Abstract
Spontaneous DNA breakage is predicted to be a frequent, inevitable consequence of DNA replication and is thought to underlie much of the genomic change that fuels cancer and evolution1–3. Despite its importance, there has been little direct measurement of the amounts, types, sources and fates of spontaneous DNA lesions in living cells. We present a direct, sensitive flow cytometric assay in single living Escherichia coli cells for DNA lesions capable of inducing the SOS DNA damage response, and we report its use in quantification of spontaneous DNA double-strand breaks (DSBs). We report efficient detection of single chromosomal DSBs and rates of spontaneous breakage ~ 20- to 100-fold lower than predicted. In addition, we implicate DNA replication in the origin of spontaneous DSBs with the finding of fewer spontaneous DSBs in a mutant with altered DNA polymerase III. The data imply that spontaneous DSBs induce genomic changes and instability 20–100 times more potently than previously appreciated. Finally, FACS demonstrated two main cell fates after spontaneous DNA damage: viability with or without resumption of proliferation.
DNA DSBs are important instigators of genomic instability that provoke mutation, genome rearrangement and chromosome aberrations important in evolution, oncogenesis and genetic disease3–5. Frequent DSBs are thought to arise spontaneously and to be repaired accurately when normal DNA replication encounters damage from endogenous causes1–3. Indirect estimates of rates of spontaneous DSB formation have been derived from inviability, chromosome loss or cytogenetic phenotypes of cells lacking DNA repair proteins, some of which affect processes other than repair1–3. These estimates are ambiguous, ranging from 0.2–1 per genome replication in Escherichia coli1,2 and 50 per human genome replication3, and the extent to which the phenotypes underlying these estimates are attributed to DSBs versus other lesions also varies1–3. More direct measurement of spontaneous DNA breakage in wild-type living cells has not been achieved.
We describe a direct flow cytometric assay in single living E. coli cells for rapid quantification of cells with DNA damage that induces the SOS DNA damage response (including single-stranded (ss) DNA gaps, DSBs and double-strand ends (DSEs)4). RecA bound to ssDNA promotes proteolysis of the LexA transcriptional repressor, increasing expression of more than 40 SOS damage response genes4. We engineered wild-type E. coli to carry a chromosomally located gfp gene encoding green fluorescent protein (GFP) controlled by the SOS-inducible sulA promoter6, which causes cells with SOS-inducing DNA damage to fluoresce green (that is, these cells are GFP+). Because it is not plasmid borne and does not inactivate any gene (including sulA), damage is detected without perturbing levels of proteins that affect DNA damage processing (Supplementary Methods online). The emission wavelength, rapid expression (8 min after induction) and ≥24-h half-life of this GFP7 allow sensitive flow cytometric detection (~ 105 cells per minute) and isolation of living cells in which SOS has been induced.
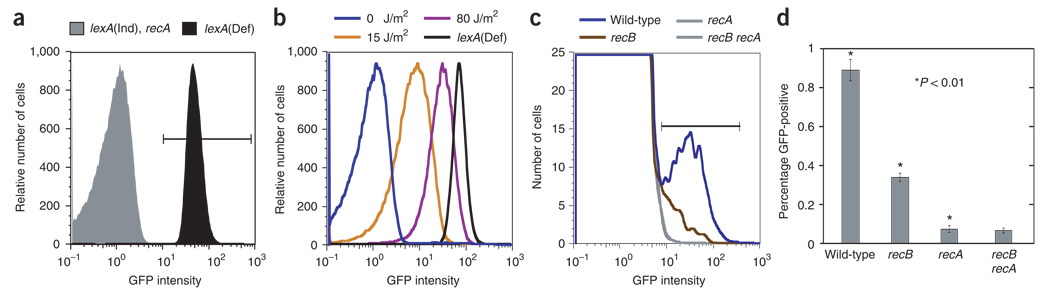
We show that in this system, green fluorescence in cells results from SOS induction (Fig. 1). In one positive control, lexA(Def) (SOS repressor–null) cultures fluoresce green quantitatively (Fig. 1a)6, and two negative controls, SOS-uninducible lexA(Ind−) or ΔrecA cells, show 2 × 10−4 ± 0.1 × 10−4 and 7 × 10−4 ± 4 × 10−4 green cells (mean ± s.e.m.), respectively (Fig. 1a,d). Thus, green cells observed at frequencies above 2 × 10−4 to 7 × 10−4 per culture reflect genuine SOS induction, not spurious promoter firing. Moreover, fluorescence intensity correlated with exposure to ultraviolet C light (Fig. 1b), demonstrating a dose response to a known SOS-inducing, DNA-damaging treatment. Thus, this assay is sensitive and responsive to SOS induction.
