Abstract
M-type (KCNQ) potassium channels play an important role in regulating the action potential firing in neurons. Here, we investigated the effect of cholesterol on M current in superior cervical ganglion (SCG) sympathetic neurons, using the patch clamp technique. M current was inhibited in a dose-dependent manner by cholesterol loading with a methyl-β-cyclodextrin-cholesterol complex. This effect was prevented when membrane cholesterol level was restored by including empty methyl-β-cyclodextrin in the pipette solution. Dialysis of cells with AMP-PNP instead of ATP prevented cholesterol action on M currents. Protein kinase C (PKC) inhibitor, calphostin C, abolished cholesterol-induced inhibition whereas the PKC activator, PDBu, mimicked the inhibition of M currents by cholesterol. The in vitro kinase assay showed that KCNQ2 subunits of M channel can be phosphorylated by PKC. A KCNQ2 mutant that is defective in phosphorylation by PKC failed to show current inhibition not only by PDBu but also by cholesterol. These results indicate that cholesterol-induced inhibition of M currents is mediated by PKC phosphorylation. The inhibition of M currents by PDBu and cholesterol was completely blocked by PIP2 loading, indicating that the decrease in PIP2-channel interaction underlies M channel inhibition by PKC-mediated phosphorylation. We conclude that cholesterol specifically regulates M currents in SCG neurons via PKC activation.
Keywords: Cell/Neuron, Channels/Potassium, Membrane/Channels, Membrane/Lipids, Signal Transduction/Protein Kinases, Patch Clamp
Introduction
Cholesterol is probably one of the most notorious natural substances because of its crucial role in major human diseases such as arteriosclerosis and coronary heart disease (1–3). On the other hand, cholesterol is an essential component of the membranes that determines their biophysical properties such as fluidity, lateral pressure profile, and bilayer thickness (4–6). In addition to these structural roles in the membrane, it serves as a cofactor of signaling molecules (7, 8) and as a precursor for steroid hormones (9, 10).
Recently, lipid molecules have been found to act as signaling molecules (11–13). One of the most famous molecules in this respect is phosphatidylinositol 4,5-bisphosphate (PIP2).2 PIP2 was originally known as a membrane component (14). However, now, PIP2 is also known as a signal mediator in the regulation of numerous ion channels including ATP-sensitive K+ channel (15, 16), M-type (KCNQ) (17, 18), and G protein-gated inwardly rectifying K+ (GIRK) channels (19, 20). Even though cholesterol has multiple roles, however, it is unclear whether it also acts as a signaling molecule. Recently, it was shown that cholesterol, itself, modulated inwardly rectifying K+ (IRK) channel activity in vascular endothelial cells (21, 22) and delayed rectifier K+ channels in cultured hippocampal neurons (23). These observations raised these questions: how does cholesterol regulate channel activity and does it also regulate other ion channels? Until now, the mechanism underlying cholesterol action on ion channels has not been clarified (24).
KCNQ channels are abundant in neurons and play a crucial role in regulating neuronal excitability (25, 26). This time- and voltage-dependent K+ current has a threshold for activation at typical neuronal resting potentials, with greater activity upon depolarization. This characteristic makes the M channel function as a brake on repetitive action potential discharges and, as such, has a key role in regulating the excitability of various central and peripheral neurons, including sympathetic neurons, hippocampal pyramidal cells, and striatal neurons (25).
In vivo, a number of neurotransmitters and other endogenous and exogenous factors can close M channels, releasing the brake and allowing undiminished action potential firing (25, 27). Several potential messengers were suggested to lead to closure of M channels. Considerable evidence showed that acetylcholine and angiotensin II regulate M channels by depleting PIP2 in the membrane (25, 26), whose interaction with the channels is thought necessary for their function (17, 18, 28). On the other hand, bradykinin and purinergic receptors were suggested to suppress M current via calmodulin (26). As other potential messengers, PKC also have been suggested to be involved in receptor-mediated inhibition of M currents (25, 29–31). PKC and calmodulin was thought to decrease channel affinity for PIP2 (25, 26).
The aim of the present study is to investigate the role of cholesterol in the regulation of M channels in sympathetic neurons of the rat superior cervical ganglion (SCG). The results of the present study show that cholesterol inhibited M channel activity in a dose-dependent manner. We found several lines of evidence for PKC involvement in inhibition of M channel by cholesterol. We then found that exogenous PIP2 application effectively abolished the M current inhibition induced not only by a PKC activator but also by cholesterol. From these results, we suggest that the inhibition of M current by cholesterol in SCG neurons is via decrease in channel-PIP2 interaction. Taken together, these data support the hypothesis that cholesterol is acting as a signal molecule in the regulation of KCNQ channels through PKC pathway.
EXPERIMENTAL PROCEDURES
Plasmids
Plasmids encoding human KCNQ2 (GenBankTM accession number AF110020) were kindly given to us by Mark Shapiro (University of Texas Health Science Center, San Antonio, TX). To construct the Myc-tagged KCNQ2 expression vector, the entire coding region of KCNQ2 by performing PCR with the following oligonucleotides: 5-TAGAATTCATGGTGCAGAAGTCGCGCAACGGC-3 and 5-TCACTTCCTGGGCCCGGCCCA-3. The PCR product was digested with EcoRI and cloned into a pENTR3C vector (Invitrogen). Myc-KCNQ2 was constructed in the pDEST-CS3-MT vector using the Gateway LR reaction (Invitrogen). The QuikChange site-directed mutagenesis kit (Stratagene) was used to generate KCNQ2 mutant (S527A). For expression in Escherichia coli, fragment of KCNQ2 (amino acids 491–633) was amplified by Pfu polymerase with the following oligonucleotides: 5-TAGGATCCAGCTGCCCCTGCGAGTTTGTGACC-3 and 5-CGGCTCTTTGGCCCCAAAGTA-3. The amplified PCR product was digested with BamHI and was subcloned into the pGEX-4T-1 plasmid (Amersham Biosciences).
Cell Culture, Transfection, and SCG Isolation
HEK293 cells were handled as previously described (32). Transfections were made using Lipofectamine 2000 reagents (Invitrogen) and green fluorescent protein (GFP) was used as a reporter. SCG neurons were cultured from juvenile rats as previously described (33). Protocols were approved by the Animal Care Committee at Sungkyunkwan University.
Electrophysiological Recordings
Current measurements were made with the whole cell patch-clamp technique. Voltage-clamp was performed by using an EPC-10 amplifier (HEKA Instruments) and filtered at 10 kHz. The patch pipettes (World Precision Instruments, Inc.) were made by a Narishige puller (PP-830, Narishige, Tokyo). The patch pipettes used had a resistance of 2–3 MΩ when filled with the pipette solutions listed in the next sections. The pipette capacitance was compensated after formation of a gigaohm seal. Access resistance was typically 2.8–3.2 MΩ, and it was not significantly altered by cholesterol loading. Series-resistance compensation was not used. Because IM amplitudes were typically 0.1 nA, this implies a series-resistance voltage error of ∼0.5 mV. For the dialysis, we waited >5 min before starting the experiment. Because M currents were obtained by difference currents revealed by XE-991 and those procedures could eliminate the possible contamination of leak current, we did not use leak current subtraction. Data were not corrected for the liquid junction potential (−9 mV).
