Abstract
The ability of thymocytes to assess T cell receptor (TCR) signaling strength and initiate the appropriate downstream response is crucial for determining their fate. We have previously shown that a c-Cbl RING finger mutant knock-in mouse, in which the E3 ubiquitin ligase activity of c-Cbl is inactivated, is highly sensitive to TCR-induced death signals that cause thymic deletion. This high intensity signal involves the enhanced tyrosine phosphorylation of the mutant c-Cbl protein promoting a marked increase in the activation of Akt. Here we show that this high intensity signal in c-Cbl RING finger mutant thymocytes also promotes the enhanced induction of two mediators of TCR-directed thymocyte apoptosis, Nur77 and the pro-apoptotic Bcl-2 family member, Bim. In contrast, a knock-in mouse harboring a mutation at Tyr-737, the site in c-Cbl that activates phosphatidylinositol 3-kinase, shows reduced TCR-mediated responses including suppression of Akt activation, a reduced induction of Nur77 and Bim, and greater resistance to thymocyte death. These findings identify tyrosine-phosphorylated c-Cbl as a critical sensor of TCR signal strength that regulates the engagement of death-promoting signals.
Keywords: Akt PKB, Cell Death, E3 Ubiquitin Ligase, Phosphatidylinositol 3-Kinase, T-cell Receptor, Bim, Nur77, c-Cbl
Introduction
The development of T cells in the thymus involves stringent selection processes to ensure the generation of T cells that are not strongly self-reactive (1–3). Thus thymocytes expressing T cell receptors (TCRs)2 that recognize self-peptide·major histocompatibility complex ligands with high affinity trigger strong signaling responses that result in cell death. Intriguingly, similar TCR signaling pathways are also employed for the survival and differentiation of thymocytes that interact with self-peptide·major histocompatibility complex complexes with lower affinity. The TCR is therefore an exceptional example of a receptor whose engagement directs signaling responses that can lead to opposing cell fates of survival or death. For example, in mature T cells a strong signal stimulates proliferation and cytokine production, whereas the same signal received by immature thymocytes induces an apoptotic response. In contrast, engagement of a weaker TCR signal in immature thymocytes is ineffective in producing a death signal but promotes survival. As a consequence there has been much interest in identifying the signaling molecule or molecules that can sense TCR signal strength and act as a switch to direct signaling pathways toward either a survival or a death pathway.
It has been proposed that because both low and high intensity TCR signals in thymocytes activate the Ras and calcium pathways, yet only the high intensity signal activates a death program, the TCR signal may be re-directed or “split” to activate an additional pathway by a molecule that senses the intensity of the signal (4). Recently we showed that whereas either the complete loss of c-Cbl or inactivation of its RING finger domain markedly enhances thymocyte signaling from the TCR, only the RING finger mutant mouse exhibits thymic deletion and increased sensitivity to thymocyte death (5). These differences between c-Cbl knock-out (KO) and RING finger knock-in mice have provided a unique opportunity to further characterize signaling pathways that direct TCR-mediated deletion of thymocytes.
c-Cbl is a multiadaptor proto-oncogene that possesses E3 ubiquitin ligase activity by virtue of its RING finger domain, which recruits ubiquitin conjugating enzymes (E2s). It is abundantly expressed in the thymus where it functions as a negative regulator of TCR signaling (6, 7). c-Cbl KO mice have elevated levels of TCR and CD3 on the surface of CD4+CD8+ double positive (DP) thymocytes, increased levels of Lck and Fyn, and enhanced activity of the ZAP-70 tyrosine kinase (8–11). However, despite the resultant increase in the intensity of TCR signals, c-Cbl KO thymi appear to develop normally, although experiments with major histocompatibility complex class II-restricted TCR transgenic mice do show that, in the absence of c-Cbl, the selection of CD4+ thymocytes is enhanced on a weakly selecting background (9).
To better understand the mechanisms involved in the regulation of thymocyte signaling and fate by c-Cbl we studied a knock-in mouse with a loss-of-function mutation in the c-Cbl RING finger domain (5). This mutation of the N-terminal cysteine in the RING finger at position 379 (i.e. C379A) disrupts the interactions of c-Cbl with E2 enzymes and abolishes its E3 ligase activity (12–15). Mice with this mutation have many characteristics identical to the c-Cbl KO such as increased expression of TCR, CD3, Lck, and Fyn in DP thymocytes (5). Remarkably, however, unlike the c-Cbl knock-out, the RING finger mutation results in a progressive loss of the thymus even though DP thymocytes from both mutants show equivalent increases in TCR-directed activation of ZAP-70, the Ras and Rac pathways, c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases, and calcium mobilization (5). We showed that thymic loss is not caused by a developmental block, a lack of thymic progenitors, or peripheral T cell activation (5). Rather the phenotype correlates with a marked increase in expression of CD5 and CD69 activation markers on DP thymocytes and increased sensitivity to cell death when cultured with plate-bound anti-CD3, a signal that in WT thymocytes is insufficient to induce a death response. In addition, expression of a Bcl-2 transgene rescues thymic loss and blocks anti-CD3-mediated thymocyte apoptosis (5). These findings support the hypothesis that thymic deletion in the c-Cbl RING finger mutant mouse is caused by an abnormally intense TCR-directed apoptotic signal.
Surprisingly the only signaling event in c-Cbl(C379A) thymocytes that we found to differ from the c-Cbl KO involved the markedly elevated activation of Akt in response to anti-CD3 cross-linking (5). Furthermore, we demonstrated that the mutant c-Cbl protein itself is responsible for the enhanced Akt activation through an increased interaction with the p85 regulatory subunit of PI3K (5). This is caused by high levels of TCR, CD3, and Fyn in the mutant thymocytes that results in a greater proportion of c-Cbl phosphorylation on tyrosine 737, the site recognized by the SH2 domains of p85 (16–19). Thus analysis of this mouse has provided evidence that tyrosine-phosphorylated c-Cbl can play a positive role in directing thymocyte apoptosis through its interaction with p85 and the resultant activation of the PI3K/Akt pathway.
Here we expand the study of this pathway by examining the regulation of Nur77 and Bim, two key proteins involved in thymocyte negative selection. Our analysis of the c-Cbl RING finger mutant mouse and a c-Cbl knock-in mouse with a phenylalanine substitution of tyrosine 737 has identified a novel apoptotic pathway directed by c-Cbl that activates Akt and promotes enhanced levels of Bim and Nur77.
EXPERIMENTAL PROCEDURES
Mice
The generation of c-Cbl−/−, c-Cbl(C379A), and c-Cbl(Y737F) mice have been previously described (5, 8, 30). Mice were maintained on a mixed C57BL/6J x129Sv/J background and experiments were performed in compliance with the Animal Ethics Committee at the University of Western Australia (approvals 03/100/275 and 07/100/578).
Inhibitors
Pharmacological inhibitors used and their working concentrations are: Akt inhibitor VIII, Akti-1/2, 10 μm (Calbiochem, 124018); histone deacetylase inhibitor trichostatin A, 33 or 400 nm (Sigma, T 8552); PI3K inhibitors wortmannin, 100 nm, and LY 294002, 25 μm (Alomone Labs, W-400 and L-300 respectively); PKC inhibitor Ro 31-8220, 200 nm (Alexis Biochemicals, 270-020-M001); PKC/PKD inhibitor Go6976, 400 nm (Alexis Biochemicals, 270-021-MC05); and MEK inhibitor PD98059, 40 μm (Calbiochem, 513000).
