Abstract
The ability to produce apolipoprotein (apo) B-containing lipoproteins enables hepatocytes, enterocytes, and cardiomyocytes to export triglycerides. In this study, we examined secretion of apoB-containing lipoproteins from mouse kidney and its putative impact on triglyceride accumulation in the tubular epithelium. Mouse kidney expressed both the apoB and microsomal triglyceride transfer protein genes, which permit lipoprotein formation. To examine de novo lipoprotein secretion, kidneys from human apoB-transgenic mice were minced and placed in medium with 35S-amino acids. Upon sucrose gradient ultracentrifugation of the labeled medium, fractions were analyzed by apoB immunoprecipitation. 35S-Labeled apoB100 was recovered in ∼1.03–1.04 g/ml lipoproteins (i.e. similar to the density of plasma low density lipoproteins). Immunohistochemistry of kidney sections suggested that apoB mainly is produced by tubular epithelial cells. ApoB expression in the kidney cortex was reduced ∼90% in vivo by treating wild type mice with apoB-antisense locked nucleic acid oligonucleotide. Inhibition of apoB expression increased fasting-induced triglyceride accumulation in the kidney cortex by 20–25% (p = 0.008). Cholesterol stores were unaffected. Treatment with control oligonucleotides with 1 or 4 mismatching base pairs affected neither the triglyceride nor the cholesterol content of the kidney cortex. The results suggest that mammalian kidney secretes apoB100-containing lipoproteins. One biological effect may be to dampen excess storage of triglycerides in proximal tubule cells.
Keywords: DNA/Antisense, Lipid/Triacylglycerol, Lipoprotein, Lipoprotein/Metabolism, Organisms/Mouse, Tissue/Organ Systems/Kidney, Antisense DNA, Apolipoprotein Genes
Introduction
Apolipoprotein (apo)2 B is the principal structural protein in triglyceride-rich lipoproteins when secreted from the liver and intestine. The human liver produces the full-length apoB100 protein, whereas the intestine mainly secretes a truncated version of the apoB protein, i.e. apoB48 (1). The formation of apoB-containing lipoproteins depends on microsomal triglyceride transfer protein (MTP), which transfers lipids onto the newly synthesized apoB polypeptide during its translation and translocation into the endoplasmic reticulum (2). Formation of apoB-containing lipoproteins provides cells with the capacity to efficiently export triglycerides and other lipids, including cholesterol. For instance, the human intestine packs and secretes ∼70 g/day of fat in apoB-containing chylomicrons. Export of triglycerides in the form of apoB-containing lipoproteins also affects lipid homeostasis in tissues other than liver and intestine. Thus, apoB100-containing particles are made in the placenta, where they might participate in lipid transport between mother and fetus (3, 4). The heart produces apoB100-containing lipoproteins that prevent deleterious lipid accumulation, e.g. as induced by type I diabetes or obesity (5–8). Recent data also suggest a role of apoB in formation of age-related macular degeneration of the eye (9).
The chicken kidney proximal tubule cells produce apoB100-containing lipoproteins. Thus, ultrastructural studies demonstrated very low density lipoprotein and low density lipoprotein (LDL)-sized lipoproteins in secretory granulae and suggested that these are secreted to the basolateral lumen of proximal tubule cells (10). In other studies, slices of chicken kidney were incubated with 35S-labeled amino acids, and secretion of newly formed apoB100 in very low density lipoprotein, LDL, intermediate density lipoprotein, and even high density lipoprotein-like lipoproteins was seen (11). Even though the lipoprotein metabolism is markedly different in birds and mammals, indications that the mammalian kidney might also produce apoB-containing lipoproteins came from early studies showing triglyceride transfer activity in microsomal extracts of rat kidney (12).
Lipid accumulation in the kidney is associated with damage to proximal tubule cells, e.g. following ischemia or rhabdomyolysis (13). Also, cisplatin-induced tubular damage involves free fatty acid and triglyceride accumulation in the proximal tubules (14). Cisplatin deactivates the transcription factor peroxisome proliferator-activated receptor-α (15), which controls fatty acid oxidation and lipid and lipoprotein metabolism (16). Interestingly, deleterious effects of cisplatin and ischemia-reperfusion were alleviated by treating mice with peroxisome proliferator-activated receptor-α agonists (14, 17). These seminal studies have supported the hypothesis that lipid accumulation during acute kidney injury might involve blockade of fatty acid oxidation with deleterious lipid accumulation as one result. Acute renal failure affects as much as 5% of hospitalized patients and is often fatal (18). Irrespective of the mechanism, proximal tubule lipid accumulation may be important for the tubular damage in the critically ill patients.