Figure 1.
Steady-state levels of spontaneous RecB-dependent SOS induction demonstrate the presence of spontaneous DSBs and/or DSEs in a small cell subpopulation. (a–c) Representative flow cytometry histograms. (a) Positive control, GFP+ lexA51(Def) cells, and negative controls, lexA3(Ind−) and ΔrecA control cells. (b) Wild-type cells after exposure to ultraviolet C light. (c) Steady-state levels of SOS induction in wild-type, recA, recB and recB recA cells (full genotypes of all strains are given in Supplementary Methods and Supplementary Table 1). Horizontal bars represent the GFP+ gate. For b, ~ 0.9% of these cells are spontaneously green (see c,d), but the y-axis scale hides this shoulder to the main peak. For c, the recA and recB recA curves are both shown in gray, as the lines superimpose. (d) Quantification of steady-state levels of green wild-type cells (within the GFP+ gate) (mean ± s.e.m. of 18, 15, 7 and 4 experiments for wild-type, recB, recA and recB recA cells, respectively). In all figures (except where specified), sample groups with stars indicate statistical significance among all other starred sample groups using single-factor ANOVA and Tukey test, P < 0.01. Additionally, in Figure 1d, all strains are significantly different from all others (P < 0.01), except for recA and recB recA, which are not.
We examined logarithmic-phase wild-type cells grown aerobically (Supplementary Methods) for spontaneous SOS-inducing DNA damage. The previous estimate of 0.2–1 DSB or DSE per genome replication (for example, see refs. 1,2) might have been expected to produce a unimodal distribution of cells shifted toward higher GFP intensity from the position of the peaks from SOS-uninducible lexA(Ind−) and ΔrecA (non-green) control cultures, as was seen with varying doses of ultraviolet C light (Fig. 1b). Notably, untreated, log-phase wild-type cells show a small subpopulation (~ 0.9%) of spontaneously green cells (Fig. 1c,d). This is well above the limit established in our controls of fluorescence independent of the SOS response, so it does not reflect spurious promoter firing (Fig. 1a,c). Moreover, this green signal is RecA dependent, indicating a genuine SOS response (Fig. 1c,d).
To determine the fraction of this spontaneous SOS signal caused by DSBs or DSEs, we quantified the fraction dependent on functional RecBCD enzyme. RecB is required for SOS induction resulting from treatments that produce DSEs but not for SOS induction in the absence of DSEs4. RecB was required for 62% ± 3% of spontaneous SOS induction in wild-type cells (Fig. 1c,d). The remaining GFP signal in recB cells was RecA dependent (Fig. 1c,d), as expected, implying that other non-DSE lesions (such as ssDNA gaps) account for the remaining ~ 38% of spontaneous SOS induction.
To determine the assay’s sensitivity to DSBs, we created single, chromosomal DSBs in vivo using a regulatable I-SceI double-strand endonuclease and a chromosomal cut site shown to linearize chromosomal DNA efficiently8 (Supplementary Methods and Fig. 2). Notably, essentially all of the cells fluoresced green (Fig. 2a) after I-SceI induction, with 87% ± 5% of cells falling within a stringently green ‘gate’ (Fig. 2a,d and Supplementary Methods). This response requires endonuclease induction, a cut site, RecA and RecB (Fig. 2d). Thus, the assay was able to detect a model chromosomal DSB efficiently.
Figure 2.
