Abstract
AMP-activated protein kinase (AMPK) is a sensor of cellular energy state and a regulator of cellular homeostasis. In endothelial cells, AMPK is stimulated via the upstream kinases LKB1 and Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ). Previously, AMPK has been reported to activate endothelial nitric-oxide synthase (eNOS). Using genetic and pharmacological approaches, we show that vascular endothelial growth factor (VEGF) stimulates AMPK in human and mice endothelial cells via CaMKKβ. VEGF-induced AMPK activation is potentiated under conditions of energy deprivation induced by 2-deoxyglucose. To investigate the role of AMPK in endothelial function, CaMKKβ, AMPKα1, or AMPKα2 was down-regulated by RNA interference, and studies in AMPKα1−/− mice were performed. We demonstrate that AMPK does not mediate eNOS phosphorylation at serine residue 1177 or 633, NO- dependent cGMP generation, or Akt phosphorylation in response to VEGF. Using inhibitors of eNOS or soluble guanylyl cyclase and small interfering RNA against eNOS, we show that NO does not act upstream of AMPK. Taken together, these data indicate that VEGF-stimulated AMPK and eNOS pathways act independently of each other. However, acetyl-CoA carboxylase, a key enzyme in the regulation of fatty acid oxidation, was phosphorylated in response to VEGF in an AMPKα1- and AMPKα2-dependent manner. Our results show that AMPKα1 plays an essential role in VEGF-induced angiogenesis in vitro (tube formation and sprouting from spheroids) and in vivo (Matrigel plug assay). In contrast, AMPKα2 was not involved in VEGF-triggered sprouting. The data suggest that AMPKα1 promotes VEGF-induced angiogenesis independently of eNOS, possibly by providing energy via inhibition of acetyl-CoA carboxylase.
Keywords: AMP-activated Protein Kinase (AMPK), Endothelium, Energy Metabolism, Nitric Oxide, Nitric-oxide Synthase, Serine Threonine Protein Kinase, Vascular Endothelial Growth Factor
Introduction
AMP-activated protein kinase (AMPK)2 is a heterotrimeric serine/threonine kinase composed of a catalytic α-subunit and regulatory β- and γ-subunits (1, 2). AMPK acts as a sensor of cellular energy status and maintains the balance between ATP production and ATP consumption. It is activated by a rise in the AMP:ATP ratio following cellular ATP depletion and initiates processes aimed at restoring the energy balance such as the inhibition of ATP-requiring pathways and the stimulation of ATP-generating pathways, as well as changes in gene and protein expression (3–7). In addition, AMPK is involved in the regulation of cellular homeostasis and in signaling and control processes such as growth, proliferation, and apoptosis (4, 5).
In endothelial cells, a major function of AMPK is to maintain an anti-inflammatory and anti-atherogenic phenotype and to support endothelial functions involved in angiogenesis (8). AMPK protects endothelial cells from various cellular stresses, including hypoxia and oxidative stress as well as excessive exposure to free fatty acids and glucose. It also mediates responses to oxidants, shear stress, vascular mediators (thrombin, histamine, extracellular nucleotides, sphingosine-1-phosphate, and bradykinin), hormones (adiponectin, estradiol, and ghrelin), and drugs (metformin, statins, and rosiglitazone) (8–23). Some of the protective actions of AMPK are related to the activation of endothelial NO synthase (eNOS) and the formation of NO, which is a central signaling molecule in the vasculature. AMPK has been shown to phosphorylate eNOS serine 1177 in vitro and in endothelial cells (8, 10–12, 15–17, 19–22, 24). In addition, the AMPKα2 isoform has recently been reported to phosphorylate serine 633 of eNOS (25). On the other hand, AMPK activation and eNOS phosphorylation are dissociated in some situations (13, 14, 23, 26, 27), and the signals linking AMPK to eNOS are not well understood.
AMPK activation requires phosphorylation of threonine 172 (Thr172) in the activation loop of the α-subunit (28). LKB1, Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ), and transforming growth factor-β-activated kinase 1 (TAK1) have been identified as responsible upstream kinases (29–36). Importantly, the activation of AMPK via CaMKKβ is independent of changes in the AMP:ATP ratio (9, 13, 14, 16, 18) and is initiated by agonists, which leads to a receptor-coupled increase of intracellular Ca2+. In endothelial cells, the CaMKKβ/AMPK pathway is stimulated by thrombin, extracellular nucleotides, sphingosine-1-phosphate, bradykinin, or ghrelin (14, 18, 20, 21, 23). In addition, recent studies have shown that vascular endothelial growth factor (VEGF) stimulates the activation of AMPK via a CaMKK-dependent pathway (19, 20).
VEGF is a key regulator of angiogenesis and controls the proliferation, migration, differentiation, and survival of endothelial cells through binding to VEGF receptor-2 (VEGFR2) (37). VEGFR2 is a receptor tyrosine kinase that autophosphorylates and initiates a variety of signaling pathways including the phospholipase Cγ/protein kinase C/Ca2+ pathway and the phosphoinositide 3-kinase/Akt pathway (38, 39). VEGF has also been reported to activate eNOS via Ca2+- and Akt-dependent mechanisms (40–45). Moreover, AMPK has been suggested to be involved in VEGF-induced eNOS activation (19, 20).
The present study was aimed at further investigating VEGF-induced AMPK activation and its relation to eNOS phosphorylation and angiogenesis. We employed two different in vitro models, human umbilical vein endothelial cells (HUVEC) and microvascular lung endothelial cells (MLEC) from wild type and AMPKα1 knock-out mice, as well as an in vivo model of angiogenesis in both mouse species. Our data show that VEGF activates AMPK via a VEGFR2/phospholipase C/Ca2+/ CaMKKβ-dependent pathway. The effect of VEGF on AMPK is potentiated under conditions of energy deprivation induced by 2-deoxyglucose. Using different experimental approaches (CaMKK inhibition by STO-609; RNA interference for CaMKKβ, AMPKα1, and AMPKα2 as well as AMPKα1−/− endothelial cells), we have demonstrated that AMPK does not contribute to eNOS phosphorylation in VEGF-stimulated cells. Importantly, our data show that AMPKα1 mediates VEGF-induced angiogenesis in vitro (tube formation and spheroid assay) and in vivo (Matrigel plug assay). In contrast, AMPKα2 had no significant effects on angiogenesis in vitro (spheroid assay). Our data suggest that AMPK affects VEGF-induced angiogenesis, an energy-consuming and yet vital process, independently of eNOS.
EXPERIMENTAL PROCEDURES
Materials
Cell culture media and sera were from Lonza (Verviers, Belgium), and endothelial mitogen was from Sanbio Deutschland GmbH (Beutelsbach, Germany). Rabbit polyclonal antibodies reacting with AMPKα1, AMPKα2, AMPKα, Akt, phospho-acetyl-CoA carboxylase (ACC), phospho-Akt (serine 473), and phospho-eNOS (serine 1177) as well as rabbit monoclonal antibodies against ACC, phospho-AMPKα (Thr172), and LKB1 were acquired from Cell Signaling Technology (Frankfurt, Germany). The rabbit polyclonal antibody recognizing eNOS phosphorylated at serine 633 was from Millipore GmbH (Schwalbach, Germany). Monoclonal antibodies against human eNOS (clone 3) and mouse CD102, the basement membrane matrix, MatrigelTM (growth factor-reduced and phenol-red free), and the IHC zinc fixative were purchased from BD Transduction, BD Pharmingen, and BD Biosciences, respectively. The polyclonal antibody against platelet endothelial adhesion molecule-1 (PECAM-1/CD31) was from Acris Antibodies GmbH (Herford, Germany), and Cy3-labeled goat anti-rabbit IgG (H+L) was from Dianova (Hamburg, Germany). Peroxidase-labeled anti-mouse and anti-rabbit IgG were from Kirkegaard and Perry Laboratories, Inc. (Gaithersburg, MD), and M-450 sheep anti-rat beads were acquired from Dynal Biotech (Hamburg, Germany). Proteinase inhibitor mixture complete, EDTA-free, was from Roche Diagnostics, and stock solution was prepared as described by the manufacturer. Recombinant human VEGF-165 was acquired from R&D Systems GmbH (Wiesbaden, Germany). Calmodulin and inhibitors for phospholipase C (U-73122), VEGFR2 (SU-5614), calmodulin-dependent kinase II (CaMKII, KN-93), conventional protein kinases C (PKC; Gö-6976), and soluble guanylyl cyclase (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ)) were obtained from Merck Biosciences/Calbiochem (Darmstadt, Germany). STO-609 was from Tocris (Ellisville, MO) and S-nitrosoglutathione (GSNO) from Axxora Deutschland GmbH (Lörrach, Germany). A 3H-cGMP Biotrak radioimmunoassay was purchased from Amersham Biosciences. l-Nitroarginine methyl ester (l-NAME), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), phorbol 12-myristate 13-acetate, and other reagents were purchased from Sigma.
Animals
AMPKα1−/− mice were obtained as reported previously (46, 47). Experiments were performed with 3–4-month-old wild type (AMPKα1+/+) and AMPKα1−/− mice. The study conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
Cell Culture
HUVEC were cultured in M199 containing 15% fetal calf serum (FCS), 5% human serum, and 7.5 μg/ml endothelial mitogen as described (48) and characterized by flow cytometric staining for CD31 (>98% positive). Experiments were performed with cells from the first or second passage.
MLEC were isolated from mouse lungs with collagenase A and selected twice in a magnetic field after the cultures were incubated with magnetic beads coated with anti-mouse CD102 antibody (49, 50). The culture medium contained 35% DMEM, 35% F-12, 20% FCS, 50 μg/ml endothelial mitogen, 2 mm l-glutamine, and 100 μg/ml heparin. Cultures from wild type and AMPKα1−/− mice were started simultaneously. 87.0 ± 6.4% of AMPKα1+/+ and 87.1 ± 5.4% of AMPKα1−/− mice-derived cell preparations, respectively, were CD31-positive as measured by flow cytometry (n = 5).