In this study, we used mice to examine whether the mammalian kidney has the capacity to secrete apoB-containing lipoproteins. Inhibition of lipoprotein formation in vivo was achieved with an apoB-antisense oligonucleotide to test the hypothesis that lipoprotein formation affects triglyceride storage in proximal tubule cells.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 mice (Taconic M&B A/S, Ry, Denmark) and human apoB transgenic mice (Taconic Europe) were fed a standard mouse chow (Altromin no. 1314; Altromin, Rugarden, Denmark) and housed at the Panum Institute, University of Copenhagen, in a temperature-controlled (21−23 °C) facility with a 12-h light/dark cycle for at least 5 days before any treatment. The animal experiments were performed according to the principles of the Danish law on animal experiments and approved by the Animal Experiments Inspectorate, Ministry of Justice, Denmark.
mRNA Quantification
Frozen tissue biopsies (10–50 mg) were homogenized in TRIzol reagent (Invitrogen) using a tissue-lyzer (Retsch/Qiagen catalog no. 85220, Haan, Germany). Total RNA was extracted according to the manufacturer's instructions and suspended in RNase-free H2O. The RNA concentration was calculated from the absorbance at 260 nm (A260) using a Nanodrop (Thermo Scientific, Denmark). The RNA integrity was ensured with a Standard 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany).
cDNA was made with Moloney murine leukemia virus reverse transcriptase (Roche Applied Science) from 1 μg of total RNA as described previously (19). Real time PCR was performed with a Lightcycler (7) or using 384-well clear plates, Fast SYBR Green Master Mix and the ABI 7900 HT sequence detection system (Applied Biosystems, Foster City, CA). Reaction volumes were 20 μl using 2 μl of template cDNA. The specificity and sequences of primers have been reported previously (7, 20–22). All analyses were done in duplicate, and gene expression data were normalized with the amount of hypoxanthine-guanine phosphoribosyltransferase in the same cDNA preparation. Similar results were obtained using 18 S as the housekeeping gene.
Histological Analyses
ApoB was detected with immunohistochemistry in sections of formalin/perfusion-fixed and paraffin-embedded kidneys from human apoB transgenic mice as described previously (23). Lipid accumulation in the kidney was visualized with Oil-red O staining of formalin-fixed and frozen 10-μm sections (8).
Secretion of ApoB-containing Lipoproteins from Mouse Kidney
Neoformation of apoB-containing lipoproteins in the kidney was examined as described previously (4). Briefly, human apoB transgenic mice were anesthetized with Hypnorm/Dormicum (Boehringer Ingelheim, Ingelheim, Germany) and perfused with 0.9% saline. The kidney was minced into pieces of ∼0.5 mm3 and incubated with [35S]Met and -Cys at 37 °C for ∼16 h. Lipoproteins in the medium were separated by sucrose gradient ultracentrifugation at 35,000 rpm for 71 h. Fractions (the upper 1 ml and then 1.5 ml each) were collected from the top and weighed to determine the density. ApoB-containing lipoproteins in the collected fractions were immunoprecipitated with a polyclonal anti-human apoB antibody (DAKO A/S, Glostrup, Denmark). The immunoprecipitates were separated in 4–20% polyacrylamide gels under denaturing and reducing conditions. Human LDL was always included on the gels to identify the migration of the apoB100 band. 35S-Labeled apoB was visualized with a PhosphorImager.
In Vivo Knockdown of Apolipoprotein B Expression
To knock down apoB expression, mice were injected intraperitoneally with a single dose of a 14-mer locked nucleic acid antisense oligonucleotide against apoB (antisense apoB-LNA, 5′-AGmCattggtatTmCA-3′, where the uppercase letters denote LNA monomers; lowercase letters denote DNA monomers, and superscript m indicates methylation, e.g. mC stands for LNA-5-methylcytidine). For controls, we used a 13-mer LNA with four mismatching bases (antisense control-4, 5′- GmCatacgaaaTCA-3′) and a 14-mer oligonucleotide with one mismatching base (antisense control-1; 5′-AGmCataggtatTmCA-3′). Each mouse received 5 mg/kg body weight of oligonucleotide dissolved in ∼100 μl of 0.9% NaCl or 0.9% NaCl (100 μl). The LNA oligonucleotides were provided by Santaris Pharma, Horsholm, Denmark. Four days after the injection of LNA, a blood sample was collected from the eye before the mice were anesthetized with Hypnorm/Dormicum (Boehringer Ingelheim) and perfused with ice-cold 0.9% NaCl through the left ventricle of the heart prior to removal of kidney, heart, and liver biopsies. The kidney cortex was carefully dissected from the medulla. Tissues were immediately frozen in liquid N2 and stored at −80 °C. In some experiments, the mice were fasted for 12 h before sacrifice.
Plasma Lipids
Blood samples were collected in tubes containing Na2-EDTA and centrifuged at 4000 × g for 10 min at 4 °C. Plasma was stored at −80 °C. Enzymatic kits were used for measurements of free fatty acids (Wako NEFA C kit, TriChem Aps, Frederikssund, Denmark), cholesterol (CHOD-PAP; Roche Applied Science), and triglycerides (TR0100, serum triglyceride determination kit, Sigma).
