Abstract
Glucagon-like peptide-1 (GLP-1) protects β-cells against apoptosis, increases their glucose competence, and induces their proliferation. We previously demonstrated that the anti-apoptotic effect was mediated by an increase in insulin-like growth factor-1 receptor (IGF-1R) expression and signaling, which was dependent on autocrine secretion of insulin-like growth factor 2 (IGF-2). Here, we further investigated how GLP-1 induces IGF-1R expression and whether the IGF-2/IGF-1R autocrine loop is also involved in mediating GLP-1-increase in glucose competence and proliferation. We show that GLP-1 up-regulated IGF-1R expression by a protein kinase A-dependent translational control mechanism, whereas isobutylmethylxanthine, which led to higher intracellular accumulation of cAMP than GLP-1, increased both IGF-1R transcription and translation. We then demonstrated, using MIN6 cells and primary islets, that the glucose competence of these cells was dependent on the level of IGF-1R expression and on IGF-2 secretion. We showed that GLP-1-induced primary β-cell proliferation was suppressed by Igf-1r gene inactivation and by IGF-2 immunoneutralization or knockdown. Together our data show that regulation of β-cell number and function by GLP-1 depends on the cAMP/protein kinase A mediated-induction of IGF-1R expression and the increased activity of an IGF-2/IGF-1R autocrine loop.
Keywords: Insulin, Insulin-like Growth Factor (IGF), Pancreatic Islet, Receptors, Translation Control, GLP-1, Gluco-incretin, IGF-1 Receptor, Proliferation, Secretion
Introduction
Glucagon-like peptide-1 acutely potentiates glucose-induced insulin secretion and is also a growth and differentiation factor for pancreatic β-cells (1, 2). Its action is initiated by binding to a G protein-coupled receptor that activates adenylate cyclase and production of cAMP (3). In β-cells, cAMP activates the classical protein kinase A (PKA) pathway, but also the MAP2 kinase cascade leading to rapid phosphorylation of extracellular signal-regulated kinase (ERK) 1/2, the PI 3-kinase pathway inducing phosphorylation of Akt, and the cAMP binding protein Epac2 (4–7). These different events then control the acute and trophic effects of GLP-1. For instance, phosphorylation of various elements of the glucose signaling pathway by PKA is required for the insulinotropic effect of GLP-1, and activation of Epac2 controls the release of Ca2+ from the endoplasmic reticulum as well as the activity of small G proteins regulating insulin granule exocytosis (8–10). The trophic effects of GLP-1 are mediated by transcriptional control of gene expression, for instance, the PKA-dependent phosphorylation of the transcription factor CREB, which activates IRS-2 expression (11), or the PI 3-kinase/Akt-dependent nuclear exclusion of the transcription factor FoxO1 (12), which then leads to increased expression of PDX-1 (13), a critical regulator of mature β-cell function.
Intracellular cAMP levels have been recognized over two decades ago to be critical for normal glucose-stimulated insulin secretion (14). More recently, it has been shown that relatively higher intracellular cAMP levels are required to induce gene transcription than to stimulate secretion (15), and activation of the Epac2 pathways also requires higher intracellular cAMP concentrations than activation of PKA (10).
In a recent study we established a new link between the GLP-1 and IGF-1 receptor signaling pathways (16). We showed that GLP-1 induced a robust up-regulation of IGF-1 receptor expression, which was associated with a parallel increase in Akt phosphorylation. Induction of Akt phosphorylation was dependent on IGF-1 receptor expression, which was activated by the autocrine secretion of IGF-2. We further demonstrated that the protection against cytokine-induced apoptosis conferred by GLP-1 was dependent on IGF-1 receptor up-regulation and IGF-2 action. These data provided evidence for the existence in β-cells of an IGF-2/IGF-1 receptor autocrine loop, whose activity could be increased by up-regulating IGF-1 receptor expression, a mechanism that is the basis for the anti-apoptotic effect of GLP-1.
Here, we further demonstrate that the cAMP/PKA pathway induces IGF-1 receptor up-regulation by transcriptional and translational control, with GLP-1 increasing only translation and IBMX increasing both translation and transcription. We then demonstrate that the control by GLP-1 of β-cell glucose competence and proliferation also depends on up-regulation of IGF-1 receptor expression and autocrine production of IGF-2. Thus, the biological processes regulated by GLP-1 to control β-cell mass and function, protection against apoptosis, glucose competence, and proliferation appear to be secondary to the activation of an IGF-2/IGF-1 receptor autocrine loop. This may serve as a general model for the mode of action of all ligands modulating β-cell function through activation of the cAMP signaling pathway.
EXPERIMENTAL PROCEDURES
Mice and Reagents
C57BL/6 mice were used between 8 and 10 weeks of age. All of the experimental procedures received approval from the Service Vétérinaire du Canton de Vaud.
Forskolin, PD98059, LY 294002, wortmannin, nimodipine, and rabbit polyclonal antibody against actin were purchased from Sigma. Sp-CPT-cAMPs and 8-CPT-2′-0-Me-cAMP were from Biolog (Bremen, Germany). Rabbit polyclonal antibodies against the IGF1-R (C20; sc-713) was from Santa Cruz Biotechnology (Nunningen, Switzerland). IGF-2 goat antibodies were from R & D Systems.
Quantitative PCR Analyses
The islets were isolated (17) and kept in culture overnight before RNA extraction (18). Real time quantitative PCR was performed using Light Cycler technology (Roche Applied Science). Primer sets were chosen to amplify products of ∼200 bp. cDNAs were obtained from 2.5 μg of total RNA with SuperScript II RNase H− reverse transcriptase (Invitrogen) and 50 pmol of random hexamer (Applied Biosystems). cDNA amplification was performed in a 20-μl reaction mixture including 1× QuantiTectTM SYBR Green PCR Master Mix (Qiagen) and 10 pmol of each primer. The primers for mouse IGF1-R were: sense, 5′-TGGTGACCGGCTACGTGAAG-3′; antisense, 5′-CAAAGTACATCTTTCCGGACC-3′, and those for mouse cyclophilin were: sense, 5′-TCCATCGTGTCATCAAGGAC-3′; antisense, 5′-CTTGCCATCCAGCCAGGAG-3′. All of the measurements were normalized to cyclophilin.