HUVEC or MLEC were seeded on 24-well plates (for cell counting, flow cytometry, tube formation, and spheroid assay), 30-mm wells (for nucleotide and transfection experiments), or 60-mm-diameter dishes (for protein analysis). Short-term experiments were carried out after a 5-h incubation in serum-free M199 or DMEM/F12 containing 0.25% human serum albumin (M199/HSA or DMEM/F12/HSA for HUVEC or MLEC, respectively). Pretreatment with inhibitors and stimulation were performed in Hepes/HSA buffer (10 mm Hepes (pH 7.4), 145 mm NaCl, 5 mm KCl, 1 mm MgSO4, 10 mm glucose, 1.5 mm CaCl2, 0.25% HSA). SU-5614, U-73122, STO-609, BAPTA-AM, KN-93, Gö-6976, and ODQ were dissolved in dimethyl sulfoxide. Control cells received the same volume of solvent, and the final concentration did not exceed 0.1%. VEGF was used at a concentration of 50 ng/ml (10 ng/ml in spheroid assays) for the indicated times or for 5 min if not specified. Growth and survival experiments were started after a 5-h incubation in serum-depleted medium (DMEM/F12 containing 0.1% FCS), and VEGF was added to the same medium.
Small Interfering RNA (siRNA)-mediated Knockdown of AMPK, CaMKKβ, and LKB1
siRNA duplex oligonucleotides used in this study were based on the human cDNAs encoding AMPKα1, AMPKα2, CaMKKβ, LKB1, and eNOS. CaMKKβ and LKB1 siRNAs as well as a nonsilencing control siRNA were obtained from Qiagen GmbH (Hilden, Germany). For CaMKKβ, 5′-CGAUCGUCAUCUCUGGUUAdTdT-3′ (sense) and 5′-UAACCAGAGAUGACGAUCG-3′ (antisense) were used; and for LKB1, 5′-GGCUCUUACGGCAAGGUGAdTdT-3′ (sense) and 5′-UCACCUUGCCGUAAGAGCCdTdT-3′ (antisense) were used. The siRNA sequences employed as a negative control in CaMKKβ and LKB1 silencing experiments were 5′-UUCUCCGAACGUGUCACGUdTdT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAAdTdT-3′ (antisense). AMPKα1- and AMPKα2-specific SMARTpool siRNA reagents (M-005027 and M-005361, respectively) and the respective nonspecific control SMARTpool siRNA (D-001206-13-05) were purchased from Millipore (Schwalbach, Germany). For down-regulation of eNOS, ON-TARGETplus SMARTpool siRNA for human NOS3 (L-006490-00-0005) was obtained from Thermo Fisher Scientific (Lafayette, CO), and ON-TARGETplus Non-targeting Pool (D-001810-10-0005, Thermo Fisher Scientific) was used as a control.
1.8 × 105 HUVEC plated on 30-mm-diameter dishes 24 h prior to transfection were 50% confluent when siRNA was added. The amount of siRNA duplexes applied was 1 μg/dish for CaMKKβ, LKB1, and eNOS and 0.5 μg/dish for AMPKα1 and AMPKα2. Transfection was performed using the amphiphilic delivery system SAINT-RED (Synvolux Therapeutics B.V., Groningen, The Netherlands) according to the manufacturer's instructions and as described previously (14). Briefly, siRNA was complexed with 15 nmol of transfection reagent, diluted with M199/HSA to 1 ml, and added to the cells for 4 h. Subsequently, 2 ml of culture medium was added, and incubation proceeded to 72 h.
Western Blot Analysis
MLEC were lysed in ice-cold Tris buffer (50 mm Tris (pH 7.4), 2 mm EDTA, 1 mm EGTA) containing 1% Triton X-100, 0.1% SDS, 50 mm NaF, 10 mm Na4P2O7, 1 mm Na3VO4, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride, and 10 μl/ml protease inhibitor mixture stock solution. Lysate proteins were solubilized in Laemmli buffer and separated by SDS-PAGE (50 μg lysate protein/lane). Blots were subjected to immunostaining with primary antibodies overnight and peroxidase-conjugated secondary antibodies for 1 h, and proteins were visualized using the ECL technique. Protein bands were evaluated by densitometry. Phosphospecific signals, normalized against the amount of total protein, are shown as arbitrary units. Changes in phosphorylation were calculated by comparing the differences between basal and stimulated values of specifically treated samples and their respective controls.
Sample Preparation for AMPK, CaMKKβ, and LKB1 Assays
Following experimental treatments, HUVEC were rinsed with Hepes buffer (50 mm Hepes (pH 7.4) including 50 mm NaF, 1 mm EDTA, 1 mm dithiothreitol, 5 mm Na4P2O7) and lysed in Hepes buffer containing 0.1 mm phenylmethylsulfonyl fluoride, 0.157 mg/ml benzamidine, 4 mg/ml trypsin inhibitor, 1% Triton X-100, and 10% glycerol.
AMPK Immunoprecipitation and Assay
AMPK was immunoprecipitated from 50–100 μg of cell lysate protein using a rabbit anti-pan-β-antibody (51) and protein A-Sepharose or sheep anti-α1-antibodies (52) and protein G-Sepharose. AMPK activity was determined by phosphorylating the synthetic substrate peptide HMRSAMSGLHLVKRR (SAMS peptide) (53) in the presence of 5 mm MgCl2, 0.2 mm [γ-32P]ATP (specific radioactivity ∼200 cpm/pmol), and 0.2 mm AMP.
CaMKKβ Immunoprecipitation and Assay
CaMKKβ was immunoprecipitated from 100 μg of cell lysate protein using 2 μl of rabbit sera raised against bacterially expressed CaMKKβ bound to protein A-Sepharose. Activity was measured by the activation of purified recombinant AMPK complexes in the presence of 2 mm CaCl2, 2 μm bovine brain calmodulin, and 0.2 mm AMP (34).
LKB1 Immunoprecipitation and Assay
LKB1 was immunoprecipitated from 100 μg of cell lysate protein using 2 μl of rabbit sera raised against bacterially expressed LKB1 bound to protein A-Sepharose. LKB1 activity was measured in the same way as CaMKKβ activity by activation of AMPK but in the absence of CaCl2 and calmodulin.
Determination of cGMP
HUVEC monolayers were incubated for 30 min in Hepes/HSA containing 0.5 mm isobutylmethylxanthine and stimulated with agonists for 20 min. The reaction was stopped with 96% ethanol, and after evaporation 50 mm Tris/4 mm EDTA (pH 7.5) was added. The cGMP content of cellular extracts was measured by radioimmunoassay as described previously (48). Cells in parallel dishes were lysed with 100 mm NaOH, 2% Na2CO3, and 1% SDS, and the protein content was determined according to the Lowry method. The intracellular cGMP concentration was expressed in pmol/mg cell protein.
Survival Studies
MLEC were seeded and grown in normal culture medium overnight. After 5 h of serum starvation in medium containing 0.1% FCS, initial cell numbers and initial cell cycle analysis were obtained. The cells were grown for a further 24 h in the absence or presence of VEGF and subjected to counting or flow cytometry analysis afterward.
Detachment of endothelial cells was achieved by trypsin-EDTA (0.05/0.02% v/v). Cell counting was carried out in aliquots of trypsinized monolayers using a Neubauer chamber. Cell cycle analysis was performed in a pooled suspension of trypsinized cells and cells obtained from culture supernatants. Cells were permeabilized by adding cold ethanol (70%) in phosphate-buffered saline for 30 min, RNA was decomposed with ribonuclease A (110 ng/ml, 30 min), and DNA was stained with propidium iodide (11 ng/ml, 30 min). The DNA fluorescence of 10,000 cells/sample was analyzed in a FACScan flow cytometer (BD Biosciences) using CellQuest software. The proportion of cells in the sub-G1 fraction reflecting the number of cells with fragmented DNA (apoptotic cells) was calculated from FL-2 histograms and given as the percentage of gated cells.
Tube Formation
Differentiation of endothelial cells in vitro into capillary-like tubules was measured in a Matrigel assay. Growth factor reduced Matrigel matrix was added to wells (100 μl/well of a 24-well plate) and allowed to polymerize for 2 h at 37 °C. MLEC preincubated for 5 h in serum-depleted medium before detachment were seeded on Matrigel and grown in the presence or absence of VEGF for 20 h. Subsequently, cells were fixed with 1% paraformaldehyde, washed, and kept in phosphate-buffered saline. After the washing, tube formation was observed using a light microscope, and pictures were captured with a computer system. The images were subjected to pixel analysis using analySIS software (Soft Imaging System GmbH, Münster, Germany), and the total tube length per image was calculated from five independent pictures of each condition.
Spheroid Assay
Spheroids containing 3000 cells were generated by mixing an endothelial cell suspension with 0.2 volume of methyl cellulose (stock solution 12 mg/ml) and culturing the cells overnight in 96-well round-bottom plates. 25–30 spheroids were seeded into wells of a 24-well plate containing 150 μl of fibrinogen (2 mg/ml) plus aprotinin (1.8 mg/ml). Subsequently, thrombin (0.66 unit/well) was added to induce the formation of a three-dimensional fibrin gel. Spheroids were cultured in the presence of M199 containing 3% fetal calf serum and 10 ng/ml VEGF as indicated. After 48 h, spheroids were fixed with 4% paraformaldehyde, viewed by light microscopy, photographed with a computer system, and analyzed using analySIS software. The number of sprouts per spheroid of each condition was calculated as the mean value of five spheroids/well in duplicates.
