Abstract
The catalytic domain of Bordetella pertussis adenylate cyclase toxin (ACT) translocates directly across the plasma membrane of mammalian cells to induce toxicity by the production of cAMP. Here, we use electrophysiology to examine the translocation of toxin into polarized epithelial cells that model the mucosal surfaces of the host. We find that both polarized T84 cell monolayers and human airway epithelial cultures respond to nanomolar concentrations of ACT when applied to basolateral membranes, with little or no response to toxin applied apically. The induction of toxicity is rapid and fully explained by increases in intracellular cAMP, consistent with toxin translocation directly across the basolateral membrane. Intoxication of T84 cells occurs in the absence of CD11b/CD18 or evidence of another specific membrane receptor, and it is not dependent on post-translational acylation of the toxin or on host cell membrane potential, both previously reported to be required for toxin action. Thus, elements of the basolateral membrane render epithelial cells highly sensitive to the entry of ACT in the absence of a specific receptor for toxin binding.
Keywords: Bacterial Toxins, Cyclic AMP (cAMP), Epithelial Cell, Membrane, Receptors, Electrophysiology
Introduction
The adenylate cyclase toxin (ACT),3 a multifunctional, single polypeptide toxin, is expressed by six of the eight members of the genus Bordetella and was discovered by detection of adenylate cyclase enzymatic activity in a commercial pertussis vaccine (1). ACT derives its cytotoxic effects from delivery of its 400-amino acid adenylate cyclase enzymatic domain into the cell cytoplasm, resulting in the unregulated, calmodulin-dependent conversion of ATP into cAMP (2–4). Translocation, the process by which the catalytic domain is delivered across the cytoplasmic membrane, is unaffected by cytochalasin D or ammonium chloride and is dependent on the interaction of the ∼1000-amino acid cell-binding domain with the cell membrane (5).
This cell-binding domain is homologous to the members of the RTX (repeat-in-toxin) family of pore-forming bacterial toxins, such as Escherichia coli hemolysin, HlyA, and several leukotoxins from organisms such as Pasteurella hemolytica and Actinobacillus actinomycetemcomitans (6, 7). The RTX region of ACT oligomerizes in the cell membrane independently of translocation, forming cation-selective pores and causing hemolysis of erythrocytes and nonapoptotic death of nucleated cells (8–14). Membrane interaction and pore formation by ACT can occur in artificial lipid bilayers and liposomes and are influenced by lipid and glycolipid composition (11, 15–18). Although ACT intoxicates a broad range of cells and is able to associate with artificial lipid membranes containing no proteins, the β2-integrin, CD11b/CD18 (Mac-1), which is expressed on phagocytic leukocytes, has been shown to increase the potency of ACT by an order of magnitude and has been considered a receptor for ACT (19). Accordingly, most of the studies on the functional, cytotoxic effects of ACT have focused on CD11b/CD18-positive cells, beginning with the initial observation that the oxidative burst of human neutrophils is inhibited by ACT (20). Mucosal epithelial cells that initially defend against infection with Bordetella pertussis, however, do not express CD11b/CD18. The functional and physiologic consequences of intoxication of epithelial cells, or any cell type lacking CD11b/CD18, are not well characterized.
In the present work, we use electrophysiology to measure cAMP-dependent chloride secretion across an epithelial monolayer as a model to investigate the ACT-cell interaction leading to intoxication. Monolayers of polarized T84 epithelial cells exhibit a cAMP-dependent Cl− secretory response that is quantified as short circuit current (Isc). The cell line has been used extensively to study the mechanisms and consequences of intoxication by cholera toxin, as well as to define the physiology of Cl− secretion in the intestinal epithelium (21–23). The responsiveness of T84 monolayers to real time changes in cAMP provides the opportunity to examine the mechanism of intoxication by ACT with a high level of sensitivity and temporal resolution not provided by biochemical assays for cAMP. It also provides a model of ACT activity at mucosal surfaces where infection with B. pertussis starts.
We find, in these studies, that the apical membrane of polarized T84 cells almost completely resists intoxication by ACT. The basolateral membrane, however, allows for efficient translocation of the catalytic domain of ACT into the cytosol. The same polarity of intoxication is seen in human respiratory epithelium organ cultures, showing that the efficiency of basolateral entry can be generalized to other mucosal surfaces. We also find that toxicity does not require membrane pore formation, membrane potential as a driving force, or post-translational acylation. Remarkably, basolateral sensitivity is not explained by the polarized expression of a specific membrane receptor.
EXPERIMENTAL PROCEDURES
Materials
All of the reagents, unless otherwise stated, were purchased from Sigma. Cholera toxin was obtained from Calbiochem-Behring Corp. (San Diego, CA). Protective antigen and edema factor from Bacillus anthracis were graciously provided by Dr. R. John Collier (Harvard Medical School). The monoclonal antibody to ACT, 3D1, was produced as described previously (24), as was the polyclonal antibody to ACT. The monoclonal antibody to CD11b (M1/70) for the blocking experiment in supplemental Fig. S2 and isotype control were purchased from BD Pharmingen. The monoclonal antibody to CD11b clone 44a (ATCC) was used for Western blotting.
Production and Purification of ACT
E. coli XL-1 Blue cells (Stratagene, La Jolla, CA) containing the appropriate plasmid construct (wild type ACT, nonacylated ACT, ΔN489, detoxAC) were used for toxin production as described previously (24, 25). Cultured bacteria were centrifuged, and the resulting pellet was resuspended in 50 mm Tris, pH 7.5, sonicated, and extracted with 8 m urea. Urea-extracted ACT was purified on a DEAE ion exchange column and a calmodulin affinity column as described previously (26). ACT was stored at −70 °C in 8 m urea, 10 mm Tricine, 0.5 mm EDTA, 0.5 mm EGTA, pH 8.0. For experiments containing deletion mutant ΔN489 urea-extracted toxin was used (24).