Efficient SOS induction by chromosomal DSBs. (a–c) Representative flow cytometry histograms and (d–f) quantification of multiple experiments, showing GFP+ cells after induction of I-SceI endonuclease in the presence of a single chromosomal I-SceI cut site. Horizontal bars in a–c represent GFP+ gate (Supplementary Methods). (a) SOS induction by I-SceI in log-phase cells with a single chromosomal cut site. (b) Efficient detection of a chromosomal DSB repaired (and removed) by homologous recombination with an F′ episome. (c) Detection of cells with only two DSEs (a single chromosome with an I-SceI-induced DSB) in dnaA(TS) cells with chromosome counts reduced to one (Supplementary Fig. 1 and Supplementary Note). Isogenic dnaA+ cells assayed in parallel produced profiles similar to those in a. Here and in a, there is a small enzyme- and cut site–dependent contribution to green cells independent of intentional induction, which probably reflects difficulty of repressing I-SceI expression8. In b, there is a low-level, repeatable F′-specific and I-SceI induction–specific SOS induction in all cells, perhaps suggesting F′-specific binding of I-SceI that induces a low-level response. (d–f) Multiple experiments assessing SOS and GFP induction and their dependence on RecA and RecB in response to I-SceI-mediated DSBs. Strains as in a–c, carrying a single chromosomal I-SceI cut site in the absence (d) or in the presence (e) of the F′ episome carrying the 20-kb homologous region for recombinational repair or in dnaA46(TS) cells (f) at restrictive temperature (one chromosome per cell; Supplementary Fig. 1). White and gray bars indicate percentages of cells within the green gate (shown in a–c) after repression and induction, respectively, of I-SceI. ‘DSB’ indicates that both I-SceI and a cut site were present. Shown are mean ± s.e.m. of three (d), three to seven (e) and three (f) experiments. Strain designated ‘F′ cut site’ (e) has a cut site disrupting the homology on the F′. This control shows that the reduction of green signal when homology is supplied for repair is caused by repair, not extraneous aspects of the F′.
Most log-phase cells have two or more chromosomes9 (Supplementary Fig. 1 online), both of which might have been cleaved by I-SceI (Fig. 2a). To address whether a single DSB per cell is detected, we quantified fluorescent cells after induction of DSBs by I-SceI in cells with their chromosome number reduced to one using a dnaA(TS) mutation. dnaA(TS) cells cannot initiate replication at the restrictive temperature but continue to divide until most carry only one chromosome (Supplementary Fig. 1 and references therein). First, a temperature shift reduced the chromosome number to one in 61% ± 4% of cells (Supplementary Fig. 1 and Supplementary Note online). Second, 84 ± 4% of shifted cells fell within the green gate after DSB induction (Fig. 2c,f), compared with 95% ± 2% of dnaA+ cells (Fig. 2f). Thus, the assay was able to detect a single DSB (two DNA ends or DSEs) per cell efficiently.
Because most growing cells have two or more chromosomes9, most spontaneous DSBs should be repaired via homology-mediated DSB repair using a sister chromosome. Thus, most cells might have triggered the SOS response after I-SceI induction (Fig. 2a,c,d,f) because attempted repair using the sister restores an I-SceI cut site, making these DSBs irreparable, in effect. We modeled reparable DSBs using I-SceI in cells carrying an F′ episome with ~ 20 kb of DNA identical to the region spanning the chromosomal I-SceI cut site but with no I-SceI site itself 8 (Supplementary Methods). This allows healing of the chromosomal break upon repair, as expected for natural DSBs. Of these cells, 27% ± 2% were GFP+ upon I-SceI induction (Fig. 2b,e); this response to I-SceI requires RecA and RecB (Fig. 2e). Thus, the assay was able to detect a transient, reparable DSB with ~ 27% efficiency. The decreased efficiency of detection of these reparable DSBs (27%, Fig. 2e) versus irreparable DSBs (87–84%; Fig. 2d,f) results from repair, not from the F′ episome per se (Fig. 2e and Supplementary Note), and might occur either because (i) most or all DSBs induce SOS but at a level lower than that which activates the sulA promoter controlling GFP (a mid-range SOS promoter10), or (ii) many DSBs are repaired quickly and do not induce SOS. Either way, the 27% figure indicates that about four times more (1 / 0.27) cells with reparable DSBs are present than are detected, thus providing a correction factor with which to calculate spontaneous DSB levels from the spontaneous green signal.
