Abstract
The telomeric complex, shelterin, plays a critical role in protecting chromosome ends from erosion, and disruption of these complexes can lead to chromosomal instability culminating in cell death or malignant transformation. We reported previously that dominant-negative mutants of one of the telomeric proteins called TIN2 cause death of androgen receptor (AR)-negative but not AR-positive prostate cancer cells, raising the question of a possible role of AR in the structural stability of telomeric complexes. Consistent with this possibility, in the present study, we observed that the AR antagonist Casodex (bicalutamide) disrupted telomeric complexes in AR-positive LNCaP cells but not in AR-negative PC-3 cells. Immunofluorescent studies revealed colocalization of TIN2 and AR. Reciprocal immunoprecipitation studies showed association of AR with telomeric proteins. Furthermore, telomeric proteins were overexpressed in prostate cancer cells compared with normal prostate epithelial cells, and sucrose density gradient analysis showed co-sedimentation of AR with telomeric proteins in a shelterin-like mega complex. Together, these observations suggest an allosteric role of AR in telomere complex stability in prostate cancer cells and suggest that AR-antagonist Casodex-mediated cell death may be due to telomere complex disruption.
Keywords: DNA Damage, Protein-Protein interactions, Steroid Hormone Receptor, Telomere, Tumor, Androgen Receptor, Prostate Cancer
Introduction
Telomeres are the DNA-protein structures that cap the ends of linear chromosomes and protect them from fusing end-to-end. Maintaining the integrity and length of telomeres is essential for genomic stability, normal growth, and survival of mammalian cells (1). Although telomerase is known to maintain telomere length by adding telomeric DNA repeats to chromosome ends, a host of proteins that bind to telomeric DNA either directly or indirectly (through protein-protein interactions) are known to be important for regulation of telomere length and capping. Among the proteins that bind directly are the telomeric repeat-binding factors TRF1 and TRF2. Factors that bind indirectly to the telomeric DNA include TIN2 (TRF1-interacting protein 2), which binds directly to TRF1 and TRF2, and indirectly to protector of telomerase 1 (POT1) (2–5). These proteins, together with TPP1 and hRap1, form a core telomere maintenance complex called shelterin (6). Other proteins involved in cellular processes such as DNA repair, including RAD50 (7) and Ku (8, 9), also interact with TRF1 and TRF2 in shelterin. TRF1, TRF2, and TIN2 regulate telomere length (6), and overexpression of these proteins occurs in several cancers, including lung cancer, lymphomas, and hepatocarcinoma (10–12). However, the level of expression of these proteins and their role in the structural and functional stability of shelterin or its related subcomplexes (13) in prostate cancer cells remain to be determined.
We reported previously that TIN2 mutants TIN2–15C (with a C-terminal deletion) and TIN2–13 (with an N-terminal deletion) abolish TIN2 interaction with TRF1 and TRF2, respectively, and induce apoptosis in estrogen receptor-negative MDA-MB-231 and MDA-MB-157 but not in estrogen receptor-positive MCF-7, breast cancer cells (13). TIN2–15C and TIN2–13 also cause death of AR-negative PPC-1 but not AR-positive LNCaP, prostate cancer cells (13). Failure to kill receptor-positive cells by disrupting telomeric complexes with TIN2 mutants suggests that the structure of telomeric complexes may differ in receptor-positive versus receptor-negative cells and raises an intriguing question of whether AR2 stabilizes telomeric complexes in prostate cancer cells. To test this hypothesis, we examined the effect of the AR antagonist bicalutamide (Casodex). Casodex caused a dramatic disruption of telomeric complexes, as measured by recruitment of 53BP1 to telomeres (14), in AR-positive LNCaP cells but not in AR-negative PC-3 cells. Furthermore, immunofluorescence staining and biochemical fractionation studies revealed AR interaction with telomeric complexes in LNCaP cells. These studies indicate that AR is a structural component of telomeric complexes and its inhibition by Casodex disrupts telomeric complexes required for the viability of prostate cancer cells.