The M currents in SCG neurons were fully activated by setting the holding potential (Vh) at −60 mV and further deactivated by 1-s pulses from −20 mV to −60 mV, every 5 s. The KCNQ2 currents from HEK cells were studied by holding the cell at −20 mV, and 1-s hyperpolarizing steps to −60 mV were applied, followed by 1-s pulses back to −20 mV. The amplitude of the current was usually defined as the difference between the holding current at −20 mV, and the current at the beginning (after any capacity current had subsided) of the 1-s pulse back to −20 mV. In some cells, a more precise measurement was the XE991-sensitive current at the holding potential of −20 mV. The perfusion system was a homemade 100-μl perfusion chamber through which solution flowed continuously at 5 ml/min. All recordings were carried out at room temperature.
In Vitro Kinase Assay
In vitro kinase assays for PKC were performed as described earlier (34). Briefly, cell extracts were prepared from 293T cells, which had been transfected with PKC expression vector (kindly provided by Dr. I. B. Weinstein, Columbia University, New York) or control vector pcDNA3 by resuspending cells in PKC extraction buffer (50 mm HEPES, pH 7.5, 150 mm NaCl, 0.1% Tween 20, 1 mm EDTA, 2.5 mm EGTA, and 10% glycerol) containing protease inhibitors, (10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.1 mm phenylmethylsulfonyl fluoride) and phosphatase inhibitors (5 mm NaF, 1 mm Na3VO4). After brief sonication, the lysates were centrifuged for 10 min at 16,000 × g. Cleared supernatants were immunoprecipitated with anti-HA antibody (Roche) and protein A/G-agarose (Calbiochem.), the beads were washed three times with NETN buffer (25 mm Tris-HCl, pH 7.5, 1 mm EDTA, 150 mm NaCl, 0.5% Nonidet P-40) and then twice with kinase reaction buffer (50 mm HEPES, pH 7.5, 10 mm MgCl2, 1 mm dithiothreitol, 2.5 mm EGTA, 1 mm NaF, and 1 mm Na3VO4). The immunoprecipitate was resuspended in 20 μl of kinase reaction buffer containing 10 μCi of [γ-32P]ATP and 1 μg of GST-KCNQ2 wild-type or S527A mutant proteins. The kinase reaction was conducted at 30 °C for 30 min and stopped by the addition of SDS-polyacrylamide gel electrophoresis loading buffer. Proteins were separated on SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Immunoprecipitated HA-PKCs were confirmed by Western blotting with α-HA antibody. Radiolabeled proteins were visualized and quantified on BAS (Fuji). Recombinant GST-KCNQ2 wild-type or S527A mutant proteins were expressed in E. coli strain BL21(DE3) and purified to homogeneity using glutathione S-Sepharose beads (Amersham Biosciences).
Solutions and Drugs
The normal external solution for HEK293 cell and rat SCG neuron recording was as follows (in mm): 143 NaCl, 5.4 KCl, 5 HEPES, 0.5 NaH2PO4, 11.1 glucose, 0.5 MgCl2, 1.8 CaCl2, pH 7.4 adjusted with NaOH. The pipette solution was as follows (in mm): 140 KMeSO4, 20 KCl, 20 HEPES, 0.5 Na-GTP, 5 Mg-ATP, 4 Vit C, 10 1,2-bis(2-aminophenoxy)ethane N,N,N′,N′-tetraacetic acid (BAPTA), pH 7.4 adjusted with KOH.
Drugs were obtained as follows: water soluble-cholesterol (MβCD/Chol), MβCD, AMP-PNP (Sigma); calphostin C, phorbol-12,13-dibutyrate (PDBu), 4α-PDBu (Biomol); diC8-PIP2 (Echelon); Fura 2-AM (Invitrogen); and XE991 (Tocris).
Confocal Imaging
HEK293 cells were grown on glass coverslips and transfected with phospholipase C (PLC)δ-PH-GFP and M1 receptor. One day after transfection, the coverslips were placed into a flow-through chamber. The fluorescence images were obtained in a Zeiss 510 confocal laser scanning microscope with a 488-nm excitation line and 505–545-nm emission filter. Determination of the fluorescence was accomplished by assigning regions of interest in the cytosol and membrane.
Cholesterol Determination
Cells grown in 100-mm plates were assayed in duplicate for cholesterol using the Amplex red cholesterol kit from Molecular Probes. Cell monolayers were washed twice with ice-cold phosphate-buffered saline, and homogenized with hypotonic buffer (10 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 2 mm EGTA, 25 mm β-glycerolphosphate, 5 mm sodium fluoride, 2 mm sodium pyrophosphate, and 1 mm sodium orthovandate) using a 22-gauge needle. The samples were then centrifuged at 1,000 × g for 10 min to remove nuclei and cell debris. Membranes were pelleted from the postnuclear supernatants by centrifugation for 1 h at 100,000 × g, and assayed according to the supplier's instructions.
Statistical Analysis
Currents were analyzed and fitted using PULSE (HEKA Instrument) and Origin 6.1 (Originlab) software. Data were expressed as the mean or percentage change ± S.E. Statistical analyses were performed using the Student's t test. The difference between two groups was considered to be significant with p < 0.01 and not significant with p > 0.05.
RESULTS
Cholesterol Inhibits M Currents in Sympathetic Neurons
M current was measured using a standard voltage-clamp protocol (Fig. 1A, inset) (35). Application of the hyperpolarizing test pulse resulted in a slowly deactivating whole cell current, characteristic of the M current, which was inhibited by the specific M channel blocker, XE991 (20 μm) (36). In subsequent voltage-clamp experiments, we termed the M current the XE991-sensitive component of the slowly deactivating current produced by stepping the membrane voltage from −20 to −60 mV.
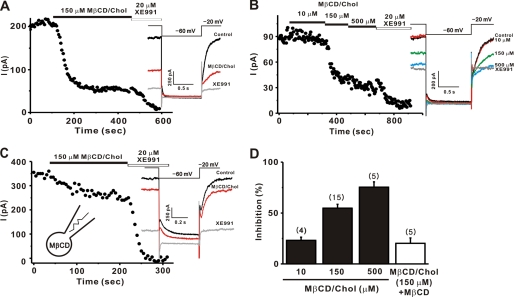
FIGURE 1.
Cholesterol inhibited M currents in SCG neurons. SCG neurons were studied under whole cell patch clamp. M currents were monitored by 1-s hyperpolarizing steps to −60 mV from a holding potential of −20 mV at 5-s intervals as shown in the inset. A–C, time course for the effect of 150 μm MβCD/Chol on M currents. MβCD/Chol was applied to the bath. Representative current traces are shown on the right (A). The time course of M current amplitude during cumulatively increasing concentrations of MβCD/Chol are indicated (B). In the presence of empty MβCD (3 mm) in the pipette solution, inhibition of M channel by 150 μm MβCD/Chol was significantly reduced (C). Insets denote the pulse protocol and representative current traces recorded during experiments. D, summary of the percent inhibitions of M currents. Error bars indicate S.E. The numbers in parentheses indicate the number of cells tested.