Flow Cytometry and Antibody Staining
Antibody-stained thymocyte, spleen, and lymph node cell suspensions were acquired on a BD Biosciences FACSCanto and the data were analyzed using FlowJo software (Tree Star Inc). Antibodies used were against: TCRβ (H57-597), CD4 (RM4–5), CD8 (53-6.7), CD5 (53-7.3), CD69 (H1.2F3), and B220 (RA3-6B2) (BD Biosciences). To detect intracellular levels of Nur77, thymocytes were stained with anti-CD4 and anti-CD8, fixed, and permeabilized in Cytofix/Cytoperm (BD Biosciences) for 20 min at room temperature and washed in FACS buffer. Thymocytes were then incubated with ice-cold methanol for 30 min at 4 °C and washed twice in FACS buffer before incubation with mouse anti-Nur77 (BD Biosciences Transduction Labs) for 45 min at 4 °C. Following two washes in FACS buffer thymocytes were incubated with fluorescein isothiocyanate-labeled anti-mouse IgG1 (BD Biosciences). For the detection of Bim, thymocytes were fixed and permeabilized in Cytofix/Cytoperm, washed twice in FACS buffer + 0.03% saponin, and incubated on ice for 45 min with rat anti-Bim (3C5/10B12 from Andreas Strasser) in FACS buffer containing 0.3% saponin. Bim was detected by sequential incubations with biotinylated anti-rat IgG2a (BD Biosciences) followed by streptavidin phycoerythrin (Molecular Probes), both in FACS buffer containing 0.3% saponin and on ice for 45 min. Between each step thymocytes were washed twice with FACS buffer + 0.03% saponin and the thymocytes were finally stained with anti-CD4-fluorescein isothiocyanate and anti-CD8-allophycocyanin. For the detection of intracellular levels of Lck and Fyn in thymocytes, antibodies were purchased from Santa Cruz Technologies (sc-433 and sc-434, respectively). The cells were fixed and permeabilized in Cytofix/Cytoperm (BD Biosciences) and all procedures were carried out in FACS buffer containing 0.1% saponin as previously described (5). The Lck and Fyn antibodies were detected with allophycocyanin-conjugated goat anti-mouse IgG (BD Biosciences) and the thymocytes finally stained with anti-CD4-allophycocyanin-Cy7 and anti-CD8-phycoerythrin (BD Biosciences).
Thymocyte Stimulation, Immunoprecipitation, and Immunoblotting
Thymocytes were incubated with biotinylated antibodies against CD3 (500A2), CD4 (GK1.5), or CD28 (37–51) (BD Biosciences), and stimulated by streptavidin cross-linking at 37 °C. Cells were lysed in 0.2% Nonidet P-40, 60 mm n-β-d-glucopyranoside containing buffer and lysates were analyzed by immunoprecipitation and immunoblotting as previously described (10). PLCγ-1 and ERK antibodies were purchased from Santa Cruz Technology, anti-ZAP-70 antibodies from Transduction Labs, anti-p85 and c-Cbl from Upstate Cell Signaling Solutions, and anti-actin from Sigma. Anti-phosphotyrosine (4G10) was provided by Brian Druker and anti-LAT by Larry Samelson. Antibodies to Bim, Akt, p-Akt(S473), p-ERK(Thr-202/Tyr-204), p-c-Cbl(Tyr-731 and Tyr-774), p-LAT(Tyr-191), p-PKD(Ser-916), and PKD were from Cell Signaling and p-PLCγ-1(Tyr-783) antibodies were from BIOSOURCE. Quantitation of protein bands was determined by densitometric analysis of scanned x-ray films using Kodak Molecular Imaging Software version 4.0 (Eastman Kodak Co.).
Plate-bound Antibody-mediated Assays to Determine Thymocyte Apoptosis and the Induction of Nur77 and Bim
To determine the extent of antibody-mediated thymocyte apoptosis, culture plates (96-well) were coated overnight at 4 °C with 10 μg/ml of anti-CD3 (2C11) with or without varying amounts of anti-CD28 (37.51) antibodies (BD Biosciences). Thymocytes at 2 × 106/ml were cultured in triplicate in Iscove's modified Dulbecco's medium, 10% fetal calf serum for 16 h at 37 °C, then harvested and stained with 0.2 μg/ml of propidium iodide (PI) for analysis by flow cytometry. For Nur77 and Bim analysis the thymocytes were cultured as above but in 48-well antibody-coated dishes and harvested after 2 and 4 h, respectively.
Real Time PCR Quantitation of Bim mRNA
Thymocytes (2 × 107 cells/time point) were cultured in Iscove's modified Dulbecco's medium, 10% fetal calf serum for 2 or 4 h at 37 °C in 96-well culture plates that had been coated overnight at 4 °C with 10 μg/ml of anti-CD3 (2C11) with or without 10 μg/ml anti-CD28 (37.51) of antibodies. At the end of each time point, cells were harvested and RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. First strand cDNA was prepared from 2 μg of RNA using TaqMan Reverse Transcription Reagent (Roche Applied Science). Real time PCR was performed using the ABI Prism 7900 (Applied Biosystems) and the Power SYBR Green PCR Master Mix (Applied Biosystems) in 15-μl reaction volumes. Data analyses were performed with the CT method using β-actin as an internal control.
Quantitative reverse transcription-PCR was performed using the following forward and reverse primers (a kind gift from Dr. D. C. S. Huang): Bim, 5′-GAGTTGTGACAAGTCAACACAAACC-3′ (sense) and 5′-GAAGATAAAGCGTAACAGTTGTAAGATAACC-3′ (antisense); Nur77, 5′-CCTGTTGCTAGAGTCTGCCTTC-3′ (sense) and 5′-CAATCCAATCACCAAAGCCACG-3′ (antisense); and actin, 5′-TATTGGCAACGAGCGGTTC-3′ (sense) and 5′-CCATACCCAAGAAGGAAGGCT-3′ (antisense).
RESULTS
Enhanced Nur77 and Bim Induction in c-Cbl RING Finger Mutant Thymocytes
As previously described c-Cbl RING finger mutant mice were generated through matings between heterozygous c-Cbl C379A mice (termed +/A mice) and c-Cbl−/− mice, which produce c-Cbl A/− and c-Cbl+/− littermates (5). The c-Cbl A/− offspring carry only a single copy of the mutated allele and have improved survival rates compared with homozygous c-Cbl A/A mutant mice (5).