MIN6 Cell Culture
MIN6 cells (19) were grown in Dulbecco's modified Eagle's medium containing 15% heat-inactivated fetal calf serum, 2 mm glutamine, and 50 μm β-mercaptoethanol and used between passages 19 and 30. For pharmacological treatments, 106 MIN6 cells were plated in 6-cm tissue culture dishes for 4 days, the medium was replaced, and the cells were incubated for 2 h at 37 °C before the addition of various reagents at the concentrations and for the periods of time indicated in the figure legends.
Gel Electrophoresis and Immunoblotting
Western blot analysis was performed as described (18) with antibodies diluted in TBS, 5% nonfat dry milk (1/500 dilution for IGF1-R and 1/1000 for actin) and revealed using horseradish peroxidase-conjugated donkey anti-rabbit IgG or horseradish peroxidase-conjugated sheep anti-mouse IgG as secondary antibodies (Amersham Biosciences). The band intensities were determined using a Bio-Rad densitometer (Strasbourg, France).
cAMP Assay
On day one, 200′000 MIN6 cells were seeded in each well of a 24-well plate. On day 3, the cells were incubated in Krebs-Ringer bicarbonate Hepes buffer supplemented with 2 mm glucose for 2 h. The cells were then incubated in Krebs-Ringer bicarbonate Hepes (KRBH) buffer (120 mm NaCl, 4 mm KH2PO4, 20 mm Hepes, 1 mm MgCl2, 1 mm CaCl2, 5 mm NaHCO3, and 0.5% bovine serum albumin, pH 7.4) containing 20 mm glucose in the presence or absence of GLP-1 (100 nm), or IBMX (10 μm) for the indicated time at 37 °C. The medium was aspirated, and cells were lysed in 200 μl of HCl (0.1 m). The direct cAMP enzyme immunoassay kit (Assay Designs, Ann Arbor, MI) was used to determine intracellular cAMP content.
Pulse-Chase Experiments
200 mouse islets kept overnight in culture or 1 million MIN6 cells in culture were washed three times with phosphate-buffered saline and then starved for 1 h in 3 ml of Dulbecco's modified Eagle's medium lacking l-methionine and l-cysteine (Invitrogen; catalog number 21013) supplemented with 2 mm l-glutamine (Sigma; catalog number G7513) and 15% fetal bovine serum dialyzed 24 h in PBS at 4 °C using a 6000-Da cut-off membrane. The cells were incubated in 1 ml of methionine-cysteine free medium supplemented with 100 μCi of l-[35S]methionine and l-[35S]cysteine (Hartmann Analytic; IS-103; 2 mCi, 200 μl, 74 MBq) for the indicated times and in the presence or absence of GLP-1 (100 nm). The cells were then washed twice with PBS and lysed in immunoprecipitation lysis buffer (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, 10% glycerol, 1% Nonidet P-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm orthovanadate). The lysates were precleared with 1 μg of goat IgG purified Immunoglobulin (Sigma; catalog number I5256) for 15 min and then protein A-agarose beads (Roche Applied Science; catalog number 11134515001) for an additional 15 min at 4 °C with gentle rocking. The beads were pelleted at 9300 rpm for 1 min at 4 °C, and the supernatant was transferred into a new Eppendorff tube containing 1 μg of an anti-IGF1-R antibody. After 2 h of gentle rocking at 4 °C, 40 μl of protein A-agarose beads were added for an additional hour at 4 °C with gentle rocking. The beads were then washed three times and boiled 5 min at 95 °C in 30 μl of Laemmli buffer, and a 10% SDS-PAGE was performed.
Islet Proliferation
Twenty islets from C57BL/6 or Igf1rlox/lox (20) mice were plated on tissue culture dishes coated with an extracellular matrix derived from bovine corneal endothelial cells (Novamed, Jerusalem, Israel) for 7 days. Monolayers of Igf1rlox/lox islets were infected for 12 h with a GFP (Ad-GFP) or Cre (Ad-Cre) expressing adenovirus with a multiplicity of infection of 100 and then cultured for 36 h prior to proliferation test. The mouse islets were incubated for 48 h in the presence of GLP-1 (100 nm), and for the last 24 h 5′-bromo-2′-deoxyuridine (BrdUrd, 10 μm) was added. The cells were washed with PBS and fixed in ethanol and incubated for 1 h at 37 °C with an anti-BrdUrd antibody (BrdUrd labeling and detection kit; Roche Applied Science). The cells were then washed with PBS and incubated for 30 min at 37 °C with the secondary antibodies (Cy3-conjugated goat anti-mouse; Jackson Immunoresearch) and fluorescein isothiocyanate-conjugated goat anti-rabbit (Calbiochem). The cells were mounted with Vectashield medium containing 4′,6′-diamino-2-phenylindole (Reactolab, Servion, Switzerland). The percentage of BrdUrd-positive cells was analyzed under a Leica confocal fluorescence microscope using Image J software. For IGF-2 immunoneutralization experiments, the IGF-2 or unspecific antibodies were added for the 48-h GLP-1 incubation period at a concentration of 10 μg/ml. For knockdown of IGF-2 expression in islets, mouse islet monolayers were infected with an adenovirus expressing and IGF-2 shRNA at a multiplicity of infection of 100, as described above.
siRNAs
siRNAs for IGF-1R and IGF-2 knockdown were as previously described (16). For Ad-shIGF-2 preparation, the shRNA was obtained from pLKO1 parent vector (Open Biosystem), then subcloned into the pSUPER vector (21), and finally cloned with H1 promoter into the pADTrack vector. Purified adenoviruses were produced by Vira Quest, Inc (North Liberty, IA).
