Abstract
Cyclotides are a family of macrocyclic peptides that combine the unique features of a head-to-tail cyclic backbone and a cystine knot motif, the combination of which imparts them with extraordinary stability. The prototypic cyclotide kalata B1 is toxic against two economically important gastrointestinal nematode parasites of sheep, Haemonchus contortus and Trichostrongylus colubriformis. A lysine scan was conducted to examine the effect of the incorporation of positive charges into the kalata B1 cyclotide framework. Each of the non-cysteine residues in this 29-amino acid peptide was successively substituted with lysine, and the nematocidal and hemolytic activities of the suite of mutants were determined. Substitution of 11 residues within kalata B1 decreased the nematocidal activity dramatically. On the other hand, six other residues that are clustered on the surface of kalata B1 were tolerant to Lys substitution, and indeed the introduction of positively charged residues into this region increased nematocidal activity. This activity was increased further in double and triple lysine mutants, with a maximal increase (relative to the native kalata B1) of 13-fold obtained with a triple lysine mutant (mutated at positions Thr-20, Asn-29, and Gly-1). Hemolytic activity correlated with the nematocidal activity of all lysine mutants. Our data clearly highlight the residues crucial for nematocidal and hemolytic activity in cyclotides, and demonstrate that the nematocidal activity of cyclotides can be increased by incorporation of basic amino acids.
Keywords: Parasitology, Peptide Chemical Synthesis, Peptide Conformation, Peptide Interactions, Protein Structure, Haemonchus contortus, Trichostrongylus colubriformis, Cyclic Peptides, Cyclotides, Structure-Activity Relationships
Introduction
Gastrointestinal nematodes cause major losses to livestock industries worldwide. The control of these pests, until now, has been via synthetic anthelmintics, including benzimidazoles (e.g. thiabendazole), nicotinic acetylcholine receptor agonists (e.g. levamisole), and macrocyclic lactones (e.g. ivermectin and moxidectin). However, resistance of sheep and cattle nematodes to these broad-spectrum anthelmintics is a serious issue for livestock production globally (1–3). Thus, the development of novel agents with potent anthelmintic activity is of great economic and agricultural importance.
Cyclotides are circular miniproteins discovered originally in plants of the Rubiaceae, Violaceae, and Cucurbitaceae families (4, 5). Kalata B1, the first cyclotide to be structurally characterized, is abundant in the tropical African plant Oldenlandia affinis (6, 7) but has also been reported in the European Sweet Violet (Viola odorata) (8) and several other Viola species (9–13). Members of the cyclotide family possess a cyclic peptide backbone and a cystine knot motif, which is formed by three disulfide bonds at the core of their three-dimensional structure. The sequence and structure of kalata B1 is illustrated in Fig. 1, which highlights the six backbone loops between the six conserved cysteine residues that make up the cystine knot (14). The cyclic cystine knot motif is thought to be responsible for the extraordinary enzymatic and chemical stability (15) of the cyclotides. Cyclotides have a wide range of bioactivities, including anti-HIV (16–19), neurotensin antagonism (20), hemolytic (21), antimicrobial (22), anti-fouling (23), and pesticidal activities (24–29).
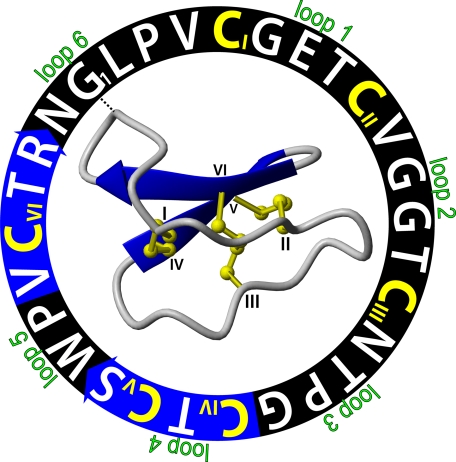
FIGURE 1.
Representation of the sequence and three-dimensional structure of kalata B1 (PDB ID 1nb1). The amino acid sequence of this 29-residue peptide is shown in a circle starting from G1. The backbone segments between the six cysteine residues (colored yellow with Roman numerals I-VI) are marked as loops 1–6. The blue arrows are β-strands in the cyclic backbone, indicating the direction of the peptide chain from amino to carboxyl ends. The cystine knot is formed by a disulfide bond (CIII to CVI) that penetrates a ring made by two more disulfide bonds (CI to CIV and CII to CV), and their connecting backbone segments in loops 1 and 4. The starting points of the sequence and ribbon structure are connected with a dashed line.
In a recent study (30), the anthelmintic activity of a range of cyclotides was demonstrated. Kalata B1, B2, B6, and B7, cyclotides extracted from O. affinis, significantly inhibited the development of the larval life stages of two sheep gastrointestinal nematodes Haemonchus contortus (H. contortus) and Trichostrongylus colubriformis, as well as inhibiting motility of adult H. contortus. A subsequent study reported enhanced nematocidal activity of other natural cyclotides possessing multiple basic residues (31). Cycloviolacin O2 (containing three basic residues) was found to be the most potent cyclotide tested against sheep parasites. The removal of charge through acetylation of lysine residues resulted in reduced anthelmintic activity, highlighting the importance of the positively charged residues in the anthelmintic activity of cyclotides.
Cyclotides have been proven to be amenable to chemical synthesis (22, 32, 33), and as a result, mutated cyclotides can be readily synthesized, enabling detailed studies into their structure-activity relationships. In the current study, a “lysine scan” of the prototypic cyclotide kalata B1 was conducted to understand the structural and functional role of positive charge at different sites within cyclotides. Each of the non-cysteine residues in this 29-amino acid peptide was successively replaced with lysine using solid-phase peptide synthesis, and the suite of mutants was assayed against H. contortus and T. colubriformis. In addition, select double and triple lysine mutants were designed and assayed. Many of the substitutions resulted in a total loss of activity, thus helping to define residues implicated in bioactivity, but some substitutions resulted in increased anthelmintic activity, which was further increased in some double and triple mutants.