Cell Culture and Electrophysiology
T84 cells obtained from ATCC were cultured on permeable supports, and measurements of (Isc) were performed with 0.33-cm2 monolayers as described previously (22, 23). Transepithelial resistance of >1000 Ω were achieve in ∼8 days when 3.5 × 104 were seeded onto a support membrane. The measurements of monotypic channel functions in amphotericin permeabilized T84 monolayers were as described previously (27). All of the measurements in the illustrated time courses are representative of at least three independent experiments. Urea, added at the concentration equivalent to 10 μg/ml ACT, did not change the Isc (not shown).
Human tracheobronchial epithelial cells were obtained from specimens resected during lung transplantation, using a protocol approved by the University of North Carolina Institutional Review Board and the University of North Carolina Cystic Fibrosis Center Tissue Culture Core, and then cultured as described previously (28). The cells were derived from normal single patient sources and were expanded onto permeable Transwell-Col (12-mm diameter) supports. At confluency, human airway epithelium cultures, also referred to as human respiratory epithelium organ cultures, were grown with an air-liquid interface for 4–6 weeks to generate well differentiated, polarized cultures that resembled in vivo pseudostratified mucociliary epithelium. Human airway epithelia were mounted in Ussing chambers, and chloride secretion was measured as the transepithelial potential difference (PD/Rt = Isc) across the monolayers (29), where Rt represents transepithelial resistance. The cultures were studied under open circuit conditions using a Physiologic Instruments Voltage Clamp (San Diego, CA). PD was recorded continually, and a constant current pulse of 2–10 μA was applied at 1-min intervals to calculate resistance. The serosal side of human airway epithelium was bathed in Krebs bicarbonate ringer solution, and the lumenal side in high potassium low chloride solution. The CHO cells expressing CD11b/CD18 or containing a neomycin resistance vector were obtained as a kind gift from Dr. Douglas Golenbock and grown as described (30).
Measurement of Intracellular cAMP
T84 cells were grown on permeable supports under the conditions described for electrophysiologic measurements. Growth medium was removed, and ACT in Hanks' balanced salt solution with 0.1% human albumin was added at the indicated concentrations to either the apical or basolateral chamber of the support. The cells were then incubated for 30 min at 37 °C and washed, and cAMP was measured using the Tropix enzyme-linked immunosorbent assay-based cAMP assay kit. Raw cAMP values were adjusted for total cellular protein.
For experiments testing inhibition of intoxication, an ACT mutant protein lacking enzymatic activity (detoxAC) at the indicated concentrations was added to the indicated surface of the monolayer and incubated at 37 °C for 15 min. Wild type ACT was then added in the continuous presence of detoxAC. The cells were washed, and intracellular cAMP was measured as described above. When measuring intoxication, an equal volume of urea buffer (used for extraction of ACT during preparation as described above) was added to cells in place of detoxAC in the control samples because the urea buffer affects the absolute value of intoxication of some cell types, whereas it does not affect enzymatic activity, cell association, or electrophysiologic measurements (data not shown).
Immunofluorescence
T84 cells were grown on permeable supports under the conditions described for electrophysiologic measurements. Growth medium was exchanged for Hanks' balanced salt solution with 10% heat-inactivated fetal bovine serum with the indicated concentration of ACT added. The cells were incubated at 37 °C for 30 min, then moved to 4 °C, and washed, and polyclonal antibody to ACT (31) was added at 1:200. The cells were fixed in 4% paraformaldehyde. After permeabilizing with 0.2% saponin and blocking with goat serum, anti-human ZO-1 antibody (Invitrogen) was added at 1:200. Alexafluor 488-conjugated goat anti-rabbit and Alexafluor 555-conjugated goat anti-mouse secondary antibodies were used as secondary antibodies at 1:200. 4′,6′-Diamino-2-phenylindole (Invitrogen) was used for counterstaining, and inserts were cut and mounted on glass slides using Slowfade Gold mounting reagent (Invitrogen). The cells were imaged by confocal microscopy on a Nikon TE2000 inverted microscope (Nikon Instruments, Melville, NY) coupled to a PerkinElmer Life Sciences, spinning disk confocal unit, using a Nikon PlanFluor 40 × (1.3 NA) oil immersion objective lens and an Orca AG scientific-grade cooled CCD camera (Hamamatsu Photonics K.K., Japan). For confocal images requiring visualization of 4′,6′-diamino-2-phenylindole, a Zeiss LSM510 Axiovert inverted microscope containing a purple diode laser was used, and the images were analyzed by Zeiss LSM Image Browser software and Image J. Adjustments to brightness, contrast, and color balance were applied to the entire image.
Immunoblotting
T84 cells were grown as a confluent monolayer for >8 days (time for differentiation on Transwell inserts), or CHO cells were grown to confluence on tissue culture-treated flasks, and each cell type was scraped into lysis buffer (10 mm HEPES, pH 7.4, 150 mm NaCl, 10 mm EDTA, 4 mm EGTA, 1% Triton X-100). The protein was measured, and then reducing sample buffer was added followed by boiling. Whole cell lysates were added in equal protein amounts and separated by 10% SDS-PAGE gel, followed by transfer to polyvinylidene difluoride membranes. The membranes were blocked (phosphate-buffered saline, 1% Tween, 5% skim milk) and then incubated with primary mouse anti-human monoclonal antibody to CD11b (44a; ATCC). The membrane was washed, incubated with secondary antibody conjugated to horseradish peroxidase at 1:1000, and developed by chemiluminescence assay system (Pierce).
Adenylate Cyclase Enzymatic Activity
Enzymatic activity was measured by conversion of [α-32P]ATP to [32P]cAMP in a cell-free assay as described previously (2). Briefly, the reaction was carried out at 30 °C and continued for 10 min in a final volume of 60 μl. Each assay contained 60 mm Tricine, pH 8.0, 10 mm MgCl, 1 mm ATP (with 2 × 105–5 × 105 cpm of [α-32P]ATP), and 1 μm calmodulin. The reaction was terminated by the addition of 100 μl of a solution containing 1% SDS, 20 mm ATP, and 6.25 mm cAMP (including 15,000–20,000 cpm of [3H]cAMP/tube for calculation of recovery).