Tissue Lipids
Tissue cholesterol and triglycerides were quantified with TLC. Lipids were extracted from heart (11–30 mg), liver (10–60 mg), and kidney cortex (9–30 mg) biopsies with chloroform/methanol and dissolved in toluene (24). TLC plates (20 × 20 cm, DC-Fertigplatten SIL G-25, Macherey-Nagel) were washed with acetone by ascending development, dried, and activated at 110 °C for 30 min before 1 μl of lipid extracts and a dilution series of a standard with known amounts of free cholesterol, triglycerides, and cholesterol esters (Sigma) were applied. The plates were developed in n-hexane/diethyl ether/acetic acid (70:30:2) followed by n-hexane before placed in 10% cupric sulfate (w/v) in 8% phosphoric acid (v/v) for 10 s, dried with a hair dryer, and baked for 2 min at 200 °C. Digitalized images of plates were analyzed with the ImageJ software (W. Rasband, National Institutes of Health, Bethesda, rsb.info.nih.gov). All samples were analyzed in duplicate on different TLC plates.
Statistical Analyses
Statistical analyses were performed with GraphPad Prism 4.00 (GraphPad Software Inc., San Diego). Two group comparisons were done with Student's t test. p < 0.05 was considered significant.
RESULTS
Mouse Kidney Secretes ApoB100-containing Lipoproteins
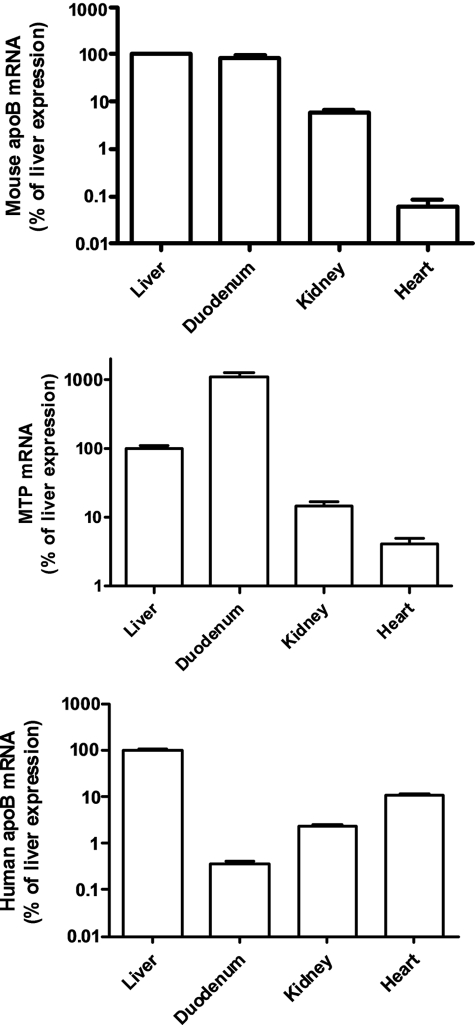
Both apoB and MTP mRNA were easily detected in the mouse kidney. On real time PCR analyses, the kidney expression of the mouse apoB and MTP genes was ∼3–5% that in the liver. We also examined the kidney expression of a human apoB mRNA in human apoB transgenic mice generated with an ∼80-kb genomic transgene with ∼19 kb of 5′-flanking sequences (25) and found robust amounts of human (and mouse) apoB mRNA in the kidney (Fig. 1). Immunohistochemistry showed human apoB protein staining in the kidney tubular epithelial cells in the cortex, whereas there was no apoB staining in glomerular cells (Fig. 2, A and B).
FIGURE 1.
Expression of mouse apoB, human apoB, and MTP mRNA in the kidney cortex, liver, duodenum, and heart of human apoB transgenic mice. Data are expressed as percent of the value in the liver and are means ± S.E., n = 4–5. Note that the human apoB transgene, as described previously (23), does not confer apoB mRNA expression in the intestine but robust expression in the kidney.
FIGURE 2.
ApoB100-containing lipoproteins are produced by mouse kidney. A, laser confocal microscopy (combined fluorescent and differential interference contrast micrograph) showing human apoB protein staining (red) in kidney tubular epithelial cells in a human apoB-transgenic mouse. B, control-stained section without primary antibody. C, kidney tissue from a human apo-B transgenic mouse was incubated with 35S-labeled amino acids. The medium was subsequently subjected to sucrose density gradient ultracentrifugation. ApoB was isolated from each density fraction by immunoprecipitation and analyzed by SDS-PAGE and filmless autoradiographic analysis. 35S-ApoB100 was seen in the d ∼1.03–1.04 g/ml lipoprotein fractions.
To examine kidney lipoprotein secretion in mice, we incubated minced kidney tissue from human apoB-transgenic mice with 35S-labeled amino acids, isolated lipoprotein fractions from the conditioned medium with density gradient ultracentrifugation, immunoprecipitated human apoB-containing particles, and separated them with SDS-PAGE. We saw apoB100-sized 35S-labeled bands in lipoproteins with a density of ∼1.030–1.040 g/liter, i.e. similar to plasma LDL particles (Fig. 2C). These data suggest that mouse kidney secretes apoB-containing lipoproteins.