Overall, this biochemical mutagenesis study has identified key residues in the prototypic cyclotide kalata B1 important for anthelmintic activity and demonstrated the ability to enhance activity through incorporation of positively charged residues into a localized region of the peptide. We refer to this localized region as the “amendable” face of cyclotides and describe a mechanistic model explaining its role in modulating cyclotide bioactivity.
EXPERIMENTAL PROCEDURES
Native Protein Purification
Native kalata B1 was isolated from the above ground parts of O. affinis and purified as described previously (34). The concentrations of peptide samples were determined spectrophotometrically using an extinction coefficient of 5875 m−1 cm−1 at 280 nm.
Synthesis of Lysine Mutants
The linear precursors of 23 lysine mutants of kalata B1 containing an N-terminal Cys residue were synthesized using manual solid-phase peptide synthesis with an in situ neutralization/HBTU protocol (35) for Boc chemistry on a 0.5 mm scale, using a procedure described previously (27, 33). Precursor peptides were assembled on PAM resin via a thioester linker and cleaved from the resin with hydrogen fluoride. In this approach, a linear precursor with a C-terminal thioester undergoes an irreversible S,N acyl migration to form a native peptide bond in the resultant cyclic protein (32, 36). Cyclization and oxidation of the mutants were performed by dissolving them (0.5 mg/ml) in a standard folding buffer comprising 50% isopropyl alcohol (v/v) in 0.1 m ammonium bicarbonate (pH 8.2) with 2 mm reduced glutathione and 0.4 mm oxidized glutathione added. The mixture was stirred for 48 h at room temperature and the peptide solutions were diluted with 0.1% trifluoroacetic acid and purified by reversed phase high performance liquid chromatography (RP-HPLC) on a Phenomenex C18 column. A 1%/min gradient of solvent B (90% acetonitrile/0.045% trifluoroacetic acid) against solvent A (100% H2O/0.05% trifluoroacetic acid) was used, and peptides were detected by monitoring UV absorbance at 215 nm.
The correctly folded mutants were identified by their late elution under reversed phase conditions and characterized by electrospray ionization mass spectrometry (ESI-MS). The purity of fractions was assessed using analytical HPLC with the same gradient of the solvents used for the previous steps. Correct folding of the peptides was confirmed by 1H NMR spectrometry. Samples for 1H NMR measurement contained 1–2 mm peptide in 90% H2O/10% D2O (v/v). One- and two-dimensional NMR spectra of mutants were recorded on Bruker Avance 500 MHz or 600 MHz spectrometers at 298 K as described previously (37, 38). An additional four mutants with two or three residues substituted with lysines were synthesized using the method described above. The names of these mutants are abbreviated for simplicity; for example, G6K refers to substitution of the Gly at position 6 with Lys.
Egg Extraction
Nematode embryos were prepared as described previously (30). Sheep infected with H. contortus (Kirby isolate) or T. colubriformis (McMaster isolate) were maintained at the CSIRO McMaster laboratory at Armidale, NSW, Australia. Both were susceptible strains to all commercial anthelmintics and had no history of exposure to anthelmintics. Sheep faeces containing eggs of H. contortus or T. colubriformis were sent by overnight courier to Brisbane, the eggs were recovered by passing the faeces through fine sieves (250 μm and 75 μm), and the sieved material was then centrifuged in a stepwise sucrose gradient (10, 25, and 40% sucrose). The eggs were collected from the interface between the 10 and 25% sucrose layers, and washed with water over a 25-μm sieve to remove residual sucrose. The eggs were diluted to obtain 50–60 eggs per 30 μl after the addition of fungizone (30 mg per ml of egg solution).
The Larval Development Assay
Larval development assays (LDAs)4 were conducted in a 96-well plate as described by Lacey et al. (39). After 200 μl of 2% agar was placed into each well and allowed to solidify, 30 μl of nematode egg solution was added to each well. Cyclotide solution was added into each of the wells in 10-μl aliquots at varying concentrations (2-fold serial dilutions). Each concentration was conducted in at least triplicate and controls for water (10 μl) were included in each assay. The final concentration range was 0.26–33 μg/ml (0.09–11.53 μm). LDA plates were incubated for ∼20 h at 25 °C to allow the eggs to hatch, and then 10 μl of growth medium was added to each well. The growth medium consisted of Earle's salt solution (10% v/v), yeast extract (1% w/v), sodium bicarbonate (1 mm), and saline solution (0.9% sodium chloride w/v) (40). The nematodes were allowed to feed and develop for 4 days, after which they were killed using Lugol's iodine solution and scored for the number of fully developed infective stage larvae (L3) present in each well.
Calculations of Percentage Inhibition
The percentage inhibition of larval development was determined using Equation 1.
Here A is the number of larvae that had grown into the infective stage (L3) in control incubations, and B is the number of L3 larvae in incubations treated with different concentrations of cyclotides. To compare the degree of inhibition of lysine mutants, the IC50 values (the concentration at which 50% of larvae develop to the L3 stage) of tested peptides were calculated using nonlinear regression (sigmoidal dose-response, GraphPad Prism). The 95% confidence intervals (95% CI) were also determined. The relative activity of lysine mutants was estimated using the IC50 value of kalata B1 divided by the IC50 value of mutants individually.
Hemolytic Assay
A hemolytic assay was conducted using a method described earlier (21, 41). Human red blood cells were separated from serum and washed in phosphate-buffered saline (PBS, pH 7.4) with 4–5 times centrifugation at 4000 rpm. Erythrocytes were then resuspended in phosphate-buffered saline as a 0.25% (v/v) solution. Stock solutions (300 μm) of lysine mutants and wild-type kalata B1 were prepared. 2-Fold serial dilutions were prepared yielding eight solutions in total, which were aliquoted (20 μl) into a 96-well plate. The erythrocyte solution (100 μl) was dispensed into each well and incubated with diluted peptide solution at 37 °C for 1 h. Triton X-100 solution (1%, v/v, 20 μl) was used as a positive control to represent the 100% lysis, and PBS solution (20 μl) was used as a negative control (spontaneous lysis after 1 h of incubation). The 96-well plate was centrifuged to pellet the intact red blood cells, and the supernatant of each well was measured by visual absorption spectroscopy at 415 nm. All peptide solutions were assayed in triplicate.