Some eukaryotes preferentially repair from sister chromosomes rather from than homologs11, as modeled here. If present in E. coli, sister preference might prolong the time before productive repair using the F′ episome, possibly artificially elevating the green signal from reparable DSBs. In eukaryotes, sister preference is thought to result from sister cohesion after replication12. In E. coli, not all investigators observe chromosome cohesion during growth13,14, and when observed14, cohesion is minimal in cells with rapid, 30- to 60-min generation times (as in this study) during which only ~ 10% of each sister is predicted to be cohered, with cohesion at DNA near the replication origin (ref. 14 and D. Bates, personal communication). Thus, given the current data, sister preference (at least caused by chromosome cohesion) seems unlikely, although more data are needed to address this point conclusively. Moreover, the repair partner used, an F′, is carried in 1.4- to 2.3-fold excess of the chromosome15. Thus, repair might be biased toward the F′ and toward more rapid repair (a lower green signal) than when spontaneous chromosomal DSBs repair using a sister chromosome.
The signal from the model reparable DSB also is unlikely to result from repeated cycles of nonhomologous end-joining (NHEJ) and recleavage, because NHEJ is exceedingly rare to absent in E. coli (Supplementary Note16). Supporting the idea that our model of a reparable DSB is realistic, we note efficient RecA-dependent, RecB-dependent and homology-dependent repair and survival of the reparable DSB (Fig. 3b).
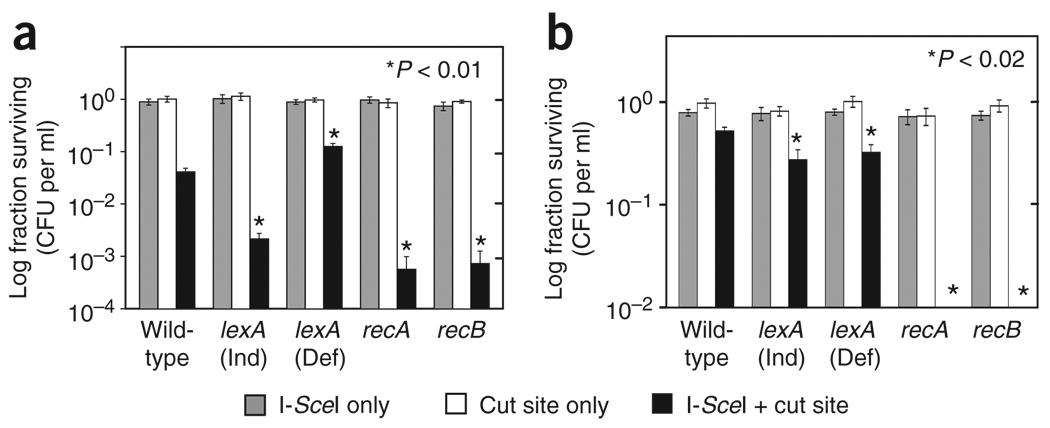
Figure 3.
Induction of the LexA regulon of the SOS response is required for survival of cells (as determined by colony formation) with induced DSBs. (a) Irreparable DSBs. (b) Reparable DSBs. Note the much greater survival with homologous DNA present for repair (b). The fraction of cells surviving I-SceI induction in the presence of a single chromosomal I-SceI site is reduced in cells unable to induce expression of SOS genes, carrying the lexA3(Ind−) allele, and in cells lacking RecA and RecB proteins required for DSB repair (in addition to their roles in SOS induction). When irreparable DSBs are induced with I-SceI (a), constitutive high expression of LexA and SOS genes in lexA(Def) repressor-null mutant cells also improves survival (to 13% ± 1%) relative to wild-type (4% ± 0.4%). Data represent mean ± s.e.m. from four or five experiments. Stars indicate statistical significance calculated as in Figure 1 but versus wild-type cells only. In addition, the surviving fraction of wild-type cells with both I-SceI and cut site differs significantly from the surviving fraction of I-SceI-only cells and the surviving fraction of cut site–only cells, in both a and b. CFU, colony-forming units.
Reparable DSBs have been thought not to activate the DNA damage response in yeast17, but they did activate it here (Fig. 2b,e). This apparent difference might result from different quantitative levels of detection in the yeast assay versus the E. coli assay, rather than a qualitative difference (Supplementary Note). SOS is required for maximum survival of DSBs, including reparable DSBs (Fig. 3), suggesting that SOS is a normal response to DSBs.