Knockdown of ApoB Expression Augments Fasting-induced Lipid Accumulation in the Kidney
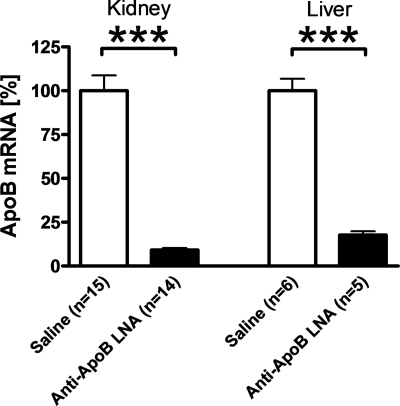
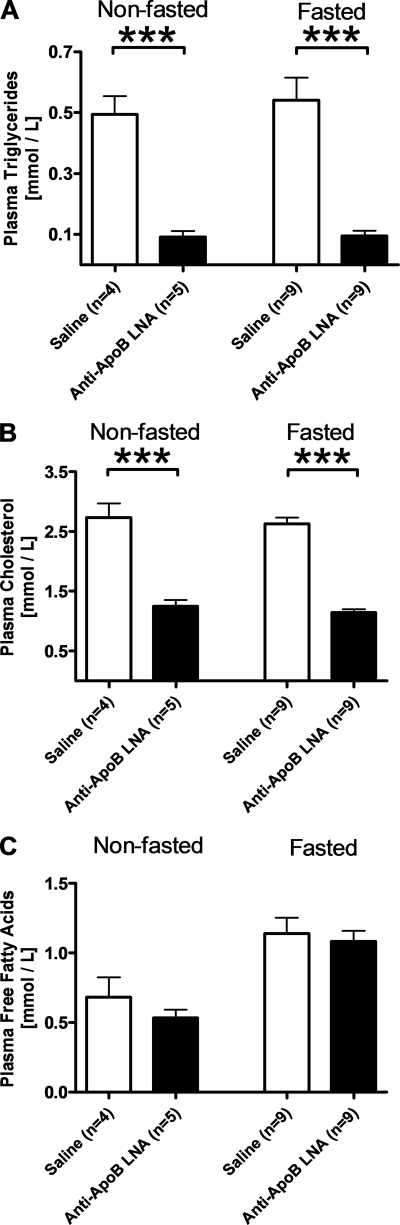
To determine the impact of kidney lipoprotein formation in vivo, we inhibited apoB expression in the kidney of wild type mice with an LNA antisense oligonucleotide (antisense apoB-LNA). Four days after an intraperitoneal injection of antisense apoB-LNA (5 mg/kg), the kidney cortex apoB mRNA expression was ∼9% that in saline-injected control mice (Fig. 3). The antisense apoB-LNA also effectively reduced apoB mRNA expression in the liver (Fig. 3), but we did not see effects on intestinal or cardiac apoB mRNA expression (data not shown). In accordance with the marked effect on hepatic apoB mRNA expression, plasma triglyceride and cholesterol concentrations were reduced by ∼82% (p < 0.0001) and ∼56% (p < 0.0001), respectively, in antisense apoB-LNA-treated mice (Fig. 4, A and B). The effect of antisense apoB-LNA on plasma triglycerides and cholesterol was similar in fasted and fed mice (Fig. 4, A and B). Plasma free fatty acids increased during fasting (p < 0.0001) but were unaffected by antisense apoB-LNA treatment (Fig. 4C).
FIGURE 3.
ApoB-antisense LNA oligonucleotide reduces apoB mRNA expression in kidney and liver. C57Bl/6 mice received an intraperitoneal injection of apoB-antisense LNA oligonucleotide (filled bars) or saline (open bars). Four days later, the RNA was isolated from the kidney cortex and a liver biopsy and used for mouse apoB mRNA quantification. Data are expressed as percent of the average value in the saline group and are means ± S.E. The numbers of mice in each group are shown in the figure. ***, p < 0.0001.
FIGURE 4.
ApoB-antisense LNA oligonucleotide reduces plasma triglycerides and cholesterol. Plasma triglycerides (A), cholesterol (B), and free fatty acids (C) were determined in C57Bl/6 mice 4 days after an intraperitoneal injection of apoB-antisense LNA oligonucleotide (filled bars) or saline (open bars). Mice were fasted for 12 h or allowed free access to chow prior to collecting blood for plasma analyses in the morning. Values are means ± S.E. The numbers of mice in each group are shown in the figure. ***, p < 0.0001.