For experiments addressing blocking of cell association by detoxAC, the conditions were as described for measurement of inhibition of intoxication as described above. Again, wild type ACT was added in the continuous presence of detoxAC. Under these conditions, urea did not affect the degree of cell association.
RESULTS
Rapid Induction of Isc by ACT at the Basolateral Surface but Not the Apical Surface of the T84 Monolayer
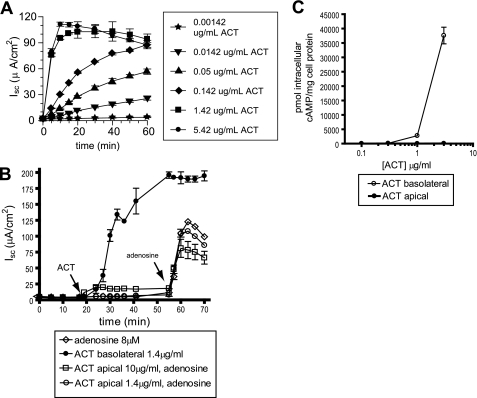
Application of ACT to the basolateral surface of a polarized T84 monolayer results in concentration-dependent, transepithelial chloride secretion, measured as short circuit current, Isc (Fig. 1A). There is little or no response to ACT applied to the apical surface of the monolayer (Fig. 1B). The subsequent addition of adenosine causes a robust increase in Isc, demonstrating that these cells are intact and able to secrete Cl− (32). The differential response of the two surfaces to ACT can also be measured biochemically as ACT-catalyzed intracellular cAMP accumulation (Fig. 1C). ACT added at 1.42 μg/ml (8.5 nm) induces a maximal Isc because the toxin-generated intracellular cAMP saturates the cellular mechanisms of chloride secretion in T84 cells.
FIGURE 1.
Effects of concentration, time, and monolayer polarity on the ACT-induced chloride secretory response and ACT-generated intracellular cAMP in T84 monolayers. A, Isc measured after ACT added to the basolateral surface at the indicated concentrations. B, Isc measured after ACT added to the apical or basolateral surface or adenosine to the apical surface at the indicated concentrations. C, ACT-induced intracellular cAMP when ACT is added to either the apical or basolateral surface of the monolayer.
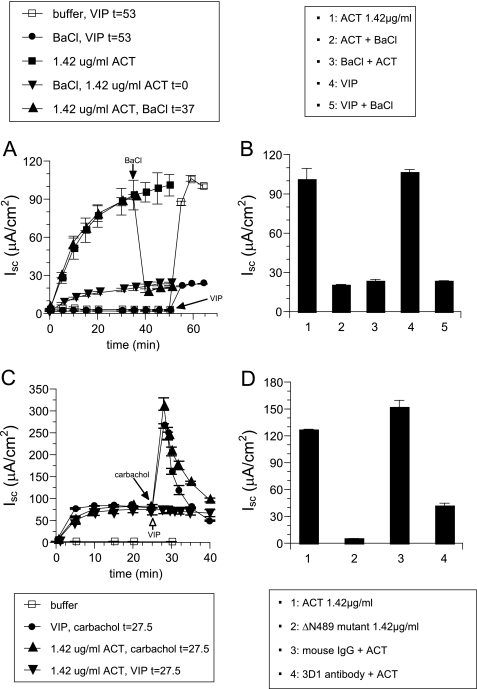
To show that ACT-induced Isc results from translocation of the ACT catalytic domain into the cytosol and subsequent production of intracellular cAMP and not from other functions of ACT that could induce Isc, such as oligomeric pore formation or Ca2+ influx (33), we examined the dependence of toxin-induced Isc on the cAMP-gated K+ channel KCNQ1/KCNE3. This channel is required for the cAMP-induced Isc and is strongly inhibited by Ba2+ (34). Fig. 2(A and B) shows that Ba2+ inhibits Isc induced by both ACT and the cAMP-agonist vasoactive intestinal peptide (VIP), which is used as a positive control (22). Ba2+ inhibits ACT-induced Isc even when applied after the addition of toxin. The inability of VIP to increase the Isc elicited by a maximal concentration of ACT (Fig. 2C) is further evidence that increased intracellular cAMP is responsible for the ACT-induced chloride response. On the other hand, carbachol, which induces Isc by increasing the intracellular Ca2+ concentration (21), acts synergistically with cAMP and causes a rapid and robust increase in maximal Isc after the response from the addition of ACT or VIP (Fig. 2C). These data show that the Cl− secretion induced by ACT results from toxin-generated intracellular cAMP and not from transmembrane pore formation or Ca2+ influx.
FIGURE 2.
Increases in Isc specifically caused by translocation of the catalytic domain of ACT and stimulation of cAMP-dependent chloride secretion. A, Isc measured after addition of ACT (1.42 μg/ml) or VIP (1 nm) with or without BaCl2 (3 mm). B, measurement at final time point of Isc stimulated by ACT (1.42 μg/ml) alone (column 1), ACT added before BaCl2 (column 2), ACT added after BaCl2 (column 3), VIP (1 nm; column 4), and VIP added before BaCl2 (column 5). C, Isc measured after addition of ACT (1.42 μg/ml) or VIP (1 nm) with or without subsequent addition of carbachol (10 μm). D, Isc measured after addition of ACT (1.42 μg/ml; column 1), ΔN489 (1.42 μg/ml; column 2), ACT incubated with mouse whole IgG control (column 3), and ACT incubated with mouse mAb 3D1 (column 4).
As an additional control to assure that the ACT-generated transmembrane pore is not contributing to the Isc elicited by toxin addition, we used an ACT protein (ΔN489) that lacks the catalytic domain and is unable to catalyze the production of cAMP from ATP (24). This mutant toxin also exhibits greater pore-forming activity than native ACT, as measured by its hemolytic potency. As shown in Fig. 2D, ΔN489 does not induce Isc when applied to the basolateral membranes of T84 cells. Similarly, the monoclonal antibody 3D1, which prevents delivery of the catalytic domain, also blocks the ACT-induced Isc (24). Together, these data establish definitively that it is the production of cAMP by ACT, not any of its other functions, that is responsible for the resultant Isc.