We estimated the rate of cells experiencing one or more spontaneous DSBs as follows. Considering that 0.9% of growing cells experienced SOS induction (Fig. 1c,d), 62% of which is RecB dependent (Fig. 1c,d), and given that this assay can detect 27% of reparable DSBs (Fig. 2b,e), the fraction of cells with one or more DSBs at steady state is approximately 0.9% × 62% / 27% = 2.1%. We assume that neither dilution by cell division nor degradation of GFP contributes significantly to the net change in GFP+ cells per generation because (i) the GFP half-life is ≥24 h (ref. 7) (which is long, relative to the E. coli generation time of 0.5–1 h; Supplementary Methods); (ii) only ~ 35% of GFP+ cells can divide to form colonies (below) and (iii) cell division normally lags after SOS induction4. Because the frequency of GFP+ cells is unchanged during early, late and mid-log phase (Supplementary Fig. 3 online), we can assume a constant rate of addition of new green cells each generation. Thus, the maximum number of GFP+ cells added per cell doubling is half the total number of GFP+ cells in culture (to achieve a steady state). Therefore, the maximum rate of addition of cells with one or more DSBs is 2.1% × 0.5 = 1% of cells per generation. This rate is 20- to 100-fold lower than previous estimates for E. coli1,2 and, proportionately to DNA content, approximately fourfold lower than the 50 per generation estimated for human cells3. These previous estimates probably reflect real DNA problems, but our data imply that most are not DSBs.
Breakage of DNA replication forks is postulated to underlie most spontaneous DSBs1,18. To test this idea, after trying several imperfect strategies (Supplementary Note and Supplementary Fig. 2 online), we examined an altered function allele affecting the catalytic subunit of DNA polymerase (Pol) III, the main replicative polymerase. The dnaE915 allele causes a reduced spontaneous mutation rate19. Although DnaE915 has not been characterized biochemically, genetic evidence suggests that it may exclude lower-fidelity DNA polymerases, possibly owing to increased processivity20, which might reduce replication fork breakage. We observed a 48% ± 4% decrease in levels of spontaneous SOS induction in dnaE915 cells, mostly in the RecB-dependent component (Fig. 4), implying a reduction of spontaneous DSBs or DSEs, or a reduction in their detection, in this mutant. These data implicate the activity of the wild-type Pol III catalytic subunit, and thus implicate replication, in generation of most of the spontaneous DSB- or DSE-induced SOS response.
Figure 4.
Reduction of spontaneous SOS induction from DSEs in cells having DNA Pol III with altered function. Labels are as in Figure 1. (a) Representative flow cytometry histograms of the isogenic strains indicated (Supplementary Table 1 for full genotypes). The dnaE915 antimutator allele19 of the gene encoding the DNA Pol III catalytic subunit is reviewed in the text. (b) Data represent mean ± s.e.m. from four experiments. As in Figure 1, sample groups marked with an asterisk differ significantly from all other sample groups with an asterisk (P < 0.01). Additionally, here, all sample groups differ significantly from all others (P < 0.01) except for recB versus dnaE915 recB, and recA versus dnaE915 recA, which do not differ significantly.
Replication by Pol III could either promote DNA breakage by any of several hypothesized routes involving replication into sites of DNA damage and/or fork stalling1,2,21, or it could, in principle, affect SOS induction after breakage. Either way, association of the replisome with spontaneous DSBs and/or DSEs is supported. The data also suggest an alternative hypothesis for the dnaE915 ‘antimutator’ phenotype: reduction of spontaneous SOS induction and, thus, SOS-associated mutagenesis4.
Finally, we used FACS to explore the fates of cells that sustained spontaneous DNA damage. We isolated spontaneously green cells (97% ± 0.5% purity; Supplementary Note) and assayed for colony formation. Approximately 35% of GFP+ cells produced colonies (29% ± 0.6% of green cells forming colonies, normalized by 82% ± 1% of non-green cells forming colonies, which controls for FACS-induced effects on colony formation; values represent mean ± range from two experiments). The GFP+ cells that did not formcolonies seemed to be viable (as determined by their exclusion of propidium iodide; Supplementary Methods, Supplementary Note and Supplementary Fig. 4 online) but non-culturable. Therefore, many of the SOS-induced cells detected were not en route to death, but rather at least 35% (and perhaps most) of them succeeded at repair and survived. In addition, the remaining ~ 65% (non–colony formers) appear to be analogous to senescent human cells—alive but not proliferating—and, similarly, seem to have entered this state in response to DNA damage22. We excluded alternative possibilities, such as that these cells were under typical cell-cycle arrest with recovery or that they were in a transient growth slowdown called ‘persistence’ in bacteria (Supplementary Note and Supplementary Fig. 4).