Secretion Test
MIN6 cells were seeded at 200,000 cells in 12-well plates and 24 h later co-transfected with a plasmid encoding human growth hormone (hGH) and either an IGF1-R-encoding plasmid (pcDNA3-IGF1-R) or an empty vector (pCDNA3), or with various siRNA, using Lipofectamine. Two days later, the cells were washed and preincubated for 2 h in KRBH buffer supplemented with 2 mm glucose. Then the medium was replaced with KRBH containing 2 or 20 mm glucose for an additional hour. Secreted and cellular hGH (extracted in PBS with 0.5% Triton X-100) was assessed by enzyme-linked immunosorbent assay (Roche Applied Science) (22, 23). Secretion was calculated as the ratio of secreted over total intracellular hGH, and this ratio was set at 1 for cells in control conditions and in the presence of 2 mm glucose.
Islet secretion activity was assessed using 20 islets incubated in 1 ml of KRBH. Insulin content was assessed from islets lysed in ethanol acid. Each experiment was performed in triplicate. Insulin was determined using an enzyme-linked immunoassay (SpiBio; catalog number A05105).
Statistical Analyses
All of the experiments were performed at least three times. The results are expressed as the means ± S.D. Comparisons were performed using unpaired Student's t test, one-way or two-way analysis of variance for the different groups followed by post hoc pairwise multiple-comparison procedures (Tukey Test or Bonferroni, respectively). Single, double, and triple asterisks indicate statistically significant differences (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
RESULTS
Transcriptional and Translational Induction of IGF-1R Expression
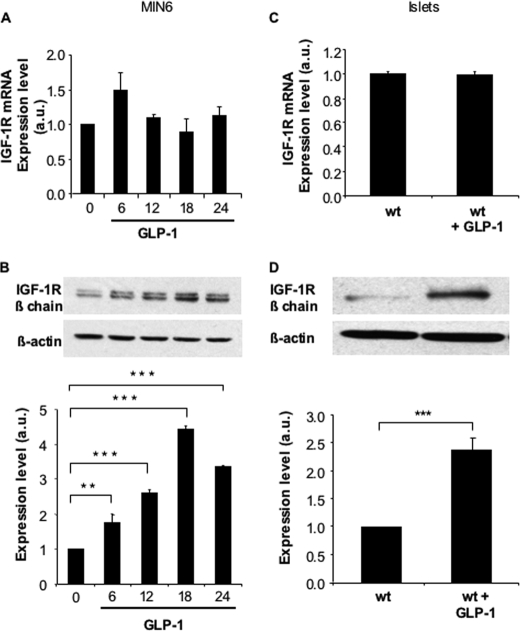
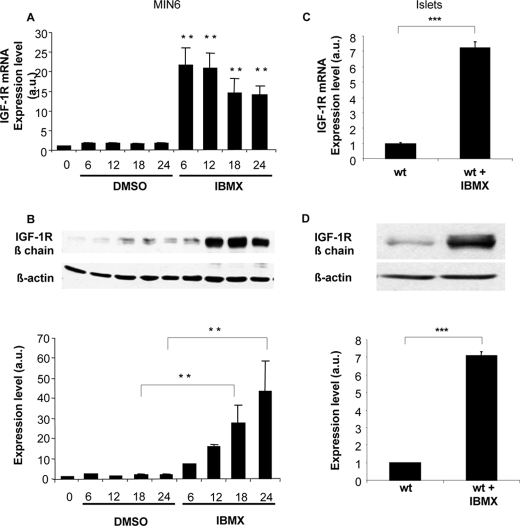
Exposing MIN6 cells to saturating concentration of GLP-1 (100 nm, Kd = ∼0.6 nm) induced a time-dependent increase in IGF-1 receptor expression with a maximum reached at 18 h (Fig. 1B), as previously described (16). This was, however, not associated with an increase in receptor mRNA levels (Fig. 1A). Similarly, GLP-1 induced IGF-1R protein but not mRNA in mouse islets (Fig. 1, C and D). When MIN6 cells were exposed to the phosphodiesterase inhibitor IBMX, a strong induction of IGF-1R protein was observed, which was associated with a parallel increase in expression of its mRNA (Fig. 2, A and B). Using primary mouse islets, a similar induction of IGF-1R expression by IBMX was observed both at the mRNA and protein levels (Fig. 2, C and D).
FIGURE 1.
GLP-1 increases IGF-1R protein but not mRNA expression in MIN6 cells and primary mouse islets. MIN6 cells were exposed to GLP-1 (100 nm) for the indicated periods of time and IGF-1R mRNA (A) as well as protein (B) expressions were quantitated by qRT-PCR and by Western blot analyses, respectively. Mouse control islets were exposed to GLP-1 (100 nm) for 18 h, and IGF-1R mRNA (C) as well as protein (D) expressions were quantitated by qRT-PCR and by Western blot analyses, respectively. The levels are expressed as arbitrary units (a.u.). The data are the means ± S.D. from three independent experiments. **, p < 0.01; ***, p < 0.001.
FIGURE 2.
IBMX induces a strong increase in both IGF-1R mRNA and protein expression in MIN6 cells and primary mouse islets. MIN6 cells were exposed to IBMX (10 μm) for the indicated periods of time, and IGF-1R mRNA (A) as well as protein (B) expressions were quantitated by qRT-PCR and by Western blot analyses, respectively. Mouse control islets were exposed to IBMX (10 μm) for 18 h and IGF-1R mRNA (C) as well as protein (D) expressions were quantitated by qRT-PCR and by Western blot analyses, respectively. The levels are expressed as arbitrary units (a.u.). The data are the means ± S.D. from three independent experiments. **, p < 0.01; ***, p < 0.001. DMSO, dimethyl sulfoxide; wt, wild type.