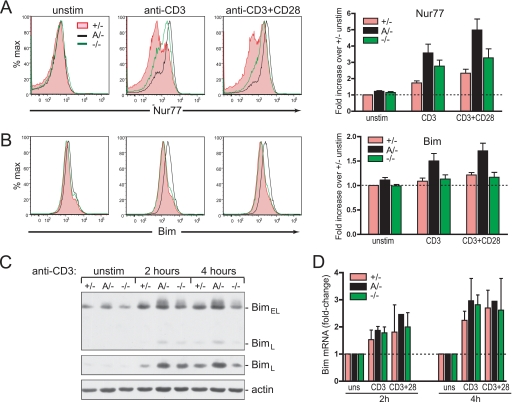
Because Nur77 and Bim are key mediators of negative selection in DP thymocytes (20–22) we tested whether the greater susceptibility of c-Cbl A/− thymocytes to TCR-mediated death may be linked to an enhanced induction of these proteins. Time course analyses found that the optimal expression of Nur77 and Bim proteins occurred after 2 and 4 h of culture, respectively (Ref. 23 and supplemental Fig. S1). Levels of Nur77 and Bim were therefore examined after culturing thymocytes with plate-bound anti-CD3 or anti-CD3 + anti-CD28 antibodies for 2 and 4 h, respectively. Flow cytometry of fixed, permeabilized, and antibody-stained DP thymocytes revealed that Nur77 protein levels are induced to markedly higher levels in c-Cbl A/− thymocytes compared with c-Cbl+/− thymocytes, and to slightly higher levels than in c-Cbl−/− thymocytes (Fig. 1A). The high level of Nur77 induction in both mutant mice by anti-CD3 stimulation was surprising because we had previously shown that this activation alone is unable to mediate c-Cbl−/− thymocyte death, whereas it produces a potent death signal in c-Cbl A/− thymocytes (5). This finding suggests that additional signaling events, other than a high level of Nur77 induction, are likely contributing to thymocyte death.
FIGURE 1.
Nur77 and Bim protein induction is enhanced in DP thymocytes from c-Cbl RING finger mutant mice. Thymocytes from WT (+/−), c-Cbl RING finger mutant mice (A/−), and c-Cbl KO mice (−/−) were cultured with plate-bound anti-CD3 or anti-CD3 + anti-CD28 antibodies (at 10 μg/ml) for 2 or 4 h before fixation, permeabilization, and intracellular staining with (A) anti-Nur77 antibody (12.14), or (B) anti-Bim antibody (3C5/10B12). Representative flow cytometry profiles are from the CD4+CD8+-gated population. The bar graphs with S.E. show the mean level of induction relative to unstimulated WT thymocytes. The quantitation was determined using geometric mean fluorescence from four independent experiments. C, cell lysates of thymocytes from c-Cbl+/−, A/−, and −/− mice were left unstimulated or cultured with plate-bound anti-CD3 for 2 or 4 h and immunoblotted with anti-Bim antibody. The alternatively spliced forms of Bim, i.e. BimEL and BimL are indicated. The middle panel shows a longer exposure to more clearly reveal the relative levels of BimL between the three genotypes. The anti-actin blot was used as a loading control. D, the c-Cbl RING finger mutation does not enhance Bim mRNA induction following anti-CD3 or anti-CD3 + anti-CD3 + anti-CD28 stimulation. mRNA prepared from thymocytes cultured for 2 and 4 h was measured by real time quantitative PCR. The bar graphs represent Bim mRNA levels in stimulated cells relative to unstimulated controls (± S.E.) for each genotype from three independent experiments.
Analysis of Bim induction in DP thymocytes in response to anti-CD3 and anti-CD3 + anti-CD28 stimulation showed that Bim levels were also induced to much higher levels in c-Cbl A/− thymocytes (Fig. 1B). Interestingly, however, in contrast to Nur77, no significant difference was seen in the extent of Bim induction between c-Cbl+/− and c-Cbl−/− DP thymocytes. Both genotypes showed similar modest but consistent increases of 10–20% in the levels of Bim in response to culturing with the plate-bound antibodies (Fig. 1B, bar graph). These findings suggest that the enhanced induction of Bim in c-Cbl A/− thymocytes may be an important factor in mediating thymic deletion. The enhanced levels of Bim protein in c-Cbl A/− thymocytes was further confirmed by immunoblotting lysates from anti-CD3-stimulated thymocytes with an anti-Bim antibody (Fig. 1C). These results showed that both BimEL and BimL isoforms were induced to higher levels in c-Cbl A/− thymocytes compared with WT and c-Cbl−/− thymocytes.
Enhanced Levels of Bim Protein in c-Cbl RING Finger Mutant Mice Are Caused by Post-transcriptional Events
Since the enhanced sensitivity to a stimulatory death signal correlated with increased Bim protein levels in c-Cbl A/− compared with c-Cbl−/− or c-Cbl+/− thymocytes (Fig. 1, B and C), we performed quantitative real time PCR to determine whether this effect was due to increased transcription of Bim in c-Cbl A/− thymocytes. Thymocytes from c-Cbl A/−, c-Cbl+/−, and c-Cbl−/− mice were cultured for 2 or 4 h in medium alone or in the presence of plate-bound anti-CD3 or anti-CD3 + anti-CD28 antibodies. At each time point cells were harvested and total RNA extracted for analysis. PCR quantitation showed that whereas stimulation with either anti-CD3 or anti-CD3 + CD28 antibodies induced an increase in the levels of Bim mRNA, there was no significant difference in the extent of this increase among the three genotypes (Fig. 1D). Thus the higher level of Bim protein in c-Cbl A/− thymocytes appears to be due to post-transcriptional events.
Pharmacologic Inhibitors Reveal Differing Requirements for MEK and Akt in the Induction of Nur77 and Bim
We have previously shown that the PI3K/Akt pathway is markedly enhanced in thymocytes from the RING finger knock-in mouse but not the c-Cbl−/− mouse. In contrast, all other pathways examined, including the ERK pathway, are equivalently enhanced in both mutants compared with thymocytes from WT mice (5). In addition we have shown that culturing c-Cbl A/− thymocytes in the presence of the PI3K inhibitor LY294002 suppresses anti-CD3-induced death (5). In this study we examined the roles of the PI3K/Akt and ERK/MEK pathways in the induction of Nur77 and Bim through the use of pharmacological inhibitors. The inhibitors included wortmannin, a potent inhibitor of the PI3K superfamily that shows excellent specificity except for its inhibition against the smooth muscle isoform of myosin light chain kinase (IC50 of 260 nm) (24), and Akti, a specific inhibitor against the Akt1 and Akt2 isoforms of Akt that exhibits no inhibitory effects against the closely related AGC family kinases, PKA, PKC, and serum- and glucocorticoid-induced kinase (25). We found that Nur77 and Bim protein induction in thymocytes from c-Cbl A/− mice were inhibited by both wortmannin and Akti treatment (Fig. 2, A–D), although of the two compounds Akti consistently showed a more inhibitory effect on Bim than Nur77. We found similar levels of inhibition of Nur77 and Bim when we examined the effects of LY 294002, another commonly used inhibitor of PI3K (supplemental Fig. S2). LY 294002 does not show activity against the smooth muscle isoform of myosin light chain kinase but is active against casein kinase II, whereas wortmannin is not (24).
FIGURE 2.
Pharmacological inhibitors reveal differential regulation of Nur77 and Bim in c-Cbl RING finger mutant DP thymocytes. Thymocytes were cultured with or without plate-bound anti-CD3 in the presence of DMSO, 100 nm PI3K inhibitor wortmannin, 10 μm Akt inhibitor Akti-1/2, 40 μm MEK inhibitor PD98059, or 33 nm histone deacetylase inhibitor trichostatin A. Nur77 protein levels were determined after 2 h of culture in (A) and Bim protein levels were determined after 4 h (B). Flow cytometry profiles are of the CD4+CD8+-gated population and the numbers represent the degree of stimulation in inhibitor-treated cultures normalized to DMSO-treated controls, with the increase in geometric mean fluorescence of anti-CD3 stimulated versus unstimulated cultures treated with DMSO taken as 1.00. C and D, fold-change in the levels of Nur77 and Bim protein relative to the anti-CD3 DMSO controls. The quantitation was determined by geometric mean fluorescence and the data represent three independent experiments. E, WT DP thymocytes show the same pattern of inhibition as c-Cbl RING finger thymocytes to treatment with Akt and MEK inhibitors. Thymocytes from WT mice were cultured with plate-bound anti-CD3 in the presence of DMSO, Akti-1/2, or PD98059 and the levels of Nur77 and Bim were determined by flow cytometry. The fold-changes are normalized to the change in geometric mean fluorescence of anti-CD3 versus unstimulated DMSO-treated thymocytes.