Antisense apoB-LNA treatment did not affect total kidney cortex triglyceride or cholesterol stores in fed mice (Fig. 5, A and B). However, when deposition of lipids in the kidney was induced by fasting mice for 12 h, apoB knockdown further increased kidney triglyceride accumulation. Fasting of saline-treated control mice on average increased kidney cortex triglycerides ∼10-fold (Fig. 5A), whereas the free cholesterol content only increased marginally by ∼6% (p = 0.04) (Fig. 5B). The fasting-induced increase of triglyceride stores in tissues such as kidney, skeletal muscle, and heart probably to some extent reflects the fasting-induced increase of plasma free fatty acids (Fig. 4A). Oil-red-O staining of neutral lipids showed that the fasting-induced triglyceride accumulation in the kidney cortex mainly occurred in tubular epithelial cells (Fig. 6). In three independent experiments, antisense apoB-LNA treatment increased kidney cortex triglycerides by ∼20–25% (p = 0.008) in fasted mice but did not consistently affect the cholesterol content (Fig. 5, A and B). Notably, the effect of apoB-antisense LNA treatment on kidney cortex apoB mRNA expression was similar in fasted and fed mice (data not shown). As expected, antisense apoB-LNA treatment increased liver triglyceride stores both in fed and in fasted mice (Fig. 5C). Interestingly, apoB-LNA treatment slightly reduced the cardiac triglyceride stores in fasted mice (Fig. 5E).
FIGURE 5.
ApoB-antisense LNA oligonucleotide reduces kidney triglyceride stores in fasted mice. Triglycerides (A, C, and E) and cholesterol (B, D, and F) were determined in kidney cortex (A and B), liver (C and D), and heart (E and F) 4 days after an intraperitoneal injection of apoB-antisense LNA oligonucleotide (filled bars) or saline (open bars). Mice were fasted for 12 h or allowed free access to chow prior to removal of tissues. Values are mean ± S.E. The numbers of mice in each group are shown in the figure. *, p < 0.05; **, p < 0.01; ***, p < 0.0001.
FIGURE 6.
Fasting induces neutral lipid accumulation in kidney tubule epithelial cells. Histological sections of kidneys from 12-h fasted (A) and a fed (B) mice were stained with Oil-red-O visualizing neutral lipids (red) in tubular epithelial cells (white arrow) but not in glomeruli (black arrow) of fasted mice.
To ensure that the effect of apoB-antisense LNA did not result from off-target or nonspecific effects in the kidney cortex, we compared the antisense apoB-LNA with two control LNA oligonucleotides (antisense control-1 and -4). Although antisense control-1 reduced apoB mRNA kidney and liver expression marginally, it was not statistically significant (supplemental Fig. 1). Antisense control-4 also did not affect apoB mRNA expression (supplemental Fig. 1). Neither of the two control oligonucleotides increased kidney triglycerides (supplemental Fig. 1). Accordingly, compared with the two control oligonucleotides, antisense apoB-LNA reduced apoB mRNA expression and increased triglyceride stores in the kidney cortex and the liver of fasted mice (supplemental Fig. 1).
Effect of Reducing ApoB Expression on Kidney Expression of Genes Involved with Lipid Metabolism
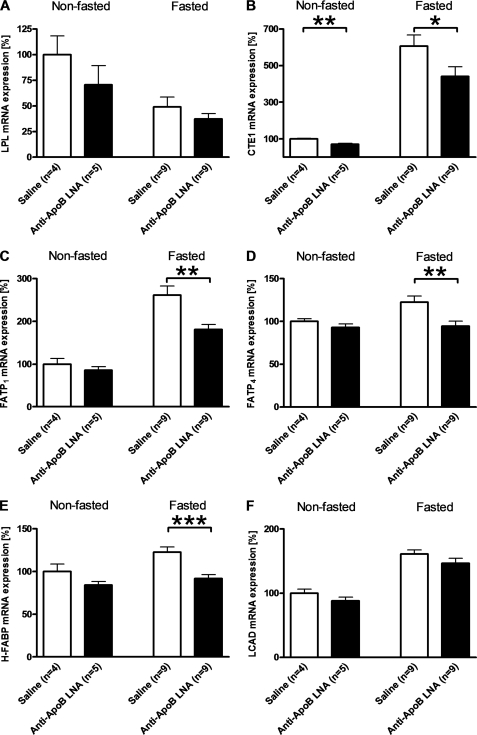
Antisense apoB-LNA treatment both reduced lipid substrate availability (i.e. plasma triglycerides) and lipoprotein secretion capacity in the kidney cortex. To further examine the combined effect of inhibiting apoB expression on lipid metabolism in the kidney, we measured the kidney cortex expression of genes controlling lipid metabolism. Fasting decreased the expression of lipoprotein lipase mRNA. It increased the expression of cytosolic acyl-CoA thioesterase 1 (CTE1), fatty acid transport protein 1 and 4 (FATP1 and FATP4), and heart-type fatty acid-binding protein all involved with intracellular fatty acid transport, and the expression of long chain acyl-CoA dehydrogenase, which affects fatty acid oxidation (Fig. 7). Antisense apoB-LNA treatment attenuated the fasting-induced increases of FATP1, FATP4, heart-type fatty acid-binding protein, and CTE1 mRNA in the kidney cortex (Fig. 7). In fed mice, antisense apoB-LNA treatment significantly decreased CTE1 mRNA expression, whereas the other genes measured were unaffected (Fig. 7).