Adult Motility Assays
To assess the effect of lysine mutants on motility of adult H. contortus in vitro, a method modified from Colgrave et al. (30) was utilized. The recovery and culture methods for adult nematode were described by Kotze and McClure (42). Adult nematodes were harvested from the sheep abomasa and were recovered in culture medium (RPMI 1640 medium containing 0.8% glucose, 10 mm N-(2-hydroxy-ethyl)-piperazine-N′-2-ethanesulfonic acid, 20% bovine serum, antibiotics, and fungicide) for 1–2 h at 37 °C. Peptide solutions (kalata B1, T20K, V25K, N29K, G1K, T20K/N29K, and T20K/G1K) were prepared using serial dilution (two times dilution starting at 34.6 μm) with culture medium. Groups of 10 females were transferred to separate glass tubes containing peptide solution at different concentrations, and incubated at 37 °C in an atmosphere of 20% CO2, 5% O2, and 75% N2. The control groups were incubated in culture medium only, under the same atmospheric conditions. The degree of motility of nematodes was scored at 24-h intervals for 3 days. Before scoring, each of the assay tubes was swirled vigorously to disturb the nematodes. Their degree of motility was determined according to the 0–3 scoring system described by O'Grady and Kotze (43): 3: most individuals showing similar movement (smooth sinusoidal motion) as at the start of culture period; 2: at least one individual able to move in a normal sinusoidal fashion; and 1: only very limited movement (twitching) apparent in a small number of individuals, no movement in most worms, no sinusoidal motion observed; 0: no movement at all.
RESULTS
Synthesis of Lysine Mutants
Twenty-seven linear precursors of single (23), double (3), or triple (1) point-mutated lysine mutants of kalata B1 were synthesized using manual solid-phase peptide synthesis. After cleavage from the resin and purification with RP-HPLC, the lysine mutants were cyclized and oxidized in a standard folding buffer and all except one (P24K) folded correctly. The precursor of the P24K mutant proved difficult to dissolve in the standard folding buffer and gave an insufficient yield of cyclic product for subsequent assays. In attempts to improve the yield of this mutant, buffers with varying percentages of isopropyl alcohol (0, 25, 50, 80, and 100%) were used but the yield of cyclic P24K could not be improved. The yields and HPLC retention times of the 26 correctly folded mutants of kalata B1 are listed in supplemental Table S1. The yield was determined by comparing the area of the peak of correctly folded peptide to total area from the analytical HPLC trace. NMR α-proton chemical shifts of the lysine mutants were determined using two-dimensional NMR spectra and showed no significant differences from kalata B1 (supplemental Table S2), indicating that the lysine mutants had similar three-dimensional folds to the wild-type peptide.
The Larval Development Assay
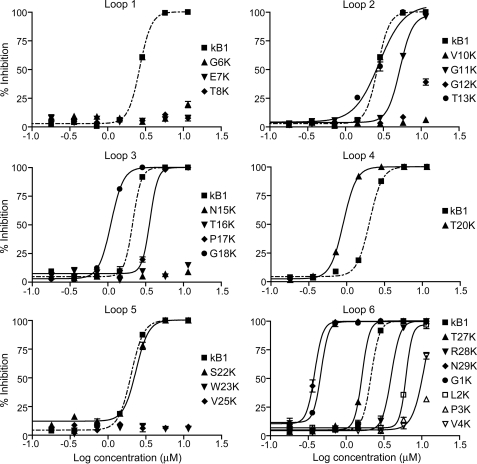
The LDA examines the ability of an anthelmintic compound to disrupt the development of nematode eggs to infective stage L3 larvae. Eggs were placed onto a layer of agar in wells of a 96-well plate, allowed to hatch, and provided with a growth medium. The numbers of larvae that developed to the L3 stage were counted after 5 days. The 22 correctly folded single point lysine mutants were assayed against the larvae of H. contortus and T. colubriformis. Fig. 2 shows the dose-response curves for inhibition of the development of H. contortus larvae by lysine mutants of kalata B1, presented separately for the six backbone loops. Kalata B1 was assayed along with all mutants for comparison. The half-maximal inhibitory concentrations (IC50) of the 22 single point mutated lysine analogues toward both nematode species are shown in Table 1. The IC50 values determined for T. colubriformis were ∼2-fold higher than those for H. contortus for most of the mutants. The full dose-response curves for the 22 lysine mutants causing inhibition of development of T. colubriformis larvae are shown in supplemental Fig. S1.
FIGURE 2.
Dose-response curves for the inhibition of larval development of eggs to third instar (L3) larvae of H. contortus of the 22 correctly folded lysine mutants of kalata B1. Each dose was tested in triplicate, and the error bars represent the S.D. per treatment.
TABLE 1.
IC50 values and relative activities of lysine mutants against H. contortus and T. colubriformis
| Mutant |
H. contortus |
T. colubriformis |
|||
|---|---|---|---|---|---|
| IC50 | Relative activitya | IC50 | Relative activitya | ||
| μm | μm | ||||
| Wild-type | kB1 | 2.7 | 1.0 | 4.5 | 1.0 |
| Loop 1 | G6K | >11.5 | <0.2 | >11.5 | <0.4 |
| E7K | >11.5 | <0.2 | >11.5 | <0.4 | |
| T8K | >11.5 | <0.2 | >11.5 | <0.4 | |
| Loop 2 | V10K | >11.5 | <0.2 | >11.5 | <0.4 |
| G11K | 5.3 | 0.5 | 6.1 | 0.7 | |
| G12K | >11.5 | <0.2 | >11.5 | <0.4 | |
| T13K | 2.8 | 1.0 | 4.0 | 1.1 | |
| Loop 3 | N15K | >11.5 | <0.2 | >11.5 | <0.4 |
| T16K | >11.5 | <0.2 | >11.5 | <0.4 | |
| P17K | 3.6 | 0.7 | 4.9 | 0.9 | |
| G18K | 1.1 | 2.4 | 1.8 | 2.5 | |
| Loop 4 | T20K | 0.9 | 3.0 | 1.7 | 2.6 |
| Loop 5 | S22K | 2.3 | 1.1 | 2.7 | 1.7 |
| W23K | >11.5 | <0.2 | >11.5 | <0.4 | |
| P24K | N/Db | N/D | N/D | N/D | |
| V25K | >11.5 | <0.2 | >11.5 | <0.4 | |
| Loop 6 | T27K | 1.6 | 1.7 | 3.4 | 1.3 |
| R28K | 3.9 | 0.7 | 7.7 | 0.6 | |
| N29K | 0.4 | 7.0 | 1.2 | 3.8 | |
| G1K | 0.5 | 5.8 | 1.5 | 2.9 | |
| L2K | 6.2 | 0.4 | 6.6 | 0.7 | |
| P3K | >11.5 | <0.2 | >11.5 | <0.4 | |
| V4K | 11.1 | 0.2 | >11.5 | <0.4 | |
| Double-lysine | T20K/S22K | 0.7 | 4.1 | 1.0 | 4.5 |
| T20K/N29K | 0.4 | 6.5 | 0.7 | 6.8 | |
| T20K/G1K | 0.4 | 6.5 | 0.7 | 6.8 | |
| Triple-lysine | T20K/N29K/G1K | 0.2 | 13.0 | 0.7 | 6.2 |
a Expressed relative to wild-type kalata B1.