Matrigel Plug Assay
The in vivo angiogenesis assay was performed in 12–15-week-old female wild type and AMPKα1−/− mice. 0.5 ml of Matrigel containing 400 ng/ml VEGF and 400 μg/ml (12.7 units/ml) heparin or heparin alone was injected subcutaneously into the left and right flanks. Mice were sacrificed after 7 days. The plugs were carefully dissected from the host tissue, fixed overnight in zinc fixative, and subsequently embedded in paraffin. Two 5-μm sections were prepared from each plug. After deparaffinization in xylene and rehydration, sections were treated with blocking solution (phosphate-buffered saline containing 0.01% Triton and 10% goat serum) and subjected to immunofluorescence staining using a polyclonal anti-CD31 antibody and a Cy3-labeled goat anti-rabbit secondary antibody. Analysis of CD31-positive cells was performed by fluorescence microscopy using the BX61 microscope (Olympus, Hamburg, Germany; equipped with cell⋀D software) and the TCS SP5 laser scanning microscope (Leica, Wetzlar, Germany). From each section four images (magnification ×400) were evaluated with a standard imaging software (ImageJ, version 1.43b). The CD31-positive area of each plug, calculated as a mean value from four images/section and two sections/plug, is expressed as a percentage of the total area.
Statistical Analysis
All data are given as means ± S.E. of three to five independent experiments or as means ± S.E. of the number of Matrigel plugs per treatment group. To determine the statistical significance of the described results, analysis of variance with Bonferroni's correction for multiple comparisons, t tests, or Mann-Whitney rank sum tests were performed as appropriate. A p value of <0.05 was accepted as statistically significant.
RESULTS
VEGF Stimulates AMPK via a CaMKKβ-dependent Pathway
In an initial series of experiments we tested the effect of VEGF on AMPK activation in HUVEC. We found that VEGF (50 ng/ml, 1–45 min) led to a time-dependent increase of Thr172 phosphorylation that is known to be essential for enzyme activation (Fig. 1A). In parallel, AMPK activity was increased (Fig. 1A). AMPK activation was transient, peaking at 2–5 min and returning to control levels at 30 min. Similar results were obtained when MLEC were stimulated with VEGF (data shown in Fig. 5).
FIGURE 1.
VEGF activates AMPK in endothelial cells via a CaMKKβ-mediated pathway. HUVEC were stimulated with 50 ng/ml VEGF for the indicated times (A) or for 5 min (B and C). B, cells were preincubated with STO-609 for 30 min. C, cells were pretreated with a transfection reagent (control), control-siRNA, or specific CaMKKβ-siRNA (1 μg/30-mm-diameter dish, 72 h). A–C, cell lysates were subjected to Western blot analysis using antibodies against phosphorylated AMPKα (Thr172) and total AMPKα. In parallel, AMPK activity was determined in AMPKα1 immune complexes using the SAMS peptide assay and calculated as nmol/min/mg lysate protein. C, CaMKKβ activity (nmol/min/mg AMPK) was measured in anti-CaMKKβ immune complexes by the activation of recombinant AMPK using the SAMS peptide assay to demonstrate down-regulation of the enzyme. Because Ca2+ and calmodulin were present in the test, the data may not reflect in vivo activation of CaMKKβ. A–C, representative blots, densitometric analyses, and enzyme activities are shown (mean ± S.E., n = 3). +, p < 0.05 versus unstimulated controls; *, p < 0.05 versus non-inhibitor-treated (B) or control-siRNA-treated (C) VEGF-stimulated cells.
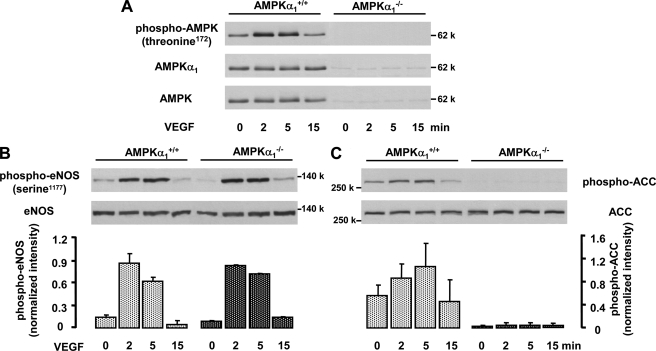
FIGURE 5.
VEGF-induced ACC phosphorylation but not eNOS phosphorylation is inhibited in endothelial cells from AMPKα1−/− mice. MLEC from AMPKα1+/+ and AMPKα1−/− mice were stimulated with 50 ng/ml VEGF for the indicated times. Cell lysates were subjected to Western blot analysis using antibodies against phosphorylated AMPKα (Thr172), AMPKα1, or pan-AMPKα (A); phosphorylated (serine 1177) or total eNOS (B); and phosphorylated or total ACC (C). Representative blots and densitometric analysis from two experiments for each treatment are shown (mean ± S.E.). In addition to the kinetic studies shown here, three experiments with a single VEGF stimulation (5 min) were performed. Statistical analysis for the 5-min time points is given under “Results”. A significant inhibition of ACC but not of eNOS phosphorylation was observed.
To examine whether VEGFR2 was involved in VEGF-induced AMPK activation, we performed experiments with SU-5614, a specific inhibitor of VEGFR2 (54). Supplemental Fig. S1 shows that Thr172 phosphorylation was largely prevented when HUVEC were pretreated with SU-5614 (78% inhibition). Similarly, preincubation of HUVEC with U-73122, a phospholipase C inhibitor (55), or BAPTA, a chelator of intracellular calcium, led to a significant reduction of VEGF-induced phosphorylation at Thr172 (66 and 85% inhibition, respectively). These data indicate that VEGF activates AMPK in endothelial cells via a VEGFR2/phospholipase C/calcium-dependent pathway and point to CaMKKβ as the responsible upstream kinase.
To confirm the role of CaMKKβ, we performed experiments in HUVEC in which the enzyme was pharmacologically inhibited or down-regulated by RNA interference. STO-609 (5–20 μg/ml), a CaMKK inhibitor (56), inhibited both Thr172 phosphorylation and AMPK activity in immune complexes in a dose-dependent manner up to 100 and 87%, respectively (Fig. 1B). Similar results were seen when CaMKKβ was targeted with a specific siRNA. This treatment led to a down-regulation of CaMKKβ activity by 78% compared with control siRNA (Fig. 1C). Cells pretreated with CaMKKβ-specific siRNA showed an inhibition of VEGF-induced AMPK phosphorylation and activation by 88 and 78%, respectively (Fig. 1C), indicating that VEGF stimulates AMPK via CaMKKβ. On the contrary, down-regulation of LKB1 had no effect on VEGF-initiated AMPK phosphorylation and activation (supplemental Fig. S2), demonstrating that this reaction is LKB1-independent.
VEGF-triggered AMPK Activation Does Not Mediate eNOS Phosphorylation but Leads to ACC Phosphorylation
To understand the functional relevance of AMPK activation by VEGF, we investigated the phosphorylation of eNOS and ACC, two potential downstream targets of AMPK (2, 8), in HUVEC and MLEC. VEGF stimulation (50 ng/ml, 1–45 min) induced phosphorylation of eNOS at serine 1177 with a maximum at 2 min and at serine 633 with a plateau between 10 and 45 min (supplemental Fig. S3, A and B). In addition, ACC was time-dependently phosphorylated with a peak at 5 min (supplemental Fig. S3C). Interestingly, eNOS phosphorylation at serine 1177 in response to VEGF was completely blocked by the inclusion of BAPTA (supplemental Fig. S4A), indicating that this process was essentially dependent on Ca2+. To further elucidate the role of Ca2+ in VEGF-triggered eNOS phosphorylation, we employed inhibitors of Ca2+-dependent protein kinases. The CaMKII inhibitor KN-93 (57) was not able to affect VEGF-induced serine 1177 phosphorylation of eNOS (supplemental Fig. S4B). In contrast, treatment of cells with Gö-6976, an inhibitor of the conventional PKC isoforms α and β (58), or with phorbol 12-myristate 13-acetate under conditions known to deplete PKC (59) induced an almost complete inhibition of serine 1177 phosphorylation in response to VEGF (supplemental Fig. S4, C and D). These data suggest that conventional PKCs mediate Ca2+-dependent eNOS phosphorylation in response to VEGF.
To investigate the role of AMPK in VEGF-stimulated eNOS and/or ACC phosphorylation, we first performed experiments in HUVEC in which CaMKKβ, the AMPK upstream kinase that triggers the effect of VEGF, was inhibited or down-regulated. Our data demonstrate that STO-609, an inhibitor of CaMKKs, did not affect VEGF-induced eNOS phosphorylation at serine 1177 or eNOS activation measured as cGMP formation (Fig. 2, A and C). Similarly, down-regulation of CaMKKβ expression by a specific siRNA did not alter eNOS phosphorylation at serine 1177 or serine 633 or cGMP formation in response to VEGF (Fig. 2, B and D, and supplemental Fig. S5). However, both STO-609 and CaMKKβ-siRNA led to a significant reduction of VEGF-induced ACC phosphorylation in HUVEC (up to 80 and 68%, respectively) (Fig. 2, E and F).
FIGURE 2.
CaMKKβ inhibition or down-regulation impairs VEGF-induced ACC phosphorylation but not eNOS phosphorylation. HUVEC were either preincubated for 30 min with STO-609, a CaMKK inhibitor, at the indicated concentrations (A, C, and E) or pretreated with CaMKKβ-specific or control-siRNA (1 μg/30-mm-diameter dish, 72 h) (B, D, and F) and subsequently stimulated with 50 ng/ml VEGF for 5 min (A, B, E, and F) or 20 min (C and D). A, B, E, and F, cells were lysed and subjected to immunoblotting using antibodies against phosphorylated eNOS (serine 1177) or total eNOS and against phosphorylated ACC or total ACC. Typical experiments and the densitometric analysis of three experiments for each staining are shown (mean ± S.E.). +, p < 0.05 versus unstimulated controls; *, p < 0.05 versus non-inhibitor-treated VEGF-stimulated cells. C and D, cells were denaturated with ethanol and processed for cGMP formation. cGMP was determined in ethanolic extracts by means of radioimmunoassay and calculated as pmol/mg cell protein, which was assayed in parallel samples (mean ± S.E., n = 4). +, p < 0.05 versus unstimulated controls.