The increase in Isc begins within seconds after the addition of ACT, an observation made possible by the real time measurements of the T84 monolayer system. The rapidity is consistent with the concept of direct translocation of the ACT catalytic domain across the membrane and is almost equivalent in tempo to the response to VIP, a stimulator of endogenous adenylyl cyclase. In contrast, the Isc response to other cAMP-producing toxins, cholera toxin of Vibrio cholerae and edema toxin of B. anthracis, is delayed because of the intracellular trafficking required for the activity of these proteins (supplemental Fig. S1).
Polarized Sensitivity of Human Respiratory Epithelial Cultures to ACT
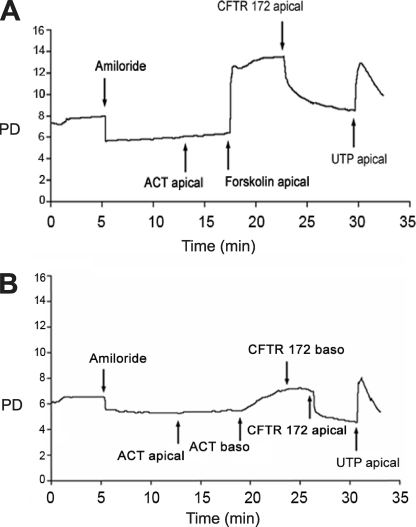
To test whether the finding of selective sensitivity of the basolateral membrane of T84 monolayers to ACT could be generalized to the respiratory epithelium, we measured the electrophysiologic response of human airway epithelial cultures to ACT. To prepare the airway epithelial layers, cells from human tissue specimens are grown on collagen-coated semi-permeable supports, resulting in an in vitro pseudostratified mucociliary epithelium that recapitulates in vivo morphology (28). Supports are mounted in Ussing chambers in which chloride secretion is measured as PD. The respiratory epithelium is first treated with amiloride to block Na+ transport, resulting in a decrease in PD to a new steady state (35). The addition of ACT (0.95 μg/ml) to apical membranes does not induce a change in PD (Fig. 3A). Subsequent addition of the cAMP agonist, forskolin, however, does cause an increase in PD. The addition of CFTR(inh)-172, a thiazolidinone that blocks transport across the apical cAMP-dependent chloride channel, CFTR, confirms that PD reflects chloride current (36). The nucleotide UTP activates Ca2+-dependent/CFTR-independent Cl− secretion and is added as an additional control. Fig. 3B shows a similar experiment in which there is no response to apically added ACT (0.95 μg/ml), but basolateral addition of ACT results in an increase in PD. As confirmed by apical (but not basolateral) addition of CFTR(inh)-172, the change in PD results from cAMP-dependent Cl− secretion. Thus, at this concentration, ACT intoxicates human respiratory epithelium only when added at the basolateral surface.
FIGURE 3.
Polarized sensitivity of human respiratory epithelium organ cultures to ACT. Treatments were added to the specified sides of the epithelia. Forskolin (1 × 10−5 m), a cell-permeable activator of cellular adenylyl cyclase, was used as a positive control. Amiloride (1 × 10−4 m), a sodium channel blocker, was used as a control for specificity of PD to chloride secretion. UTP (1 × 10−4 m) activates calcium dependent/CFTR-independent chloride secretion and was used as a positive control. CFTR(inh)-172 (1 × 10−5 m) is a specific inhibitor of the apically located, cAMP-dependent CFTR. A, no chloride response after apical addition of ACT (0.95 μg/ml) but response to apically added forskolin. B, no chloride response after apical addition of ACT (0.95 μg/ml) but response to basolaterally added ACT.
Association of ACT with the Basolateral and Apical Surfaces of the T84 Monolayer
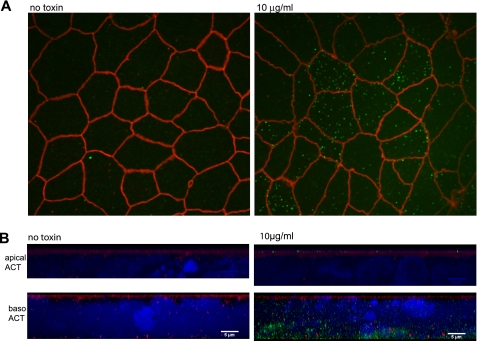
We next used epifluorescence confocal microscopy to determine whether the differential sensitivity of apical and basolateral membranes of the T84 monolayer to ACT can be explained by a failure of toxin to associate with the apical surface. Here, live cells were incubated with ACT applied either apically or basolaterally at 37 °C for 30 min. After immersion in fresh buffer at 4 °C, the monolayers were immunostained for ACT with a rabbit polyclonal anti-ACT antibody. The cells were washed again at 4 °C, fixed, permeabilized with 0.2% saponin, and immunostained for the tight junction associated protein ZO-1 to mark the apical membrane. Because the cells were intact during immunostaining, ACT identified by labeling with the antibody can only be associated with the outer surfaces of the cells. Fig. 4A shows that ACT applied to the apical surface (10 μg/ml) appears as distinct puncta at the apical surface of the T84 monolayer. There was no association detectable by epifluorescence microscopy at 1 μg/ml (data not shown). Shown in Fig. 4B are lateral views of the monolayer with full depth-of-field focused reconstructions generated from compilation of 0.3-μm Z-sections of the monolayer. These collapsed views show that ACT associates with the cell membranes whether added apically (Fig. 4B, top panels) or basolaterally (Fig. 4B, bottom panels). The signal from ACT associated with the basolateral membrane is greater than that associated with the apical membrane. This may be either due to enhancement of association of ACT with cell membranes by components of the basolateral surface or due to the larger cell membrane surface area exposed to ACT when added to the basolateral surface. On both surfaces, ACT forms clusters under these staining conditions.