MβCD is a water-soluble cyclic oligosaccharide that enhances the solubility of cholesterol by incorporating it into a hydrophobic cavity (37). Treating cells with cholesterol-free or cholesterol-loaded MβCD (MβCD/Chol) has been widely used to selectively and efficiently reduce or enhance the cholesterol content of the membrane (22–24). We applied an MβCD/Chol complex to increase the cholesterol content of the cell membrane. Application of 150 μm MβCD/Chol to the bath solution resulted in a dramatic and sustained reduction in M current (Fig. 1A). We then examined the dose-inhibition relationship for cholesterol in Fig. 1B. Various concentrations of cholesterol were applied in a cumulative manner. The extent of current inhibition became larger as cholesterol concentration increased (Fig. 1, B and D); the extent of inhibition induced by 10, 150, and 500 μm MβCD/Chol was 23.1 ± 3.1% (n = 4), 54.8 ± 3.7% (n = 15), and 75.4 ± 5.2% (n = 5), respectively. The effect of MβCD/Chol on M currents usually reached a plateau within 5 min of application (Figs. 1 and 3F). Because 500 μm MβCD/Chol was not easily dissolved into the bath solution, 150 μm MβCD/Chol was used in subsequent experiments.
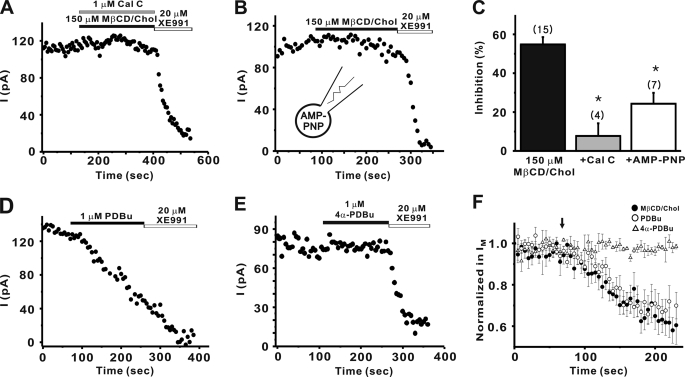
FIGURE 3.
Cholesterol-mediated inhibition of M current requires PKC. A, PKC inhibitor, calphostin C (1 μm) significantly blocked the inhibition of M current by MβCD/Chol. B, in the presence of AMP-PNP (4 mm) in the internal solution instead of ATP, cholesterol-induced inhibition of M current was significantly abolished. C, summary of the percent inhibition of M currents. Values are expressed as means ± S.E. *, p < 0.01 versus control condition. D and E, 1 μm PDBu, a PKC activator (D) inhibited M current, but 1 μm 4α-PDBu, an inactive analog (E), did not affect M current. F, averaged time courses of normalized M current amplitude during application of MβCD/Chol (●, n = 15) or PDBu (○, n = 4), or 4α-PDBu (▵, n = 5). The perfusion of each drug started at the time indicated by the arrow. Data are shown as means ± S.E.
Because cholesterol molecules readily “flip-flop” between inner and outer membrane leaflets with half times on the order of second (38), cholesterol would be expected to affect M currents via the internal side of the membrane. We confirmed this point by showing that application of 150 μm MβCD/Chol to the internal surface of the membrane induced 47.1 ± 10.1% (n = 4), compatible with those induced by external application of 150 μm MβCD/Chol (p > 0.05, supplemental Fig. S1).
Cholesterol-induced inhibition was abolished when the level of cholesterol in the membrane was restored by including empty MβCD in the pipette solution (Fig. 1C). The inhibitory effect of MβCD/Chol was reduced to 20.2 ± 5.2% (n = 5, p < 0.01, Fig. 1D). MβCD itself did not significantly affect M currents. After a 5-min dialysis with pipette solution containing MβCD, the relative amplitude of M currents was not changed much from the current level obtained immediately after dialysis (104.4 ± 9.9%, n = 6). Bath-applied MβCD did not affect M currents either (supplemental Fig. S2).
To verify the concept that MβCD/Chol-induced current inhibition was due to cholesterol loading of the cells, we monitored the cholesterol level using HEK cell system. We found that total cholesterol level in HEK cells that were not exposed to cholesterol was 28.9 ± 2.0 μg/mg protein (n = 8), similar to total cholesterol levels reported earlier for sympathetic neurons (39) and for other cell types (37, 40). Exposure of HEK cells to MβCD/Chol for 10 min resulted in a dose-dependent increase of cholesterol content; 10, 150, 500 μm MβCD/Chol increased the total cholesterol level by about 8%, 28 and 42%, respectively (n = 4–6). Incubation of cells with empty MβCD for 10 min decreased cholesterol content by 18% (n = 4).
PIP2 Depletion Is Not Involved in Cholesterol-induced Inhibition of M Currents
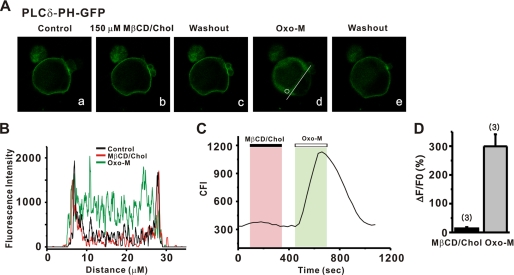
M channels require a certain level of PIP2 in the cell membrane to open (17, 18, 25, 26, 28). It was therefore possible that channel inhibition by cholesterol might result from the depletion of membrane PIP2 as a result of its hydrolysis. To examine whether cholesterol can induce PIP2 hydrolysis, we transfected HEK cells (using an effectene system, Molecular Probes) with an optical probe for PIP2, PLCδ-PH-GFP (41). The probe contained the PH domain of PLCδ that binds with high affinity to PIP2 and IP3. After transfection, we examined whether cholesterol induced membrane-to-cytosol translocation of the probe using a confocal microscope (Fig. 2A). Under basal conditions, PLCδ-PH-GFP was predominantly localized to the plasma membrane as shown by an intensity line plot (Fig. 2B). Application of cholesterol did not significantly change fluorescence intensity or its distribution in the cell. In contrast, stimulation of co-transfected M1 receptors caused robust translocation of PLCδ-PH-GFP from the plasma membrane to the cytosol (Fig. 2, A–C). Cytosolic fluorescence during muscarinic stimulation increased by 299.4 ± 41.8% (n = 3), whereas during cholesterol application, the fluorescence intensity in cytosol increased by 13.8 ± 4.6% (n = 3, p < 0.01, Fig. 2D). This result indicates that cholesterol addition caused very little PIP2 hydrolysis, and therefore PIP2 depletion was not a major mechanism in the inhibition of M current by cholesterol.
FIGURE 2.