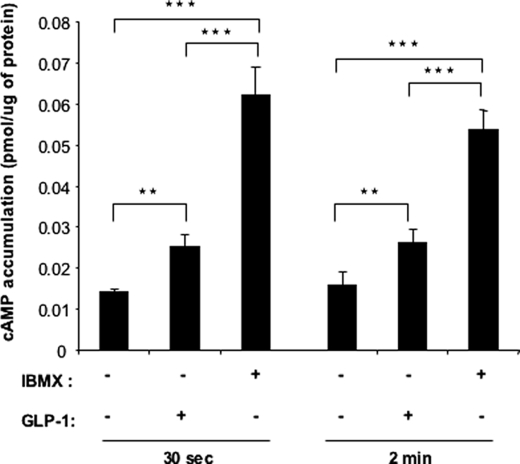
The differential effect of GLP-1 and IBMX on IGF-1R expression could be related to the differential induction of intracellular cAMP levels by both treatments. Indeed, GLP-1 induces oscillatory increases in intracellular cAMP levels, whereas IBMX induces relatively higher and stable cAMP concentrations, conditions that are required to induce gene transcription (15, 24). We thus measured cAMP in MIN6 cells treated with GLP-1 or IBMX for 30 s or 2 min. Fig. 3 shows that IBMX indeed induced a higher intracellular cAMP concentration that GLP-1.
FIGURE 3.
GLP-1 and IBMX differentially increase cAMP levels in MIN6 cells. MIN6 cells were incubated in the presence or absence of GLP-1 (100 nm) or IBMX (10 μm) for 30 s or 2 min, and intracellular cAMP contents were measured by radioimmunoassay. The data are the means ± S.D., n = 3 independent experiments. **, p < 0.01 (treatments compared with control); §§, p < 0.01 (IBMX treatment compared with GLP-1 treatment).
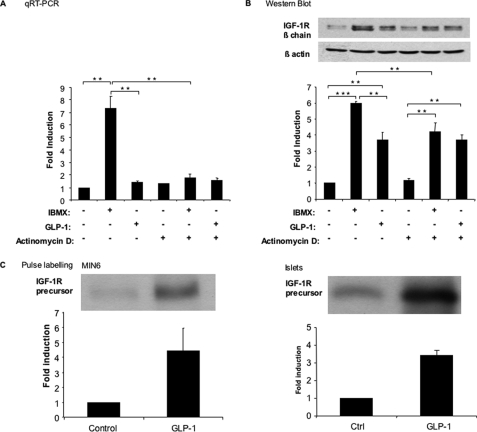
To determine whether IBMX induced transcriptional up-regulation of the IGF-IR, we analyzed IGF-1R mRNA and protein expression in MIN6 cells stimulated by IBMX, in the presence or absence of the transcriptional inhibitor actinomycin D. Fig. 4A shows that induction of IGF-1R mRNA by IBMX was suppressed by actinomycin D; IGF-1R mRNA expression was then similar to that present in nontreated or GLP-1-treated cells. To determine whether IBMX also induced IGF-1R expression by post-transcriptional control, we performed the same experiment as in Fig. 4A to determine IGF-1R expression level. The data of Fig. 4B shows that IBMX-induced up-regulation of IGF-1R protein was only partially reduced upon actinomycin D treatment, reaching the same level as that obtained upon GLP-1 treatment of the cells. On the other hand, GLP-1-induced increase in IGF-1R expression was insensitive to transcription inhibition.
FIGURE 4.
IBMX and GLP-1 regulate IGF-1R expression by transcriptional and post-transcriptional mechanisms. A and B, MIN6 cells were exposed to either IBMX (10 μm) or GLP-1 (100 nm) for 18 h and in the presence or absence of actinomycin D (1 μg/ml). IGF1-R mRNA (A) as well as protein (B) expression was quantitated by qRT-PCR and Western blot analysis, respectively. C, MIN6 cells (left panel) or mouse islets (right panel) were biosynthetically labeled for 1 h with [35S]methionine and [35S]cysteine in the presence or absence of GLP-1 (100 nm). IGF-1R was immunoprecipitated from the cell lysates and separated by gel electrophoresis; the amount of newly synthesized precursor receptor was quantitated by densitometric analysis of the autoradiograms. The data are the means ± S.D. from three independent experiments. **, p < 0.01; ***, p < 0.001. Ctrl, control.
To evaluate whether GLP-1 increased IGF-1R expression by a translational control mechanism, we performed biosynthetic labeling experiments. MIN6 cells or mouse islets were incubated for 60 min in the presence of [35S]methionine and [35S]cysteine and in the presence or absence of GLP-1. IGF-1R was then immunoprecipitated from identical amounts of protein extracts. Incubation of MIN6 cells or pancreatic islets in the presence of GLP-1 robustly increased the rate of IGF-1R biosynthesis (Fig. 4C). Together the above data indicate that GLP-1 induces IGF-1R expression by a translational mechanism, whereas IBMX induces IGF-1R up-regulation by both transcriptional and translational regulations.
GLP-1 Induces IGF-1R Expression through the cAMP/PKA Pathway
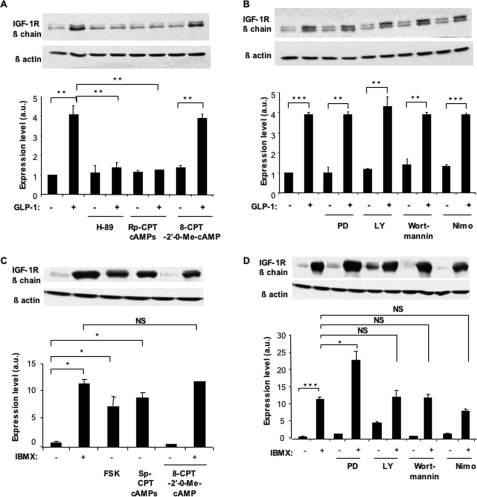
To determine which intracellular signaling pathway activated by GLP-1 receptor activation leads to IGF-1R up-regulation, we treated MIN6 cells for 18 h with GLP-1 and the PKA inhibitors H-89 or Rp-CPT-cAMPs. As shown in Fig. 5A, both PKA inhibitors suppressed GLP-1-induced IGF-1R expression; the same figure also shows that exposing cells to the Epac2 activator 8-CPT-2′-0-Me-cAMP in the absence of GLP-1 did not induce IGF-1R expression. In Fig. 5B, we show that GLP-1-induced IGF-1R expression was not suppressed by incubation with the inhibitors of MAP kinases (PD98059) or PI 3-kinase (Ly-294002 or wortmannin) or by the Ca2+ channel blocker nimodipine. We performed similar analysis of signaling in IBMX-treated cells. As shown in Fig. 5 (C and D) IBMX-induced IGF-1R expression was mimicked by forskolin or by the cAMP agonist Sp-CPT cAMPs; as above, the Epac2 activator had no effect on IGF-1R expression. Inhibiting the MAP kinase pathway by PD98059 unexpectedly increased IGF-1R expression, but the PI 3-kinase inhibitors or the Ca2+ channel antagonist did not modify IBMX-induced IGF-1R expression. Together these data indicate that GLP-1 and IBMX up-regulate IGF-1R expression through protein kinase A-dependent mechanisms.