FIGURE 4.
ACT associates with both surfaces of the T84 monolayer. A, immunofluorescent staining of the apical surface of a polarized T84 monolayer grown on Transwell insert. Tight junctions (red) are labeled by mouse monoclonal antibody to ZO-1. ACT 10 μg/ml is labeled with rabbit polyclonal antibody to ACT (green). Shown is a Z-section of the apical surface of the monolayer. B, shown are full-transparency, lateral views of three-dimensional reconstructions of monolayers imaged by 0.3-μm Z-sections. Immunofluorescent staining of monolayers after either apical (apical ACT) or basolateral (baso ACT) addition of ACT. ACT 10 μg/ml was added to either side of the monolayer (right) or not added (left) and stained with polyclonal antibody to ACT (green) prior to fixation or permeabilization. Tight junctions are labeled by monoclonal antibody to ZO-1 (red). The nuclei are stained with 4′,6′-diamino-2-phenylindole.
Association of ACT with apical or basolateral membranes was not detected by epifluorescence microscopy when ACT was added to cells at 4 °C, suggesting either a low affinity binding mechanism or a requirement for membrane rearrangement that is inhibited by low temperatures. Cell-associated ACT was also not detected when fixed and permeabilized with saponin prior to immunostaining for ACT. This suggests that saponin extracts membrane-associated toxin, possibly because of interaction of ACT with membrane lipids.
To test whether ACT interacts with a specific receptor at the apical membrane, we used detoxAC (13, 37) as a competitive inhibitor. In these studies, detoxAC was applied to the apical surface of the T84 monolayer at 37 °C for 15 min. Subsequently, a range of concentrations of wild type ACT was added at 37 °C for 30 min in the continuous presence or in the absence of detoxAC. The cells were washed to remove free toxin, and cell association was measured by in vitro assay for adenylate cyclase enzymatic activity from whole cell lysates (the values were corrected for background endogenous cellular adenylyl cyclase activity). This sensitive technique has been used as a quantitative measure of cell association of ACT with multiple other cell types (25, 38, 39), and cell-associated ACT is detectable by this assay when cells are exposed to 300 ng/ml of toxin. In the presence of excess detoxAC (30 μg/ml), apically associated ACT is not displaced. Thus, toxin association with the apical surface is not consistent with binding to a specific receptor.
To test for specific binding of ACT to the basolateral surface, we first confirmed that T84 cells do not express CD11b (supplemental Fig. S2). We then tested whether detoxAC inhibits intoxication of polarized T84 cells when ACT is added to the basolateral monolayer surface. Intoxication (intracellular cAMP) was measured because ACT adheres to the polyester support underlying the basolateral cell surface, preventing accurate measurement of enzymatic activity when ACT was added basolaterally. We found that in the continuous presence of excess detoxAC (or urea as a vehicle control), intoxication by ACT is not competitively inhibited (Fig. 5B). The results are represented, here, in log scale for clarity of comparison, rather than linear scale as shown in Fig. 1C.
FIGURE 5.
detoxAC does not compete for association of ACT with the apical surface nor intoxication of the basolateral surface by ACT. A, association of ACT with the apical surface measured by retained enzymatic activity after addition of ACT followed by washing. ACT was added in the continuous presence or absence of detoxAC (30 μg/ml). B, intracellular cAMP measured after addition of ACT to the basolateral surface in the continuous presence of detoxAC (20 μg/ml) or urea solvent. C, intracellular cAMP was measured after addition of ACT to CHO cells expressing CD11b/CD18 in the continuous presence or absence of detoxAC (20 μg/ml) or urea.
As a positive control for the competition experiments represented in Fig. 5B, we show that detoxAC does inhibit intoxication of CHO cells expressing CD11b/CD18 (Fig. 5C). As a positive control for the experiments represented in Fig. 5A, we have also confirmed that inhibition of intoxication of these CHO cells is due to displacement of cell-associated enzymatic activity (not shown). Thus, although ACT appears to associate with both apical and basolateral membranes of T84 cells, we believe that the very limited quantity detected on the apical surface is not pathophysiologically relevant. Most importantly, the mechanism of association of ACT with the basolateral surface, in contrast to that seen in CHO cells expressing CD11b/CD18, is not consistent with binding to a specific receptor.
Translocation across the Basolateral Surface Independent of Acylation of the Toxin or Membrane Potential
Translocation of ACT has been shown, in prior studies, to be driven by membrane potential and affected by post-translational acylation of the toxin molecule. Otero et al. (41) have demonstrated by patch clamping of frog cardiac myocytes and measurement of calcium current through cell type-specific cAMP-stimulated calcium channels that translocation by ACT depends on an inwardly directed, negative membrane potential. Similarly, insertion of a negatively charged peptide into the catalytic domain of ACT prevents translocation across the cytoplasmic membrane of sheep erythrocytes (42). To address the charge dependence of ACT translocation across the basolateral membrane of polarized T84 cells, we applied a voltage clamp to T84 cell basolateral membranes at the desired membrane potential and measured the Isc response to ACT. The basolateral membrane was electrically isolated by selectively permeabilizing the apical membrane of the T84 monolayer to monovalent ions by application of amphotericin B using buffers containing K+ as the primary charge-carrying cation (see “Experimental Procedures”). Under these conditions, the transepithelial Isc recorded represents K+ transport across the basolateral membrane (termed Isc-blK) (27). Because the ACT-induced Isc in T84 cells requires the activation of the basolateral cAMP-gated K+ channel (Fig. 2), translocation of toxin across the basolateral membrane can be measured in this permeabilized cell system under defined basolateral membrane potential as a toxin-induced Isc-blK. We find that ACT translocates across the basolateral membranes to induce an increase in Isc-blK with transmembrane potential held over the full range of physiologic potentials: −35, 0, or 35 mV (Fig. 6, A–C). Thus, the basolateral membranes of polarized T84 cells allow translocation even under depolarized conditions.