FIGURE 7.
Effect of apoB-antisense LNA oligonucleotide and fasting on kidney expression of genes involved in fatty acid metabolism. Kidney cortex mRNA expression of lipoprotein lipase (LPL) (A), cytosolic acyl-CoA thioesterase 1 (CTE1) (B), fatty acid transport protein (FATP)1 (C), FATP4 (D), heart-type fatty acid-binding protein (H-FABP) (E), and long chain acyl-CoA dehydrogenase (LCAD) (F) was measured 4 days after an intraperitoneal injection of apoB-antisense LNA oligonucleotide (filled bars) or saline (open bars). Mice were fasted for 12 h or allowed free access to chow prior to removal of tissues. Values are means ± S.E. The numbers of mice in each group are shown in the figure. *, p < 0.05; **, p < 0.01; ***, p < 0.0001.
DISCUSSION
This study demonstrates that the mouse kidney produces apoB-containing lipoproteins and that inhibition of apoB expression increases fasting-induced lipid accumulation in the kidney cortex. We detected newly formed apoB100-containing particles in the supernatant after incubation of minced kidney tissue in the cell culture medium. This result was obtained in two separate experiments where labeled amino acids were incubated ex vivo with minced kidney tissue. Both experiments yielded relatively faint apoB bands, which might reflect that the kidney proximal tubules are notoriously sensitive to ischemic or toxic insults. As such, the results shown in Fig. 2C cannot be used to judge the quantity of lipoproteins secreted from the kidney in vivo. Immunohistochemistry studies suggested that the lipoproteins might be produced by tubular epithelial cells rather than glomerular or vascular cells. However, it should be kept in mind that the apoB staining seen in the proximal tubules might at least to some extent reflect plasma-derived apoB rather than local synthesis. The secreted apoB100-containing particles had a density similar to plasma LDL. We cannot exclude that the kidney actually secretes more buoyant triglyceride-enriched particles and that our observations reflect the effect of residual lipoprotein lipase activity in the minced kidney biopsies. Nevertheless, the density of the apoB100-containing particles was similar to that of lipoproteins secreted by the heart. In the avian kidney, Walzem et al. (10) saw intracellular lipoproteins of varying size along the tubular epithelium, and Tarugi et al. (11) detected secretion of apoB-containing particles from chicken kidney slices that varied from dense high density lipoprotein to buoyant very low density lipoprotein. Thus, Walzem et al. (10) suggested that the density of the kidney-derived apoB-containing lipoproteins may depend on the lipid availability, which may be higher in the proximal than in the more distal tubule cells of the kidney.
Treatment of mice with an apoB-antisense LNA oligonucleotide effectively reduced apoB mRNA expression by ∼90% in the kidney cortex. Although this did not affect the triglyceride stores in the kidney cortex in fed mice (indicating that inhibition of apoB in the kidney is not lipotoxic in itself), the fasting-induced lipid accumulation in the tubular epithelium was augmented by the inhibition of apoB expression. This result supports the idea that apoB-containing lipoproteins can export excess triglycerides from the tubular epithelium when the supplies exceed the utilization in β-oxidation.
The apoB-antisense LNA treatment also reduced apoB expression in the liver and consequently decreased plasma cholesterol and triglycerides. Thus, in addition to inhibiting local lipoprotein formation in the kidney, the apoB-antisense LNA treatment at the same time reduced the availability of plasma triglycerides for peripheral tissues. Indeed, in the heart, where apoB expression was not affected by the apoB-antisense LNA, the lowering of plasma triglycerides was associated with a decrease in cardiac triglyceride stores in fasted mice. Also, the fasting-induced changes in kidney cortex expression of genes involved with lipid metabolism tend to be attenuated by apoB-antisense LNA treatments. Therefore, it is possible that the triglyceride-increasing effect of inhibited local lipoprotein secretion in the kidney at the same time was counteracted by a decreased delivery of plasma triglycerides to the kidney. As such, the present studies may have underestimated the importance of local secretion of apoB-containing lipoproteins from the kidney.
The kidney cortex triglyceride content increased ∼10-fold, whereas kidney cortex cholesterol essentially was unchanged upon overnight fasting of the mice. Interestingly, fasting also induced the kidney cortex expression of genes involved in the intracellular metabolism of fatty acids. The changes in gene expression are similar to those associated with increased delivery of free fatty acids and accumulation of triglycerides in the heart of obese mice (8). Thus, we suspect that the fasting-induced triglyceride accumulation reflects increased delivery of fatty acids to the kidney but cannot exclude that decreased fuel utilization also plays a role. Plasma free fatty acids, which were doubled by fasting, are bound to albumin, which is filtered in the glomeruli and taken up by the tubular epithelium (26). Indeed, Oil-red-O staining showed that the fasting-induced lipid accumulation in the kidney cortex was confined to the tubule cells. Thus, the fasting-induced triglyceride accumulation may reflect increased glomerular filtration and tubular re-uptake of albumin-bound fatty acids. It remains to be explored to what extent the fasting-induced triglyceride accumulation in the kidney also reflects increased uptake of lipoprotein-bound triglycerides. Fatty acids can be delivered to tissues via the blood and by lipoprotein lipase acting on triglyceride-rich lipoproteins. Whereas fasting decreases the activity of lipoprotein lipase in adipose tissue, the lipoprotein lipase activity is increased in cardiac and skeletal muscle in rodents (27–29). It is unknown how fasting affects lipoprotein lipase activity in the mouse kidney.