In contrast to inhibitors of the PI3K/Akt pathway, MEK inhibitor PD98059 only minimally affected the induction of Bim, whereas Nur77 was markedly inhibited (Fig. 2, A–D). PD98059 is a selective inhibitor of MEK and to date no other protein kinases have been identified that are inhibited by PD98059 (24). These findings indicate that Bim and Nur77 are both regulated by a TCR-activated PI3K/Akt pathway, but the enhanced induction of Bim in c-Cbl RING finger thymocytes is largely independent of activated MEK. This finding supports our earlier findings that the PI3K/Akt pathway, but not the ERK pathway, is a key component in directing the strong death signal in c-Cbl A/− thymocytes (5). Consistent with these findings we found that Nur77 and Bim induction in WT thymocytes showed a similar pattern of dependence for MEK and Akt, respectively (Fig. 2E). We found a small inhibitory effect of Akti on Nur77 induction, whereas Bim was markedly suppressed. In contrast, inhibition of MEK by PD98059 was very effective in blocking Nur77, whereas Bim levels remained unaffected.
Trichostatin A Inhibits Rather Than Induces Nur77 in DP Thymocytes
Because histone deacetylase 7 (HDAC7) is highly expressed in DP thymocytes and has been found to inhibit activity of the Nur77 promoter in the T cell hybridoma DO11.10 (26), we tested the effect of the HDAC inhibitor trichostatin A (TSA) on Nur77 induction in c-Cbl A/− thymocytes. TSA is a potent inhibitor of HDACs, which is highly active at nanomolar concentrations. In marked contrast to findings in DO11.10 T cells, where TSA was found to induce Nur77 expression, we found that TSA markedly inhibited the induction of Nur77 in c-Cbl A/− thymocytes (Fig. 2, A and C). In addition, we also found that TSA had a similar inhibitory effect on the induction of Bim (Fig. 2, B and D). The reason for the conflicting results of the effect of TSA on Nur77 is not clear, however, Dequiedt and colleagues (26) only examined the effects of TSA on DO11.10 T cells, not primary thymocytes, and used TSA at a higher concentration of 400 nm, compared with 33 nm in our experiments. However, even when we treated WT thymocytes with 400 nm TSA for up to 6 h we found no evidence of Nur77 induction (Fig. 3A). In addition, 400 nm TSA was found to inhibit Nur77 induction in anti-CD3 and anti-CD3 + anti-CD28-stimulated WT thymocytes to a level equivalent to that observed with 33 nm (Fig. 3B). These findings indicate that DO11.10 hybridoma cells differ in their regulation of Nur77 compared with primary thymocytes and as such may not be an appropriate model for examining thymocyte signaling.
FIGURE 3.
TSA inhibits rather than induces Nur77 in DP thymocytes. A, WT thymocytes were cultured in the presence of DMSO or 400 nm TSA for 2, 4, or 6 h without plate-bound antibodies before analysis by flow cytometry to determine the levels of Nur77 protein. CD4+CD8+ DP thymocyte-gated profiles are shown. B, the inhibition of Nur77 is equivalent in the presence of either 33 or 400 nm TSA. WT thymocytes were cultured for 2 h with plate-bound anti-CD3 or anti-CD3 + anti-CD28 in the presence or absence of the indicated concentrations of TSA. Unstimulated thymocytes cultured in the absence of plate-bound antibodies are indicated by the shaded green histogram. Nur77 levels in DP thymocytes were determined as in A.
An additional observation in DO11.10 T cells relating to the regulation of Nur77 that is relevant to this study is the finding that the induction of Nur77 by phorbol 12-myristate 13-acetate is suppressed by Gö6976, an inhibitor that targets both calcium-dependent isoforms of protein kinase C (PKC) and protein kinase D (PKD) (27–29). Consistent with the findings for phorbol 12-myristate 13-acetate we found that Gö6976 also had an inhibitory effect on the induction of Nur77 by anti-CD3, whereas no inhibition was seen with Ro 31-8220, a selective inhibitor of PKC that does not affect PKD (27, 28) (Fig. 4, A and B). It has been found in DO11.10 T cells that PKD participates in the induction Nur77 by phosphorylating HDAC7, which results in its nuclear export, thus removing its inhibitory presence (29). However, because our findings with TSA indicate that HDACs are not inhibitory in thymocytes, but appear to be required for promoting the induction of Nur77, this suggests that another mechanism for the activity of PKD is involved. An analysis of this mechanism is beyond the scope of this study, however, because PKD clearly has a role in regulating Nur77 we investigated whether the induction of phospho-PKD differed between c-Cbl A/− and c-Cbl−/− thymocytes. As shown in Fig. 4C, although anti-CD3 + anti-CD4-stimulated thymocytes from both mutants show a greater induction of phospho-PKD than WT, each were activated to equivalent levels and with similar kinetics. This finding is similar to that observed for phospho-PLCγ1 and phospho-ERK, but in marked contrast to the marked activation of Akt seen only in the RING finger mutant (Fig. 4C and Ref. 5). Thus, as with PLCγ1 and ERK, a perturbation to PKD signaling activity does not provide an explanation for the marked differences in sensitivity to thymocyte death that exists between the two c-Cbl mutants.
FIGURE 4.
A, the induction of Nur77 in c-Cbl A/− thymocytes is inhibited by a PKC/PKD inhibitor but not by an inhibitor of PKC. Flow cytometry profiles of Nur77 protein expression induced by culturing thymocytes for 2 h in the presence of plate-bound anti-CD3 antibodies plus DMSO, Gö6976 (a dual PKC/PKD inhibitor), or Ro 31-8220 (a specific PKC inhibitor) is shown in A. The level of inhibition relative to the anti-CD3 DMSO control is indicated by numbers in each flow cytometry profile and in B, which represents data from two independent experiments. C, the activation of PKD is equivalently enhanced in c-Cbl A/− and c-Cbl−/− thymocytes. Thymocytes from WT (+/−), c-Cbl RING finger mutant mice (A/−), and c-Cbl KO mice (−/−) were left unstimulated or stimulated by cross-linking with anti-CD3 + anti-CD4 antibodies for 5 or 15 min at 37 °C. Cell lysates were immunoblotted with the indicated phosphospecific antibodies or anti-actin as a loading control.