FIGURE 5.
GLP-1 and IBMX induce IGF-1R expression through activation of the cAMP/PKA pathway. A, MIN6 cells were exposed for 18 h to GLP-1 (100 nm) to induce IGF-1R expression. This induction was blocked in the presence of the protein kinase A inhibitor H-89 (10 μm) or the cAMP antagonist Rp-CPT-cAMP (100 μm). Activation of Epac2 by 8-CPT-2′-O-Me-cAMP (50 μm) did not induce IGF-1R expression in the absence of GLP-1. B, induction of the IGF-1R by GLP-1 in MIN6 cells expression, which was not suppressed by the MAP kinase inhibitor PD98059 (PD, 50 μm), the PI 3-kinase inhibitors LY294002 (LY, 50 μm), or wortmannin (50 nm) nor by the Ca2+-channel blocker nimodipine (Nimo, 1 μm). C, induction of IGF-1R by IBMX (10 μm) was mimicked by exposing the cells to forskolin (FSK, 10 μm), or the protein kinase A activator SP-CPT-cAMP (100 μm). However, induction of IGF-1R expression was not increased by Epac2 activator 8-CPT-2′-O-Me-cAMP (50 μm) in the absence of IBMX. D, induction of IGF-1R expression by IBMX was not suppressed by PD98059, by LY294002, wortmannin (50 nm), nor by nimodipine. The levels are expressed as arbitrary units (a.u.). The data are the means ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; NS, not significant.
Activation of the IGF-2/IGF-1R Autocrine Loop Increases β-Cell Glucose Competence
We previously showed that GLP-1-induced activation of IGF-1R signaling required autocrine secretion of IGF2 and that this pathway was required for GLP-1 protection against cytokine-induced apoptosis. The IGF-1R signaling pathway also regulates glucose competence, i.e. the magnitude of the insulin secretion response to a rise in glucose concentration (20, 25).
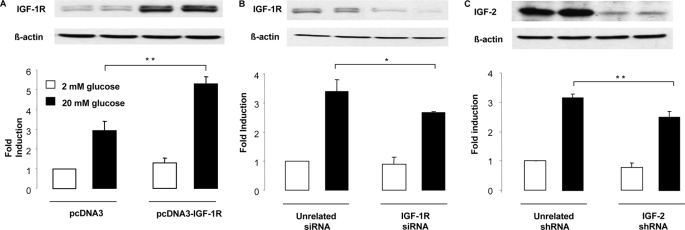
Here, we evaluated the impact of IGF-1R overexpression or knockdown on glucose-stimulated secretion as well as the impact of IGF-2 knockdown on secretion in MIN6 cells co-transfected with a hGH expression plasmid and detection of intracellular and secreted hGH. First, we showed that overexpression of the IGF-1R by transient transfection of MIN6 cells increased glucose-stimulated secretion (Fig. 6A). Next, we determined that siRNA-mediated reduction of IGF-1R expression decreased stimulated secretion (Fig. 6B). To evaluate whether IGF-2 was involved in regulating glucose-stimulated secretion, IGF-2 expression was reduced by siRNA-mediated knockdown, which markedly reduced IGF-2 expression (Fig. 6C). This led to a reduction in secretion, which was of similar magnitude as that obtained by IGF-1R knockdown (Fig. 6B).
FIGURE 6.
MIN6 glucose competence is modulated by IGF-1R and IGF-2 expression. A, MIN6 cells were co-transfected with a control (pcDNA3) or IGF-1R (pcDNA3-IGF1-R) expression plasmid together with a plasmid encoding hGH. Two days later the cells were exposed to 2 or 20 mm glucose for 1 h, and secreted and intracellular hGH were measured. Secretion was calculated as the ratio of secreted over intracellular hGH, and this ratio was set at 1 for cells treated in control conditions exposed to 2 mm glucose. B, MIN6 cells were co-transfected with an unrelated or an IGF-1R-specific siRNA together with hGH-expressing plasmid. Secretion experiments were performed and expressed as above. C, MIN6 cells were co-transfected with an unrelated or an IGF-2-specific shRNA together with hGH-expressing plasmid. Secretion experiments were performed and expressed as above. The data are the means ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01.