b N/D, not determined.
Relative to native kalata B1, the incorporation of a positive charge in any of the positions in loop 1, in Val-10 or Gly-12 of loop 2, in Asn-15 or Thr-16 of loop 3, in Trp-23 or Val-25 of loop 5, or in Pro-3 or Val-4 of loop 6 either dramatically decreased or abolished the activity, i.e. mutation of any of these residues to lysine is detrimental to the nematocidal activity of kalata B1. In contrast, incorporation of a lysine into any of the positions corresponding to Gly-1, Gly-18, Thr-20, Ser-22, Thr-27, and Asn-29 resulted in enhanced anthelmintic activity. The N29K mutant was the most potent of the single point mutants, with an increase in activity relative to kalata B1 of 7-fold for H. contortus and 4-fold for T. colubriformis. The five remaining single point mutants showed equipotent (T13K) or slightly decreased (G11K, P17K, R28K, and L2K) activity to the wild-type peptide.
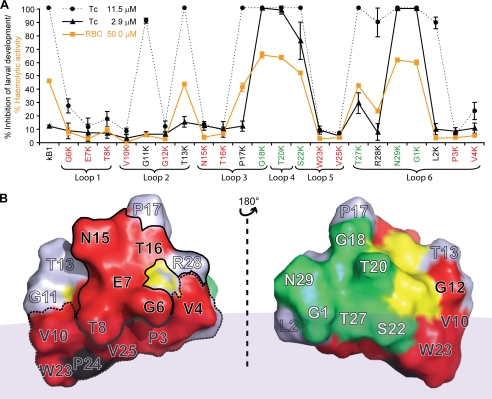
Fig. 3A shows the effect of two selected concentrations of the lysine mutants on larval development of this nematode. The higher concentration (11.5 μm) resulted in 100% inhibition of development for wild-type kalata B1, whereas the lower concentration (2.9 μm) had minimal effect (∼10% inhibition) on larval development. At the higher concentration, 11 mutants showed comparable (85–100%) anthelmintic activity to wild-type kalata B1 (G11K and T13K in loop 2, P17K and G18K in loop 3, T20K in loop 4, S22K in loop 5, T27K, R28K, N29K, G1K, and L2K in loop 6), whereas at the lower concentration the percentage inhibition was significantly higher than that of wild-type kalata B1 in six cases: G18K (100%), T20K (98.3%), S22K (75.4%), T27K (28.9%), N29K (99.3%), and G1K (99.4%). The mutants G11K and T13K in loop 2, P17K in loop 3, and R28K and L2K in loop 6 had a similar toxicity to kalata B1. Overall the trends in anthelmintic activity were similar for the two species of nematodes tested. The hemolytic activity of each of the single point Lys mutants was also determined (Fig. 3A) and displayed a similar trend to that observed for larval inhibition.
FIGURE 3.
A, comparison of percentage inhibition of 22 lysine mutants with a single point mutation against T. colubriformis at concentrations of 11.5 μm (solid circle with dashed line) and 2.9 μm (solid triangle with solid line). The percentage of hemolytic activity of these lysine mutants at 50 μm is also shown in the same panel (RBC (red blood cell), solid square with orange line). Each dose was tested in triplicate, and the error bars represent the S.D. per treatment. Residues that, when substituted with lysine, resulted in a dramatic decrease in nematocidal and hemolytic activities are colored red. Residues that resulted in increases in nematocidal and hemolytic activity when substituted with lysine are colored green. B, two-sided view of the three-dimensional structure of the kalata B1 molecule. The surface is colored according to A. The six cysteine residues are colored yellow. All residues in loop 1 (Gly-6, Glu-7, and Thr-8), Asn-15, Thr-16, and the cationic residue (Arg-28) in the sequence responsible for the bioactivity of kalata B1 (27) are surrounded by a solid circle (the bioactive patch). The majority of the hydrophobic residues (except Pro-17) form a surface-exposed patch and are outlined by a dashed line (the hydrophobic patch). The faint gray area represents the surface of dodecylphosphocholine (DPC) micelle to depict the interaction of the hydrophobic patch of kalata B1 with the model membrane (44).
Fig. 3B shows a surface representation of kalata B1, and maps the positions of the residues in mutants where substitution resulted in either decreased or increased activity relative to kalata B1. The separate clustering of the residues that increase or decrease activity is readily apparent. The mutants (G6K, E7K, T8K, V10K, G12K, N15K, T16K, W23K, V25K, P3K, and V4K) that cause lower relative activity (<0.2) are clustered on one face of the three-dimensional structure of kalata B1, and the mutants (G18K, T20K, S22K, T27K, N29K, and G1K) that caused higher relative activity (>1.0) are on the opposite side.