EXPERIMENTAL PROCEDURES
Cell Culture
LNCaP, PC-3, and PPC-1 cells obtained from ATCC were grown in RPMI medium containing 10% fetal bovine serum, 2.5 mm glutamine, 100 μg/ml streptomycin, and 100 units/ml penicillin. Primary normal prostate epithelial cells were purchased from Lonza, Inc. (Riverside, CA) and cultured following the manufacturer's instructions.
Immunoprecipitation and Western Blot Analysis
LNCaP cell lysates were prepared as described (15) using radioimmune precipitation assay buffer containing 0.5 m NaCl and diluted with 2 volumes of buffer A (50 mm Tris, pH 7.6, 1% Nonidet P-40, and 10% glycerol). AR, TRF2, and hemagglutinin (HA)-tagged TRF1 in diluted cell lysates were immunoprecipitated with 2–4 μg/ml of AR-441 (Santa Cruz Biotechnology), TRF2 (IMG-124A, Imgenex), or HA (sc-7392, Santa Cruz Biotechnology) antibodies, respectively. For Western blot analysis, membranes were probed with antibodies against AR (AR-N20, Santa Cruz Biotechnology), TRF2 (IMG-124A), TRF1 (H-242, Santa Cruz Biotechnology), HA (sc-805, Santa Cruz Biotechnology), TIN2 (15), TPP1 (Abcam), or glyceraldehyde-3-phosphate dehydrogenase (Chemicon). Immunoreactive bands were developed using horseradish peroxidase conjugated secondary antibodies and SuperSignal WestPico chemiluminescent substrate (Pierce) and visualized using x-ray film.
Indirect Immunofluorescence
The immunofluorescence staining of cells grown on glass slides was performed as described (15). Permeabilized cells fixed with 4% paraformaldehyde were incubated with antibodies against TIN2 (15), AR (AR-N20, AR-441, Santa Cruz Biotechnology), 53BP1 (Abcam), γ-H2AX (Upstate), or TRF2 (Imgenex). After washing, cells were stained with goat anti-rabbit fluorescein isothiocyanate- and goat anti-mouse Texas Red-labeled (Molecular Probes) secondary antibodies. Images of cells were acquired on an LSM-410 confocal microscope (Zeiss) or fluorescent microscope (Olympus).
Sucrose Density Gradient Analysis
Nuclei prepared as described (16) were extracted with buffer B (50 mm Tris, pH 7.6, 0.5 m NaCl, 0.25 m sucrose, 5 mm MgCl2, 8 mm dithiothreitol, and protease inhibitor mixture) on ice for 30 min. The nuclear extracts were cleared by centrifugation (30 min, 40,000 × g) and dialyzed against buffer C (50 mm Tris, pH 7.6, 150 mm NaCl, 0.2 mm EDTA, 0.025% Nonidet P-40, 0.5 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride). Nuclear extract (0.6 ml) was layered onto a 15–65% sucrose density gradient and centrifuged in a Beckman SW50.1 rotor at 35,000 rpm, 4 °C, for 16 h as described (16). The gradient was resolved into 0.5-ml fractions, and the protein in 200 μl of each fraction was precipitated with 8% (final concentration) trichloroacetic acid for Western blotting.