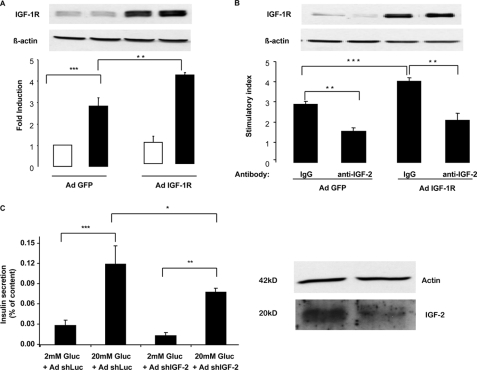
We next evaluated whether IGF-1R and IGF-2 regulated insulin secretion in mouse islets. Primary islets were infected with recombinant adenoviruses expressing either a green fluorescent protein (Ad-GFP) or the IGF-1R (Ad-IGF-1R). Overexpression of the IGF-1R significantly increased glucose-stimulated insulin secretion (GSIS) (Fig. 7A). The effect of immunoneutralizing IGF-2 was then tested. As shown in Fig. 7B, the IGF-2 blocking antibody reduced GSIS in both Ad-GFP- and Ad-IGF-1R-infected cells. Finally, we tested the effect of down-regulating IGF-2 expression in primary islets on GSIS. Primary islets were infected with adenoviruses expressing a control or an IGF-2-specific siRNA. Fig. 7C shows, by Western blot analysis, the reduction in IGF-2 expression achieved by adenovirus-mediated shRNA transduction and the parallel reduction in GSIS. This reduction was similar to that obtained by immunoneutralization of IGF-2 or reducing IGF-1R expression. Together these data indicate that the glucose competence of the β-cells can be modulated by increasing or decreasing the activity of the IGF-2/IGF-1R autocrine loop.
FIGURE 7.
Mouse islet glucose competence is modulated by IGF-1R and IGF-2 expression. A, mouse islets were infected with a control adenovirus (Ad GFP) or an IGF-1R-expressing adenovirus (AdIGF1-R) and 2 days later exposed to either 2 or 20 mm glucose, and insulin secretion was measured and expressed as in Fig. 6. B, mouse islets were infected as in A and 2 days later exposed to either 2 or 20 mm glucose in the presence of a control IgG or an IGF-2 blocking antibody. Insulin secretion is reported relative to secretion at 2 mm glucose (stimulatory index). C, mouse islets were infected with an adenovirus expressing a unrelated (Luc) or an IGF-2-specific shRNA, and the islets were then processed as above for measurement of glucose-stimulated insulin secretion. The Western blot in the right panel shows the reduction in ProIGF-2 expression in AdshIGF-2-infected cells. IGF-1R, IGF-2, and β-actin expression was detected by Western blot analysis. The data are the means ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
GLP-1-induced β-Cell Proliferation Depends the IGF-2/IGF-1R Autocrine Loop
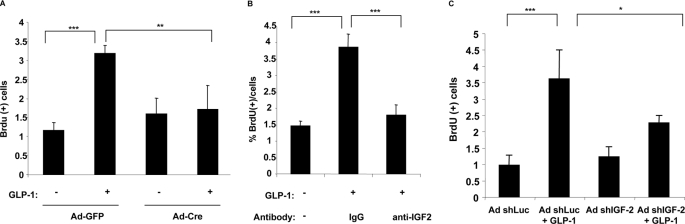
To assess whether GLP-1-induced β-cell proliferation was dependent on the IGF-2/IGF-1R autocrine loop, we first evaluated whether inactivation of the Igf-1r gene prevented the proliferation effect of GLP-1. Islets from Igf-1rlox/lox mice were cultured on an extracellular matrix to form cell monolayers. These were infected with recombinant adenoviruses allowing expression of the CRE recombinase (Ad-CRE) or an Ad-GFP. Preliminary experiments were performed to assess the infection efficiency, and we determined that with a multiplicity of infection of 100, ∼80–90% of the cells were infected. Two days after infection, the monolayers were exposed to GLP-1 for 48 h, and BrdUrd was added for the last 24 h of incubation. Fig. 8A shows that GLP-1 induced a ∼3-fold increase in BrdUrd incorporation in Ad-GFP-infected cells, whereas there was no induction of proliferation when the Igf-1r gene was inactivated following Ad-CRE infection. Next, to determine whether secretion of IGF-2 was required for GLP-1-induced proliferation, primary islet cell monolayers were stimulated with GLP-1 for 48 h, and BrdUrd was added for the last 24 h. GLP-1-induced proliferation was suppressed by the blocking antibody but not by a nonspecific IgG fraction (Fig. 8B). Finally, GLP-1-induced proliferation was also evaluated after adenovirus-mediated transduction of primary islets with a control or an IGF-2-specific shRNA. Reducing IGF-2 expression led to a significant reduction in GLP-1-induced β-cell proliferation (Fig. 8C). Together these data indicate that GLP-1-induced β-cell proliferation depends on IGF-1R expression and autocrine secretion of IGF-2.
FIGURE 8.
GLP-1-mediated β-cell proliferation depends on the IGF-2/IGF-1R autocrine loop. A, islets from Igf-1Rlox/lox mice were plated on extracellular matrix-coated dishes for 7 days to form monolayers. They were then infected with Ad-GFP or an Ad-CRE. Two days later they were exposed to GLP-1 (100 nm) for 48 h and then with BrdUrd for the last 24 h. BrdUrd-positive cells were then recorded and are expressed as percentages of BrdUrd-positive cells. B, mouse islets monolayers were exposed to GLP-1 for 48 h in the presence of nonspecific IgG fraction or a neutralizing IGF-2 antibody, and BrdUrd was added for the last 24 h. Proliferation was recorded as BrdUrd-positive cells. C, mouse islet monolayers were infected with an adenovirus expressing an unrelated (Luc) or an IGF-2-specific shRNA. GLP-1-induced proliferation was measured as above. The data are the means ± S.D. from three independent experiments. ***, p < 0.001.
DISCUSSION
Here, we confirmed our previous studies indicating that an IGF-2/IGF-1 receptor autocrine loop operates in pancreatic β-cells and that its activity can be increased by GLP-1 through an up-regulation of IGF-1 receptor expression (16). We further extended these observations by showing that IGF-1 receptor expression can be increased by transcriptional and translational controls and that glucose competence and GLP-1-induced proliferation are also regulated by increased IGF-1R expression and autocrine secretion of IGF-2.