The data reported provide four important conclusions. First, SOS is a relatively robust detector of reparable DSBs (Fig. 2b,e). Second, the data support the hypothesis1–3,21 that DNA replication contributes to formation of most spontaneous DSBs and/or DSEs (Fig. 4). Third, the rate of spontaneous double-strand breakage is 20- to 100-fold lower than predicted. Fourth, the main fates awaiting cells that sustain spontaneous SOS-inducing DNA damage, including DSBs, are viability with rapid proliferation or viability without rapid proliferation.
Previous studies that predicted 20- to 100-fold higher rates of spontaneous DNA breakage in bacteria and approximately fourfold higher rates of breakage per human DNA equivalent than estimated here relied on viability or cytogenetic phenotypes of repair-defective mutants: E. coli recA and recB and human blm1–3. These estimations assume that loss of the repair protein merely allows accumulation of spontaneous DSBs and DSEs and does not cause additional DNA damage. This assumption is likely to be incorrect or incomplete. If these mutant backgrounds increase DNA damage levels, this might partially account for the discrepancies between previous estimates and ours; for example, RecA promotes replication fork regression, which could exacerbate or ameliorate damage23. In addition, cleft, regressed replication forks are more abundant in cells lacking RecBCD, such that RecBCD might prevent fork cleavage and DSE formation normally21. Moreover, all of these proteins affect processes other than repair, including, at the very least, damage-response induction (RecA and RecB4) and probably DNA replication as well (BLM24). Thus, in their absence, DNA damage might arise and produce the phenotypes noted in earlier studies1–3 as a result of lesions other than DSBs and DSEs being processed in unusual ways. Our estimates are based more specifically on the contribution of DSBs and are from wild-type cells, thereby avoiding potential damage generated only in repair mutants. Additionally, many different kinds of DNA lesions should cause viability and cytogenetic phenotypes. Whereas it is likely that replication is perturbed frequently1,18, our data establish an upper limit on how often the lesion resulting from replication arrest is a DSB.
Likewise, our measurements pertain specifically to DSBs. RecBCD enzyme is expected to respond to a single DSE in addition to a DSB, which can be regarded as two DSEs in a cell. Whereas I-SceI experiments established that the SOS-GFP assay detects as few as two DNA ends in a cell (Fig. 2c,f), it remains formally possible that a single DSE per cell is detected inefficiently. The data cannot rule out the possibility that one replication fork collapse per replication produces one DSE2, but they can exclude the possibility that an equivalent frequency of replication fork stoppages or disruptions creates DSBs (reviewed in ref. 18). Such disruptions probably occur18 but generate DNA lesions or structures other than DSBs and thus have different consequences for genome instability.
These data alter our view of DNA replication and the origins of spontaneous genomic change in two ways. First, the apparent potency of DSBs in inducing spontaneous genome rearrangements is 20–100 times higher than estimated previously. For example, DSBs probably underlie spontaneous duplicated chromosome segments8,25 present in ~ 10−3 to 10−4 bacteria. If that frequency approximates the formation rate, with ~ 10−2 cells with one or more DSBs per genome replication, this implies that as many as 10% of DSBs are repaired mutagenically, producing rearrangements. Each DSB has an extremely high probability of destabilizing the genome. Because similar methods have been used previously to estimate spontaneous DSBs in humans3, these are also likely to be overestimates. DSB repair underlies some breast cancers4, highlighting the importance of more accurate estimates. The strategy used in our assay could be adapted for use in eukaryotic cells.
Second, the lower rate of DSBs implies that the first defense against their genotoxic effects is break avoidance, not break repair, at replisomes. Break avoidance is probably an active function, and we suggest that many good candidates for its enforcement are already identifiable. Many conditional replication mutants show chromosome breakage21, implying that altering the encoded proteins negates the break prevention functions that they normally possess. Replication appears dangerous, but break prevention may be a first defense limiting genome instability, and repair may be a second, riskier stratagem used when prevention fails.