RESULTS
Anti-androgen Bicalutamide Disrupts Telomeric Complexes in Prostate Cancer Cells
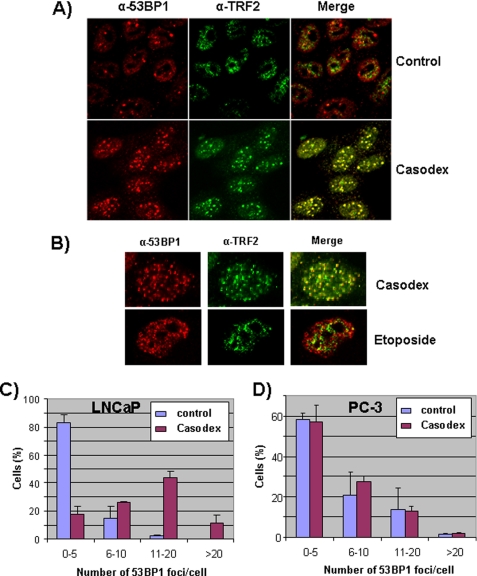
The disruption of telomeric complexes has been shown to elicit a DNA damage response, which leads to the recruitment of 53BP1 (a p53-binding protein) to, and phosphorylation of histone H2AX at, telomeres (14). This DNA damage response can be monitored by colocalization of 53BP1 or phosphorylated γ-H2AX with one of the telomeric proteins, such as TRF2 or TIN2 (2, 14). We examined the potential role of AR in the maintenance of telomeric complexes by studying the effect of bicalutamide (Casodex) on the recruitment of 53BP1 to, or phosphorylation of γ-H2AX at, telomeres in AR-positive LNCaP cells. As shown in Fig. 1A, we observed a dramatic increase in the number of immunofluorescent 53BP1 foci in Casodex-treated cells as compared with controls. These 53BP1 foci in Casodex-treated cells colocalized with TRF2. We observed a similar colocalization of γ-H2AX foci with TIN2 foci in Casodex-treated cells (data not shown). By comparison, there was very little co-localization of TRF2 foci with the few 53BP1 foci that were present in control cells (Fig. 1A), indicating that 53BP1 foci in control cells are not located at telomeres.
FIGURE 1.
Casodex disrupts telomeric complexes in AR-positive prostate cancer cells. A and B, LNCaP cells treated with 100 μm Casodex for 48 h (A and B) or with 20 μg/ml etoposide for 1 h (B) were co-immunostained with antibodies against 53BP1 and TRF2. LNCaP (C) or PC-3 (D) cells were treated with or without 100 μm Casodex for 48 h and then immunostained with 53BP1 antibody. 53BP1 foci were counted, and data are presented as the percentage of cells with 0–5, 6–10, 11–20, or >20 foci/cell. Immunostaining and confocal microscopy were performed as described under “Experimental Procedures.” 80 cells in each treatment group were scored in three separate experiments.
To determine whether the DNA damage response (recruitment of 53BP1 to telomeres) induced by Casodex was due to disruption of telomere complexes or induction of DNA double strand breaks, we compared the effect of Casodex with that of etoposide. Etoposide induces random DNA double-strand breaks and causes 53BP1 recruitment to sites of DNA damage throughout the chromosomes (17). As shown in Fig. 1B, etoposide caused extensive DNA damage as indicated by the increase in the number of 53BP1 foci. However, very few 53BP1 foci in etoposide-treated cells were colocalized with TRF2 (Fig. 1B). Thus, whereas Casodex-induced 53BP1 foci were located predominantly in telomeres (Fig. 1, A and B), etoposide-induced 53BP1 foci were located throughout the chromatin (Fig. 1B). These observations suggest that a structural effect of Casodex on AR led to disruption of telomeric complexes that protect chromosome ends and initiated a DNA damage response at telomeres.
We then reasoned that if the DNA damage response caused by Casodex was due to an effect on AR, then Casodex treatment should have no effect on 53BP1 foci formation in AR-negative prostate cancer cells. We compared the effect of Casodex on the number of 53BP1 foci/cell in AR-positive LNCaP versus AR-negative PC-3 cells. In LNCaP cells, Casodex caused a substantial increase in the percentage of cells with >5 (6–10, 11–20, or >20) 53BP1 foci/cell, and a corresponding decrease in the percentage of cells with ≤5 53BP1 foci/cell (Fig. 1C). By contrast, Casodex had no significant effect on the number of 53BP1 foci/cell in PC-3 cells (Fig. 1D). Thus, Casodex had a selective effect on the disruption of telomeres, as indicated by the recruitment of 53BP1 to telomeres, in AR-positive prostate cancer cells.