Characterization of Mice with a c-Cbl(Y737F) Mutation
Previously we showed that a greater proportion of c-Cbl protein in RING finger mutant thymocytes is phosphorylated on tyrosine 737, which promotes a greater association with the p85 regulatory subunit of PI3K resulting in enhanced Akt activation (5). Tyr-737, together with Tyr-706 and Tyr-780, represent the three major tyrosine phosphorylation sites in mouse c-Cbl (equivalent to Tyr-731, Tyr-700, and Tyr-774 of human c-Cbl, respectively). To further investigate the involvement of phosphotyrosine 737 in the activation of the PI3K/Akt pathway we generated a c-Cbl knock-in mouse where tyrosine 737 has been mutated to a phenylalanine (i.e. c-Cbl(Y737F)) (30). We found that homozygous c-Cbl Y737F mice (termed F/F) were viable and born at close to the expected frequencies from heterozygous matings (i.e. of 250 mice 23% were genotyped as F/F, 48% as +/F, and 29% as +/+). Thymocytes from homozygous F/F mutant mice were found to be present in normal numbers (18.35 ± 1.77 × 106 for +/+, n = 27, versus 22.03 ± 2.11 × 106 for F/F, n = 25; mean ± S.E.), showed no developmental perturbations, and the cell surface expression of TCR, CD3, CD4, and CD8 were unaltered compared with WT littermates (Fig. 5, A and B). In addition, levels of CD5 and CD69 activation markers were unchanged compared with normal littermates, as were levels of protein-tyrosine kinases, Lck, Fyn, and ZAP-70 (Fig. 5B and supplemental Fig. S3). Furthermore, no changes in T or B cell numbers were evident in lymph nodes or spleen (Fig. 5C) nor were spleen sizes affected (87.9 ± 5.0 mg for +/+, n = 17, versus 84.5 ± 4.2 mg for F/F, n = 18; mean ± S.E.).
FIGURE 5.
Thymocyte development in c-Cbl(Y737F) mice is normal. Thymocytes from 7-week-old WT (+/+) and homozygous c-Cbl(Y737F) (F/F) littermates were examined by flow cytometry for: A, the proportions of CD4 and CD8 single positive, DP, and double negative cells, and B, for the expression of TCR, CD5, and CD69 on total and gated DP thymocytes. C, analysis of lymph node and spleen cells using the anti-TCR and B220 antibodies shows that the proportion of T and B cells in the periphery are not affected by the c-Cbl(Y737F) mutation.
c-Cbl(Y737F) Mutation Abolishes Binding to the p85 Regulatory Subunit of PI3K
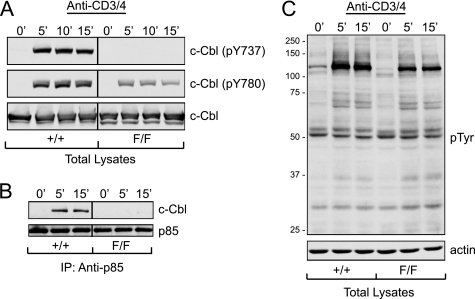
The lack of phosphorylation at Tyr-737 in c-Cbl F/F thymocytes following anti-CD3 + anti-CD4 stimulation was shown by immunoblotting with a phosphospecific antibody directed against this residue (Fig. 6A). In addition, the mutation also reduced the level of phosphorylation of Tyr-780, one of the other major tyrosine phosphorylation sites in c-Cbl (18, 31). Thus Tyr-737 plays an important role in determining the level of c-Cbl tyrosine phosphorylation. However, the tyrosine phosphorylation of other substrates in thymocytes did not appear affected by the Y737F mutation when total lysates were examined by immunoblotting with anti-phosphotyrosine antibodies (Fig. 6C). Significantly the immunoprecipitation of lysates with anti-p85 antibodies showed that the Y737F mutation completely abolishes the inducible association between c-Cbl and p85 (Fig. 6B). This finding demonstrates that phosphorylation of Tyr-737 is an absolute requirement for this interaction.
FIGURE 6.
A, the c-Cbl(Y737F) mutation reduces the level of phosphorylation of Tyr-780 in c-Cbl. Thymocytes from 6-week-old littermates were left unstimulated or stimulated by cross-linking with anti-CD3 + anti-CD4 antibodies for the indicated times at 37 °C. Lysates were immunoblotted with anti-c-Cbl(pY737), anti-c-Cbl(pY780), or anti-c-Cbl antibodies. B, the c-Cbl(Y737F) mutation abolishes the induced association between c-Cbl and the p85 regulatory subunit of PI3K. Thymocytes were stimulated as in A and the lysates immunoprecipitated (IP) with anti-p85 antibodies and immunoblotted with either anti-c-Cbl or anti-p85 antibodies. C, the total phosphotyrosine signal is not affected by the c-Cbl(Y737F) mutation. Thymocytes from WT (+/+) and c-Cbl(Y737F) (F/F) mice were stimulated as above and total lysates were immunoblotted with anti-phosphotyrosine (4G10) or anti-actin antibodies.
The Activation of Akt Is Dependent on the Phosphorylation of Tyr-737
To further characterize the effects of the Y737F mutation on thymocyte signaling we immunoblotted lysates with a range of phosphospecific antibodies following their stimulation by cross-linking with anti-CD3 + anti-CD4 or anti-CD3 + anti-CD28 antibodies. Anti-phospho-Akt immunoblotting showed that phosphorylation of Ser-473 was markedly reduced in c-Cbl F/F thymocytes compared with thymocytes from normal littermates, most notably at the later time points of 10 and 15 min (Fig. 7, A and B, top panels). This finding is consistent with our observations that hyperphosphorylation of Tyr-737 is responsible for the marked enhancement in Akt activation seen in c-Cbl(C379A) RING finger mutant thymocytes (5). Additional analysis showed that the activation-induced phosphorylation of PLCγ1, ERK, and LAT were also suppressed in c-Cbl F/F thymocytes, with the marked effects being evident at later time points (Fig. 7). Interestingly we always observed an increase in pERK induction in F/F thymocytes compared with WT thymocytes at 5 min, but this activation was rapidly diminished by 10 and 15 min. This is consistent with ERK activation being regulated by multiple signaling molecules but the data clearly demonstrates that the sustained activation of ERK is largely reliant on c-Cbl tyrosine phosphorylation.
FIGURE 7.
The activation of Akt in thymocytes is dependent on the phosphorylation of Tyr-737. Thymocytes from c-Cbl+/+ and F/F littermates were stimulated with anti-CD3 + anti-CD4 (A) or anti-CD3 + anti-CD28 (B) antibodies for the indicated times at 37 °C before lysis and immunoblotting with phosphospecific or protein-specific antibodies. The numbers under each stimulated track refer to densitometry readings that have been normalized for protein loading and expressed as values relative to the phosphoantibody signal for +/+ thymocytes after 5 min of stimulation.