FIGURE 6.
Voltage independence of translocation and response to nonacylated ACT at the basolateral surface of the T84 monolayer. A–C, Isc measured following addition of ACT (1.42 μg/ml) after clamping at +35 (A), 0 (B), and −35 mV (C). For all figures, the apical membrane was permeabilized with 20 μm amphotericin B in buffer containing K+ as the primary charge-carrying ion, and then the voltage across the basolateral membrane was clamped as indicated. D, Isc measured after addition of ACT (1.42 μg/ml) or nonacylated ACT (1.4 μg/ml). VIP was added to calibrate the response.
Post-translational, covalent acylation by the acyl-transferase CyaC is required for hemolytic activity and for fully functional translocation/intoxication by ACT, although it is not required for in vitro enzymatic activity (13, 25, 43–45). J774 cells express CD11b/CD18 and are intoxicated by nonacylated ACT, but with a 50-fold reduction in toxin potency. Cell lines that lack CD11b/CD18 expression are resistant to intoxication by nonacylated ACT (13, 25, 44). When applied to basolateral membranes of T84 cells, however, nonacylated ACT induces chloride secretion (Fig. 6D). Thus, unlike other cell lines lacking CD11b/CD18 expression, T84 cell monolayers are susceptible to intoxication by nonacylated ACT added to the basolateral surface, providing further evidence that the basolateral surface is atypically permissive to toxin translocation.
DISCUSSION
The results of these studies show that ACT can intoxicate polarized epithelial cells, including those that line the human respiratory tract. Although the mucosal surface of epithelial monolayers resists intoxication, the basolateral surface is highly sensitive. Differential sensitivity across epithelial monolayers has been observed for other bacterial toxins. Intoxication of epithelial cells by edema toxin has been shown, using the T84 monolayer system, to occur selectively from the basolateral surface (46) because of basolateral sequestration of the edema toxin receptor. Glycophosphatidylinositol-linked proteins are greatly enriched at the apical surface (47); some toxins, such as aerolysin of Aeromonas hydrophila and α-toxin of Clostridium septicum (48, 49), use these glycophosphatidylinositol-linked proteins as their receptors and have different effects on polarized epithelial monolayers (Madin-Darby canine kidney and Fisher rat thyroid cells) depending on the side of the monolayer exposed to toxin and the distribution of glycophosphatidylinositol-linked proteins (50). The enterotoxin of Clostridium perfringens, which associates with and changes the function of claudins, decreases transepithelial resistance from the basolateral surface of polarized Madin-Darby canine kidney monolayers but not from the apical surface (51).
As our findings demonstrate, however, the interaction between ACT and the components of the T84 monolayer basolateral membrane cannot be explained by basolateral sequestration of a specific receptor. Our results suggest that ACT may interact with membrane lipids to translocate its catalytic domain into the cytoplasm. An interaction with lipids would be consistent with our findings that ACT association with the membrane was reduced by cold temperatures and by application of saponin. It is known that ACT is functional in artificial lipid bilayers and liposomes in the absence of proteins, and the interaction is affected by lipid/glycolipid composition (11, 15, 16). Lipid membrane composition of whole cells also affects the function and surface distribution of other pore-forming RTX toxins, such as the leukotoxins of Mannheimia hemolytica (52) and A. actinomycetemcomitans (53). There are dramatic differences in lipid composition of the apical and basolateral membranes of epithelial cells (54), with the apical membrane characterized by different lipid species and a more ordered, or rigid, lipid composition.
Extracellular factors may also contribute to the polarity of intoxication by ACT. The luminal surfaces of respiratory and gastrointestinal epithelia are coated by a mass of glycoproteins, the glycocalyx, which is present on the apical surface of mature T84 monolayers (55) and respiratory epithelium organ cultures. The glycocalyx serves as a barrier to multiple pathogens in the gastrointestinal tract (56) and to adenovirus in the respiratory tract (57, 58). It has recently been reported by Morova et al. (59) that N-linked glycosylation is required for ACT toxicity in CHO cells expressing CD11b/CD18 and that excess sialic acid and other oligosaccharides strongly inhibit the association of ACT with the surfaces of cells expressing CD11b/CD18. Thus, it is possible that complex oligosaccharides of the glycocalyx may bind to ACT and block intoxication by presenting “nonfunctional” binding sites distant from the apical membrane.
ACT is secreted at the luminal surface of the airway epithelium during infection with B. pertussis, which adheres to the bases of cilia (60–64). Human pathologic specimens from children who have died from severe pertussis show extensive denudation of the epithelium, filling of the alveoli with copious fluid, and a robust inflammatory response to infection (65, 66). The disruption of the mucosal surface would expose the basal side of the epithelium to ACT. Subsequent intoxication of the epithelial cells, resulting in chloride secretion, would then contribute to the accumulation of bronchoalveolar fluid that is characteristic of severe pertussis. Furthermore, intoxication of epithelial cells by ACT may contribute to pulmonary inflammation. B. pertussis, only when expressing ACT, stimulates release of the proinflammatory cytokine, interleukin-6, from nonpolarized human tracheal epithelial cells (67).
Multiple cell types will be exposed to ACT during infection with B. pertussis. We show, for the first time, that mucosal epithelial cells are a physiologically relevant target of ACT-induced toxicity in the absence of CD11b/CD18 or another specific receptor. Although CD11b/CD18 expression greatly augments the intoxication of cells by ACT, it is clearly not required for effective intoxication. We suggest that CD11b/CD18 enhances translocation by altering an underlying noncompetitive mechanism of cell association, and studies are underway to further characterize the interaction of ACT with other cell types not expressing CD11b/CD18.
Supplementary Material
Acknowledgments
We greatly appreciate expert assistance with microscopy by Dr. Ramiro Massol and Jessica Wagner.