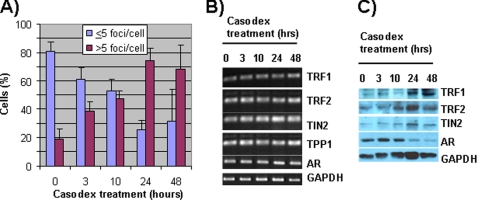
Casodex-induced Disruption of Telomeric Complexes Was Not Associated with a Decrease in the Level of Telomeric Proteins
The time course of the DNA damage response to Casodex treatment of LNCaP cells is shown in Fig. 2. The increase in the percentage of LNCaP cells with >5 53BP1 foci/cell peaked at ∼24 h (Fig. 2A). There was no concomitant decrease in the level of TRF1, TRF2, TIN2, or TPP1 either at the mRNA (Fig. 2B) or protein (Fig. 2C) level during the 48-h treatment. However, the AR protein level decreased starting 10 h after initiation of treatment with Casodex (Fig. 2C). This effect of Casodex on AR protein levels has been seen previously (18). These data suggest that Casodex disrupts telomere complexes not by decreasing the expression of telomeric proteins (an indirect effect of inhibiting AR transcriptional activity) but by disrupting AR structure (a direct effect of binding to AR). These data thus implicate a structural role of AR in telomere complexes.
FIGURE 2.
Casodex disrupts telomeric complexes without affecting the expression of telomeric proteins. A, LNCaP cells treated with 100 μm Casodex for 0, 3, 10, 24, or 48 h were immunostained for 53BP1. 53BP1 foci were counted, and cells were categorized as having ≤5 or >5 foci/cell. ∼200 cells in each treatment group were scored in three independent experiments. B, total RNA from LNCaP cells treated with Casodex was extracted using TRIzol (Invitrogen), and RT-PCR was performed as described previously (32) to measure mRNA levels. Sequence-specific primers for TIN2, TRF1, TRF2, TPP1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as described previously (11), and for AR, 5-tcagttcacttttgacctgctaa-3′ (forward) and 5′-gtggaaatagatgggcttga-3′ (reverse) primers were used. C, whole cell lysates of LNCaP cells treated with Casodex were subjected to Western blot analysis to determine TIN2, TRF1, TRF2, AR, and glyceraldehyde-3-phosphate dehydrogenase (loading control) protein levels.
AR Is Associated with Telomeric Proteins in LNCaP Cells
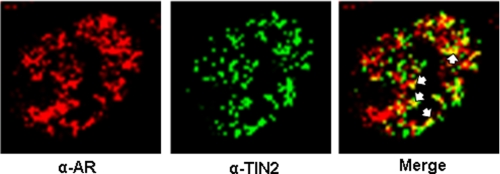
We designed experiments to test the hypothesis that Casodex-induced disruption of telomeric complexes is a consequence of AR interaction with telomeric proteins. As shown in Fig. 3, immunofluorescence staining studies of LNCaP cells revealed colocalization of AR with TIN2, which is known to interact with both TRF1 and TRF2 in telomeric complexes (2, 15). Although both AR and TIN2 were present throughout the nucleus, a subset of these proteins colocalized (Fig. 3, arrows), consistent with the possibility that a distinct subset of AR interacts with telomeric proteins in telomeres.
FIGURE 3.
AR is colocalized with TIN2 in telomeres. LNCaP cells were co-immunostained with antibodies against AR and TIN2. The yellow spots identified by white arrows in the “Merge” panel represent AR and TIN2 co-localization.
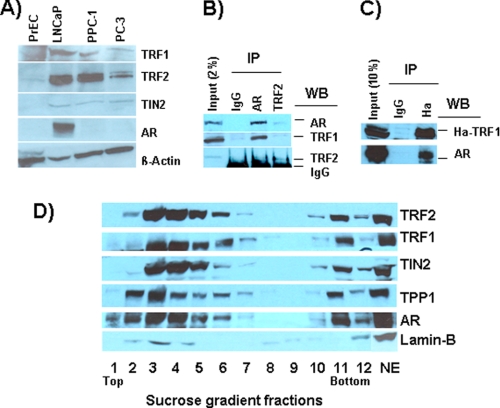
Interestingly, we observed that several telomeric proteins, including TRF1, TRF2, and TIN2 were present at a much higher level in prostate cancer cells than in normal prostate epithelial cells (Fig. 4A). However, there was a noticeable difference in the level of some of these proteins between different prostate cancer cell lines irrespective of their AR status (Fig. 4A). We further tested the possibility of AR interaction with telomeric proteins by performing biochemical fractionation of nuclear extracts prepared from LNCaP cells. In reciprocal immunoprecipitation studies, TRF1 and TRF2 were associated with immunoprecipitates prepared using AR monoclonal antibodies, and AR was associated with immunoprecipitates prepared using TRF2 (Fig. 4B) or TRF1 (Fig. 4C) antibodies. These experiments indicate that AR is associated with telomeric proteins; further studies are needed to determine whether AR interacts directly or indirectly with these telomeric proteins. We then used sucrose density gradient centrifugation to determine whether AR is associated with a shelterin-like mega complex in LNCaP cells. As shown Fig. 4D, a subset of AR in nuclear extracts sedimented with shelterin components, viz. TRF1, TRF2, TIN2, and TPP1, in a much higher molecular weight fraction (fraction 11) than expected for its molecular weight (fractions 2 to 4). Together, these observations support the possibility that AR interacts with telomeric proteins to preserve structural and/or functional stability of telomeric complexes, and agents that interfere with AR may disrupt telomeric complexes required for the viability of prostate cancer cells.