As a second approach we looked at eNOS and ACC phosphorylation in HUVEC in which AMPKα1, the predominant AMPK isoform in endothelial cells (24), had been down-regulated using RNA interference (88 ± 2.68% reduction of protein expression compared with control cells, n = 4, p < 0.05; see also Fig. 3A). VEGF-induced AMPK phosphorylation at Thr172, which reflects the phosphorylation of both AMPKα1 and -α2 isoforms, was markedly inhibited when AMPKα1 was knocked down (76 ± 5.5, 77 ± 3.6, and 69 ± 5.8% inhibition at 1, 2, and 5 min, respectively; p < 0.05, n = 4), indicating a major contribution of AMPKα1 (Fig. 3A).
FIGURE 3.
AMPKα1 down-regulation inhibits VEGF-induced ACC phosphorylation but not eNOS or Akt phosphorylation. siRNA targeted to human AMPKα1 or containing an unrelated sequence (control-siRNA) was added to HUVEC for 72 h (0.5 μg/30-mm-diameter dish). Subsequently, HUVEC were stimulated with 50 ng/ml VEGF for the indicated times. Cells were lysed and subjected to immunoblotting using antibodies against phosphorylated AMPK (Thr172) or AMPKα1 (A); phosphorylated eNOS (serine 1177, serine 633) or total eNOS (B); phosphorylated Akt (serine 473) or total Akt (C); and phosphorylated or total ACC (D). Typical experiments and the densitometric analysis of four experiments for each staining are shown (mean ± S.E.). B, the gray and black columns represent eNOS phosphorylated at serine 1177 or serine 633, respectively. +, p < 0.05 versus unstimulated controls; *, p < 0.05 versus control-siRNA-pretreated VEGF-stimulated cells.
AMPKα1 down-regulation did not alter the phosphorylation of eNOS at serine 1177 or serine 633 in response to VEGF (Fig. 3B). Further, AMPKα1 deficiency did not affect VEGF-induced phosphorylation of Akt, which had been reported previously to mediate AMPK effects on eNOS (11, 20, 60). As shown in Fig. 3C, VEGF led to a comparable Akt phosphorylation at serine 473 in control cells and in AMPKα1-siRNA-treated cells; this was time-dependent and reached a plateau between 10 and 30 min. In contrast, VEGF-triggered ACC phosphorylation was largely prevented at all time points when AMPKα1 was down-regulated (57, 78, and 60% inhibition at 1, 2, and 5 min, respectively) (Fig. 3D).
We also down-regulated AMPKα2 in endothelial cells using specific siRNA (89 ± 5.9% reduction of protein expression compared with control cells, n = 4, p < 0.05; see also Fig. 4A). Under these conditions, VEGF-induced AMPK phosphorylation at Thr172 was also reduced although to a lower extent compared with cells in which the AMPKα1 isoform was knocked down (20 ± 14.8, 24 ± 15.7, and 45 ± 20.5% inhibition at 1, 2, and 5 min, respectively; p < 0.05, n = 4) (Fig. 4A). These data indicate that both AMPKα1 and AMPKα2 isoforms are activated by VEGF. Similar to the results obtained in AMPKα1-siRNA-treated cells, no effect of AMPKα2 down-regulation on VEGF-induced eNOS phosphorylation at serine 1177 or serine 633 was observed (Fig. 4B). In contrast, VEGF-induced ACC phosphorylation was inhibited when AMPKα2 expression was low (34, 66, and 52% inhibition at 1, 2, and 5 min, respectively) (Fig. 4C), indicating that AMPKα2 is also involved in ACC regulation in endothelial cells.
FIGURE 4.
AMPKα2 down-regulation inhibits VEGF-induced ACC phosphorylation but not eNOS phosphorylation. siRNA targeted to human AMPKα2 or containing an unrelated sequence (control-siRNA) was added to HUVEC for 72 h (0.5 μg/30-mm-diameter dish). Subsequently, HUVEC were stimulated with 50 ng/ml VEGF for the indicated times. Cells were lysed and subjected to immunoblotting using antibodies against phosphorylated AMPK (Thr172) or AMPKα2 (A); phosphorylated eNOS (serine 1177, serine 633) or total eNOS (B); and phosphorylated or total ACC (C). Typical experiments and the densitometric analysis of four experiments for each staining are shown (mean ± S.E.). B, the gray and black columns represent eNOS phosphorylated at serine 1177 or serine 633, respectively. +, p < 0.05 versus unstimulated controls; *, p < 0.05 versus control-siRNA-pretreated VEGF-stimulated cells.
Finally, we compared eNOS and ACC phosphorylation in MLEC from wild type and AMPKα1−/− mice. Fig. 5A shows that MLEC obtained from AMPKα1−/− mice did not express AMPKα1 protein, as expected. Almost no staining was detected with a pan-AMPKα antibody or a phosphospecific antibody recognizing Thr172 phosphorylation of both AMPKα isoforms, suggesting that the expression of AMPKα2 is low in these cells. Fig. 5B demonstrates that eNOS phosphorylation in response to VEGF was not different between MLEC derived from AMPKα1+/+ and AMPKα1−/−, mice indicating that AMPKα1 is not involved in eNOS activation. A statistical evaluation of eNOS phosphorylation at 5 min after the addition of VEGF revealed comparable increases in both conditions (7.6- ± 2-fold and 9- ± 1.1-fold, respectively, n = 5). In contrast, AMPKα1 is an essential upstream kinase for ACC in MLEC. VEGF (5 min) led to a significant ACC phosphorylation in wild type MLEC (2.5- ± 0.13-fold, n = 5, p < 0.05), which was not observed when AMPKα1 was absent (99 ± 1.3% inhibition, n = 5, p < 0.05). Moreover, basal ACC phosphorylation was also inhibited in AMPKα1−/− cells (90.2 ± 2.9%, n = 5, p < 0.05; see also Fig. 5C). Taken together, our data obtained with different approaches and in different endothelial cell types demonstrate that AMPK does not mediate VEGF-induced eNOS phosphorylation but is clearly responsible for VEGF-induced ACC phosphorylation.
VEGF-induced AMPK Activation Is Not Mediated by the NO/Guanylyl Cyclase Pathway
Recent studies have pointed to the importance of endogenous NO in AMPK activation (9, 61–63). Indeed, VEGF-induced eNOS phosphorylation slightly precedes AMPK activation (supplemental Fig. S3 and Fig. 1), pointing to the possibility that NO released by VEGF potentiates AMPK activity. To address this question, we first performed studies with the eNOS inhibitor l-NAME. Fig. 6 shows that pretreatment of HUVEC with l-NAME had no effect on Thr172 phosphorylation in response to VEGF, indicating that the eNOS/NO pathway was not involved. We next selectively knocked down eNOS in HUVEC by RNA interference. Compared with control siRNA, eNOS-specific siRNA suppressed eNOS expression by 77 ± 6% (n = 5, p < 0.05) but had no effect on VEGF-induced AMPK phosphorylation confirming that AMPK activation by VEGF is NO-independent (Fig. 6).
FIGURE 6.
VEGF-induced NO/cGMP formation is not required for VEGF-mediated AMPK activation. HUVEC were pretreated with l-NAME (0.5 mm, 30 min), eNOS-specific or control-siRNA duplexes (1 μg/30-mm-diameter dish, 72 h), or ODQ (10 μm, 30 min) and subsequently stimulated with VEGF (50 ng/ml, 5 min). Cells were lysed and subjected to immunoblotting using antibodies against AMPKα phosphorylated at Thr172 or total AMPKα. Down-regulation of eNOS was confirmed using anti-eNOS antibodies (middle panel). Typical experiments and densitometric analyses for each staining are shown (mean ± S.E., n = 5 for eNOS-siRNA experiments; n = 3 for others). +, p < 0.05 versus unstimulated controls.
We also tested whether inhibition of the soluble guanylyl cyclase, a downstream effector of NO, influences AMPK activation by VEGF. Pretreatment of HUVEC with the guanylyl cyclase inhibitor ODQ, however, had no effect on Thr172 phosphorylation (Fig. 6). Finally, we investigated whether an NO-releasing compound, GSNO, which was able to stimulate cGMP formation in HUVEC, would induce AMPK activation. We stimulated HUVEC with GSNO in a time-dependent manner for up to 45 min but were not able to detect significant AMPK phosphorylation (supplemental Fig. S6A), although cGMP values were clearly increased (supplemental Fig. S6B). In contrast, a significant AMPK phosphorylation in response to VEGF treatment was seen in the same cells. Together, these results indicate that the NO/cGMP pathway is not required for VEGF-induced AMPK activation.