Mutation of the p85 Binding Site in c-Cbl Inhibits TCR-mediated Induction of Nur77 and Bim
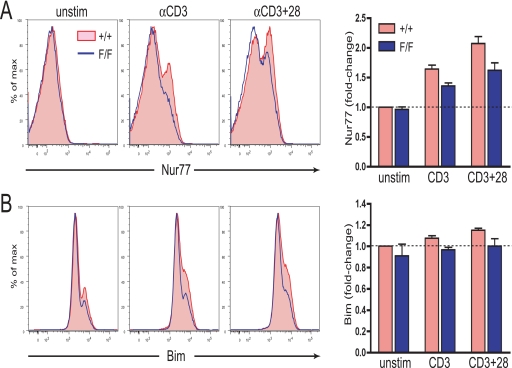
To further characterize the downstream effects of blocking the phosphorylation of Tyr-737 in c-Cbl we examined the induction of Nur77 and Bim following incubation of thymocytes with plate-bound anti-CD3 and anti-CD3 + CD28 antibodies. Analysis by flow cytometry of DP thymocytes from WT and c-Cbl(Y737F) mutant mice revealed a reduced level of induction of both Nur77 and Bim in thymocytes from c-Cbl F/F mice (Fig. 8, A and B). This finding firmly establishes a link between the extent of c-Cbl tyrosine phosphorylation and the degree of induction of two key mediators of thymocyte apoptosis. The wide ranging effects of this mutation on signaling pathways other than the PI3K/Akt pathway, including ERK, may explain the reduced induction of Nur77, which from our inhibitor studies is less dependent on Akt signaling but highly dependent on the activity of MEK. Although we were aiming for a mutation that would specifically perturb the PI3K/Akt pathway the findings support the hypothesis that c-Cbl tyrosine phosphorylation has a profound impact on the signaling events in thymocytes that regulate Nur77 and Bim.
FIGURE 8.
Nur77 and Bim induction is suppressed in c-Cbl(Y737F) thymocytes. Thymocytes from +/+ and F/F littermates were cultured in the absence of plate-bound antibody (i.e. unstimulated thymocytes) or with plate-bound anti-CD3 or anti-CD3 + anti-CD28 antibodies for 2 h for Nur77 induction (A) and 4 h for Bim induction (B). The bar graphs represent data from three independent experiments with the numbers calculated from the geometric mean fluorescence relative to unstimulated +/+ thymocytes that have been normalized to 1.00.
Mutation of the c-Cbl Tyr-737 Site Reduces Thymocyte Sensitivity to CD3 + CD28-induced Cell Death
c-Cbl A/− thymocytes are more sensitive to induction of cell death (5) and in this study we have shown that this correlates with increased induction of pro-apoptotic proteins Nur77 and Bim (Fig. 1). Because mutation of the c-Cbl Tyr-737 site has a marked effect in inhibiting TCR-mediated Nur77 and Bim expression (Fig. 8), we examined whether this mutation also reversed the sensitivity of thymocytes to in vitro cell death.
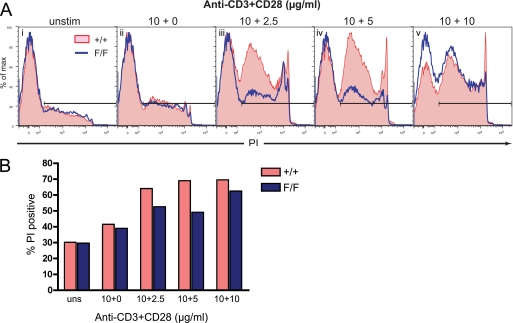
Thymocytes from WT or c- Cbl(Y737F) mutant mice were cultured in medium alone or in the presence of plate-bound anti-CD3 antibodies (10 μg/ml) and varying amounts of anti-CD28 antibodies. The extent of cell death induced by each treatment was measured as the percentage of PI positive cells at the end of a 16-h culture period (Fig. 9). Efficient thymocyte apoptosis in vitro requires triggering of both CD3 and CD28 receptors and, consistent with this, we observed negligible death when cells were cultured with anti-CD3 alone (Fig. 9A, ii). However, when cultured with anti-CD3 and anti-CD28 a lower proportion of c-Cbl(Y737F) thymocytes became PI positive compared with WT thymocytes (Fig. 9, A, iii and iv, and B). Compared with cells stimulated with anti-CD3 alone, the addition of 2.5 μg/ml of anti-CD28 antibody induced a 23% increase in PI positive WT thymocytes but only a 14% enhancement of PI positive thymocytes from c-Cbl(Y737F) mice (Fig. 9, A, iii, and B). Similarly, addition of 5 μg/ml of anti-CD28 led to a 27% increase in PI positive WT thymocytes over CD3 stimulation alone, compared with a 10% increase in c-Cbl(Y737F) thymocytes (Fig. 9, A, iv, and B). These results demonstrate that the phosphorylation of c-Cbl Tyr-737 plays a role in determining the potency of signaling responses that direct thymocyte death. We also observed that the Y737F mutation was not as effective in suppressing thymocyte death when cultured in the presence of 10 μg/ml of both anti-CD3 and anti-CD28 (Fig. 9A, v), suggesting the c-Cbl-directed death pathway can be bypassed or overwhelmed by a very high intensity signal.
FIGURE 9.
c-Cbl(Y737F) thymocytes are less susceptible to an anti-CD3 + anti-CD28 death signal. A, thymocytes from 7-week-old +/+ and F/F littermates were cultured for 16 h with or without plate-bound anti-CD3 (10 μg/ml) and 0, 2.5, 5, or 10 μg/ml of anti-CD28 antibodies as indicated above (panels i–v). After harvesting, the cells were incubated with PI at a final concentration of 0.2 μg/ml and analyzed by flow cytometry. The gates indicate the PI+ populations and the findings shown are representative of three independent experiments. B, bar graph showing the % PI positive cells under each stimulation condition shown in A.
DISCUSSION
The T cell receptor is a rare example of a receptor whose engagement directs signaling responses that can lead to opposing fates of cell survival or death. In mature T cells, a strong signal, such as that delivered by co-activation of the CD3 and CD28 receptors, stimulates proliferation and cytokine production. In contrast, the same signal received by DP thymocytes will induce an apoptotic response. However, engagement of the CD3 receptor alone, a weaker signal, is ineffective in promoting a death signal in DP thymocytes. As a consequence there has been a great deal of investigation to identify the signaling molecule or molecules that can act as a switch to activate signaling pathways toward either survival or death. Neilson and colleagues (4) proposed a model for thymocyte fate determination whereby a key molecule acts as a “signal splitter” that senses the strength of a signal initiated at the TCR and hence whether a cell death program should be engaged. Our findings here, and in a previous study (5), suggest that c-Cbl may be a point at which this “signal splitting” can occur.
We previously showed that thymocytes lacking a functional c-Cbl RING finger domain were induced to die when stimulated by anti-CD3 cross-linking, and that this signal correlated with greatly increased c-Cbl tyrosine phosphorylation and the activation of Akt (5). This enhanced susceptibility to TCR-directed death demonstrated the importance of the c-Cbl RING finger domain in regulating thymocyte signaling, however, other domains of c-Cbl were clearly required as the complete loss of c-Cbl did not cause thymic deletion or hyperactivation of Akt (5).
To further examine the role of c-Cbl in determining the fate of thymocytes we first sought to identify proteins that might be responsible for thymic loss in c-Cbl RING finger mutant thymocytes. Nur77 and Bim were identified as two such proteins whose induction was found to be increased in c-Cbl RING finger mutant thymocytes compared with WT and c-Cbl−/− thymocytes under conditions that promote apoptosis (Fig. 1). This was an important finding as the involvement of Nur77 and Bim in thymic deletion has been demonstrated in numerous studies (20, 21, 32, 33). Bim best fits the criteria as a key causative molecule that distinguishes the c-Cbl A/− and c-Cbl KO phenotypes because c-Cbl KO and WT thymocytes show an equivalent minimal induction of Bim compared with the large Bim induction found in c-Cbl A/− thymocytes (Fig. 1). In contrast, although Nurr77 levels are enhanced in c-Cbl A/− thymocytes, they are also elevated in c-Cbl KO compared with WT thymocytes. The possibility that Bim may be a major player in the proposed c-Cbl/Akt death pathway was further supported by a greater dependence of Bim protein induction on the activity of Akt, while showing little dependence on MEK (Fig. 2). In contrast, Nur77 was found to be highly sensitive to MEK inhibition but less dependent on the activity of Akt (Fig. 2). These findings, as well as data shown in Fig. 4B, are consistent with our previous findings that ERK activation is enhanced in c-Cbl A/− and KO thymocytes equivalently, and is therefore unlikely to be causing thymocyte death, whereas it is only c-Cbl A/− thymocytes that show a marked activation of Akt (5).
