [Google Scholar]
- 53.Zhong Q., Greenberg M. L. (2003) J. Biol. Chem. 278, 33978–33984 [DOI] [PubMed] [Google Scholar]
- 54.Gohil V. M., Hayes P., Matsuyama S., Schägger H., Schlame M., Greenberg M. L. (2004) J. Biol. Chem. 279, 42612–42618 [DOI] [PubMed] [Google Scholar]
- 55.Bulawa C. E. (1993) Annu. Rev. Microbiol. 47, 505–534 [DOI] [PubMed] [Google Scholar]
- 56.Klis F. M., Boorsma A., De Groot P. W. (2006) Yeast 23, 185–202 [DOI] [PubMed] [Google Scholar]
- 57.Klis F. M., Mol P., Hellingwerf K., Brul S. (2002) FEMS Microbiol. Rev. 26, 239–256 [DOI] [PubMed] [Google Scholar]
- 58.Cabib E. (2004) Arch. Biochem. Biophys. 426, 201–207 [DOI] [PubMed] [Google Scholar]
- 59.DeMarini D. J., Adams A. E., Fares H., De Virgilio C., Valle G., Chuang J. S., Pringle J. R. (1997) J. Cell Biol. 139, 75–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gladfelter A. S., Pringle J. R., Lew D. J. (2001) Curr. Opin. Microbiol. 4, 681–689 [DOI] [PubMed] [Google Scholar]
- 61.Longtine M. S., Bi E. (2003) Trends Cell Biol. 13, 403–409 [DOI] [PubMed] [Google Scholar]
- 62.Versele M., Thorner J. (2005) Trends Cell Biol. 15, 414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lew D. J., Reed S. I. (1995) J. Cell Biol. 129, 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudner A. D., Murray A. W. (1996) Curr. Opin. Cell Biol. 8, 773–780 [DOI] [PubMed] [Google Scholar]
- 65.Weinert T. A., Hartwell L. H. (1988) Science 241, 317–322 [DOI] [PubMed] [Google Scholar]
- 66.Weinert T. A., Kiser G. L., Hartwell L. H. (1994) Genes Dev. 8, 652–665 [DOI] [PubMed] [Google Scholar]
- 67.Booher R. N., Deshaies R. J., Kirschner M. W. (1993) EMBO J. 12, 3417–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russell P., Moreno S., Reed S. I. (1989) Cell 57, 295–303 [DOI] [PubMed] [Google Scholar]
- 69.Gladfelter A. S., Kozubowski L., Zyla T. R., Lew D. J. (2005) J. Cell Sci. 118, 1617–1628 [DOI] [PubMed] [Google Scholar]
- 70.Sia R. A., Bardes E. S., Lew D. J. (1998) EMBO J. 17, 6678–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNulty J. J., Lew D. J. (2005) Curr. Biol. 15, 2190–2198 [DOI] [PubMed] [Google Scholar]
- 72.Attardi G., Schatz G. (1988) Annu. Rev. Cell Biol. 4, 289–333 [DOI] [PubMed] [Google Scholar]
- 73.Gbelská Y., Subík J., Svoboda A., Goffeau A., Kovác L. (1983) Eur. J. Biochem. 130, 281–286 [DOI] [PubMed] [Google Scholar]
- 74.Hermann G. J., Shaw J. M. (1998) Annu. Rev. Cell Dev. Biol. 14, 265–303 [DOI] [PubMed] [Google Scholar]
- 75.Boldogh I. R., Yang H. C., Pon L. A. (2001) Traffic 2, 368–374 [DOI] [PubMed] [Google Scholar]
- 76.Jensen R. E., Hobbs A. E., Cerveny K. L., Sesaki H. (2000) Microsc. Res. Tech. 51, 573–583 [DOI] [PubMed] [Google Scholar]
- 77.Yaffe M. P. (1999) Science 283, 1493–1497 [DOI] [PubMed] [Google Scholar]
- 78.Kirchman P. A., Kim S., Lai C. Y., Jazwinski S. M. (1999) Genetics 152, 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berger K. H., Sogo L. F., Yaffe M. P. (1997) J. Cell Biol. 136, 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sogo L. F., Yaffe M. P. (1994) J. Cell Biol. 126, 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kornmann B., Currie E., Collins S. R., Schuldiner M., Nunnari J., Weissman J. S., Walter P. (2009) Science 325, 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pruyne D., Bretscher A. (2000) J. Cell Sci. 113, 365–375 [DOI] [PubMed] [Google Scholar]
- 83.Pruyne D., Bretscher A. (2000) J. Cell Sci. 113, 571–585 [DOI] [PubMed] [Google Scholar]
- 84.Roberts R. L., Bowers B., Slater M. L., Cabib E. (1983) Mol. Cell. Biol. 3, 922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neupert W. (1997) Annu. Rev. Biochem. 66, 863–917 [DOI] [PubMed] [Google Scholar]
- 86.DeVay R. M., Dominguez-Ramirez L., Lackner L. L., Hoppins S., Stahlberg H., Nunnari J. (2009) J. Cell Biol. 186, 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gebert N., Joshi A. S., Kutik S., Becker T., McKenzie M., Guan X. L., Mooga V. P., Stroud D. A., Kulkarni G., Wenk M. R., Rehling P., Meisinger C., Ryan M. T., Wiedemann N., Greenberg M. L., Pfanner N. (2009) Curr. Biol. 19, 2133–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poyton R. O., McEwen J. E. (1996) Annu. Rev. Biochem. 65, 563–607 [DOI] [PubMed] [Google Scholar]
- 89.Rothermel B. A., Shyjan A. W., Etheredge J. L., Butow R. A. (1995) J. Biol. Chem. 270, 29476–29482 [DOI] [PubMed] [Google Scholar]
- 90.Cabib E., Roberts R., Bowers B. (1982) Annu. Rev. Biochem. 51, 763–793 [DOI] [PubMed] [Google Scholar]
- 91.Lesage G., Bussey H. (2006) Microbiol. Mol. Biol. Rev. 70, 317–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suzuki M., Igarashi R., Sekiya M., Utsugi T., Morishita S., Yukawa M., Ohya Y. (2004) Nat. Cell Biol. 6, 861–871 [DOI] [PubMed] [Google Scholar]
- 93.Vaz F. M., Houtkooper R. H., Valianpour F., Barth P. G., Wanders R. J. (2003) J. Biol. Chem. 278, 43089–43094 [DOI] [PubMed] [Google Scholar]
- 94.Chen S., He Q., Greenberg M. L. (2008) Mol. Microbiol. 68, 1061–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmidt M., Varma A., Drgon T., Bowers B., Cabib E. (2003) Mol. Biol. Cell 14, 2128–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Casamayor A., Snyder M. (2003) Mol. Cell. Biol. 23, 2762–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richman T. J., Sawyer M. M., Johnson D. I. (1999) J. Biol. Chem. 274, 16861–16870 [DOI] [PubMed] [Google Scholar]