VEGF Activates AMPK in the Presence of 2-Deoxyglucose
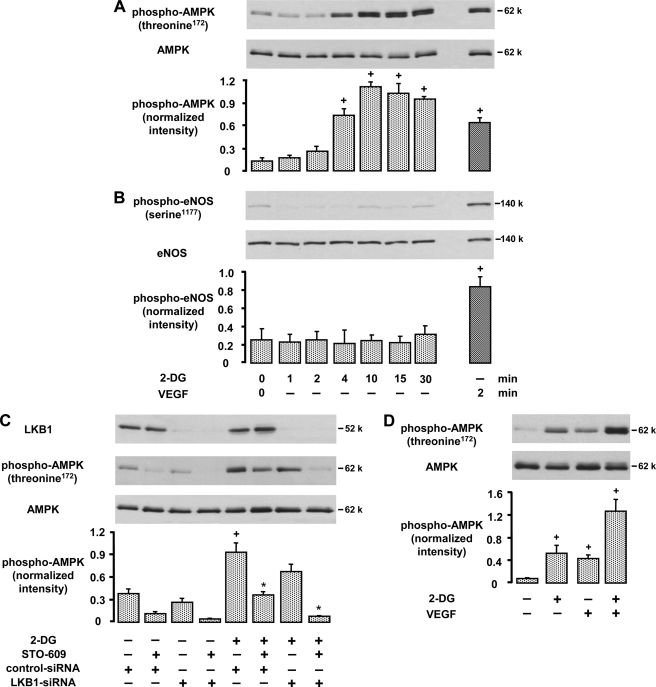
The non-metabolizable glucose analog 2-deoxyglucose is a potent glycolytic inhibitor that mimics the effects of energy starvation (64). We found that 2-deoxyglucose (20 mm, 1–45 min) caused a rapid phosphorylation of AMPK at Thr172 in a time-dependent manner (Fig. 7A). The effect of 2-deoxyglucose was suppressed when cells were pretreated with STO-609 (55%) or LKB1-specific siRNA (23%), indicating that both CaMKK and LKB1 activities were involved. Accordingly, the combined action of STO-609 and LKB1-specific siRNA led to a complete inhibition of AMPK phosphorylation induced by 2-deoxyglucose (93%, Fig. 7C).
FIGURE 7.
2-Deoxyglucose activates AMPK via CaMKKβ- and LKB1-dependent pathways and acts synergistically with VEGF. A, B, and D, HUVEC were stimulated with 20 mm 2-deoxyglucose (2-DG) or 50 ng/ml VEGF for the indicated times (A and B) or with 2-DG for 4 min followed by VEGF for 2 min (D). Cell lysates were subjected to immunoblotting using antibodies against phosphorylated (Thr172) or total AMPKα (A and D) and against phosphorylated (serine 1177) or total eNOS (B). Representative blots and densitometric analyses are shown (mean ± S.E., n = 3). C, HUVEC were pretreated with LKB1-specific or control-siRNA (1 μg/30-mm-diameter dish, 72 h), additionally preincubated for 30 min with 20 μg/ml STO-609 as indicated, and subsequently stimulated with 2-deoxyglucose (20 mm, 4 min). Cell lysates were subjected to immunoblotting using antibodies against LKB1 and phosphorylated (threonine 172) or total AMPK. Representative blots and densitometric analyses are shown (mean ± S.E., n = 3). +, p < 0.05 versus unstimulated controls; *, p < 0.05 versus 2-deoxyglucose-stimulated, control-siRNA-pretreated cells.
To test whether VEGF activates AMPK under the condition of energy depletion, we treated HUVEC with 2-deoxyglucose (20 mm, 4 min) and additionally stimulated them with VEGF. Fig. 7D shows that the VEGF effect on AMPK phosphorylation is maintained in the presence of 2-deoxyglucose and that both compounds act synergistically on AMPK activation (160 ± 19.8% in comparison with the sum of individual effects, n = 6, p < 0.05).
Interestingly, although AMPK was strongly activated by 2-deoxyglucose, eNOS was not phosphorylated under these conditions (Fig. 7B). These data indicate that AMPK activation and eNOS phosphorylation may be dissociated when AMPK is activated by metabolic stress. Moreover, eNOS did not become an AMPK substrate when cells were stimulated with VEGF in the presence of 2-deoxyglucose as suggested previously (65). The combined addition of LKB1-siRNA and STO-609 completely prevented the phosphorylation of AMPK induced by VEGF plus 2-deoxyglucose but had no effect on eNOS phosphorylation at serine 1177 (supplemental Fig. S7).
AMPKα1 Mediates VEGF-induced Angiogenesis but Not VEGF-induced Cell Survival
AMPK activation has been shown to protect endothelial cells from apoptosis induced by high glucose, palmitate, or mitogen deprivation (66–68). Because VEGF is known to be a potent anti-apoptotic factor for endothelial cells, we investigated whether AMPK activation also contributes to VEGF-induced cell survival. To this end, we compared apoptosis induced by serum depletion in MLEC derived from AMPKα1+/+ or AMPKα1−/− mice. Fig. 8A shows that serum depletion for 24 h led to a significant detachment of cells (49 and 54% of AMPKα1+/+ and AMPKα1−/− cells, respectively), which was partially prevented when VEGF was added to the medium. The protective effect of VEGF, however, did not differ between AMPKα1 wild type and knock-out cells. A similar result was seen when the percentage of apoptotic cells was determined by the number of cells with fragmented DNA (sub-G1). Serum depletion for 24 h led to an increase of apoptotic cells from 6.2 to 25.5% in AMPKα1+/+ cells and from 4.7 to 30.5% in AMPKα1−/− cells. This increase was reduced by a comparable extent in both cell types when VEGF was added to the medium (12.7 and 11.2% apoptotic cells in knock-out and wild type cells, respectively), indicating that the survival effect of VEGF was AMPK-independent (Fig. 8B).
FIGURE 8.
AMPKα1 mediates tube formation of MLEC on Matrigel and in vivo angiogenesis in Matrigel plugs but is not involved in VEGF-mediated cell survival. A and B, MLEC from AMPKα1+/+ and AMPKα1−/− mice were grown in serum-depleted medium in the absence or presence of 50 ng/ml VEGF. Cells were counted (A) or subjected to cell cycle analysis (B) at the time of VEGF addition and 24 h later. Apoptotic cells were determined as cells with fragmented DNA (sub-G1 fraction) and are shown as a percentage of gated cells (B). Data are shown as mean ± S.E., n = 5 (A) or n = 4 (B); +, p < 0.05 versus initial values; *, p < 0.05 versus 24-h incubations without VEGF. C and D, MLEC from wild type and AMKα1 knock-out mice were seeded on a Matrigel matrix and grown in serum-depleted medium for 20 h in the absence or presence of 50 ng/ml VEGF, and tube formation was evaluated by light microscopy. Representative images (C) and an analysis of tube length (D) are shown. The average tube length per image was calculated from five independent pictures per condition in duplicates (mean ± S.E., n = 4). E and F, 500 μl of Matrigel mixed with 200 ng of VEGF plus 200 μg of heparin or with heparin alone was injected subcutaneously into wild type or AMPKα1 knock-out mice. Plugs were removed after 7 days, fixed in zinc fixative, and embedded in paraffin. Tissue sections were stained with anti-CD31 antibody and evaluated by fluorescence microscopy. Representative images (×400 (E)) and an analysis of the vascularized area (F) are shown. The average CD31-positive area was calculated from four images/section and two sections/plug using a standard imaging software (mean ± S.E.; n = 4 for control plugs, n = 13 or 14 for VEGF-containing plugs in wild type or AMPKα1 knock-out mice, respectively). D and F, +, p < 0.05 versus non-VEGF-treated controls; *, p < 0.05 versus the respective values in wild type cells (D) or mice (F).
AMPK has already been shown to mediate angiogenesis in response to different stimuli including VEGF, but thus far AMPK-mediated eNOS activation and NO formation have been thought to be the underlying mechanism. Because AMPK was not involved in VEGF-induced eNOS phosphorylation under our conditions, we asked whether it would still contribute to the angiogenic effect of VEGF. To address this question we first performed tube formation assays on Matrigel using MLEC from AMPKα1+/+ and AMPKα1−/− mice. The seeding of wild type MLEC on a Matrigel matrix led to the formation of capillary-like tubules, which was increased in the presence of VEGF (50 ng/ml, 20 h) (Fig. 8, C and D). When AMPKα1−/− cells were grown on Matrigel, the generation of tubes was significantly lower. Moreover, VEGF was not able to induce additional tube formation in AMPKα1 knock-out cells, suggesting that AMPKα1 was required for the angiogenic function of VEGF independently of an effect on eNOS. These data were confirmed by using another in vitro approach in HUVEC, the spheroid assay. Spheroids were prepared from HUVEC after treatment with siRNAs against AMPKα1 or AMPKα2 or with a nonspecific control siRNA and embedded in fibrin gels. The addition of VEGF caused significant sprouting of capillary-like structures from control spheroids (Fig. 9). The formation of sprouts was inhibited by ∼45% when AMPKα1 was down-regulated. Interestingly, the effect of VEGF on sprout formation was not affected when AMPKα2 expression was reduced, pointing to a distinct role for AMPKα1 in the regulation of angiogenesis (Fig. 9).
FIGURE 9.
AMPKα1 but not AMPKα2 mediates sprout formation from endothelial cell spheroids. Synthetic RNA duplexes targeted to human AMPKα1 or AMPKα2 or containing an unrelated sequence (control-siRNA) were added to HUVEC for 48 h (0.5 μg/30-mm-diameter dish). Subsequently, spheroids were generated, embedded in fibrin gels, and stimulated with 10 ng/ml VEGF for a further 48 h. Spheroids were viewed by light microscopy. Representative images (A) and an analysis of the number of sprouts per spheroid (B) are shown. The average sprout number per spheroid was calculated from five independent pictures per condition in duplicates. Means ± S.E. from five different experiments are shown; +, p < 0.05 versus unstimulated controls.
To confirm the role of AMPKα1 in VEGF-induced blood vessel formation in vivo, we performed Matrigel plug assays in wild type and AMPKα1−/− mice. Matrigel plugs retrieved from wild type animals showed a low basal angiogenesis and a 3.8-fold increase in response to VEGF. In contrast, in plugs from AMPKα1−/− mice no effect of VEGF on vessel formation was seen (Fig. 8, E and F). These in vivo data highlight the essential role of AMPKα1 in VEGF-induced angiogenesis.