Cholesterol did not induce a PIP2 hydrolysis. A, translocation of membrane-localized PLCδ-PH to the cytosol reports PIP2 hydrolysis by cholesterol and muscarinic stimulation in HEK cells. B, plot of fluorescence intensity across a single line scan (A, white line) before and during the cholesterol (150 μm) or the Oxo-M (10 μm) response. C, continuous time plot of cytosolic fluorescence intensity recorded in a single region of interest (A, white circle). D, bars summarize the normalized cytosolic fluorescence increase by cholesterol and muscarinic stimulation. The change in fluorescence is given as ΔF/F0 × 100%, where ΔF = F − F0, F is peak fluorescence after drug application, and F0 is baseline fluorescence before agonist application.
Cholesterol-mediated Inhibition of M Current Requires PKC
In many cell types, cholesterol could activate protein kinases, including PKC (42–44). To unravel the intracellular signaling cascade linking cholesterol and M current in SCG neurons, we examined whether the effects of cholesterol on M channels depends on PKC activation using the specific inhibitor, calphostin C. Fig. 3A shows that calphostin C almost completely blocked the modulation of M currents by cholesterol. In the presence of calphostin C, the cholesterol-mediated inhibition of M current was 7.7 ± 6.5% (n = 4), and it was significantly different from those observed in control cells (54.8 ± 3.7%, n = 15, p < 0.01; Fig. 3C).
To confirm that the phosphorylation process was involved in cholesterol-induced modulation of M currents, the effect of AMP-PNP, a nonhydrolyzable analog of ATP, on cholesterol-induced modulation was tested (Fig. 3B). The presence of AMP-PNP (4 mm) in place of ATP in the internal solutions significantly blocked cholesterol-induced modulation of M currents, and inhibition of M currents was reduced to 24.3 ± 5.6% (n = 7, p < 0.01 versus control; 54.8 ± 3.7%, n = 15; Fig. 3C).
To determine whether PKC regulates M current, we tested if direct activation of PKC by phorbol ester inhibits M current. In the control, the PKC activator PDBu significantly inhibited M current (Fig. 3D), whereas the inactive analog, 4α-PDBu, did not inhibit M current (Fig. 3E). The extent of inhibition induced by PDBu was 40.9 ± 8.5% (n = 4), whereas that induced by 4α-PDBu was 6.9 ± 4.8% (n = 5, p < 0.01, Fig. 3F). Furthermore, the extent and time course of inhibition of M current induced by PDBu was similar to those induced by cholesterol (Fig. 3F). These data suggest that PKC regulates M channels and effects of cholesterol on M channels depend on the PKC-induced phosphorylation process.
Phosphorylation of KCNQ2 by PKC
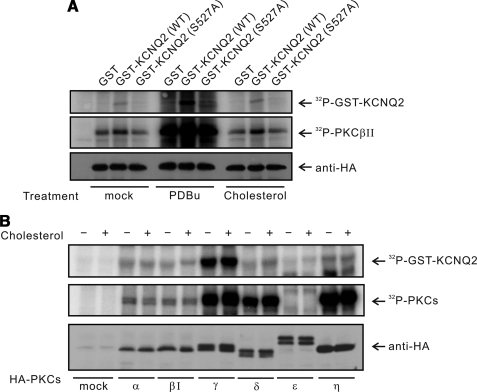
We then examined whether M channels were phosphorylated by PKC. M channels in SCG neurons are known to be composed of KCNQ2/KCNQ3 heterotetramers (45). It has been reported that KCNQ2 can interact with PKCβII (30). There are several PKC phosphorylation sites corresponding to the consensus sequence ((S/T)X(R/K)) throughout the KCNQ channel subfamily, and two of these phosphorylation sites of the KCNQ2 subunit have been implicated in muscarinic receptor-induced current inhibition (30). The alanine mutant of the Ser-527 (Ser-541 in rat counterparts) in the C-terminal tail of KCNQ2 protein reduced the agonist-induced current inhibition (30). Until now, however, there is no direct evidence showing that KCNQ2 channel is phosphorylated by PKC. To explore this possibility, we performed in vitro kinase assay using wild-type KCNQ2 or the alanine-substituted mutant of Ser-527 as substrates for PKCβII. The PDBu-triggered PKCβII activation clearly increased an incorporation of phosphate into the wild-type KCNQ2 (Fig. 4A). In contrast, the S527A mutant failed to show an increase in the phosphate incorporation in response to PDBu stimulation of PKCβII. These data indicate that PKC can directly phosphorylate KCNQ2 channels. Furthermore, the Ser-527 residue is responsible for PKC-dependent phosphorylation of KCNQ2. However, we cannot rule out the possibility that other PKC sites are involved in this process, as well.
FIGURE 4.
PKC stimulation induces phosphorylation of KCNQ2 at Ser-527. A, HEK cells were transfected with empty vector or HA-PKCβII expression vector. After 48 h, PDBu (1 μm) or MβCD/Chol (150 μm) were added to culture medium for 5 min. GST-KCNQ2 (amino acids 491–633) wild-type or mutant (S527A) protein were used as substrates in the in vitro kinase assay with immunoprecipitated HA-PKCβII. B, HEK cells were transfected with empty vector or the indicated PKC expression vectors. After treatment of MβCD/Chol (150 μm) for 5 min, HA-PKCs were immunoprecipitated and used in the in vitro kinase assay with GST-KCNQ2 wild-type proteins as substrates. The levels of HA-PKC isoforms in each reaction were assessed by immunoblotting with anti-HA antibody.
Next, we investigated whether cholesterol can directly modulate activities of PKC resulting in phosphorylation of KCNQ2. In contrast to the PDBu activation of PKCβII, cholesterol application failed to induce phosphorylation of the wild-type or the mutant channels by PKCβII in an in vitro kinase assay. We then tested whether other PKC isoforms (α, βI, γ, δ, ϵ, η) can be regulated by cholesterol. As shown in Fig. 4B, none of the tested PKC isoforms phosphorylated KCNQ2 in response to cholesterol. These data indicate that cholesterol cannot directly stimulate PKC in vitro. These results further suggest that cholesterol activates PKC not through a mechanism involving a direct stimulation but most likely via a modulation of membrane physicochemical properties (46). Thus, cholesterol-mediated PKC activation might require an intact membrane structure. We then investigated the role of PKC-dependent phosphorylation in cholesterol action by examining the effects of S527A mutation on channel modulation in vivo.