We found the apparent senescence-like state of some cells that experienced SOS-inducing damage to be unexpected. Senescence in multicellular animals is regarded as an evolutionarily selected response to DNA damage that (temporarily) allows cancer avoidance22. It and apoptosis are p53-dependent alternatives that suppress proliferation of cells that have experienced DNA damage and thus might carry mutations22. Although E. coli has no p53 homolog, it undergoes both programmed cell death, perhaps in response to DNA damage26, and also apparent senescence in response to aging27 and nutritional depletion28. The connection of DNA damage to a senescence-like state in a bacterium might, like senescence in multicellular animals, be selected to suppress proliferation of less-fit cells, which is useful to the clone. Mathematical modeling indicates an advantage for diminishing the contribution of less-fit cells to clones during rapid growth (the conditions of this study) but not during a stationary phase29. SOS might provide a mechanism that stops proliferation of less-fit cells during rapid growth (as the results of this work imply) and instead promotes mutagenesis8 in the stationary phase, both as adaptive strategies8,29. Our findings suggest that the evolution of such stasis responses preceded the evolution of p53 and p53-dependent senescence mechanisms.
METHODS
Bacterial strains, genetic constructs and mutant alleles
Bacterial strains used in each figure are described in detail in Supplementary Methods and Supplementary Table 1 online.
Flow cytometry analysis
Log-phase cells cultured in M9 glucose medium were analyzed using the Epics XL-MCL flow cytometer (Beckman Coulter) as described (Supplementary Methods and ref. 30). FlowJo software was used for data analysis (Treestar). P values were calculated by single-factor analysis of variance (ANOVA) and the Tukey test using SigmaStat software (Systat Software).
Cell sorting
GFP+ cells were sorted from wild-type cultures and counted using the Epics Altra cell sorter equipped with a Forward Fluorescence Detector (Beckman Coulter) as described in Supplementary Methods and ref. 30. Further details on sorting and sorting purity are given in the Supplementary Note.
Survival after I-SceI induction of DSBs
Survival is described in detail in Supplementary Methods.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Baylor College of Medicine Flow Cytometry Core and S.A. Sincock and J.P. Robinson (Purdue Cytometry Laboratories) for help with flow cytometry; P.J. Hastings, D.B. Magner, S. Plon and G. Weinstock for discussions and D. Bates, A. Bielinsky, M.M. Cox, C. Herman, M.N. Hersh, G. Ira, M.-J. Lombardo, G.J. McKenzie, X. Pan, R. Rothstein and J.D. Wang for comments on the manuscript. This work was supported by a US Department of Defense Breast Cancer Research Program predoctoral fellowship (J.M.P.) and by US National Institutes of Health grants R01-GM53158 and R01-CA85777, equally.
Footnotes
Note: Supplementary information is available on the Nature Genetics website.
AUTHOR CONTRIBUTIONS
S.M.R. conceived the study; J.M.P. and S.M.R. designed the study, analyzed the data and wrote the paper and J.M.P. performed the experiments.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Cox MM, et al. The importance of repairing stalled replication forks. Nature. 2000;404:37–41. doi: 10.1038/35003501. [DOI] [PubMed] [Google Scholar]
- 2.Kuzminov A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol. Mol. Biol. Rev. 1999;63:751–813. doi: 10.1128/mmbr.63.4.751-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. USA. 2003;100:12871–12876. doi: 10.1073/pnas.2135498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedberg EC, et al. DNA Repair and Mutagenesis. Washington, D.C: American Society for Microbiology; 2005. [Google Scholar]
- 5.Eichler EE, Sankoff D. Structural dynamics of eukaryotic chromosome evolution. Science. 2003;301:793–797. doi: 10.1126/science.1086132. [DOI] [PubMed] [Google Scholar]
- 6.McCool JD, et al. Measurement of SOS expression in individual Escherichia coli K-12 cells using fluorescence microscopy. Mol. Microbiol. 2004;53:1343–1357. doi: 10.1111/j.1365-2958.2004.04225.x. [DOI] [PubMed] [Google Scholar]