Mutants with Multiple Lysine Incorporation
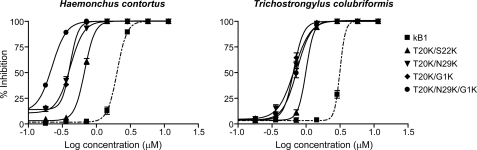
The impact of incorporation of multiple lysine residues within the framework of kalata B1 on anthelmintic activity was also investigated. On the basis of the results for the single point mutations, four residues amenable to lysine mutation (Thr-20, Ser-22, Asn-29, and Gly-1) were selected. Three double mutants (T20K/S22K, T20K/N29K, and T20K/G1K) and one triple mutant (T20K/N29K/G1K) were synthesized (see “Experimental Procedures”) and tested using LDAs with both nematode species. Fig. 4 shows the inhibition curves of these four mutants and native kalata B1 against H. contortus (left panel) and T. colubriformis (right panel) larvae. All of the multiple Lys mutants were more potent than wild-type kalata B1. The IC50 values and relative activity of these four mutants are reported in Table 1. T20K/N29K and T20K/G1K showed equipotent anthelmintic activity against both nematodes (the IC50 values are 0.4 μm for H. contortus and 0.7 μm for T. colubriformis) and T20K/S22K was slightly less potent (the IC50 value is 0.7 μm for H. contortus and 1.0 μm for T. colubriformis). The triple lysine mutant (T20K/N29K/G1K) showed comparable activity to the double lysine mutants against T. colubriformis, but showed a clear enhancement in activity against H. contortus relative to each of the double mutants.
FIGURE 4.
Dose-response curves for the inhibition of larval development of eggs to L3 stage of H. contortus and T. colubriformis larvae for the mutants incorporating double and triple lysine mutation. Kalata B1 (dashed line) was assayed along with all mutants for comparison.
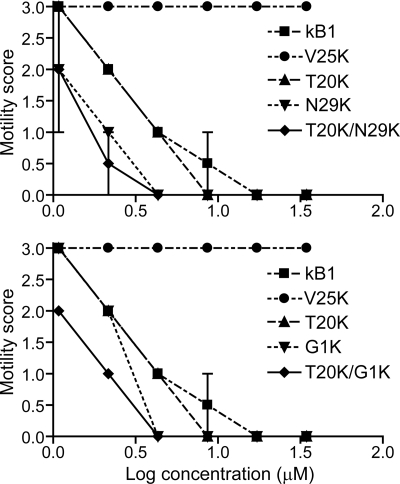
Effects of Lysine Mutants on the Motility of Adult H. contortus
The effects on motility of adult nematodes 48 h after treatment with Lys mutants are shown in Fig. 5. In all control assays (with no peptide added), the majority of the individuals showed smooth sinusoidal motion after 48 h, similar to the start of the culture period (mean motility score = 3, n = 13). The motility of V25K-treated adults after 48 h was also comparable to the degree of motion of control worms, even for those treated with the highest concentration. V25K was noted to be an inactive mutant in larval inhibition assays and was selected as a negative control. Five other lysine mutants (T20K, N29K, G1K, T20K/N29K, and T20K/G1K) and wild-type kalata B1 were tested at a range of concentrations and scored for motility every 24 h (data collected at 24 and 72 h were not shown). After 48 h, no movement of adults treated at the two highest concentrations of all tested peptides was observed. The order of potency in the adult motility assay was T20K/N29K ≈ T20K/G1K ≈ N29K > G1K > T20K > kalata B1.
FIGURE 5.
Motility scores of H. contortus adult nematodes after 48 h treatment with kalata B1 lysine mutants. Each data point represents the mean of two separate experiments (n = 2 for each experiment, error bars represent the S.D.). The mutants are shown on separate graphs for clarity, with common controls included on each graph.
DISCUSSION
In this study the structural and functional role of positive charge in the cyclotide framework was assessed by synthesis of a suite of lysine mutants, each with a single point mutation of the prototypic cyclotide kalata B1. The anthelmintic properties of the lysine mutants were evaluated by their ability to interfere with the development of eggs through to L3 larvae stage for two agriculturally important nematode species H. contortus and T. colubriformis. These nematodes are responsible for major stock and economic losses in the livestock industry. Eleven of 22 single point lysine mutants showed a dramatic decrease in anthelmintic activity, five showed equipotent or slightly decreased activity, and the remaining six mutants displayed an increased activity, which was ascribed to the incorporation of charge in a region amenable to amino acid substitution. Mutants with multiple lysine substitutions within this region further enhanced the anthelmintic activity. Overall, the results suggest that the anthelmintic potency of kalata B1 is decreased after residue substitution on one face of the molecule, maintained on substitution of another set of residues, and significantly increased by the incorporation of one or multiple basic residues in an “amendable” face. The term amendable is used here in its literal sense of “able to be modified to produce an improvement.”
Recent studies (27, 41) identified two faces of the kalata B1 molecule that are associated with its mode of insecticidal action: a hydrophilic face that appears to be involved in cyclotide self-association and a hydrophobic face that interacts with membranes. It was hypothesized that insecticidal activity is due to pore formation in membranes that results from cyclotide self-association and membrane binding. The results from the current study support this hypothesis and suggest that it is a general mechanism for other cyclotide activities, including anthelmintic activity and hemolytic activity. The biochemical mutagenesis studies undertaken here have specifically identified regions of the surface of kalata B1 that are associated with bioactivity and membrane binding.
The LDA data in the current study demonstrated that even at the highest concentration tested, the development of nematode larvae was not inhibited by 11 of the lysine mutants (G6K, E7K, T8K, V10K, G12K, N15K, T16K, W23K, V25K, P3K, and V4K). Except for G12K, 10 of the 11 residues that upon mutation profoundly reduced the anthelmintic activity of kalata B1 are co-localized on one face of this prototypic cyclotide (Fig. 3B, left). Thus, these results define a region of kalata B1 critical for anthelmintic activity. When combined with data from the recent Ala-scanning mutagenesis study that examined insecticidal activity (27), it is apparent that this region is made up of two distinct subregions. The first is centered on Glu-7 and adjacent hydrophilic residues has been described previously as the “bioactive face” (circled in Fig. 3B, solid line) (27). The second subregion comprises a surface-exposed patch of hydrophobic residues (encircled by a dashed line in Fig. 3B). The Ala-scanning mutagenesis study (27) suggested that the hydrophilic bioactive face is implicated in self-association and that the hydrophobic patch is implicated in membrane binding, and that both are essential for insecticidal activity.