DISCUSSION
The results of the present study demonstrate that VEGF stimulates AMPK in endothelial cells from different vasculature and species (HUVEC and MLEC) and identify CaMKKβ as the responsible upstream kinase. We show that inhibition of CaMKK activity or down-regulation of CaMKKβ expression by specific siRNA prevents VEGF-induced AMPK activation measured in intact cells or in isolated AMPK immune complexes. Our data confirm the findings of previous studies showing that CaMKK/CaMKKβ-dependent AMPK activation is induced by VEGF in human or bovine aortic endothelial cells (19, 20). In addition, our study indicates that both AMPKα1 and AMPKα2 isoforms are activated by VEGF. VEGF is the first stimulus demonstrated to stimulate AMPK via a receptor tyrosine kinase-mediated intracellular Ca2+ increase. The data on VEGF together with data on agonists that activate AMPK via G-protein-coupled pathways (14, 18, 20, 21, 23) emphasize the role of Ca2+ as a regulator of AMPK activation in response to physiologic endothelial stimuli. Interestingly, while VEGF is able to activate AMPK, AMPK has been shown to mediate expression of VEGF in response to glucose deprivation or hypoxia (69, 70), suggesting a positive feedback system that may amplify VEGF-induced effects on endothelial cells. In addition, because VEGF is released under hypoxic conditions it may act together with hypoxic stress to activate AMPK. Our study shows that the action of VEGF on AMPK can indeed be amplified by the addition of a relevant second stimulus. We found that 2-deoxyglucose, a potent glycolytic inhibitor that mimics the effects of energy starvation (64), was able to activate AMPK in a LKB1- and CaMKKβ-dependent way. The involvement of CaMKKβ indicates that 2-deoyxglucose not only depletes ATP but also increases intracellular Ca2+, probably because Ca2+ is no longer sufficiently pumped out of the cytoplasm by ATP-driven pumps. When VEGF and 2-deoxyglucose were added together to endothelial cells in our study, a synergistic action on AMPK activation occurred, suggesting that energy depletion may potentiate the effect of VEGF on AMPK.
To explore the functional relevance of VEGF-induced AMPK activation, we investigated potential downstream targets of AMPK. One of the substrates suggested to be phosphorylated by AMPK in endothelial cells is eNOS. eNOS is known to be activated by reversible phosphorylation of its serine residue 1177 (71, 72), and several kinases including Akt, protein kinase A, CaMKII, and AMPK have been shown to mediate this effect. In addition, phosphorylation of the eNOS serine 633 has been observed, and it has been suggested that this process accounts for a longer lasting eNOS activation (73, 74). Serine 633 or serine 635 phosphorylation of eNOS (human/rodent or bovine sequence, respectively) has recently been reported to be mediated by AMPKα2 (25).
Our data show that VEGF is a strong stimulus of eNOS phosphorylation at both residues, serine 1177 and serine 633, and of cGMP formation, as expected. However, using several independent approaches, we were not able to reveal a role of AMPKα1 or -α2 isoform on eNOS regulation in response to VEGF. We found that neither prevention of VEGF-induced AMPK activation, by inhibiting or down-regulating the upstream kinase CaMKKβ, nor knockdown of AMPKα1 or AMPKα2 had an effect on eNOS phosphorylation at serine 1177 or cGMP formation in HUVEC. Moreover, eNOS phosphorylation at serine 1177 in response to VEGF was not different in MLEC from AMPKα1−/− mice when compared with wild type cells. In addition, we did not observe any alteration of VEGF-triggered eNOS phosphorylation at serine 633 in HUVEC with down-regulated AMPKα1, AMPKα2, or CaMKKβ. Thus, although VEGF stimulated both AMPK activation and eNOS phosphorylation, these observations were not linked.
Our findings are contradictory to earlier reports, which have shown that VEGF activates eNOS via AMPK in human or bovine aortic endothelial cells (19, 20, 60). In these studies, the treatment of cells with a dominant negative AMPK mutant or with AMPKα1-specific siRNA resulted in a reduction of VEGF-triggered eNOS phosphorylation at serine 1177 and NO production. Interestingly, two of these studies suggested that AMPK may stimulate eNOS, at least in part, indirectly via Akt (20, 60). Akt activation had already been reported to be essential for eNOS phosphorylation at serine 1177 and NO release in response to VEGF (41–43, 45). In our study, however, AMPK and Akt activation upon VEGF stimulation was not related. The knockdown of AMPKα1 by RNA interference had no influence on VEGF-induced Akt phosphorylation, and conversely, inhibition of Akt phosphorylation by a specific Akt1/2 inhibitor did not affect VEGF-triggered AMPK phosphorylation (data not shown). Notably, eNOS phosphorylation at serine 1177 was an immediate response following VEGF stimulation, whereas Akt phosphorylation was delayed, suggesting that Akt may be responsible for the late phase of eNOS activation (40, 43). In contrast, the early eNOS phosphorylation may be a Ca2+-dependent process. Accordingly, eNOS phosphorylation induced by VEGF was essentially dependent on Ca2+ in our study, as it was completely prevented by the inclusion of BAPTA. In line with these observations, VEGF had previously been shown to cause eNOS activation via an early Ca2+ increase and a late Akt-mediated eNOS phosphorylation, with the latter strictly conditional on the initial Ca2+-dependent stimulation of the enzyme (43). Previous studies in bradykinin-stimulated endothelial cells suggested that CaMKII is responsible for the Ca2+-dependent serine 1177 phosphorylation (75). In our study, KN-93, an inhibitor of CaMKII, had, however, no effect on eNOS phosphorylation at serine 1177. In contrast, PKC depletion by long-term incubation with phorbol 12-myristate 13-acetate, as well as inhibition of conventional PKCs α and β by Gö-6976, led to an almost complete inhibition of VEGF-triggered eNOS phosphorylation at serine 1177. Together, these data point to a role of Ca2+-dependent conventional PKC isoforms in VEGF-induced eNOS phosphorylation at serine 1177, although the applied inhibitor lacks an absolute specificity. Our data are in line with previous studies suggesting a role of PKCα in eNOS activation (76) but disagree with other reports (77), which may be due to differences in the experimental setting.
Our study shows that AMPK activation and eNOS phosphorylation can be dissociated in some situations. We have seen this dissociation in response to VEGF in the present study and in response to thrombin in an earlier study (14). Similar observations have been made in response to ischemia, phenformin, bradykinin, shear stress, and estradiol (13, 23, 26, 27). On the other hand, eNOS phosphorylation by AMPK has been seen in a variety of conditions including hypoxic stress, peroxynitrite, hormones, drugs, shear stress, vascular mediators, and 5-aminoimidazole-4-carboxamide ribonucleoside (8, 10–12, 15–17, 19–22, 24). We suggest that AMPK activation alone may not be sufficient to trigger eNOS phosphorylation under certain conditions. Other factors such as subcellular localization of the proteins, the availability and the activation kinetics of competing eNOS kinases, and their reaction rates with eNOS likely play a role. In addition, different substrates (ACC, eNOS, 3-hydroxy-3-methyl-glutaryl-CoA reductase) may compete for AMPK phosphorylation as has been suggested previously (25), which may affect the kinetics and extent of individual substrate phosphorylation. The situation may differ depending on the cell type and the specific circumstances of stimulation. A recent study, for example, suggests that eNOS may become AMPK-responsive under conditions of ATP depletion (65); these authors show that thrombin phosphorylates eNOS via the CaMKKβ/AMPK pathway only when cellular ATP is lowered, for instance when cells are preincubated with 2-deoxyglucose. Another study reports that serine 1177 phosphorylation of eNOS by AMPK occurs only in hypoxic but not in normoxic conditions (10). In our experiments, the pretreatment of HUVEC with 2-deoxyglucose, however, did not turn VEGF-induced eNOS phosphorylation into an AMPK-dependent process. A combination of STO-609 and LKB1-siRNA did not affect VEGF-induced serine 1177 phosphorylation of eNOS in the presence of 2-deoxyglucose, although AMPK phosphorylation was clearly inhibited by this treatment. Interestingly, although 2-deoxyglucose led to a pronounced phosphorylation of AMPK it did not cause eNOS phosphorylation, indicating that even under conditions of energy deprivation AMPK activation does not necessarily lead to eNOS activation.
In addition to the question of whether AMPK acts upstream of eNOS, we also asked whether NO that is generated in response to VEGF is able to promote AMPK activation. Indeed, recent studies have shown that AMPK activation by peroxynitrite, metformin, shear stress, or Ca2+ ionophore requires an initial formation of NO, indicating that eNOS may act upstream of AMPK (61–63). Furthermore, AMPK has been shown to be activated by NO donors via a guanylyl cyclase-mediated and CaMKKβ-dependent pathway (63). In contrast, our results do not support a role of endogenous NO in VEGF-induced AMPK activation. Down-regulation of eNOS by siRNA, inhibition of eNOS activity by l-NAME, or inhibition of guanylyl cyclase activation by ODQ had no effect on AMPK phosphorylation at Thr172 in response to VEGF. Similarly, GSNO, which spontaneously released NO as evidenced by an increase of intracellular cGMP, was not able to induce AMPK phosphorylation in HUVEC. Thus, AMPK activation in response to VEGF was independent of eNOS activation.
Akt and eNOS have both been shown to be essential downstream effectors in mediating VEGF-initiated endothelial survival and angiogenesis (78) and have also been suggested to trigger AMPK effects on angiogenesis in response to several stimuli including VEGF (10, 11, 16, 20). Because AMPK was related to neither VEGF-induced eNOS activation nor Akt activation in our study, we asked whether AMPK would influence endothelial survival or angiogenesis independently of these pathways. We compared the survival effect of VEGF in endothelial cells derived from either wild type mice or AMPKα1 knock-out mice. VEGF significantly prevented apoptosis induced by serum deprivation, but this effect was present in both AMPKα1+/+ and AMPKα1−/− MLEC, indicating that AMPK was not involved. This result is probably related to the failure of AMPK to activate Akt or eNOS in response to VEGF as discussed above. Our findings, however, do not exclude an antiapoptotic role of AMPK in response to other stimuli, which has been shown previously and attributed, at least in part, to an antioxidant action of AMPK (66–68).