Intracellular cAMP levels are critical for the normal functioning of pancreatic β-cells, in particular for their basal glucose competence (26). Thus, receptors linked to cAMP production, such as the GLP-1, glucose-dependent insulinotropic polypeptide, glucagon, vasoactive intestinal polypeptide, pituitary adenylate cyclase-activating polypeptide, and many other receptors present at the β-cell surface play an important role in integrating multiple hormonal signals to control cellular cAMP levels. This integrative role is supported, for instance, by our previous data showing that inactivation of both the GLP-1 and glucose-dependent insulinotropic polypeptide receptors, but not of each receptor separately, leads to a cell autonomous defect in glucose-stimulated insulin secretion (27). Comparative transcript profiling of control and double receptor knock-out islets revealed a reduction in IGF-1 receptor expression in the mutant islets, and subsequent studies showed that GLP-1 treatment of β-cells can robustly increase IGF-1 receptor expression (16). Our present study indicates that at the relatively low cellular cAMP levels induced by GLP-1 treatment, IGF-1R up-regulation is controlled by translation of its mRNA, whereas at the higher levels induced by IBMX, IGF-1R expression is induced both at the transcriptional and translation levels. Although we do not have a formal proof from our data that this differential control of IGF-1R expression depends on graded concentrations of intracellular cAMP, this interpretation is in agreement with the work of Dyachok et al. (15), who compared the effect of IBMX and GLP-1 on intracellular cAMP levels in the insulinoma cell line INS-1. They showed that IBMX led to stable elevations in cAMP levels, whereas GLP-1 only induced oscillatory increase in cAMP. They further showed that stable elevations in cAMP are required for translocating PKA to the nucleus, an event that is necessary for transcriptional control of gene expression. In addition, although IBMX may have actions other than purely increasing cAMP, we have shown that both GLP-1 and IBMX increase IGF-1R expression by the cAMP/PKA pathway, reinforcing the possibility that the differential effect of both agents on IGF-1R expression may be related to changes in intracellular cAMP production.
Increased glucose competence and induction of proliferation of mature β-cells are important to preserve or increase β-cell functional mass. Here, we show that similar to the protection against cytokine-induced apoptosis, GLP-1 induces proliferation of β-cells through activation of IGF-1 receptor expression and signaling, which depend on IGF-2 secretion. We previously demonstrated that glucose induced IGF-2 secretion by a mechanism that could be blocked by the KATP channel agonist diazoxide and the Ca2+ channel blocker nimodipine and that can be potentiated by GLP-1 treatment (16). Thus, our data were in agreement with studies showing that IGF-2 is normally co-packaged and secreted with insulin (28–30).
Together our present data provide additional support for the existence of an IGF-2/IGF-1R autocrine loop operating in β-cells that can be modulated by increasing IGF-1R expression and secretion of IGF-2. Although we cannot exclude the possibility that the PKA/CREB pathway activates genes encoding direct regulators of β-cell mass and function, these data and our previous (16) data identify the IGF-1R/Akt pathway as a central regulator of β-cell biology, with the capacity to control proliferation, glucose competence, and apoptosis. This provides an integrated view of two signaling pathways in β-cells, the cAMP pathway activated by GLP-1 and probably by all ligands binding to Gs protein-coupled receptors expressed by β-cells and the IGF-1R/Akt pathway. Numerous investigations of mice with genetic inactivation of the IGF-1R, of IRS-2, and of Akt have provided evidence for the importance of this pathway in β-cell biological control (11, 20, 25, 31–34). Our study provides extended support for this view by demonstrating that this pathway can be enhanced by GLP-1 action. This is in agreement with the observation that GLP-1 induction of β-cell mass was blunted in IRS-2 knock-out mice and the conclusion from this study that IRS-2 signaling was required for the proliferation-inducing effect of GLP-1 (35). In addition, our present demonstration that the autocrine secretion of IGF-2 is required for activation of IGF-1R signaling suggests that this pathway may also be regulated by stimulating IGF-2 production and secretion. Further work will be required to test whether the endogenous production of IGF-2 by β-cells can be regulated by hormonal or nutritional stimuli and whether it is required for the in vivo effect of GLP-1 on β-cell mass and function. Finally, if considered to be of potential therapeutic interest, it should nevertheless be remembered that IGF-2 overexpression is a major step in the progression of hyperplastic islets to tumorigenic transformation (36). Thus, it may be an interesting therapeutic approach to transiently increase IGF-2 expression when IGF-1R expression is already increased by GLP-1 treatment. However, at the present time it is not clear whether a similar mechanism also operates in human islets, and determining this is an important goal for future research.
Acknowledgment
We thank Dr. P. Halban for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 DK67536 (to R. N. K.). This work was also supported by Swiss National Science Foundation Grant 31003A-113525, Juvenile Diabetes Research Foundation Program Project 7-2005-1158, the Integrated Project Eurodia LSHM-CT-2006 518153 Framework Programme 6 of the European Community (to B. T.), and a Juvenile Diabetes Research Foundation Fellowship (to D. K.).
- MAP
- mitogen-activated protein
- GLP-1
- glucagon-like peptide-1
- IGF-1R
- insulin-like growth factor-1 receptor
- IBMX
- isobutylmethylxanthine
- PI
- phosphatidylinositol
- PKA
- cAMP-dependent protein kinase
- PBS
- phosphate-buffered saline
- GFP
- green fluorescent protein
- BrdUrd
- 5′-bromo-2′-deoxyuridine
- shRNA
- small hairpin RNA
- siRNA
- small interfering RNA
- hGH
- human growth hormone
- GSIS
- glucose-stimulated insulin secretion
- qRT
- quantitative reverse transcription.