Why has the kidney maintained a capacity to secrete lipoproteins in evolutionary distant species such as mammals and birds? Birds need large amounts of cholesterol and lipids for yolk production, and the kidney is integrated with avian whole body lipid metabolism (10). In mammals, however, the kidney contribution to the total plasma lipoprotein pool likely is negligible. Perhaps lipoprotein formation by the kidney epithelium instead helps re-secrete fatty acids and perhaps other essential lipid molecules that are taken up from the pre-urine after filtration in glomeruli. For instance, retinol-binding protein is filtered into the pre-urine from where it is normally taken up and endocytosed by the tubule epithelial cells, thus preventing the loss of retinol in the urine. The endocytosed retinol-binding protein likely is degraded in lysosomes (30). ApoB-containing lipoproteins contain retinol and play a role in the transport of newly ingested retinol from the intestine to the liver (30). Thus, the re-secretion to plasma of retinol or other essential lipid molecules could involve formation of apoB-containing lipoproteins, but further studies are obviously needed to explore this possibility. Secretion of apoB-containing lipoproteins could also serve to attenuate triglyceride accumulation as suggested by the present results. Lipid accumulation has detrimental effects on cellular function, e.g. by inducing oxidative stress and promoting apoptosis (31, 32). Thus lipotoxicity can induce insulin resistance in skeletal muscle, decrease contractile function in the heart, and impair insulin secretion from pancreatic β-cells (33–35). The mechanisms are not completely resolved but may relate to the accumulation of ceramides and increased fluxes of free fatty acids causing oxidative stress (31, 34). Recent data suggest that the kidney proximal tubular epithelium suffers from excess triglyceride accumulation in association with tubular damage induced, e.g. by reperfusion after ischemia, rhabdomyolysis, and treatment with cisplatin (13, 30). Indeed, studies by Portilla et al. (15) have mechanistically linked cisplatin-induced kidney damage with a decrease in the fatty acid oxidation. It remains to be explored whether the capacity of the kidney to secrete apoB-containing lipoproteins plays a role in protecting the kidney from deleterious triglyceride accumulation in humans. The lipoprotein secretion capacity from the human liver and heart is affected by a polymorphism in the promoter region of the MTP gene (36). The present data suggest that it may be worthwhile to explore whether the polymorphism affects the risk of tubular affection and acute kidney injury in critically ill patients.
Supplementary Material
Acknowledgments
We thank Tina Estrup Axen and Karen Rasmussen for technical assistance and Jonas Vikeså for confocal microscopy.
This work was supported by grants from Danish National Research Council, Ingeborg and Leo Danins Foundations, Henry Hansen and Hustru's Fund, and the NovoNordisk Foundation (to L. B. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- apo
- apolipoprotein
- MTP
- microsomal triglyceride transfer protein
- LDL
- low density lipoprotein
- LNA
- locked nucleic acid.
REFERENCES
- 1.Young S. G. (1990) Circulation 82, 1574–1594 [DOI] [PubMed] [Google Scholar]
- 2.Gregg R. E., Wetterau J. R. (1994) Curr. Opin. Lipidol. 5, 81–86 [DOI] [PubMed] [Google Scholar]
- 3.Demmer L. A., Levin M. S., Elovson J., Reuben M. A., Lusis A. J., Gordon J. I. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 8102–8106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madsen E. M., Lindegaard M. L., Andersen C. B., Damm P., Nielsen L. B. (2004) J. Biol. Chem. 279, 55271–55276 [DOI] [PubMed] [Google Scholar]
- 5.Borén J., Véniant M. M., Young S. G. (1998) J. Clin. Invest. 101, 1197–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen L. B., Véniant M., Borén J., Raabe M., Wong J. S., Tam C., Flynn L., Vanni-Reyes T., Gunn M. D., Goldberg I. J., Hamilton R. L., Young S. G. (1998) Circulation 98, 13–16 [DOI] [PubMed] [Google Scholar]