Importantly, in contrast to the failure of AMPK to mediate VEGF-induced cell survival, we have presented evidence that AMPK essentially contributes to an angiogenic response in vitro and in vivo. VEGF-induced tube formation on a Matrigel matrix was inhibited in AMPKα1−/− MLEC compared with wild type cells, and similarly, sprout formation from HUVEC spheroids was reduced when AMPKα1 was down-regulated by a specific siRNA. Interestingly, down-regulation of AMPKα2 in HUVEC by RNA interference did not affect sprout formation from spheroids, pointing to a specific role of AMPKα1 in the regulation of angiogenesis. An essential role of this isoform was also demonstrated in Matrigel plug assays performed in AMPKα1−/− and AMPKα1+/+ mice. Although VEGF induced significant blood vessel formation in plugs from wild type mice, no effect was seen in mice with genetically ablated AMPKα1. Importantly, these data provide the first in vivo evidence for an angiogenic role of AMPKα1.
Our findings are in line with a recent report showing that siRNA against AMPK was able to diminish tube formation in endothelial cells and angiogenesis in Matrigel plugs in mice (79). In that study, VEGF-triggered induction of cytochrome P-450 epoxygenase and production of epoxyeicosatrienoic acids via an AMPK-dependent pathway were shown to mediate angiogenesis (79). Another possible mechanism through which AMPK may promote angiogenesis is the supply of ATP, as VEGF-induced angiogenesis is known to involve ATP-consuming processes such as migration, proliferation, and growth. AMPK regulates energy balance in part via phosphorylation and inhibition of ACC isoforms 1 and 2. The product of the reaction catalyzed by ACCs is malonyl-CoA, a major precursor of fatty acid synthesis and a crucial regulator of mitochondrial fatty acid β-oxidation through its inhibition of carnitine palmitoyltransferase 1 (80). Inhibition of ACC leads to a decreased formation of malonyl-CoA, reduced fatty acid synthesis (mediated by ACC1), increased fatty acid oxidation (mediated by ACC2), and finally ATP generation. Our study shows, by different approaches (inhibition or down-regulation of CaMKKβ and knockdown of AMPKα1 in HUVEC or mice; down-regulation of AMPKα2 in HUVEC), that VEGF initiates ACC phosphorylation via both AMPKα isoforms in endothelial cells and may stimulate fatty acid-derived ATP production. However, we cannot discriminate the ACC isoforms targeted by AMPKs because the applied phosphospecific antibody recognizes the phosphorylated forms of both ACC1 and ACC2. Interestingly, fatty acids have been shown to be a major fuel of the endothelium and to account for ∼40% of the ATP production under conditions of AMPK activation (81, 82). Moreover, AMPK activation has been shown to stimulate fatty acid oxidation in endothelial cells (23) and to lead to an increase of ATP (83). These data support the suggestion that VEGF-induced AMPK activation may provide energy for ATP-requiring processes that are vital to the process of angiogenesis. Interestingly, although both AMPKα isoforms contribute to ACC regulation, only AMPKα1 has been shown to promote angiogenesis. One possible explanation is that the AMPKα isoforms may show distinct subcellular localization (84) and interact with different ACC isoforms and thus may have a different impact on the energy balance in endothelial cells.
In summary, the data presented in this study show that VEGF activates AMPK via a Ca2+/CaMKKβ-dependent pathway. The effect of VEGF is amplified in an energy-deficient situation, i.e. when cells are pretreated with 2-deoxyglucose, which mimics a hypoxic angiogenic situation. We have shown that AMPKα1 is essential for VEGF-induced angiogenesis in vitro and in vivo, whereas AMPKα2 may not be involved in an angiogenic response. Our experiments clearly show, by different genetic and pharmacological approaches, that VEGF-induced AMPK activation is not related to the activation of eNOS or Akt. AMPK mediates, however, the phosphorylation of ACC in response to VEGF, suggesting that an increased β-oxidation of fatty acids and ATP generation may be the underlying mechanisms of the angiogenesis-promoting effect of AMPK.
Supplementary Material
Acknowledgments
We thank Gunda Guhr, Elke Teuscher, and Rose-Marie Zimmer for their excellent technical assistance.
This work was supported by grants from the Deutsche Forchungsgemeinschaft (He2304-2/1) and the Interdisziplinäres Zentrum für Klinische Forschung Jena (to R. H.). Further support was obtained from the European Commission EXGENESIS Integrated Project Grant LSHM-CT-2004-005272 (to D. C. and B. V.) and the Medical Research Council UK (to D. C. and A. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
- AMPK
- AMP-activated protein kinase
- VEGF
- vascular endothelial growth factor
- eNOS
- endothelial nitric-oxide synthase
- CaMKK
- Ca2+/calmodulin-dependent protein kinase kinase
- VEGFR2
- vascular endothelial growth factor receptor-2
- HUVEC
- human umbilical cord vein endothelial cells
- MLEC
- microvascular lung endothelial cells
- ACC
- acetyl-CoA carboxylase
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- GSNO
- S-nitrosoglutathione
- l-NAME
- l-nitroarginine methyl ester
- HSA
- human serum albumin
- BAPTA-AM
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- FCS
- fetal calf serum
- PKC
- protein kinase C
- siRNA
- small interfering RNA
- DMEM
- Dulbecco's modified Eagle's medium.
REFERENCES
- 1.Carling D. (2004) Trends Biochem. Sci. 29, 18–24 [DOI] [PubMed] [Google Scholar]
- 2.Hardie D. G. (2004) J. Cell Sci. 117, 5479–5487 [DOI] [PubMed] [Google Scholar]
- 3.Kahn B. B., Alquier T., Carling D., Hardie D. G. (2005) Cell Metab. 1, 15–25 [DOI] [PubMed] [Google Scholar]
- 4.Hardie D. G., Hawley S. A., Scott J. W. (2006) J. Physiol. 574, 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Towler M. C., Hardie D. G. (2007) Circ. Res. 100, 328–341 [DOI] [PubMed] [Google Scholar]
- 6.Hardie D. G. (2007) Annu. Rev. Pharmacol. Toxicol. 47, 185–210 [DOI] [PubMed] [Google Scholar]
- 7.Hardie D. G. (2007) Nat. Rev. Mol. Cell Biol. 8, 774–785 [DOI] [PubMed] [Google Scholar]
- 8.Zou M. H., Wu Y. (2008) Clin. Exp. Pharmacol. Physiol. 35, 535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou M. H., Hou X. Y., Shi C. M., Kirkpatick S., Liu F., Goldman M. H., Cohen R. A. (2003) J. Biol. Chem. 278, 34003–34010 [DOI] [PubMed] [Google Scholar]
- 10.Nagata D., Mogi M., Walsh K. (2003) J. Biol. Chem. 278, 31000–31006 [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thors B., Halldórsson H., Thorgeirsson G. (2004) FEBS Lett. 573, 175–180 [DOI] [PubMed] [Google Scholar]
- 13.Schulz E., Anter E., Zou M. H., Keaney J. F., Jr. (2005) Circulation 111, 3473–3480 [DOI] [PubMed] [Google Scholar]
- 14.Stahmann N., Woods A., Carling D., Heller R. (2006) Mol. Cell. Biol. 26, 5933–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis B. J., Xie Z., Viollet B., Zou M. H. (2006) Diabetes 55, 496–505 [DOI] [PubMed] [Google Scholar]
- 16.Sun W., Lee T. S., Zhu M., Gu C., Wang Y., Zhu Y., Shyy J. Y. (2006) Circulation 114, 2655–2662 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Lee T. S., Kolb E. M., Sun K., Lu X., Sladek F. M., Kassab G. S., Garland T., Jr., Shyy J. Y. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1281–1287 [DOI] [PubMed] [Google Scholar]
- 18.da Silva C. G., Jarzyna R., Specht A., Kaczmarek E. (2006) Circ. Res. 98, e39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reihill J. A., Ewart M. A., Hardie D. G., Salt I. P. (2007) Biochem. Biophys. Res. Commun. 354, 1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine Y. C., Li G. K., Michel T. (2007) J. Biol. Chem. 282, 20351–20364 [DOI] [PubMed] [Google Scholar]
- 21.Xu X., Jhun B. S., Ha C. H., Jin Z. G. (2008) Endocrinology 149, 4183–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle J. G., Logan P. J., Ewart M. A., Reihill J. A., Ritchie S. A., Connell J. M., Cleland S. J., Salt I. P. (2008) J. Biol. Chem. 283, 11210–11217 [DOI] [PubMed] [Google Scholar]
- 23.Mount P. F., Lane N., Venkatesan S., Steinberg G. R., Fraser S. A., Kemp B. E., Power D. A. (2008) Atherosclerosis 200, 28–36 [DOI] [PubMed] [Google Scholar]
- 24.Morrow V. A., Foufelle F., Connell J. M., Petrie J. R., Gould G. W., Salt I. P. (2003) J. Biol. Chem. 278, 31629–31639 [DOI] [PubMed] [Google Scholar]