KCNQ2 Phosphorylation Is Required for Cholesterol-mediated Inhibition
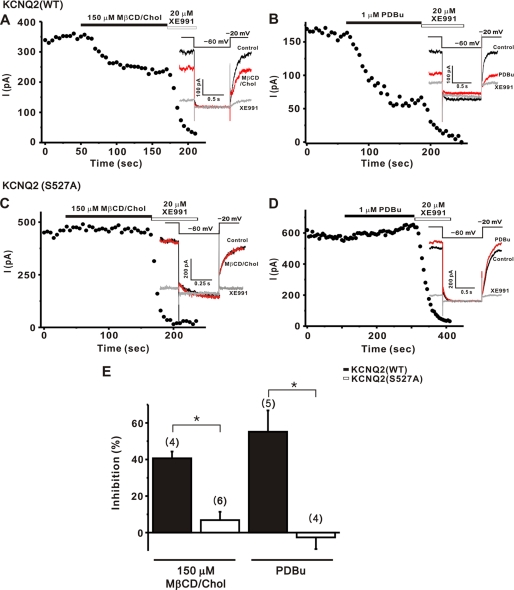
To test whether the phosphorylation of KCNQ2 correlates with cholesterol-induced modulation, wild-type KCNQ2 or the S527A mutant was expressed in HEK cells. M current amplitudes in mutant KCNQ2 (S527A)-expressing cells were not significantly different from those in wild-type KCNQ2-expressing cells. Mean amplitudes (in picoamperes) ± S.E. were as follows: wild-type, 199.9 ± 14.1 (n = 9); S527A, 368.0 ± 95.0 (n = 10, p > 0.05). We first confirmed that cholesterol-induced inhibition of wild-type KCNQ2 channels (Fig. 5A). The mean current inhibition by cholesterol was 40.6 ± 3.7% (n = 4, Fig. 5E). In contrast, the S527A mutant channels appeared to be totally insensitive to cholesterol (Fig. 5C) with the extent of inhibition being 6.8 ± 4.5% (n = 6, p < 0.01, Fig. 5E).
FIGURE 5.
S527A mutation of KCNQ2 channel abolished the inhibition of M currents by MβCD/Chol and PDBu. A–D, current inhibition induced by 150 μm MβCD/Chol (A and C) or 1 μm PDBu (B and D) were measured in HEK cells transfected with the wild-type KCNQ2 (A and B) or the mutant KCNQ2 (S527A) (C and D). The currents were elicited by 1-s steps to −60 mV from a holding potential of −20 mV at 5-s intervals as shown in the inset. E, effects of point mutation on the current inhibition of KCNQ2 channels induced by MβCD/Chol or PDBu. Values are expressed as means ± S.E. *, p < 0.01 compared with wild-type KCNQ2 channels.
In addition, PDBu-induced inhibition in cells expressing WT KCNQ2 channels was shown to be 55.2 ± 11.6% (n = 5, Fig. 5B). However, application of PDBu did not induce an inhibition of M channels in cells expressing the S527A mutant (Fig. 5D), but rather, a small increase of the current by 2.6 ± 6.3% (n = 4, p < 0.01) during the experiment was observed instead. The data are summarized in Fig. 5E. Thus, from the KCNQ2 mutagenesis results, we conclude that PKC-dependent phosphorylation of KCNQ channels is a key step for cholesterol-mediated inhibition.
Cholesterol- or PKC-induced Modulation of M Currents Does Not Change Channel Voltage Dependence
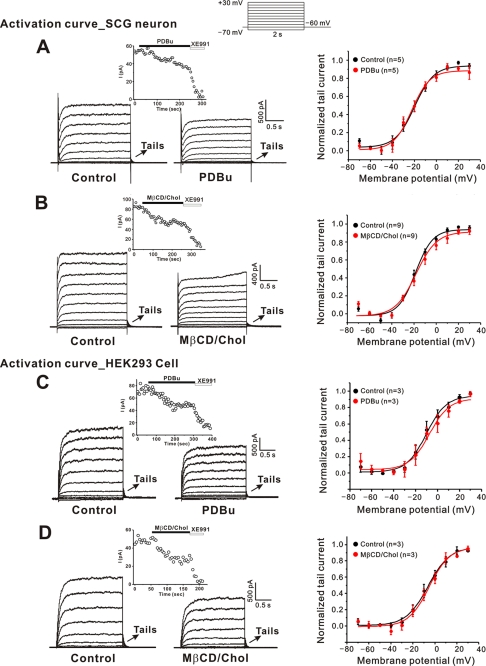
It was previously reported that PKC exerts its effects on KCNQ channels expressed in Xenopus oocytes by modulating voltage dependence of channel activation (31). To test whether the PKC action on the neuronal M currents involves a shift in the voltage dependence of channel activation, we applied a family of voltage steps from a holding potential of −60 mV, and measured the amplitude of the tail current at −60 mV following each test current (Fig. 6A). We found that PDBu did not affect the voltage dependence of channel activation while it produced about 40% inhibition (Fig. 6A, inset). Before the application of PDBu, the voltage that produces half-maximal activation of the conductance (V1/2) was −19.7 ± 1.1 mV; after PDBu application V1/2 was −22.3 ± 1.2 mV (n = 5, p > 0.05, Fig. 6A, right).
FIGURE 6.
Modulation of M currents by PDBu and cholesterol did not change voltage dependence of activation. A and B, representative current recordings from neuronal M channels before and after application of 1 μm PDBu (A) or 150 μm MβCD/Chol (B). Currents were elicited by voltage steps from −70 mV to +30 with a subsequent step to −60 mV (inset) for the tail current. Tail currents are marked by arrows. The time course of current inhibition is shown in the inset. C and D, representative current recordings from KCNQ2 channels expressed in HEK cells before and after application of 1 μm PDBu (C) or 150 μm MβCD/Chol (D). Activation curves for M current before (black symbols) and after (red symbols) application of PDBu (A, C) or MβCD/Chol (B, D) are also shown in the right column. Pooled tail current amplitudes from the experiments similar to that shown in the left are plotted against voltage and fit by Boltzmann equations. Error bars indicate S.E.
We also examined the effects of cholesterol on the conductance-voltage relationship of M channel (Fig. 6B). Similar to PKC action, cholesterol inhibited M currents without inducing a voltage shift; V1/2 was −18.6 ± 1.0 mV before cholesterol application and −15.5 ± 1.2 mV (n = 9, p > 0.05) after cholesterol application (Fig. 6B, right). Taken together, these data suggest that PKC-induced modulation of M channels is not associated with shifts in channel voltage dependence in sympathetic neurons.
We repeated similar experiments with the KCNQ2 channels expressed in HEK cells. Consistent with the data obtained from sympathetic neurons, PDBu inhibited M currents without altering voltage dependence of channel activation (V1/2: −10.1 ± 1.3 versus −7.4 ± 2 mV; p > 0.05; Fig. 6C). Cholesterol-induced M currents modulation also occurred in HEK cells without shifting channel activation (V1/2: −6.1 ± 1.0 versus −5.2 ± 1.6 mV; p > 0.05; Fig. 6D).
PIP2 Blocks M Current Inhibition by Cholesterol and Phorbol Ester
Considering that channel-PIP2 interaction is crucial for the activities of M channels, it could be speculated that the phosphorylation of KCNQ channels by PKC decreases channel affinity for PIP2, which is necessary for channel activation. It was already shown that PKC decreases the GIRK channel, another PIP2-sensitive ion channel, via a decrease in channel-PIP2 interactions (47, 48). To test this hypothesis, we examined whether the inhibitory effect of PDBu on M currents was blocked by PIP2 loading.