- 7.Nielsen L. B., Bartels E. D., Bollano E. (2002) J. Biol. Chem. 277, 27014–27020 [DOI] [PubMed] [Google Scholar]
- 8.Bartels E. D., Nielsen J. M., Hellgren L. I., Ploug T., Nielsen L. B. (2009) PLoS One 4, e5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujihara M., Bartels E., Nielsen L. B., Handa J. T. (2009) Exp. Eye Res. 88, 1115–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walzem R. L., Hansen R. J., Williams D. L., Hamilton R. L. (1999) J. Nutr. 129, 467S–472S [DOI] [PubMed] [Google Scholar]
- 11.Tarugi P., Ballarini G., Pinotti B., Franchini A., Ottaviani E., Calandra S. (1998) J. Lipid Res. 39, 731–743 [PubMed] [Google Scholar]
- 12.Wetterau J. R., Zilversmit D. B. (1986) Biochim. Biophys. Acta 875, 610–617 [DOI] [PubMed] [Google Scholar]
- 13.Zager R. A., Johnson A. C., Hanson S. Y. (2005) Kidney Int. 67, 111–121 [DOI] [PubMed] [Google Scholar]
- 14.Portilla D., Li S., Nagothu K. K., Megyesi J., Kaissling B., Schnackenberg L., Safirstein R. L., Beger R. D. (2006) Kidney Int. 69, 2194–2204 [DOI] [PubMed] [Google Scholar]
- 15.Portilla D., Dai G., McClure T., Bates L., Kurten R., Megyesi J., Price P., Li S. (2002) Kidney Int. 62, 1208–1218 [DOI] [PubMed] [Google Scholar]
- 16.Fruchart J. C. (2009) Atherosclerosis 205, 1–8 [DOI] [PubMed] [Google Scholar]
- 17.Li S., Wu P., Yarlagadda P., Vadjunec N. M., Proia A. D., Harris R. A., Portilla D. (2004) Am. J. Physiol. Renal Physiol. 286, F572–F580 [DOI] [PubMed] [Google Scholar]
- 18.Michalik L., Wahli W. (2006) J. Clin. Invest. 116, 598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartels E. D., Nielsen J. E., Lindegaard M. L., Hulten L. M., Schroeder T. V., Nielsen L. B. (2007) Atherosclerosis 195, e42–e49 [DOI] [PubMed] [Google Scholar]
- 20.Christoffersen C., Bollano E., Lindegaard M. L., Bartels E. D., Goetze J. P., Andersen C. B., Nielsen L. B. (2003) Endocrinology 144, 3483–3490 [DOI] [PubMed] [Google Scholar]
- 21.Bang C. A., Bro S., Bartels E. D., Pedersen T. X., Nielsen L. B. (2007) Am. J. Physiol. Renal Physiol. 293, F1325–F1331 [DOI] [PubMed] [Google Scholar]
- 22.Lindegaard M. L., Nielsen L. B. (2008) Metabolism 57, 766–773 [DOI] [PubMed] [Google Scholar]
- 23.Nielsen L. B., McCormick S. P., Pierotti V., Tam C., Gunn M. D., Shizuya H., Young S. G. (1997) J. Biol. Chem. 272, 29752–29758 [DOI] [PubMed] [Google Scholar]
- 24.Bartels E. D., Lauritsen M., Nielsen L. B. (2002) Diabetes 51, 1233–1239 [DOI] [PubMed] [Google Scholar]
- 25.Linton M. F., Farese R. V., Jr., Chiesa G., Grass D. S., Chin P., Hammer R. E., Hobbs H. H., Young S. G. (1993) J. Clin. Invest. 92, 3029–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birn H., Christensen E. I. (2006) Kidney Int. 69, 440–449 [DOI] [PubMed] [Google Scholar]
- 27.Tan M. H., Sata T., Havel R. J. (1977) J. Lipid Res. 18, 363–370 [PubMed] [Google Scholar]
- 28.Ladu M. J., Kapsas H., Palmer W. K. (1991) Am. J. Physiol. 260, R953–R959 [DOI] [PubMed] [Google Scholar]
- 29.Ong J. M., Simsolo R. B., Saghizadeh M., Pauer A., Kern P. A. (1994) J. Lipid Res. 35, 1542–1551 [PubMed] [Google Scholar]
- 30.Christensen E. I., Moskaug J. O., Vorum H., Jacobsen C., Gundersen T. E., Nykjaer A., Blomhoff R., Willnow T. E., Moestrup S. K. (1999) J. Am. Soc. Nephrol. 10, 685–695 [DOI] [PubMed] [Google Scholar]
- 31.Shimabukuro M., Zhou Y. T., Levi M., Unger R. H. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2498–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. (1993) Science 259, 1769–1771 [DOI] [PubMed] [Google Scholar]
- 33.Shimabukuro M., Zhou Y. T., Lee Y., Unger R. H. (1998) J. Biol. Chem. 273, 3547–3550 [DOI] [PubMed] [Google Scholar]
- 34.Unger R. H. (2002) Annu. Rev. Med. 53, 319–336 [DOI] [PubMed] [Google Scholar]
- 35.Schrauwen P. (2007) Proc. Nutr. Soc. 66, 33–41 [DOI] [PubMed] [Google Scholar]
- 36.Ledmyr H., McMahon A. D., Ehrenborg E., Nielsen L. B., Neville M., Lithell H., MacFarlane P. W., Packard C. J., Karpe F. (2004) Circulation 109, 2279–2284 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.