FIGURE 4.
AR is associated with telomeric proteins in LNCaP cells. A, telomeric proteins are overexpressed in prostate cancer cells. Whole cell lysates prepared from prostate cancer cells (LNCaP, PPC-1, and PC-3) and normal prostate epithelial cells (PrEC) were subjected to Western blot analysis to determine TRF1, TRF2, TIN2, AR, and β-actin (loading control) protein levels. B, AR and TRF2 in LNCaP cell lysates were individually immunoprecipitated (IP) using monoclonal antibodies, and immunoprecipitates and starting lysate (2% of input) were subjected to Western blot (WB) analysis of AR, TRF1, and TRF2. C, cell lysates were prepared from LNCaP cells transfected with HA-tagged TRF1 as described previously (13), and HA-TRF1 in cell lysates was immunoprecipitated using antibodies against the HA epitope. Immunoprecipitates and unprecipitated lysate (10% of input) were subjected to Western blot analysis of AR and TIN2. D, sucrose density gradient analysis of telomeric proteins and AR in LNCaP cells. Nuclear extract (NE) prepared from exponentially growing LNCaP cells was subjected to sucrose density gradient centrifugation, and the gradient was resolved into 12 fractions, which were subjected to Western blot analysis to identify the distribution of TRF1, TRF2, TIN2, TPP1, and AR in the gradient. Lamin B was used as a control. Top, top of the gradient; Bottom, bottom of the gradient; NE, Nuclear extract loaded onto the gradient.
DISCUSSION
The understanding that AR is indispensable for proliferation and viability of prostate cancer cells has led to the development of a myriad of therapies targeting AR for the treatment of prostate cancer. Such therapies, which include the AR antagonist Casodex, are effective in treating locally advanced or metastatic prostate cancer. However, the benefit is short lived in most patients, who succumb to a more aggressive castration-resistant disease for which there is no cure (19). Although the molecular events leading to the development of castration-resistant prostate cancer remain obscure, the transition from androgen-dependent to androgen-independent growth is associated with a number of chromosomal alterations (20) and an extensively altered gene expression profile (21). Interestingly, these changes, viz. chromosome instability and altered gene expression, can result from disruption of telomeric complexes (22, 23). Our observations that AR interacts with telomeric proteins (Figs. 3 and 4) and that Casodex disrupts telomeric complexes (Fig. 1) raise an alarming possibility that AR-targeted therapies may disrupt telomeric complexes and, in turn, contribute to the genomic instability and altered gene expression associated with hormone-refractory growth of prostate cancer.
How does Casodex disrupt telomeric complexes? Casodex is a nonsteroidal anti-androgen that competes with dihydrotestosterone binding to AR (24) and alters AR structure to render it transcriptionally inactive and susceptible to proteolytic breakdown (25). Casodex treatment of LNCaP cells led to a decrease in AR protein (Fig. 2C), but had no effect on the expression of telomeric proteins (Fig. 2, B and C). In addition, the Casodex-induced DNA damage response, as measured by 53BP1 recruitment to telomeres, was evident at 3 h (Fig. 2A), prior to a detectable decrease in AR protein at 10 h (Fig. 2C). Thus, the disruption of telomeric complexes by Casodex appears to precede the decrease in AR protein and appears to be independent of the inhibitory effect of Casodex on AR transcriptional activity or its destabilizing effect on AR protein levels. In light of these observations, it seems likely that Casodex-induced changes in the structural conformation of AR may hinder its interaction with telomeric proteins and thereby affect the overall stability of telomeric complexes. This is akin to an allosteric effect in which an inhibitor alters the conformation of its target protein thereby affecting interactions between the target protein and other proteins in a complex (26).