This work was supported, in whole or in part, by National Institutes of Health Grants AI18000 (to E. L. H.), 5 T32 A1007046–30 (to J. C. E.), DK48106 (to W. I. L.), DK34854 (to W. I. L.), and SCCOR 5 P50 HL084934 (to R. J. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- ACT
- adenylate cyclase toxin
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- PD
- potential difference
- CHO
- Chinese hamster ovary
- CFTR
- cystic fibrosis transmembrane regulator.
REFERENCES
- 1.Wolff J., Cook G. H. (1973) J. Biol. Chem. 248, 350–355 [PubMed] [Google Scholar]
- 2.Goldhammer A. R., Wolff J., Hope Cook G., Berkowitz S. A., Klee C. B., Manclark C. R., Hewlett E. L. (1981) Eur. J. Biochem. 115, 605–609 [DOI] [PubMed] [Google Scholar]
- 3.Pearson R. D., Symes P., Conboy M., Weiss A. A., Hewlett E. L. (1987) J. Immunol. 139, 2749–2754 [PubMed] [Google Scholar]
- 4.Hanski E., Farfel Z. (1985) J. Biol. Chem. 260, 5526–5532 [PubMed] [Google Scholar]
- 5.Gordon V. M., Leppla S. H., Hewlett E. L. (1988) Infect. Immun. 56, 1066–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch R. A. (1991) Mol. Microbiol. 5, 521–528 [DOI] [PubMed] [Google Scholar]
- 7.Lally E. T., Hill R. B., Kieba I. R., Korostoff J. (1999) Trends Microbiol. 7, 356–361 [DOI] [PubMed] [Google Scholar]
- 8.Vojtova-Vodolanova J., Basler M., Osicka R., Knapp O., Maier E., Cerny J., Benada O., Benz R., Sebo P. (2009) FASEB J. 23, 2831–2843 [DOI] [PubMed] [Google Scholar]
- 9.Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. (1983) Infect. Immun. 42, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. (1988) EMBO J. 7, 3997–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabo G., Gray M. C., Hewlett E. L. (1994) J. Biol. Chem. 269, 22496–22499 [PubMed] [Google Scholar]
- 12.Sakamoto H., Bellalou J., Sebo P., Ladant D. (1992) J. Biol. Chem. 267, 13598–13602 [PubMed] [Google Scholar]
- 13.Hewlett E. L., Donato G. M., Gray M. C. (2006) Mol. Microbiol. 59, 447–459 [DOI] [PubMed] [Google Scholar]
- 14.Basler M., Masin J., Osicka R., Sebo P. (2006) Infect. Immun. 74, 2207–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benz R., Maier E., Ladant D., Ullmann A., Sebo P. (1994) J. Biol. Chem. 269, 27231–27239 [PubMed] [Google Scholar]
- 16.Gordon V. M., Young W. W., Jr., Lechler S. M., Gray M. C., Leppla S. H., Hewlett E. L. (1989) J. Biol. Chem. 264, 14792–14796 [PubMed] [Google Scholar]
- 17.Vojtová J., Kofronová O., Sebo P., Benada O. (2006) Microsc. Res. Tech. 69, 119–129 [DOI] [PubMed] [Google Scholar]
- 18.Knapp O., Maier E., Masín J., Sebo P., Benz R. (2008) Biochim. Biophys. Acta 1778, 260–269 [DOI] [PubMed] [Google Scholar]
- 19.Guermonprez P., Khelef N., Blouin E., Rieu P., Ricciardi-Castagnoli P., Guiso N., Ladant D., Leclerc C. (2001) J. Exp. Med. 193, 1035–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Confer D. L., Eaton J. W. (1982) Science 217, 948–950 [DOI] [PubMed] [Google Scholar]
- 21.Barrett K. E. (1997) Am. J. Physiol. 272, C1069–C1076 [DOI] [PubMed] [Google Scholar]
- 22.Lencer W. I., Delp C., Neutra M. R., Madara J. L. (1992) J. Cell Biol. 117, 1197–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dharmsathaphorn K., Madara J. L. (1990) Methods Enzymol. 192, 354–389 [DOI] [PubMed] [Google Scholar]
- 24.Gray M. C., Lee S. J., Gray L. S., Zaretzky F. R., Otero A. S., Szabo G., Hewlett E. L. (2001) J. Bacteriol. 183, 5904–5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewlett E. L., Gray M. C., Ehrmann I. E., Maloney N. J., Otero A. S., Gray L., Allietta M., Szabo G., Weiss A. A., Barry E. M. (1993) J. Biol. Chem. 268, 7842–7848 [PubMed] [Google Scholar]
- 26.Lee S. J., Gray M. C., Guo L., Sebo P., Hewlett E. L. (1999) Infect. Immun. 67, 2090–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rufo P. A., Merlin D., Riegler M., Ferguson-Maltzman M. H., Dickinson B. L., Brugnara C., Alper S. L., Lencer W. I. (1997) J. Clin. Invest. 100, 3111–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickles R. J., McCarty D., Matsui H., Hart P. J., Randell S. H., Boucher R. C. (1998) J. Virol. 72, 6014–6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grubb B. R., Pickles R. J., Ye H., Yankaskas J. R., Vick R. N., Engelhardt J. F., Wilson J. M., Johnson L. G., Boucher R. C. (1994) Nature 371, 802–806 [DOI] [PubMed] [Google Scholar]
- 30.Ingalls R. R., Arnaout M. A., Golenbock D. T. (1997) J. Immunol. 159, 433–438 [PubMed] [Google Scholar]
- 31.Gray M. C., Ross W., Kim K., Hewlett E. L. (1999) Infect. Immun. 67, 4393–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strohmeier G. R., Reppert S. M., Lencer W. I., Madara J. L. (1995) J. Biol. Chem. 270, 2387–2394 [DOI] [PubMed] [Google Scholar]
- 33.Fiser R., Masín J., Basler M., Krusek J., Spuláková V., Konopásek I., Sebo P. (2007) J. Biol. Chem. 282, 2808–2820 [DOI] [PubMed] [Google Scholar]
- 34.Schroeder B. C., Waldegger S., Fehr S., Bleich M., Warth R., Greger R., Jentsch T. J. (2000) Nature 403, 196–199 [DOI] [PubMed] [Google Scholar]