One fundamental difference between the Ala-scanning study and the current Lys-scanning study is that mutation of some of the residues in the hydrophobic patch to Ala did not reduce insecticidal activity much, but their mutagenesis to Lys reported here does dramatically reduce anthelmintic activity. However, rather than being a contradictory result, this difference is readily explicable on the basis of the nature of the mutational probe residues, Ala versus Lys. Alanine is a hydrophobic amino acid, and its substitution into an existing hydrophobic patch would not be expected to produce a functional perturbation. By contrast, inclusion of lysine, a highly hydrophilic residue, into a hydrophobic patch would be expected to disrupt it and lead to loss of function (provided that the hydrophobic patch has a functional role). Thus, the current results support the previously proposed dual-component (self-association and membrane binding) mechanism of action of kalata B1. However, the new mutagenesis data significantly extends the mechanistic interpretation by identifying a third precisely defined region of the surface that is amendable to enhance bioactivity.
Substitution of Gly-18, Thr-20, Ser-22, Thr-27, Asn-29, or Gly-1 with Lys resulted in increased anthelmintic activity by up to 7-fold. These six residues form a patch on the opposite side to the face comprising the combined “bioactive” and membrane binding regions. Incorporation of charged residues into the newly identified amendable face of kalata B1 probably boosts its anthelmintic activity through electrostatic interactions between the positively charged side chains and the negatively charged phospholipid head groups of target membranes.
In a recent study (30), the substitution of individual residues in kalata B1 with alanine resulted in significant decreases in inhibition observed in LDAs. The anthelmintic activity of some mutants dropped to <25% (G6A, E7A, T8A, V10A, and G12A), some decreased to between 25 and 40% (N15A, T16A, V25A, R28A, and P3A), and others to 65–90% (P17A, L2A and V4A). Similar trends were observed for decreasing lytic ability (41) of alanine mutants, as demonstrated by the leakage of a self-quenching dye from phospholipid vesicles. Although the anthelmintic activity of single point lysine mutants broadly correlates with those of alanine mutants (30, 41), there are two major differences between these data sets. First, a larger number of lysine mutants (11 of 22) showed decreased inhibition rates to <25% in LDA than the number of alanine mutants (5 of 21, as the anthelmintic activity of W23A was not determined). Second, incorporation of a lysine residue at a number of positions (Gly-18, Thr-20, Ser-22, Thr-27, Asn-29, and Gly-1) increased the anthelmintic activity, whereas none of the alanine mutants were observed to be more potent than the wild-type peptide.
The current study has demonstrated that the anthelmintic activity of kalata B1 is lost upon replacement of any of the residues on the hydrophobic patch with a hydrophilic residue. Because the hydrophobic patch has been reported to be responsible for the binding of kalata B1 to the surface of dodecylphosphocholine (44), introduction of a different hydrophobic residue (alanine) at some positions in this region may not affect the binding significantly. However, placing a hydrophilic residue in this patch may have hindered its hydrophobic interactions with the membrane, thus resulting in a loss of activity.
The LDA results obtained for the lysine mutants in this study further emphasize the functional role of the hydrophilic patch, because incorporation of a lysine (positively charged) residue in this area silences the anthelmintic activity of kalata B1. Glu-7 is a highly conserved residue in the cyclotides, which has been proven to have an important role in stabilizing the structure through hydrogen bonding interactions between loops 1 and 3, via the side chain oxygen atoms of the glutamic acid (45). Methylation of the side chain of the highly conserved glutamic acid in cyclotide cycloviolacin O2 resulted a 48-fold decrease in its cytotoxic activity (46) and a 6-fold decrease in its anthelmintic activity against H. contortus (31). These findings substantiate the important role of the glutamic acid residue in the cyclotide function. Thus, substituting the negatively charged Glu-7 or any adjacent residues with a positively charged residue resulted in a dramatic loss of activity. The R28K mutant (a relatively minor substitution) was the only lysine mutant present in the hydrophilic patch that retained 60–70% (relative activity 0.7 for H. contortus, 0.6 for T. colubriformis, Table 1) of nematocidal activity.
The anthelmintic activity of natural variants extracted from Viola odorata was determined previously (31) and cycloviolacin O2 was reported to be the most potent cyclotide in LDAs, with activity 18-fold greater than the prototypic cyclotide kalata B1. A substantial improvement in activity was observed in all peptides that contained three to four basic residues. To investigate the correlation between the enhanced anthelmintic activities and the number of charged residues within the peptide sequences, three double lysine mutants (T20K/S22K, T20K/N29K, and T20K/G1K) and one triple lysine mutant (T20K/N29K/G1K) were produced. As illustrated in Fig. 4, all four multiple lysine mutants showed higher activity than those with single point mutations at the same positions within the cyclotide structure. However, the observed lower nematocidal activity of T20K/S22K compared with T20K/N29K and T20K/G1K suggests the distribution of the positively charged residues on the amendable face is also a determinant in the relative potency of these novel anthelmintic analogues. Similar to the results of the LDAs, the double lysine mutants affected the motility of H. contortus adults to a greater degree than did the single mutants.
The lysine mutants in the current study were also assayed for hemolytic activity to determine whether the increased potency observed in anthelmintic assays would correlate with increased efficacy of hemolysis. The trends observed for hemolytic activity correlated with those shown for anthelmintic activity of these lysine mutants. Overall, the collective results suggest that a common mechanism involving cyclotide-membrane interaction is responsible for the hemolytic, insecticidal, and anthelmintic activity of kalata B1.