Following TCR stimulation, the mutant c-Cbl protein in A/− thymocytes is more highly phosphorylated on Tyr-737 resulting in increased c-Cbl/p85 association and PI3K/Akt activation (5). These results suggest that thymic loss in the c-Cbl RING finger mutant mouse is at least in part due to enhanced activation of the PI3K/Akt pathway by phosphorylated Tyr-737. To more closely investigate the roles of Tyr-737 and p85 in thymocyte signaling we examined a mouse with a Y737F knock-in mutation (i.e. the c-Cbl F/F mouse). We found that mutation of Tyr-737 effectively abolished the TCR-induced association between p85 and c-Cbl, and as a consequence markedly suppressed the activation of Akt (see Figs. 6 and 7). The extent of this suppression indicates that c-Cbl is an important, if not the most important, activator of the PI3K/Akt pathway in TCR-stimulated thymocytes. c-Cbl has been well characterized as a prominent substrate of protein-tyrosine kinases in thymocytes and this study has definitively identified a functional role for this phosphorylation. Mutation of Tyr-737 also caused other inhibitory effects, notably the reduced phosphorylation of Tyr-780, which associates with the SH2 domain CrkL (34) (see Fig. 6A). The reduced phosphorylation of Tyr-780 may be a result of reduced interactions with Fyn and Syk, two kinases that primarily associate with c-Cbl through Tyr-737 (18, 19). This may affect other sites of c-Cbl phosphorylation and explain why we also observe reduced activation of ERK, PLCγ1, and LAT in Y737F thymocytes compared with WT thymocytes (see Fig. 7).
Examination of the effect of the Y737F mutation on Nur77 and Bim expression revealed that weaker TCR-directed signals resulted in reduced induction in the levels of both proteins (Fig. 8). This was not surprising given the widespread impact of the mutation on thymocyte signaling. This finding additionally demonstrated the central role that c-Cbl tyrosine phosphorylation plays in regulating Bim and Nur77 protein levels.
In this study we found that the enhanced induction of Bim protein in the c-Cbl RING finger mutant thymocytes did not involve an increase in Bim transcription (see Fig. 1), indicating that perturbations of post-transcriptional events are responsible for the increase in protein levels. These events may involve a greater stability of Bim through an inability of the c-Cbl RING finger mutant protein to ubiquitylate Bim, a function identified in osteoclasts (35, 36). Whether c-Cbl directs the ubiquitylation of Bim in thymocytes following TCR stimulation is not known, however, the fact that c-Cbl KO thymocytes do not have altered Bim protein levels suggests other causes are likely to be involved. It has also been proposed that the thymic phenotype in the c-Cbl A/− mouse may involve a dominant negative effect on Cbl-b, however, this is unlikely given the very low level of Cbl-b protein in thymocytes (37) and the fact that the Cbl-b KO (38, 39) and Cbl-b RING finger knock-in mice show no thymic phenotype.3 Furthermore, the enhanced signaling events observed in the c-Cbl RING finger mutant thymus are not evident in the thymus of the c-Cbl/Cbl-b double mutant mouse indicating that these effects of the c-Cbl RING finger mutation cannot be explained or recapitulated by the additional loss of Cbl-b activity (40).
In summary, the work presented here supports and expands the two main observations from our original study of the c-Cbl RING finger mutant mouse, (i) that the c-Cbl RING finger mutant protein has roles both as a gain-of-function and as a loss-of-function protein and (ii) that enhanced activation of the PI3K/Akt pathway by tyrosine-phosphorylated c-Cbl promotes thymocyte death. Interestingly this gain-of-function role for c-Cbl RING finger mutants has recently been appreciated as a key element in the activation of Akt associated with a range of human myeloproliferative neoplasms that carry c-Cbl mutations (41). However, it remains a conundrum that Akt signaling can promote thymocyte apoptosis when the universally accepted role of Akt is to promote survival (42–44). However, given that a major task of the thymus is to carry out an unparalleled program of cell death it may not be unreasonable to envisage that a unique mechanism needs to be employed. Future studies of c-Cbl RING finger and p85-binding mutant mice on TCR transgenic and Akt-deficient backgrounds should help to resolve this point.
Supplementary Material
Acknowledgments
We thank Helen Moulder for animal care, Brian Druker for anti-phosphotyrosine, and Andreas Strasser for anti-Bim antibodies.
This work was supported by National Health and Medical Research Council (NHMRC) (Canberra) Grant 458539, an infrastructure grant from the Medical and Health Research Infrastructure Fund, Department of Health, Western Australia, and NHMRC Fellowships 406675 (to C. L. S.) and 303112 (to W. Y. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
W. Langdon and C. Thien, unpublished data.
- TCR
- T cell receptor
- KO
- knock-out
- DMSO
- dimethyl sulfoxide
- DP
- double positive
- WT
- wild type
- PI3K
- phosphatidylinositol 3-kinase
- SH2
- Src homology domain 2
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- FACS
- fluorescence-activated cell sorter
- PLC
- phospholipase C
- ERK
- extracellular signal-regulated kinase
- PI
- propidium iodide
- PKC
- protein kinase C
- HDAC
- histone deacetylase
- TSA
- trichostatin A.