- 35.Clarke L. L., Chinet T., Boucher R. C. (1997) Am. J. Physiol. 272, L1084–L1091 [DOI] [PubMed] [Google Scholar]
- 36.Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002) J. Clin. Invest. 110, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osicka R., Osicková A., Basar T., Guermonprez P., Rojas M., Leclerc C., Sebo P. (2000) Infect. Immun. 68, 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bejerano M., Nisan I., Ludwig A., Goebel W., Hanski E. (1999) Mol. Microbiol. 31, 381–392 [DOI] [PubMed] [Google Scholar]
- 39.Gray M., Szabo G., Otero A. S., Gray L., Hewlett E. (1998) J. Biol. Chem. 273, 18260–18267 [DOI] [PubMed] [Google Scholar]
- 40.Deleted in proof
- 41.Otero A. S., Yi X. B., Gray M. C., Szabo G., Hewlett E. L. (1995) J. Biol. Chem. 270, 9695–9697 [DOI] [PubMed] [Google Scholar]
- 42.Karimova G., Fayolle C., Gmira S., Ullmann A., Leclerc C., Ladant D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 12532–12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basar T., Havlícek V., Bezousková S., Hackett M., Sebo P. (2001) J. Biol. Chem. 276, 348–354 [DOI] [PubMed] [Google Scholar]
- 44.Barry E. M., Weiss A. A., Ehrmann I. E., Gray M. C., Hewlett E. L., Goodwin M. S. (1991) J. Bacteriol. 173, 720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Azami-El-Idrissi M., Bauche C., Loucka J., Osicka R., Sebo P., Ladant D., Leclerc C. (2003) J. Biol. Chem. 278, 38514–38521 [DOI] [PubMed] [Google Scholar]
- 46.Beauregard K. E., Wimer-Mackin S., Collier R. J., Lencer W. I. (1999) Infect. Immun. 67, 3026–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lisanti M. P., Sargiacomo M., Graeve L., Saltiel A. R., Rodriguez-Boulan E. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 9557–9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abrami L., Fivaz M., van der Goot F. G. (2000) Trends Microbiol. 8, 168–172 [DOI] [PubMed] [Google Scholar]
- 49.Gordon V. M., Nelson K. L., Buckley J. T., Stevens V. L., Tweten R. K., Elwood P. C., Leppla S. H. (1999) J. Biol. Chem. 274, 27274–27280 [DOI] [PubMed] [Google Scholar]
- 50.Abrami L., Fivaz M., Glauser P. E., Sugimoto N., Zurzolo C., van der Goot F. G. (2003) Infect. Immun. 71, 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonoda N., Furuse M., Sasaki H., Yonemura S., Katahira J., Horiguchi Y., Tsukita S. (1999) J. Cell Biol. 147, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atapattu D. N., Czuprynski C. J. (2007) Infect. Immun. 75, 4719–4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fong K. P., Pacheco C. M., Otis L. L., Baranwal S., Kieba I. R., Harrison G., Hersh E. V., Boesze-Battaglia K., Lally E. T. (2006) Cell Microbiol. 8, 1753–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuck S., Simons K. (2004) J. Cell Sci. 117, 5955–5964 [DOI] [PubMed] [Google Scholar]
- 55.Ou G., Baranov V., Lundmark E., Hammarström S., Hammarström M. L. (2009) Scand. J. Immunol. 69, 150–161 [DOI] [PubMed] [Google Scholar]
- 56.Liévin-Le Moal V., Servin A. L. (2006) Clin. Microbiol. Rev. 19, 315–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stonebraker J. R., Wagner D., Lefensty R. W., Burns K., Gendler S. J., Bergelson J. M., Boucher R. C., O'Neal W. K., Pickles R. J. (2004) J. Virol. 78, 13755–13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pickles R. J., Fahrner J. A., Petrella J. M., Boucher R. C., Bergelson J. M. (2000) J. Virol. 74, 6050–6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morova J., Osicka R., Masin J., Sebo P. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 5355–5360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mallory F. B., Hornor A. A. (1912) J. Med. Res. 27, 115–124 [PMC free article] [PubMed] [Google Scholar]
- 61.Soane M. C., Jackson A., Maskell D., Allen A., Keig P., Dewar A., Dougan G., Wilson R. (2000) Respir. Med. 94, 791–799 [DOI] [PubMed] [Google Scholar]
- 62.Tuomanen E. I., Hendley J. O. (1983) J. Infect. Dis. 148, 125–130 [DOI] [PubMed] [Google Scholar]
- 63.Lovell M. A., Miller A. M., Hendley J. O. (1998) Arch. Pediatr. Adolesc. Med. 152, 925–926 [DOI] [PubMed] [Google Scholar]
- 64.Groathouse N. A., Heinzen R. A., Boitano S. (2003) Infect. Immun. 71, 7208–7210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawal M., Cohen M., Irazuzta J. E., Kumar R., Kirton C., Brundler M. A., Evans C. A., Wilson J. A., Raffeeq P., Azaz A., Rotta A. T., Vora A., Vohra A., Abboud P., Mirkin L. D., Cooper M., Dishop M. K., Graf J. M., Petros A., Klonin H. (2009) Pediatr. Pulmonol. 44, 970–980 [DOI] [PubMed] [Google Scholar]
- 66.Paddock C. D., Sanden G. N., Cherry J. D., Gal A. A., Langston C., Tatti K. M., Wu K. H., Goldsmith C. S., Greer P. W., Montague J. L., Eliason M. T., Holman R. C., Guarner J., Shieh W. J., Zaki S. R. (2008) Clin. Infect. Dis. 47, 328–338 [DOI] [PubMed] [Google Scholar]
- 67.Bassinet L., Fitting C., Housset B., Cavaillon J. M., Guiso N. (2004) Infect. Immun. 72, 5530–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.