- 7.Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 8.Ponder RG, Fonville NC, Rosenberg SM. A switch from high-fidelity to error-prone DNA double-strand break repair underlies stress-induced mutation. Mol. Cell. 2005;19:791–804. doi: 10.1016/j.molcel.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Withers HL, Bernander R. Characterization of dnaC2 and dnaC28 mutants by flow cytometry. J. Bacteriol. 1998;180:1624–1631. doi: 10.1128/jb.180.7.1624-1631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis LK, Harlow GR, Gregg-Jolly LA, Mount DW. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J. Mol. Biol. 1994;241:507–523. doi: 10.1006/jmbi.1994.1528. [DOI] [PubMed] [Google Scholar]
- 11.Kadyk LC, Hartwell LH. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics. 1992;132:387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RD, Jasin M. Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. EMBO J. 2000;19:3398–3407. doi: 10.1093/emboj/19.13.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Liu X, Possoz C, Sherratt DJ. The two Escherichia coli chromosome arms locate to separate cell halves. Genes Dev. 2006;20:1727–1731. doi: 10.1101/gad.388406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates D, Kleckner N. Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell. 2005;121:899–911. doi: 10.1016/j.cell.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frame R, Bishop JO. The number of sex-factors per chromosome in Escherichia coli. Biochem. J. 1971;121:93–103. doi: 10.1042/bj1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowater R, Doherty AJ. Making ends meet: repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet. 2006;2:e8. doi: 10.1371/journal.pgen.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellicioli A, Lee SE, Lucca C, Foiani M, Haber JE. Regulation of Saccharomyces Rad53 checkpoint kinase during adaptation from DNA damage-induced G2/M arrest. Mol. Cell. 2001;7:293–300. doi: 10.1016/s1097-2765(01)00177-0. [DOI] [PubMed] [Google Scholar]
- 18.Rothstein R, Michel B, Gangloff S. Replication fork pausing and recombination or “gimme a break”. Genes Dev. 2000;14:1–10. [PubMed] [Google Scholar]
- 19.Fijalkowska IJ, Dunn RL, Schaaper RM. Mutants of Escherichia coli with increased fidelity of DNA replication. Genetics. 1993;134:1023–1030. doi: 10.1093/genetics/134.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenzie GJ, Lee PL, Lombardo MJ, Hastings PJ, Rosenberg SM. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell. 2001;7:571–579. doi: 10.1016/s1097-2765(01)00204-0. [DOI] [PubMed] [Google Scholar]
- 21.Michel B, Grompone G, Flores MJ, Bidnenko V. Multiple pathways process stalled replication forks. Proc. Natl. Acad. Sci. USA. 2004;101:12783–12788. doi: 10.1073/pnas.0401586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309:886–887. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- 23.Robu ME, Inman RB, Cox MM. RecA protein promotes the regression of stalled replication forks in vitro. Proc. Natl. Acad. Sci. USA. 2001;98:8211–8218. doi: 10.1073/pnas.131022698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amor-Gueret M. Bloom syndrome, genomic instability and cancer: the SOS-like hypothesis. Cancer Lett. 2006;236:1–12. doi: 10.1016/j.canlet.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Slack A, Thornton PC, Magner DB, Rosenberg SM, Hastings PJ. On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS Genet. 2006;2:e48. doi: 10.1371/journal.pgen.0020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sat B, Reches M, Engelberg-Kulka H. The Escherichia coli mazEF suicide module mediates thymineless death. J. Bacteriol. 2003;185:1803–1807. doi: 10.1128/JB.185.6.1803-1807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart EJ, Madden R, Paul G, Taddei F. Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. 2005;3:e45. doi: 10.1371/journal.pbio.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nystrom T. Aging in bacteria. Curr. Opin. Microbiol. 2002;5:596–596. doi: 10.1016/s1369-5274(02)00367-3. [DOI] [PubMed] [Google Scholar]
- 29.Watve M, Parab S, Jogdand P, Keni S. Aging may be a conditional strategic choice and not an inevitable outcome for bacteria. Proc. Natl. Acad. Sci. USA. 2006;103:14831–14835. doi: 10.1073/pnas.0606499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sincock SA, Robinson JP. Flow cytometric analysis of microorganisms. Methods Cell Biol. 2001;64:511–537. doi: 10.1016/s0091-679x(01)64027-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.