Besides its classical role as a transcription factor, there is accumulating evidence that AR has a role in DNA replication (27, 28), repair (29), and recombination (30). AR has been shown to be associated with enzymes of DNA synthesis (28), with cell cycle regulatory proteins required for the transition of cells from G1 to S phase (28), and with some of the DNA double-strand break repair proteins such as Ku70 and Ku80 (29), which also interact with TRF1 and TRF2 in telomeric complexes (8, 9). Mechanistically, whereas AR binds to DNA in its role as a transcription factor, AR appears to interact with proteins to exert its influence on DNA replication and repair. These mechanistic differences may account for the different concentrations of Casodex required to inhibit AR transcriptional activity (1–10 μm) versus DNA replication (27) or cell viability (31) (25–100 μm). In the present study, 25–100 μm Casodex was effective in disrupting telomeric complexes, indicating that the interaction of AR with telomeric proteins (Figs. 3 and 4), rather than with DNA, is responsible for its apparent effect on the structural stability of telomeric complexes in prostate cancer cells.
Besides telomeric complexes (shelterin) that contain the six core telomere-associated proteins (TRF1, TRF2, TIN2, hRap1, POT1, and TPP1), numerous other proteins that are involved in DNA repair, recombination, and replication appear to interact with shelterin components and affect telomere structure and function (6). It remains to be determined whether AR interacts with a shelterin component(s) directly or indirectly through a shelterin-associated protein. In addition, it remains to be determined whether AR interacts with telomeric complexes similarly in human prostate cancer tissues in vivo.
This work was funded by Institutional Research Support from the Henry Ford Health System (to S. K.).
- AR
- androgen receptor
- HA
- hemagglutinin.
REFERENCES
- 1.Rodier F., Kim S. H., Nijjar T., Yaswen P., Campisi J. (2005) Int. J. Biochem. Cell Biol. 37, 977–990 [DOI] [PubMed] [Google Scholar]
- 2.Kim S. H., Beausejour C., Davalos A. R., Kaminker P., Heo S. J., Campisi J. (2004) J. Biol. Chem. 279, 43799–43804 [DOI] [PubMed] [Google Scholar]
- 3.Liu D., Safari A., O'Connor M. S., Chan D. W., Laegeler A., Qin J., Songyang Z. (2004) Nat. Cell Biol. 6, 673–680 [DOI] [PubMed] [Google Scholar]
- 4.Ye J. Z., Donigian J. R., van Overbeek M., Loayza D., Luo Y., Krutchinsky A. N., Chait B. T., de Lange T. (2004) J. Biol. Chem. 279, 47264–47271 [DOI] [PubMed] [Google Scholar]
- 5.Ye J. Z., Hockemeyer D., Krutchinsky A. N., Loayza D., Hooper S. M., Chait B. T., de Lange T. (2004) Genes Dev. 18, 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palm W., de Lange T. (2008) Ann. Rev. Genet. 42, 301–334 [DOI] [PubMed] [Google Scholar]
- 7.Zhu X. D., Küster B., Mann M., Petrini J. H., de Lange T. (2000) Nat. Genet. 25, 347–352 [DOI] [PubMed] [Google Scholar]
- 8.Hsu H. L., Gilley D., Galande S. A., Hande M. P., Allen B., Kim S. H., Li G. C., Campisi J., Kohwi-Shigematsu T., Chen D. J. (2000) Genes Dev. 14, 2807–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song K., Jung D., Jung Y., Lee S. G., Lee I. (2000) FEBS Lett. 481, 81–85 [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi K., Kawai T., Kumaki F., Hiroi S., Mukai M., Ikeda E., Koering C. E., Gilson E. (2003) Clin. Cancer Res. 9, 1105–1111 [PubMed] [Google Scholar]