- 25.Chen Z., Peng I. C., Sun W., Su M. I., Hsu P. H., Fu Y., Zhu Y., DeFea K., Pan S., Tsai M. D., Shyy J. Y. (2009) Circ. Res. 104, 496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mount P. F., Hill R. E., Fraser S. A., Levidiotis V., Katsis F., Kemp B. E., Power D. A. (2005) Am. J. Physiol. Renal Physiol. 289, F1103–F1115 [DOI] [PubMed] [Google Scholar]
- 27.Fleming I., Fisslthaler B., Dixit M., Busse R. (2005) J. Cell Sci. 118, 4103–4111 [DOI] [PubMed] [Google Scholar]
- 28.Hawley S. A., Davison M., Woods A., Davies S. P., Beri R. K., Carling D., Hardie D. G. (1996) J. Biol. Chem. 271, 27879–27887 [DOI] [PubMed] [Google Scholar]
- 29.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Mäkelä T. P., Alessi D. R., Hardie D. G. (2003) J. Biol. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S. P., Leiper F. C., Woods A., Carling D., Carlson M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8839–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. (2003) Curr. Biol. 13, 2004–2008 [DOI] [PubMed] [Google Scholar]
- 33.Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 34.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. (2005) J. Biol. Chem. 280, 29060–29066 [DOI] [PubMed] [Google Scholar]
- 35.Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. (2005) Cell Metab. 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 36.Momcilovic M., Hong S. P., Carlson M. (2006) J. Biol. Chem. 281, 25336–25343 [DOI] [PubMed] [Google Scholar]
- 37.Shibuya M., Claesson-Welsh L. (2006) Exp. Cell Res. 312, 549–560 [DOI] [PubMed] [Google Scholar]
- 38.Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. (2006) Nat. Rev. Mol. Cell Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 39.Holmes K., Roberts O. L., Thomas A. M., Cross M. J. (2007) Cell. Signal. 19, 2003–2012 [DOI] [PubMed] [Google Scholar]
- 40.He H., Venema V. J., Gu X., Venema R. C., Marrero M. B., Caldwell R. B. (1999) J. Biol. Chem. 274, 25130–25135 [DOI] [PubMed] [Google Scholar]
- 41.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. (1999) Nature 399, 601–605 [DOI] [PubMed] [Google Scholar]
- 42.Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brouet A., Sonveaux P., Dessy C., Balligand J. L., Feron O. (2001) J. Biol. Chem. 276, 32663–32669 [DOI] [PubMed] [Google Scholar]
- 44.Gélinas D. S., Bernatchez P. N., Rollin S., Bazan N. G., Sirois M. G. (2002) Br. J. Pharmacol. 137, 1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanes M. G., Oubaha M., Rautureau Y., Gratton J. P. (2007) J. Biol. Chem. 282, 10660–10669 [DOI] [PubMed] [Google Scholar]
- 46.Viollet B., Andreelli F., Jørgensen S. B., Perrin C., Geloen A., Flamez D., Mu J., Lenzner C., Baud O., Bennoun M., Gomas E., Nicolas G., Wojtaszewski J. F., Kahn A., Carling D., Schuit F. C., Birnbaum M. J., Richter E. A., Burcelin R., Vaulont S. (2003) J. Clin. Investig. 111, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jørgensen S. B., Viollet B., Andreelli F., Frøsig C., Birk J. B., Schjerling P., Vaulont S., Richter E. A., Wojtaszewski J. F. (2004) J. Biol. Chem. 279, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 48.Heller R., Unbehaun A., Schellenberg B., Mayer B., Werner-Felmayer G., Werner E. R. (2001) J. Biol. Chem. 276, 40–47 [DOI] [PubMed] [Google Scholar]
- 49.Kuhlencordt P. J., Rosel E., Gerszten R. E., Morales-Ruiz M., Dombkowski D., Atkinson W. J., Han F., Preffer F., Rosenzweig A., Sessa W. C., Gimbrone M. A., Jr., Ertl G., Huang P. L. (2004) Am. J. Physiol. Cell Physiol. 286, C1195–C1202 [DOI] [PubMed] [Google Scholar]
- 50.Heller R., Chang Q., Ehrlich G., Hsieh S. N., Schoenwaelder S. M., Kuhlencordt P. J., Preissner K. T., Hirsch E., Wetzker R. (2008) Cardiovasc. Res. 80, 96–105 [DOI] [PubMed] [Google Scholar]
- 51.Woods A., Cheung P. C., Smith F. C., Davison M. D., Scott J., Beri R. K., Carling D. (1996) J. Biol. Chem. 271, 10282–10290 [DOI] [PubMed] [Google Scholar]
- 52.Woods A., Salt I., Scott J., Hardie D. G., Carling D. (1996) FEBS Lett. 397, 347–351 [DOI] [PubMed] [Google Scholar]
- 53.Davies S. P., Carling D., Hardie D. G. (1989) Eur. J. Biochem. 186, 123–128 [DOI] [PubMed] [Google Scholar]
- 54.Sun L., Tran N., Tang F., App H., Hirth P., McMahon G., Tang C. (1998) J. Med. Chem. 41, 2588–2603 [DOI] [PubMed] [Google Scholar]
- 55.Smith R. J., Sam L. M., Justen J. M., Bundy G. L., Bala G. A., Bleasdale J. E. (1990) J. Pharmacol. Exp. Ther. 253, 688–697 [PubMed] [Google Scholar]
- 56.Tokumitsu H., Inuzuka H., Ishikawa Y., Ikeda M., Saji I., Kobayashi R. (2002) J. Biol. Chem. 277, 15813–15818 [DOI] [PubMed] [Google Scholar]
- 57.Sumi M., Kiuchi K., Ishikawa T., Ishii A., Hagiwara M., Nagatsu T., Hidaka H. (1991) Biochem. Biophys. Res. Commun. 181, 968–975 [DOI] [PubMed] [Google Scholar]
- 58.Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. (1993) J. Biol. Chem. 268, 9194–9197 [PubMed] [Google Scholar]
- 59.Young S., Parker P. J., Ullrich A., Stabel S. (1987) Biochem. J. 244, 775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youn J. Y., Wang T., Cai H. (2009) Circ. Res. 104, 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zou M. H., Kirkpatrick S. S., Davis B. J., Nelson J. S., Wiles W. G., 4th, Schlattner U., Neumann D., Brownlee M., Freeman M. B., Goldman M. H. (2004) J. Biol. Chem. 279, 43940–43951 [DOI] [PubMed] [Google Scholar]
- 62.Fisslthaler B., Fleming I., Keserü B., Walsh K., Busse R. (2007) Circ. Res. 100, e12–21 [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Xie Z., Dong Y., Wang S., Liu C., Zou M. H. (2008) J. Biol. Chem. 283, 27452–27461 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Dennis P. B., Jaeschke A., Saitoh M., Fowler B., Kozma S. C., Thomas G. (2001) Science 294, 1102–1105 [DOI] [PubMed] [Google Scholar]
- 65.Thors B., Halldórsson H., Jónsdóttir G., Thorgeirsson G. (2008) Biochim. Biophys. Acta 1783, 1893–1902 [DOI] [PubMed] [Google Scholar]
- 66.Ido Y., Carling D., Ruderman N. (2002) Diabetes 51, 159–167 [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi H., Ouchi N., Kihara S., Walsh K., Kumada M., Abe Y., Funahashi T., Matsuzawa Y. (2004) Circ. Res. 94, e27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J. E., Kim Y. W., Lee I. K., Kim J. Y., Kang Y. J., Park S. Y. (2008) J. Pharmacol. Sci. 106, 394–403 [DOI] [PubMed] [Google Scholar]
- 69.Yun H., Lee M., Kim S. S., Ha J. (2005) J. Biol. Chem. 280, 9963–9972 [DOI] [PubMed] [Google Scholar]
- 70.Zwetsloot K. A., Westerkamp L. M., Holmes B. F., Gavin T. P. (2008) J. Physiol. 586, 6021–6035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alderton W. K., Cooper C. E., Knowles R. G. (2001) Biochem. J. 357, 593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mount P. F., Kemp B. E., Power D. A. (2007) J. Mol. Cell Cardiol. 42, 271–279 [DOI] [PubMed] [Google Scholar]
- 73.Boo Y. C., Hwang J., Sykes M., Michell B. J., Kemp B. E., Lum H., Jo H. (2002) Am. J. Physiol. Heart Circ. Physiol. 283, H1819–H1828 [DOI] [PubMed] [Google Scholar]
- 74.Bauer P. M., Fulton D., Boo Y. C., Sorescu G. P., Kemp B. E., Jo H., Sessa W. C. (2003) J. Biol. Chem. 278, 14841–14849 [DOI] [PubMed] [Google Scholar]
- 75.Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. (2001) Circ. Res. 88, E68–75 [DOI] [PubMed] [Google Scholar]
- 76.Partovian C., Zhuang Z., Moodie K., Lin M., Ouchi N., Sessa W. C., Walsh K., Simons M. (2005) Circ. Res. 97, 482–487 [DOI] [PubMed] [Google Scholar]
- 77.Rask-Madsen C., King G. L. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shiojima I., Walsh K. (2002) Circ. Res. 90, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 79.Webler A. C., Michaelis U. R., Popp R., Barbosa-Sicard E., Murugan A., Falck J. R., Fisslthaler B., Fleming I. (2008) Am. J. Physiol. Cell Physiol. 295, C1292–C1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Munday M. R. (2002) Biochem. Soc. Trans. 30, 1059–1064 [DOI] [PubMed] [Google Scholar]
- 81.Dagher Z., Ruderman N., Tornheim K., Ido Y. (2001) Circ. Res. 88, 1276–1282 [DOI] [PubMed] [Google Scholar]
- 82.Ruderman N. B., Cacicedo J. M., Itani S., Yagihashi N., Saha A. K., Ye J. M., Chen K., Zou M., Carling D., Boden G., Cohen R. A., Keaney J., Kraegen E. W., Ido Y. (2003) Biochem. Soc. Trans. 31, 202–206 [DOI] [PubMed] [Google Scholar]
- 83.Dagher Z., Ruderman N., Tornheim K., Ido Y. (1999) Biochem. Biophys. Res. Commun. 265, 112–115 [DOI] [PubMed] [Google Scholar]
- 84.Viollet B., Guigas B., Leclerc J., Hébrard S., Lantier L., Mounier R., Andreelli F., Foretz M. (2009) Acta Physiol. (Oxf.) 196, 81–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.