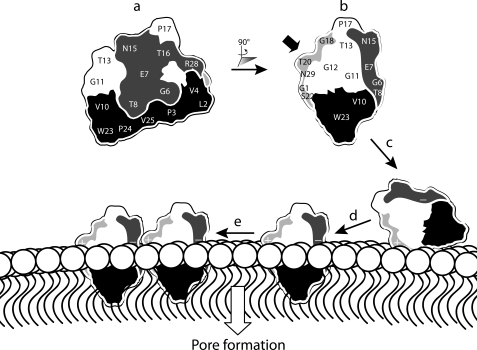
Fig. 6 shows a schematic representation of the proposed mechanism of action of kalata B1. The view shown in a highlights two important regions of the surface defined previously: the hydrophobic face and the bioactive face. Lysine incorporation into a third face, the amendable face, results in enhanced hemolytic and anthelmintic activities. The side view of kalata B1 in b clearly shows its three faces. The addition of a positive charge into the amendable face probably enhances activity by facilitating the binding of the peptide to the negatively charged head group region of the membrane, as illustrated in c. Subsequent interactions between the hydrophobic patch of kalata B1 and the membrane core would allow peptide insertion into the lipid bilayer (d). We speculate that self-association as indicated in e could then lead ultimately to pore formation. Overall, the activity of kalata B1 appears to be dependent on hydrophobic interactions with membranes, together with self-association involving residues clustered in the bioactive face, but it can be further modulated through the incorporation of a positive charge in the amendable face.
FIGURE 6.
Schematic representation of functionally important regions of kalata B1 and their role in the proposed mechanism of action. a, representation of kalata B1 displaying the bioactive (dark gray) and hydrophobic face (black). b, rotation of kalata B1 by 90° reveals the amendable face of kalata B1, which is colored light gray. The incorporation of a lysine residue in the amendable face (indicated by the bold black arrow) probably facilitates electrostatic interactions between kalata B1 and the negatively charged membrane head group region as illustrated in c. In d, interactions between the hydrophobic patch on kalata B1 and the hydrophobic core of the membrane result in peptide insertion. We speculate that peptide self-association might occur, as shown in e, ultimately leading to pore formation.
Although our focus here has been on structure-activity studies of the prototypic Möbius subfamily cyclotide kalata B1 with the aim of improving its agricultural potential, a recent report has demonstrated that a member of the trypsin inhibitor subfamily of cyclotides isolated from Momordica cochinchinensis (47, 48) also has exciting potential agricultural applications and can be re-engineered to inhibit a range of proteases other than trypsin (49). Two synthetic MCoTI-II analogues were reported to exhibit significant activity against 3C protease, a cysteine protease involved in the formation of proteins required for the RNA replication of the foot-and-mouth-disease virus (FMDV) (50, 51). This report not only discovered the first peptide-based inhibitor of this agriculturally important viral enzyme, but also demonstrated that the enzyme specificity of this class of cyclotides can be redirected with simple point mutations.
In conclusion, disruption of either the hydrophobic or bioactive patches of kalata B1 by incorporation of lysine into these regions abolished anthelmintic activity. This study has identified the presence of a third important face on the opposite side to the bioactive patch of kalata B1. Lysine substitution in this amendable face resulted in a dramatic improvement in anthelmintic activity. The structure-activity relationships described here pave the way for the further exploitation of cyclotides as valuable agents against agricultural pests.
Supplementary Material
Acknowledgments
We thank John O'Grady for extraction of embryos and preparation of adult nematodes and Chia Chia Tan for assisting in the HF cleavage of the double and triple lysine mutants.
This work was supported in part by grants from the Australian Research Council (to D. J. C.) and a grant from Commonwealth Scientific and Industrial Research Organization (to A. C. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Fig. S1.
- The abbreviation used is: LDA
- larval development assay.
REFERENCES
- 1.Besier B. (2007) Trends Parasitol. 23, 21–24 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan R. M. (2004) Trends Parasitol. 20, 477–481 [DOI] [PubMed] [Google Scholar]
- 3.Wolstenholme A. J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N. C. (2004) Trends Parasitol. 20, 469–476 [DOI] [PubMed] [Google Scholar]
- 4.Craik D. J., Daly N. L., Bond T., Waine C. (1999) J. Mol. Biol. 294, 1327–1336 [DOI] [PubMed] [Google Scholar]
- 5.Craik D. J., Daly N. L., Mulvenna J., Plan M. R., Trabi M. (2004) Curr. Protein Pept. Sci. 5, 297–315 [DOI] [PubMed] [Google Scholar]
- 6.Craik D. J. (2001) Toxicon 39, 1809–1813 [DOI] [PubMed] [Google Scholar]
- 7.Saether O., Craik D. J., Campbell I. D., Sletten K., Juul J., Norman D. G. (1995) Biochemistry 34, 4147–4158 [DOI] [PubMed] [Google Scholar]
- 8.Ireland D. C., Colgrave M. L., Craik D. J. (2006) Biochem. J. 400, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claeson P., Göransson U., Johansson S., Luijendijk T., Bohlin L. (1998) J. Nat. Prod. 61, 77–81 [DOI] [PubMed] [Google Scholar]
- 10.Göransson U., Luijendijk T., Johansson S., Bohlin L., Claeson P. (1999) J. Nat. Prod. 62, 283–286 [DOI] [PubMed] [Google Scholar]
- 11.Hallock Y. F., Sowder R. C., 2nd, Pannell L. K., Hughes C. B., Johnson D. G., Gulakowski R., Cardellina J. H., 2nd, Boyd M. R. (2000) J. Org. Chem. 65, 124–128 [DOI] [PubMed] [Google Scholar]
- 12.Broussalis A. M., Göransson U., Coussio J. D., Ferraro G., Martino V., Claeson P. (2001) Phytochemistry 58, 47–51 [DOI] [PubMed] [Google Scholar]
- 13.Trabi M., Mylne J. S., Sando L., Craik D. J. (2009) Org. Biomol. Chem. 7, 2378–2388 [DOI] [PubMed] [Google Scholar]