REFERENCES
- 1.Palmer E. (2003) Nat. Rev. Immunol. 3, 383–391 [DOI] [PubMed] [Google Scholar]
- 2.Ohashi P. S. (2003) Curr. Opin. Immunol. 15, 668–676 [DOI] [PubMed] [Google Scholar]
- 3.Siggs O. M., Makaroff L. E., Liston A. (2006) Curr. Opin. Immunol. 18, 175–183 [DOI] [PubMed] [Google Scholar]
- 4.Neilson J. R., Winslow M. M., Hur E. M., Crabtree G. R. (2004) Immunity 20, 255–266 [DOI] [PubMed] [Google Scholar]
- 5.Thien C. B., Blystad F. D., Zhan Y., Lew A. M., Voigt V., Andoniou C. E., Langdon W. Y. (2005) EMBO J. 24, 3807–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y. C. (2004) Annu. Rev. Immunol. 22, 81–127 [DOI] [PubMed] [Google Scholar]
- 7.Thien C. B., Langdon W. Y. (2005) Biochem. J. 391, 153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy M. A., Schnall R. G., Venter D. J., Barnett L., Bertoncello I., Thien C. B., Langdon W. Y., Bowtell D. D. (1998) Mol. Cell. Biol. 18, 4872–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naramura M., Kole H. K., Hu R. J., Gu H. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 15547–15552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thien C. B., Bowtell D. D., Langdon W. Y. (1999) J. Immunol. 162, 7133–7139 [PubMed] [Google Scholar]
- 11.Thien C. B., Scaife R. M., Papadimitriou J. M., Murphy M. A., Bowtell D. D., Langdon W. Y. (2003) J. Exp. Med. 197, 503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng N., Wang P., Jeffrey P. D., Pavletich N. P. (2000) Cell 102, 533–539 [DOI] [PubMed] [Google Scholar]
- 13.Joazeiro C. A., Wing S. S., Huang H., Leverson J. D., Hunter T., Liu Y. C. (1999) Science 286, 309–312 [DOI] [PubMed] [Google Scholar]
- 14.Levkowitz G., Waterman H., Ettenberg S. A., Katz M., Tsygankov A. Y., Alroy I., Lavi S., Iwai K., Reiss Y., Ciechanover A., Lipkowitz S., Yarden Y. (1999) Mol. Cell 4, 1029–1040 [DOI] [PubMed] [Google Scholar]
- 15.Thien C. B., Walker F., Langdon W. Y. (2001) Mol. Cell 7, 355–365 [DOI] [PubMed] [Google Scholar]
- 16.Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J., Neel B. G., Birge R. B., Fajardo J. E., Chou M. M., Hanafusa H., Schaffhausen B., Cantley L. C. (1993) Cell 72, 767–778 [DOI] [PubMed] [Google Scholar]
- 17.Deckert M., Elly C., Altman A., Liu Y. C. (1998) J. Biol. Chem. 273, 8867–8874 [DOI] [PubMed] [Google Scholar]
- 18.Feshchenko E. A., Langdon W. Y., Tsygankov A. Y. (1998) J. Biol. Chem. 273, 8323–8331 [DOI] [PubMed] [Google Scholar]
- 19.Grossmann A. H., Kolibaba K. S., Willis S. G., Corbin A. S., Langdon W. S., Deininger M. W., Druker B. J. (2004) FEBS Lett. 577, 555–562 [DOI] [PubMed] [Google Scholar]
- 20.Sohn S. J., Thompson J., Winoto A. (2007) Curr. Opin. Immunol. 19, 510–515 [DOI] [PubMed] [Google Scholar]
- 21.Bouillet P., Purton J. F., Godfrey D. I., Zhang L. C., Coultas L., Puthalakath H., Pellegrini M., Cory S., Adams J. M., Strasser A. (2002) Nature 415, 922–926 [DOI] [PubMed] [Google Scholar]
- 22.Strasser A., Puthalakath H., O'Reilly L. A., Bouillet P. (2008) Immunol. Cell Biol. 86, 57–66 [DOI] [PubMed] [Google Scholar]
- 23.Maltzman J. S., Kovoor L., Clements J. L., Koretzky G. A. (2005) J. Exp. Med. 202, 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnett S. F., Defeo-Jones D., Fu S., Hancock P. J., Haskell K. M., Jones R. E., Kahana J. A., Kral A. M., Leander K., Lee L. L., Malinowski J., McAvoy E. M., Nahas D. D., Robinson R. G., Huber H. E. (2005) Biochem. J. 385, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dequiedt F., Kasler H., Fischle W., Kiermer V., Weinstein M., Herndier B. G., Verdin E. (2003) Immunity 18, 687–698 [DOI] [PubMed] [Google Scholar]
- 27.Harris T. E., Persaud S. J., Jones P. M. (1996) Biochem. Biophys. Res. Commun. 227, 672–676 [DOI] [PubMed] [Google Scholar]
- 28.Gschwendt M., Dieterich S., Rennecke J., Kittstein W., Mueller H. J., Johannes F. J. (1996) FEBS Lett. 392, 77–80 [DOI] [PubMed] [Google Scholar]
- 29.Dequiedt F., Van Lint J., Lecomte E., Van Duppen V., Seufferlein T., Vandenheede J. R., Wattiez R., Kettmann R. (2005) J. Exp. Med. 201, 793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molero J. C., Turner N., Thien C. B., Langdon W. Y., James D. E., Cooney G. J. (2006) Diabetes 55, 3411–3417 [DOI] [PubMed] [Google Scholar]
- 31.Andoniou C. E., Thien C. B., Langdon W. Y. (1996) Oncogene 12, 1981–1989 [PubMed] [Google Scholar]
- 32.Calnan B. J., Szychowski S., Chan F. K., Cado D., Winoto A. (1995) Immunity 3, 273–282 [DOI] [PubMed] [Google Scholar]
- 33.Zucchelli S., Holler P., Yamagata T., Roy M., Benoist C., Mathis D. (2005) Immunity 22, 385–396 [DOI] [PubMed] [Google Scholar]
- 34.Andoniou C. E. (1996) Tumor Induction Mediated by Tyrosine Phosphorylation of the Cbl Oncogene Product. Ph.D. thesis, University of Western Australia [Google Scholar]
- 35.Akiyama T., Bouillet P., Miyazaki T., Kadono Y., Chikuda H., Chung U. I., Fukuda A., Hikita A., Seto H., Okada T., Inaba T., Sanjay A., Baron R., Kawaguchi H., Oda H., Nakamura K., Strasser A., Tanaka S. (2003) EMBO J. 22, 6653–6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purev E., Neff L., Horne W. C., Baron R. (2009) Mol. Biol. Cell. 20, 4021–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gustin S. E., Thien C. B., Langdon W. Y. (2006) J. Immunol. 177, 5980–5989 [DOI] [PubMed] [Google Scholar]
- 38.Bachmaier K., Krawczyk C., Kozieradzki I., Kong Y. Y., Sasaki T., Oliveira-dos-Santos A., Mariathasan S., Bouchard D., Wakeham A., Itie A., Le J., Ohashi P. S., Sarosi I., Nishina H., Lipkowitz S., Penninger J. M. (2000) Nature 403, 211–216 [DOI] [PubMed] [Google Scholar]
- 39.Chiang Y. J., Kole H. K., Brown K., Naramura M., Fukuhara S., Hu R. J., Jang I. K., Gutkind J. S., Shevach E., Gu H. (2000) Nature 403, 216–220 [DOI] [PubMed] [Google Scholar]
- 40.Huang F., Kitaura Y., Jang I., Naramura M., Kole H. H., Liu L., Qin H., Schlissel M. S., Gu H. (2006) Immunity 25, 571–581 [DOI] [PubMed] [Google Scholar]
- 41.Sanada M., Suzuki T., Shih L. Y., Otsu M., Kato M., Yamazaki S., Tamura A., Honda H., Sakata-Yanagimoto M., Kumano K., Oda H., Yamagata T., Takita J., Gotoh N., Nakazaki K., Kawamata N., Onodera M., Nobuyoshi M., Hayashi Y., Harada H., Kurokawa M., Chiba S., Mori H., Ozawa K., Omine M., Hirai H., Nakauchi H., Koeffler H. P., Ogawa S. (2009) Nature 460, 904–908 [DOI] [PubMed] [Google Scholar]
- 42.Datta S. R., Brunet A., Greenberg M. E. (1999) Genes Dev. 13, 2905–2927 [DOI] [PubMed] [Google Scholar]
- 43.Kandel E. S., Hay N. (1999) Exp. Cell Res. 253, 210–229 [DOI] [PubMed] [Google Scholar]
- 44.Downward J. (1998) Curr. Opin. Cell Biol. 10, 262–267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.