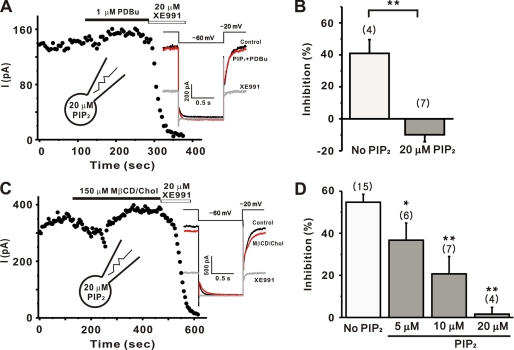
When PIP2 concentration was increased by direct application of exogenous PIP2 (up to 200 μm, Ref. 49) or by the overexpression of PI(4)5-kinase (28), the M current amplitudes were not significantly changed. Consistent with these results, including 20 μm diC8-PIP2 in the pipette solution did not affect the M current amplitudes after 5 min of dialysis (137.2 ± 28.2 pA, n = 11) compared with control cells (101.3 ± 7.7 pA, n = 55). However, the inhibitory effect of PDBu on M currents was completely abolished when 20 μm diC8-PIP2 was included in the pipette solution as shown in Fig. 7A. In the presence of 20 μm PIP2, the extent of inhibition was −9.9 ± 4.3% (Fig. 7B), which was significantly different from that in the control (40.9 ± 8.5%, n = 4, p < 0.01). These data suggest that the mechanism of action of PKC is to decrease the PIP2 affinity of the channel.
FIGURE 7.
PIP2 blocked the inhibition of M currents by cholesterol and phorbol ester. The current amplitudes were measured as the deactivation tail current induced by the pulse protocol as shown in the inset. A, inhibition M current by 1 μm PDBu was almost completely reversed when loaded with 20 μm diC8-PIP2. B, summary of the percent inhibitions of M currents by PDBu in control conditions or in cells loaded with 20 μm PIP2. C, 150 μm MβCD/Chol was almost completely reversed when loaded with 20 μm diC8-PIP2. D, summary of the percent inhibitions of M currents by MβCD/Chol in control conditions or in cells loaded with various concentrations of PIP2. Values are expressed as means ± S.E. *, p < 0.05; **, p < 0.01, compared with the current without exogenous PIP2.
Similar experiments were carried out to test whether the application of PIP2 could block the inhibitory effects of cholesterol on M currents. As shown in Fig. 7C, the inhibitory effect of cholesterol was completely blocked with 20 μm diC8-PIP2 in the patch pipette. Current inhibition was 1.6 ± 3.2% (n = 4, Fig. 7D, p < 0.01 versus control; 54.8 ± 3.7%, n = 15). To further confirm the role of the channel affinity change for PIP2 in cholesterol action, we applied various concentrations of PIP2. The effect of PIP2 was dependent on the concentration of PIP2, and the blocking was partial at lower concentrations. In the presence of 5 μm or 10 μm diC8-PIP2, the extent of inhibition was 36.7 ± 8.1% (n = 6, p < 0.05 versus control), and 20.7 ± 8.2% (n = 7, p < 0.01 versus control), respectively (Fig. 7D). Taken together, these data suggest that the PKC-mediated affinity change for PIP2 is an important mechanism of cholesterol response.
DISCUSSION
The main question addressed in the present study was whether cholesterol regulates native M channels. The results obtained can be summarized as follows: 1) direct cholesterol enhancement strongly suppresses M currents in a concentration-dependent manner; 2) when AMP-PNP was introduced in the internal solution instead of ATP, cholesterol-induced inhibition was significantly reduced; 3) the PKC inhibitor, calphostin C, blocked cholesterol-induced inhibition, and the PKC activator, PDBu mimicked the inhibition of M currents by cholesterol; 4) the PDBu-activated PKC phosphorylated the KCNQ2 subunit of M channels at the Ser-527 residue; 5) substituting the Ser-527 site by alanine (S527A) abolished the current inhibition induced by cholesterol or PDBu; and 6) inhibition of M currents by cholesterol or the direct application of PKC activator was blocked completely by exogenous application of PIP2. Taken together, the data suggest that M channel activity was inhibited by enhancement of cholesterol. The phosphorylation of M channel by PKC and consequent decrease in interaction between the channel and PIP2 underlies the current reduction.
PKC Mediates the Regulation of M Channels by Cholesterol
Cholesterol-induced M channel inhibition was reduced when ATP was replaced by AMP-PNP, the non-hydrolyzable analog. These data indicated that the cholesterol-mediated modulation of M channels involved the phosphorylation process. We found strong evidence supporting that PKC mediates cholesterol-induced modulation of M currents. First, PKC inhibitors blocked the cholesterol-induced modulation of M channels (Fig. 3). Second, the PKC activator, PDBu, inhibited KCNQ currents in both SCG neurons and HEK cells, but not the inactive analog 4α-PDBu (Figs. 3 and 5). Finally, we found that the Ser-527 residue of KCNQ2 is phosphorylated by PDBu activation of PKC. In contrast to the wild-type KCNQ2-expressing cells, the S527A mutant-expressing cells displayed an impaired cholesterol-induced channel modulation (Fig. 5). Although PKC has been implicated in the neurotransmitter-induced M channel regulation, the precise role of PKC in M channel regulation is largely unknown (25). Our present study suggests that phosphorylation of KCNQ2 by PKC is required for cholesterol-induced M channel regulation.
Cholesterol was reported to regulate several membrane-bound enzymes including PKC (42–44). It was suggested that cholesterol increases PKC activity by modulating physicochemical parameters of the membrane (46). A variety of lipophilic compounds can increase PKC activity by destabilizing the membrane bilayer rather than stimulating PKC directly (46). Conversely, compounds that stabilize the lipid bilayer attenuate PKC activity (50). Consistent with this hypothesis, cholesterol cannot stimulate PKC in vitro (Fig. 4B). However, this concept is still speculative; therefore further studies will be required to test this hypothesis. Because it was known that PKC is recruited to M channels by AKAP150, a scaffold protein (29, 30), it is possible that AKAP might be involved in the stimulatory effect of cholesterol on PKC.
PKC Modulates Channel-PIP2 Interaction
Phorbol esters have been reported to inhibit M currents (51), and previous experiments with kinase inhibitors have shown that the effect of the PKC inhibitors was to reduce sensitivity to muscarinic receptor-induced inhibition (30, 52). They suggested that a possible mechanism for PKC might be reduction of channel affinity for PIP2, thereby sensitizing the channel to receptor-induced PIP2 depletion. However, direct evidence of the linkage of PKC and reduced channel affinity for PIP2 has not been reported. In the present study, we showed that PKC- and cholesterol-mediated M channel inhibitions were blocked by exogenous PIP2 application. This result could be regarded as evidence supporting that PKC-mediated M current inhibition was mediated by decreases in channel-PIP2 interaction, because we could exclude PIP2 depletion as a major mechanism involved in cholesterol-mediated inhibition of M currents with PLCδ-PH-GFP data (Fig. 2). Note that the time course of M current modulation by PDBu or cholesterol was much slower than that of receptor-mediated inhibition (17, 51). This might be explained by the fact that PKC action decreases the affinity of M channel for PIP2 and during receptor stimulation, this factor, combined with concurrent PIP2 depletion and/or other mechanisms might cause a rapid inhibition of M current. However, the effect of exogenous PIP2 on cholesterol-mediated inhibition of M channels should be interpreted carefully. PIP2-channel interaction should be further tested by measuring the change in channel affinity for PIP2, either with biochemical measurements with purified channel proteins or by inside-out patch experiments with PIP2 or PIP2 scavengers added to the inside-facing bath solution.