- 14.Wang C. K., Hu S. H., Martin J. L., Sjögren T., Hajdu J., Bohlin L., Claeson P., Göransson U., Rosengren K. J., Tang J., Tan N. H., Craik D. J. (2009) J. Biol. Chem. 284, 10672–10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colgrave M. L., Craik D. J. (2004) Biochemistry 43, 5965–5975 [DOI] [PubMed] [Google Scholar]
- 16.Gustafson K. R., R. S. I. , Henderson L. E., Parsons I. C., Kashman Y., Cardellina J. H., II, McMahon J. B., Buckheit R. W., Jr., Pannell L. K., Boyd M. R. (1994) J. Am. Chem. Soc. 116, 9337–9338 [Google Scholar]
- 17.Daly N. L., Gustafson K. R., Craik D. J. (2004) FEBS Lett. 574, 69–72 [DOI] [PubMed] [Google Scholar]
- 18.Ireland D. C., Wang C. K., Wilson J. A., Gustafson K. R., Craik D. J. (2008) Biopolymers Peptide Science 90, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C. K., Colgrave M. L., Gustafson K. R., Ireland D. C., Goransson U., Craik D. J. (2008) J. Nat. Prod. 71, 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witherup K. M., Bogusky M. J., Anderson P. S., Ramjit H., Ransom R. W., Wood T., Sardana M. (1994) J. Nat. Prod. 57, 1619–1625 [DOI] [PubMed] [Google Scholar]
- 21.Barry D. G., Daly N. L., Clark R. J., Sando L., Craik D. J. (2003) Biochemistry 42, 6688–6695 [DOI] [PubMed] [Google Scholar]
- 22.Tam J. P., Lu Y. A., Yang J. L., Chiu K. W. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8913–8918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Göransson U., Sjögren M., Svangård E., Claeson P., Bohlin L. (2004) J. Nat. Prod. 67, 1287–1290 [DOI] [PubMed] [Google Scholar]
- 24.Jennings C., West J., Waine C., Craik D., Anderson M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 10614–10619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings C. V., Rosengren K. J., Daly N. L., Plan M., Stevens J., Scanlon M. J., Waine C., Norman D. G., Anderson M. A., Craik D. J. (2005) Biochemistry 44, 851–860 [DOI] [PubMed] [Google Scholar]
- 26.Barbeta B. L., Marshall A. T., Gillon A. D., Craik D. J., Anderson M. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1221–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonsen S. M., Sando L., Rosengren K. J., Wang C. K., Colgrave M. L., Daly N. L., Craik D. J. (2008) J. Biol. Chem. 283, 9805–9813 [DOI] [PubMed] [Google Scholar]
- 28.Colgrave M. L., Kotze A. C., Kopp S., McCarthy J. S., Coleman G. T., Craik D. J. (2009) Acta Trop. 109, 163–166 [DOI] [PubMed] [Google Scholar]
- 29.Gruber C. W., Cemazar M., Anderson M. A., Craik D. J. (2007) Toxicon 49, 561–575 [DOI] [PubMed] [Google Scholar]
- 30.Colgrave M. L., Kotze A. C., Huang Y. H., O'Grady J., Simonsen S. M., Craik D. J. (2008) Biochemistry 47, 5581–5589 [DOI] [PubMed] [Google Scholar]
- 31.Colgrave M. L., Kotze A. C., Ireland D. C., Wang C. K., Craik D. J. (2008) Chembiochem 11, 1939–1945 [DOI] [PubMed] [Google Scholar]
- 32.Tam J. P., Lu Y. A. (1998) Protein Sci 7, 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daly N. L., Love S., Alewood P. F., Craik D. J. (1999) Biochemistry 38, 10606–10614 [DOI] [PubMed] [Google Scholar]
- 34.Chen B., Colgrave M. L., Wang C., Craik D. J. (2006) J. Nat. Prod. 69, 23–28 [DOI] [PubMed] [Google Scholar]
- 35.Schnölzer M., Alewood P., Jones A., Alewood D., Kent S. B. (1992) Int. J. Pept. Protein Res. 40, 180–193 [DOI] [PubMed] [Google Scholar]
- 36.Dawson P. E., Muir T. W., Clark-Lewis I., Kent S. B. (1994) Science 266, 776–779 [DOI] [PubMed] [Google Scholar]
- 37.Daly N. L., Clark R. J., Craik D. J. (2003) J. Biol. Chem. 278, 6314–6322 [DOI] [PubMed] [Google Scholar]
- 38.Clark R. J., Daly N. L., Craik D. J. (2006) Biochem. J. 394, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacey E., Redwin J. M., Gill J. H., DeMargheriti V. M., Waller P. J. (1990) Resistance of Parasites to Antiparasitic Drugs (Boray J. C., Martin P. J., Roush R. T. eds) pp. 177–184, MSD AGVET, Rahway, NJ [Google Scholar]
- 40.Hubert J., Kerboeuf D. (1984) Can. J. Comp. Med. 48, 63–71 [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y. H., Colgrave M. L., Daly N. L., Keleshian A., Martinac B., Craik D. J. (2009) J. Biol. Chem. 284, 20699–20707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotze A. C., McClure S. J. (2001) Int. J. Parasitol. 31, 1563–1571 [DOI] [PubMed] [Google Scholar]
- 43.O'Grady J., Kotze A. C. (2004) Exp. Parasitol. 106, 164–172 [DOI] [PubMed] [Google Scholar]
- 44.Shenkarev Z. O., Nadezhdin K. D., Sobol V. A., Sobol A. G., Skjeldal L., Arseniev A. S. (2006) FEBS J. 273, 2658–2672 [DOI] [PubMed] [Google Scholar]
- 45.Rosengren K. J., Daly N. L., Plan M. R., Waine C., Craik D. J. (2003) J. Biol. Chem. 278, 8606–8616 [DOI] [PubMed] [Google Scholar]
- 46.Herrmann A., Svångard E., Claeson P., Gullbo J., Bohlin L., Göransson U. (2006) Cell Mol. Life Sci. 63, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez J. F., Gagnon J., Chiche L., Nguyen T. M., Andrieu J. P., Heitz A., Trinh Hong T., Pham T. T., Le Nguyen D. (2000) Biochemistry 39, 5722–5730 [DOI] [PubMed] [Google Scholar]
- 48.Felizmenio-Quimio M. E., Daly N. L., Craik D. J. (2001) J. Biol. Chem. 276, 22875–22882 [DOI] [PubMed] [Google Scholar]
- 49.Thongyoo P., Roqué-Rosell N., Leatherbarrow R. J., Tate E. W. (2008) Org. Biomol. Chem. 6, 1462–1470 [DOI] [PubMed] [Google Scholar]
- 50.Sweeney T. R., Roqué-Rosell N., Birtley J. R., Leatherbarrow R. J., Curry S. (2007) J. Virol. 81, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curry S., Roqué-Rosell N., Sweeney T. R., Zunszain P. A., Leatherbarrow R. J. (2007) Biochem. Soc. Trans. 35, 594–598 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.