- 11.Poncet D., Belleville A., de Roodenbeke C. T., de Climens A. R., Simon E. B., Merle-Beral H., Callet-Bauchu E., Salles G., Sabatier L., Delic J., Gilson E. (2008) Blood 111, 2388–2391 [DOI] [PubMed] [Google Scholar]
- 12.Oh B. K., Kim Y. J., Park C., Park Y. N. (2005) Am. J. Pathol. 166, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S. H., Davalos A. R., Heo S. J., Rodier F., Zou Y., Beausejour C., Kaminker P., Yannone S. M., Campisi J. (2008) J. Cell Biol. 181, 447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takai H., Smogorzewska A., de Lange T. (2003) Curr. Biol. 13, 1549–1556 [DOI] [PubMed] [Google Scholar]
- 15.Kim S. H., Kaminker P., Campisi J. (1999) Nat. Genet. 23, 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noguchi H., Prem veer Reddy G., Pardee A. B. (1983) Cell 32, 443–451 [DOI] [PubMed] [Google Scholar]
- 17.Silverman J., Takai H., Buonomo S. B., Eisenhaber F., de Lange T. (2004) Genes Dev. 18, 2108–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cifuentes E., Mataraza J. M., Yoshida B. A., Menon M., Sacks D. B., Barrack E. R., Reddy G. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 464–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debes J. D., Tindall D. J. (2004) N. Engl. J. Med. 351, 1488–1490 [DOI] [PubMed] [Google Scholar]
- 20.Legrier M. E., Guyader C., Céraline J., Dutrillaux B., Oudard S., Poupon M. F., Auger N. (2009) Int. J. Cancer 124, 1103–1111 [DOI] [PubMed] [Google Scholar]
- 21.Chen C. D., Welsbie D. S., Tran C., Baek S. H., Chen R., Vessella R., Rosenfeld M. G., Sawyers C. L. (2004) Nat. Med. 10, 33–39 [DOI] [PubMed] [Google Scholar]
- 22.Schoeftner S., Blanco R., de Silanes I. L., Muñoz P., Gómez-López G., Flores J. M., Blasco M. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 19393–19398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Else T., Trovato A., Kim A. C., Wu Y., Ferguson D. O., Kuick R. D., Lucas P. C., Hammer G. D. (2009) Cancer Cell 15, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veldscholte J., Berrevoets C. A., Brinkmann A. O., Grootegoed J. A., Mulder E. (1992) Biochemistry 31, 2393–2399 [DOI] [PubMed] [Google Scholar]
- 25.Waller A. S., Sharrard R. M., Berthon P., Maitland N. J. (2000) J. Mol. Endocrinol. 24, 339–351 [DOI] [PubMed] [Google Scholar]
- 26.veer Reddy G. P., Pardee A. B. (1983) Nature 304, 86–88 [DOI] [PubMed] [Google Scholar]
- 27.Cifuentes E., Croxen R., Menon M., Barrack E. R., Reddy G. P. (2003) J. Cell. Physiol. 195, 337–345 [DOI] [PubMed] [Google Scholar]
- 28.Murthy S., Reddy G. P. (2006) J. Cell. Physiol. 209, 711–717 [DOI] [PubMed] [Google Scholar]
- 29.Mayeur G. L., Kung W. J., Martinez A., Izumiya C., Chen D. J., Kung H. J. (2005) J. Biol. Chem. 280, 10827–10833 [DOI] [PubMed] [Google Scholar]
- 30.Lin C., Yang L., Tanasa B., Hutt K., Ju B. G., Ohgi K., Zhang J., Rose D. W., Fu X. D., Glass C. K., Rosenfeld M. G. (2009) Cell 139, 1069–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhan P., Lee E. C., Packman K., Tenniswood M. (2002) J. Steroid Biochem Mol. Biol. 83, 101–111 [DOI] [PubMed] [Google Scholar]
- 32.Bai V. U., Kaseb A., Tejwani S., Divine G. W., Barrack E. R., Menon M., Pardee A. B., Reddy G. P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 2343–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]