Another possible mechanism underlying PKC-induced inhibition of M currents might be the shift of voltage dependence of channel activation. It was supported by the fact that PKC reduced KCNQ2 currents expressed in Xenopus oocytes by inducing a depolarizing shift in the channel activation (31). However, we found that PKC had little effect on voltage dependence of activation of M channels in sympathetic neurons or KCNQ2 channels expressed in HEK cells (Fig. 5). This discrepancy might be because the relevant phosphorylation of channel is already complete in some residues in mammalian cells (31), and those residues are perhaps responsible for regulation of conductance-voltage relationship. In the same study, they showed that PIP2 depletion reduced KCNQ currents without affecting conductance-voltage relationships (31). It thus appears that PKC in sympathetic neurons and HEK cells exerts its effect on KCNQ channels by modulating channel interaction with PIP2 rather than by affecting the voltage dependence of activation.
Currently, we do not know the exact mechanism of how phosphorylation of KCNQ2 could decrease the channel affinity for PIP2. However, considering that the PKC phosphorylation site (Ser-527 in KCNQ2) is adjacent to the amino acid residues that were shown to be responsible for the channel-PIP2 interaction (Lys-452, Arg-459, Arg-461, Arg-463, and Arg-467; (26)), it could be proposed that phosphorylation may interfere with the binding of PIP2 to the channel and eventually decrease the PIP2 affinity of the M channel.
The notion that a change in the channel-PIP2 interaction could possibly regulate M channel activity has already been explored. Zhang et al. (18) showed that the mutation of the histidine residue of KCNQ2 channel lowers the interaction between M channels and PIP2, and consequently, reduced current density. So, it can be assumed that channel-PIP2 interaction is one signaling mechanism that regulates M channel activity. In this respect, M channels might be a critical point of convergence for multiple lipid and nonlipid signaling pathways. For example, if the cholesterol concentration in the cellular membrane would be up-regulated, their sensitivity to changes in PIP2 could be increased, and then the effects of PLC-induced PIP2 depletion on M channels would be potentiated. Thus, M channels can be seen as coincidence detection points that integrate signals, playing a pivotal role in adjusting synaptic efficacy (25).
Physiological Importance for Cholesterol Action on the M Channel in the Sympathetic Neuron
Sympathetic activity plays a major role in regulating normal myocardial function and cardiac pathophysiology (53). Increased sympathetic activity is prognostic of poor outcome in stroke (54) and congestive heart failure (55). In fact, hypercholesterolemia is also a major risk factor of cardiovascular events (56). Recently, it was reported that hypercholesterolemia is closely related with the increase in sympathetic activity (57, 58). However, the role of cholesterol in the development of hyperexcitability in sympathetic neuron is still controversial. Furthermore, attention was mostly focused on the nerve sprouting and sympathetic hyperinnervation (59).
In our study, we showed that the enhancement of cholesterol reduced M channel activity. It appears that the level of cholesterol is an important factor for the M channel activities, because the physiological cholesterol level under the resting condition failed to modulate activities of M channels (supplemental Fig. S2). The abnormally elevated cholesterol level induced by pathological conditions, such as hypercholesterolemia, may induce the activation of PKC leading to inhibition of M channels. Considering that M channel functions as a brake on action potential firing, reduction of M channel activity by the enhancement of cholesterol might be the mechanism leading to the increased sympathetic activity observed in hypercholesterolemia.
Despite intense research on cholesterol, its role in neuronal function is not well understood. Our study provides compelling evidence showing cholesterol-induced regulation of neuronal M channels. Although several ion channels have been shown to be regulated by cholesterol, the involvement of cholesterol in channel function and neuronal excitability are only beginning to be explored.
Supplementary Material
Acknowledgment
We thank Dr. Jong-Sun Kang for helpful discussions.
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (A080604).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- AMP-PNP
- 5′-adenylyl imidodiphosphate
- PKC
- protein kinase C
- GST
- glutathione S-transferase
- HA
- hemagglutinin
- SCG
- superior cervical ganglion
- GFP
- green fluorescent protein.
REFERENCES
- 1.Libby P., Aikawa M., Schünbeck U. (2000) Biochim. Biophys. Acta 1529, 299–309 [DOI] [PubMed] [Google Scholar]
- 2.McNamara D. J. (2000) Biochim. Biophys. Acta 1529, 310–320 [DOI] [PubMed] [Google Scholar]
- 3.Sacks F. M. (1998) Am. J. Cardiol. 82, 14T–17T [DOI] [PubMed] [Google Scholar]
- 4.Spector A. A., Yorek M. A. (1985) J. Lipid Res. 26, 1015–1035 [PubMed] [Google Scholar]
- 5.Tillman T. S., Cascio M. (2003) Cell Biochem. Biophys. 38, 161–190 [DOI] [PubMed] [Google Scholar]
- 6.Yeagle P. L. (1985) Biochim. Biophys. Acta 822, 267–287 [DOI] [PubMed] [Google Scholar]
- 7.Mann R. K., Beachy P. A. (2000) Biochim. Biophys. Acta 1529, 188–202 [DOI] [PubMed] [Google Scholar]
- 8.Tabas I. (2002) J. Clin. Invest. 110, 583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baulieu E. E., Robel P., Schumacher M. (2001) Int. Rev. Neurobiol. 46, 1–32 [DOI] [PubMed] [Google Scholar]
- 10.Stoffel-Wagner B. (2001) Eur. J. Endocrinol. 145, 669–679 [DOI] [PubMed] [Google Scholar]
- 11.Hilgemann D. W. (2004) Science 304, 223–224 [DOI] [PubMed] [Google Scholar]
- 12.Liu L., Barrett C. F., Rittenhouse A. R. (2001) Am. J. Physiol. Cell Physiol. 280, C1293–C1305 [DOI] [PubMed] [Google Scholar]
- 13.Sohn J. W., Lee D., Cho H., Lim W., Shin H. S., Lee S. H., Ho W. K. (2007) J. Physiol. 580, 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin S., Wang J., Gambhir A., Murray D. (2002) Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 [DOI] [PubMed] [Google Scholar]