REFERENCES
- 1.Egan J. M., Bulotta A., Hui H., Perfetti R. (2003) Diabetes Metab. Res. Rev. 19, 115–123 [DOI] [PubMed] [Google Scholar]
- 2.Drucker D. J. (2006) Cell Metab. 3, 153–165 [DOI] [PubMed] [Google Scholar]
- 3.Thorens B. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 8641–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez E., Pritchard C., Herbert T. P. (2002) J. Biol. Chem. 277, 48146–48151 [DOI] [PubMed] [Google Scholar]
- 5.Arnette D., Gibson T. B., Lawrence M. C., January B., Khoo S., McGlynn K., Vanderbilt C. A., Cobb M. H. (2003) J. Biol. Chem. 278, 32517–32525 [DOI] [PubMed] [Google Scholar]
- 6.Buteau J., Roduit R., Susini S., Prentki M. (1999) Diabetologia 42, 856–864 [DOI] [PubMed] [Google Scholar]
- 7.Ozaki N., Shibasaki T., Kashima Y., Miki T., Takahashi K., Ueno H., Sunaga Y., Yano H., Matsuura Y., Iwanaga T., Takai Y., Seino S. (2000) Nat. Cell Biol. 2, 805–811 [DOI] [PubMed] [Google Scholar]
- 8.Kang G., Chepurny O. G., Holz G. G. (2001) J. Physiol. 536, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto K., Shibasaki T., Yokoi N., Kashima Y., Matsumoto M., Sasaki T., Tajima N., Iwanaga T., Seino S. (2002) J. Biol. Chem. 277, 50497–50502 [DOI] [PubMed] [Google Scholar]
- 10.Kashima Y., Miki T., Shibasaki T., Ozaki N., Miyazaki M., Yano H., Seino S. (2001) J. Biol. Chem. 276, 46046–46053 [DOI] [PubMed] [Google Scholar]
- 11.Jhala U. S., Canettieri G., Screaton R. A., Kulkarni R. N., Krajewski S., Reed J., Walker J., Lin X., White M., Montminy M. (2003) Genes Dev. 17, 1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buteau J., Spatz M. L., Accili D. (2006) Diabetes 55, 1190–1196 [DOI] [PubMed] [Google Scholar]
- 13.Perfetti R., Zhou J., Doyle M. E., Egan J. M. (2000) Endocrinology 141, 4600–4605 [DOI] [PubMed] [Google Scholar]
- 14.Pipeleers D. G., Schuit F. C., in't Veld P. A., Maes E., Hooghe-Peters E. L., Van de Winkel M., Gepts W. (1985) Endocrinology 117, 824–833 [DOI] [PubMed] [Google Scholar]
- 15.Dyachok O., Isakov Y., Sågetorp J., Tengholm A. (2006) Nature 439, 349–352 [DOI] [PubMed] [Google Scholar]
- 16.Cornu M., Yang J. Y., Jaccard E., Poussin C., Widmann C., Thorens B. (2009) Diabetes 58, 1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotoh M., Maki T., Satomi S., Porter J., Bonner-Weir S., O'Hara C. J., Monaco A. P. (1987) Transplantation 43, 725–730 [DOI] [PubMed] [Google Scholar]
- 18.Klinger S., Poussin C., Debril M. B., Dolci W., Halban P. A., Thorens B. (2008) Diabetes 57, 584–593 [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki J., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y., Oka Y., Yamamura K. (1990) Endocrinology 127, 126–132 [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni R. N., Holzenberger M., Shih D. Q., Ozcan U., Stoffel M., Magnuson M. A., Kahn C. R. (2002) Nat. Genet. 31, 111–115 [DOI] [PubMed] [Google Scholar]
- 21.Brummelkamp T. R., Bernards R., Agami R. (2002) Science 296, 550–553 [DOI] [PubMed] [Google Scholar]
- 22.Iezzi M., Regazzi R., Wollheim C. B. (2000) FEBS Lett. 474, 66–70 [DOI] [PubMed] [Google Scholar]
- 23.Abderrahmani A., Cheviet S., Ferdaoussi M., Coppola T., Waeber G., Regazzi R. (2006) EMBO J. 25, 977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widmann C., Bürki E., Dolci W., Thorens B. (1994) Mol. Pharmacol. 45, 1029–1035 [PubMed] [Google Scholar]
- 25.Xuan S., Kitamura T., Nakae J., Politi K., Kido Y., Fisher P. E., Morroni M., Cinti S., White M. F., Herrera P. L., Accili D., Efstratiadis A. (2002) J. Clin. Invest. 110, 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuit F. C., Pipeleers D. G. (1985) Endocrinology 117, 834–840 [DOI] [PubMed] [Google Scholar]
- 27.Preitner F., Ibberson M., Franklin I., Binnert C., Pende M., Gjinovci A., Hansotia T., Drucker D. J., Wollheim C., Burcelin R., Thorens B. (2004) J. Clin. Invest. 113, 635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maake C., Reinecke M. (1993) Cell Tissue Res. 273, 249–259 [DOI] [PubMed] [Google Scholar]
- 29.Höög A., Hu W., Abdel-Halim S. M., Falkmer S., Qing L., Grimelius L. (1997) Ultrastruct. Pathol. 21, 457–466 [DOI] [PubMed] [Google Scholar]
- 30.Buchanan C. M., Phillips A. R., Cooper G. J. (2001) Biochem. J. 360, 431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulkarni R. N. (2005) Rev. Endocr. Metab. Disord. 6, 199–210 [DOI] [PubMed] [Google Scholar]
- 32.Dickson L. M., Rhodes C. J. (2004) Am. J. Physiol. Endocrinol. Metab. 287, E192–E198 [DOI] [PubMed] [Google Scholar]
- 33.Bernal-Mizrachi E., Wen W., Stahlhut S., Welling C. M., Permutt M. A. (2001) J. Clin. Invest. 108, 1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuttle R. L., Gill N. S., Pugh W., Lee J. P., Koeberlein B., Furth E. E., Polonsky K. S., Naji A., Birnbaum M. J. (2001) Nat. Med. 7, 1133–1137 [DOI] [PubMed] [Google Scholar]
- 35.Park S., Dong X., Fisher T. L., Dunn S., Omer A. K., Weir G., White M. F. (2006) J. Biol. Chem. 281, 1159–1168 [DOI] [PubMed] [Google Scholar]
- 36.Christofori G., Naik P., Hanahan D. (1995) Nat. Genet. 10, 196–201 [DOI] [PubMed] [Google Scholar]