- 15.Gamper N., Shapiro M. S. (2007) Nat. Rev. Neurosci. 8, 921–934 [DOI] [PubMed] [Google Scholar]
- 16.Hilgemann D. W., Ball R. (1996) Science 273, 956–959 [DOI] [PubMed] [Google Scholar]
- 17.Suh B. C., Hille B. (2002) Neuron 35, 507–520 [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Craciun L. C., Mirshahi T., Rohács T., Lopes C. M., Jin T., Logothetis D. E. (2003) Neuron 37, 963–975 [DOI] [PubMed] [Google Scholar]
- 19.Hilgemann D. W., Feng S., Nasuhoglu C. (2001) Sci. STKE 2001, re19. [DOI] [PubMed] [Google Scholar]
- 20.Huang C. L., Feng S., Hilgemann D. W. (1998) Nature 391, 803–806 [DOI] [PubMed] [Google Scholar]
- 21.Mohler E. R., 3rd, Fang Y., Shaffer R. G., Moore J., Wilensky R. L., Parmacek M., Levitan I. (2007) Biochem. Biophys. Res. Commun. 358, 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romanenko V. G., Rothblat G. H., Levitan I. (2002) Biophys. J. 83, 3211–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J., Chi S., Xu H., Jin G., Qi Z. (2008) Mol. Membr. Biol. 25, 216–223 [DOI] [PubMed] [Google Scholar]
- 24.Toselli M., Biella G., Taglietti V., Cazzaniga E., Parenti M. (2005) Biophys. J. 89, 2443–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delmas P., Brown D. A. (2005) Nat. Rev. Neurosci. 6, 850–862 [DOI] [PubMed] [Google Scholar]
- 26.Hernandez C. C., Zaika O., Tolstykh G. P., Shapiro M. S. (2008) J. Physiol. 586, 1811–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Z., Bei J., Rodat-Despoix L., Liu B., Jia Q., Delmas P., Zhang H. (2008) J. Gen. Physiol. 131, 575–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winks J. S., Hughes S., Filippov A. K., Tatulian L., Abogadie F. C., Brown D. A., Marsh S. J. (2005) J. Neurosci. 25, 3400–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higashida H., Hoshi N., Zhang J. S., Yokoyama S., Hashii M., Jin D., Noda M., Robbins J. (2005) Neurosci. Res. 51, 231–234 [DOI] [PubMed] [Google Scholar]
- 30.Hoshi N., Zhang J. S., Omaki M., Takeuchi T., Yokoyama S., Wanaverbecq N., Langeberg L. K., Yoneda Y., Scott J. D., Brown D. A., Higashida H. (2003) Nat. Neurosci. 6, 564–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajo K., Kubo Y. (2005) J. Physiol. 569, 59–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho H., Kim Y. A., Ho W. K. (2006) Mol. Cells 22, 97–103 [PubMed] [Google Scholar]
- 33.Gamper N., Reznikov V., Yamada Y., Yang J., Shapiro M. S. (2004) J. Neurosci. 24, 10980–10992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soh J. W., Weinstein I. B. (2003) J. Biol. Chem. 278, 34709–34716 [DOI] [PubMed] [Google Scholar]
- 35.Liu B., Liang H., Liu L., Zhang H. (2008) Am. J. Physiol. Cell Physiol. 295, C81–91 [DOI] [PubMed] [Google Scholar]
- 36.Zaika O., Lara L. S., Gamper N., Hilgemann D. W., Jaffe D. B., Shapiro M. S. (2006) J. Physiol. 575, 49–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christian A. E., Haynes M. P., Phillips M. C., Rothblat G. H. (1997) J. Lipid Res. 38, 2264–2272 [PubMed] [Google Scholar]
- 38.Hamilton J. A. (2003) Curr. Opin. Lipidol. 14, 263–271 [DOI] [PubMed] [Google Scholar]
- 39.Karten B., Vance D. E., Campenot R. B., Vance J. E. (2002) J. Neurochem. 83, 1154–1163 [DOI] [PubMed] [Google Scholar]
- 40.Kim J. A., Maxwell K., Hajjar D. P., Berliner J. A. (1991) J. Lipid Res. 32, 1125–1131 [PubMed] [Google Scholar]
- 41.Stauffer T. P., Ahn S., Meyer T. (1998) Curr. Biol. 8, 343–346 [DOI] [PubMed] [Google Scholar]
- 42.Sharma D. K., Brown J. C., Choudhury A., Peterson T. E., Holicky E., Marks D. L., Simari R., Parton R. G., Pagano R. E. (2004) Mol. Biol. Cell 15, 3114–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sirikçi O., Ozer N. K., Azzi A. (1996) Atherosclerosis 126, 253–263 [DOI] [PubMed] [Google Scholar]
- 44.Sun Y., Ishibashi M., Seimon T., Lee M., Sharma S. M., Fitzgerald K. A., Samokhin A. O., Wang Y., Sayers S., Aikawa M., Jerome W. G., Ostrowski M. C., Bromme D., Libby P., Tabas I. A., Welch C. L., Tall A. R. (2009) Circ. Res. 104, 455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H. S., Pan Z., Shi W., Brown B. S., Wymore R. S., Cohen I. S., Dixon J. E., McKinnon D. (1998) Science 282, 1890–1893 [DOI] [PubMed] [Google Scholar]
- 46.Armstrong D., Zidovetzki R. (2008) Biophys. J. 94, 4700–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown S. G., Thomas A., Dekker L. V., Tinker A., Leaney J. L. (2005) Am. J. Physiol. Cell Physiol. 289, C543–C556 [DOI] [PubMed] [Google Scholar]
- 48.Sohn J. W., Lim A., Lee S. H., Ho W. K. (2007) J. Physiol. 582, 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robbins J., Marsh S. J., Brown D. A. (2006) J. Neurosci. 26, 7950–7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epand R. M., Lester D. S. (1990) Trends Pharmacol. Sci. 11, 317–320 [DOI] [PubMed] [Google Scholar]
- 51.Marrion N. V. (1994) Pflugers Arch. 426, 296–303 [DOI] [PubMed] [Google Scholar]
- 52.Shen W., Hamilton S. E., Nathanson N. M., Surmeier D. J. (2005) J. Neurosci. 25, 7449–7458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manger W. M. (1982) in Catecholamines in Normal and Abnormal Cardiac Function (Advances in Cardiology) (Manger W. M. ed) pp. 71–121, Karger, Basel, Switzerland: [PubMed] [Google Scholar]
- 54.Sander D., Winbeck K., Klingelhöfer J., Etgen T., Conrad B. (2001) Neurology 57, 833–838 [DOI] [PubMed] [Google Scholar]
- 55.Francis G. S., Cohn J. N., Johnson G., Rector T. S., Goldman S., Simon A. (1993) Circulation 87, VI40–48 [PubMed] [Google Scholar]
- 56.Morikage N., Kishi H., Sato M., Guo F., Shirao S., Yano T., Soma M., Hamano K., Esato K., Kobayashi S. (2006) Circ. Res. 99, 299–306 [DOI] [PubMed] [Google Scholar]
- 57.Luo T. Y., Wu C. C., Liu Y. B., Fu Y. K., Su M. J. (2004) J. Biomed. Sci. 11, 339–345 [DOI] [PubMed] [Google Scholar]
- 58.Melenovsky V., Wichterle D., Simek J., Malik J., Haas T., Ceska R., Malik M. (2003) Am. J. Cardiol. 92, 337–341 [DOI] [PubMed] [Google Scholar]
- 59.Liu Y. B., Wu C. C., Lu L. S., Su M. J., Lin C. W., Lin S. F., Chen L. S., Fishbein M. C., Chen P. S., Lee Y. T. (2003) Circ. Res. 92, 1145–1152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.