Abstract
Background
The anterior cruciate ligament (ACL) fails to heal after traumatic rupture. Furthermore, large-animal models have recently shown that 1-month functional ACL healing is augmented after suture repair when a bioactive scaffold is placed in the tear site.
Hypothesis
At the time of suture repair, placement of a bioactive scaffold in the ACL wound site would improve the structural properties of the tissue.
Study Design
Controlled laboratory study.
Methods
Twenty-seven knees in immature pigs underwent ACL transection and suture repair. A collagen-platelet composite (CPC) was used to supplement the repair in 14 knees. Knees were harvested at 4 weeks, 6 weeks, and 3 months. Mechanical testing and histologic analysis were performed.
Results
The addition of a CPC to a suture repair resulted in improvements in yield load and linear stiffness of the repair tissue at 3 months, as well as a significant increase in cell density. A reduction in yield load and stiffness occurred at the 6-week time point in both groups, a phase when revascularization was noted.
Conclusion
The addition of a CPC to a suture repair enhanced the structural properties of the ACL, and the improvement was associated with increased cellularity within the healing ligament.
Clinical Relevance
The addition of a bioactive scaffold to the wound site improved the functional healing of the ACL after suture repair. The decreased repair strength during revascularization may indicate a need to protect the repair site through this period.
Keywords: anterior cruciate ligament, repair, scaffold, healing
Anterior cruciate ligament (ACL) injury presents a significant clinical challenge for orthopaedic surgeons and their patients.19,21,34 The current standard of care uses a tendon or ligament graft to replace the ACL within the joint space.13,15 Although ACL reconstruction provides mechanical stability to the joint, it does not fully replace the functionality of the intact ACL.17,25,38 The reason for the decrease in functionality is unknown; however, it may be secondary to graft position, the number of bundles replaced, or the graft mechanical properties. Perhaps these issues and the resulting inflammatory processes are what lead to early-onset osteoarthritis in many patients who have had an ACL injury.14,18,23,24,36,44 To lessen the lifelong challenges of an ACL tear, better techniques—such as more anatomical ACL reconstruction, improvement of the biological incorporation of the graft, improved ACL healing, and possibly, ACL regeneration—must be developed to improve the long-term outcomes of these patients.
Studies have demonstrated that the ACL will not heal in various animal models9,32,41 and that it does not heal successfully in human patients.31,40 One possible cause of poor endogenous ACL healing is the failure of fibrin clot formation, which functions as a provisional scaffold within the ACL wound site. This deficiency is likely secondary to the ACL’s location within the synovial cavity.2,16 To circumvent this problem, collagen-platelet composites (CPCs) have been used as a substitute scaffolding material.30 This scaffold simulates a fibrin clot, creating an environment conducive to healing within the gap between the torn ends of the ACL. Primary ACL repair is of particular interest because this treatment would avoid the morbidities associated with tissue harvest or allograft use, it would potentially retain the ligament insertion sites, and it could better preserve the proprioceptive nerves of the ACL.
Collagen-platelet composites are collagen scaffolds infiltrated with platelets obtained from the patient at the time of surgery. A recent study has demonstrated that these biomaterials are useful in enhancing the healing of an ACL graft, both in improving the strength of the graft and in reducing the knee laxity after ACL reconstruction.10 However, it is important to look further to see if the use of these tissue-engineering approaches can be used to heal and regenerate a torn ACL and thus avoid the need for ACL reconstruction. Additional studies have shown that CPCs can serve as a substitute provisional scaffold in the torn ACL at the time of suture repair and that they can release growth factors into the wound site over the first several weeks of healing.27 In one study, scaffold-induced growth factor release and wound site stabilization likely resulted in the improved mechanical strength found when suture repair was supplemented with a CPC scaffold, as compared with suture repair without CPCs.29 This study was the first to show augmented healing of the ACL in any animal model. Long-term success of ligament repair, rather than ACL reconstruction, could offer patients a faster recovery, enhance the longevity of the repair, and increase functionality of the knee over the patient’s life. In addition to becoming a new treatment for ACL rupture, this technique could be applied to healing tissues within the joint and general soft tissue injury.
Given the results of CPC-enhancing functional ACL healing at a 4-week time point,29 we hypothesized that the addition of CPCs to primary suture repair of the ACL would result in improvement in functional healing at a 3-month time point. Our secondary hypothesis was that this functional improvement would be associated with an increase in the cellularity of the ACL wound site, a decrease in the vascularity of the wound, and an increase in the presence of parallel collagen bundles with a normal waveform (or crimp) in the healing ACL.
MATERIALS AND METHODS
Animal Model
Institutional Animal Care and Use Committee approvals were obtained before initiating this study. Eighteen skeletally immature Yorkshire pigs (30 kg, female, 4 months old) underwent ACL transection and suture repair, as noted in Table 1. Same-gendered animals were chosen to eliminate any interanimal variability due to gender. Immature animals were selected because patients with open physes stand to have the longest period of disability after an ACL tear; thus, studying new treatments in this population is of interest. The animals were randomly assigned to 2 treatment groups. In one group, the ACLs had a complete transection in the midsubstance and a primary suture repair of the tibial stump to the femoral insertion site. Two No. 1 vicryl sutures were secured in the distal ACL, using a variable-depth technique, and an absorbable suture anchor was placed at the back of the femoral notch (TwinFix AB 5.0 Suture Anchor with DuraBraid Suture, Smith and Nephew, Inc, Andover, Massachusetts). DuraBraid sutures were then individually tied to the Vicryl sutures in the distal ACL (Figure 1A), with the knees in resting flexion. The second group had the identical procedure; however, a CPC was added to the wound site before closure of the knee (Figure 1B).
TABLE 1.
Experimental Designa
| Time Point | Treatment | Pigs, n | Suture Knees, n | CPC Knees, n |
|---|---|---|---|---|
| 4 weeks | Bilateral surgery | 5 | 5 | 5 |
| 6 weeks | Bilateral surgery | 4 | 4 | 4 |
| 3 months | Unilateral surgery | 9 | 4 | 5 |
For the 4- and 6-week time points, the operations were bilateral, with 1 knee receiving suture treatment and the other, CPC treatment. For the 3-month time point, unilateral surgeries were performed with a single pig receiving either the suture treatment or the CPC treatment. CPC, collagen-platelet composite.
Figure 1.
Schematic representation of the suture (A) and collagen-platelet composite (B) techniques. The sutures (arrows) are fixed proximally with a suture anchor and attached to a second set of sutures placed in a variable depth fashion in the tibial stump. The collagen-platelet composite is threaded on the proximal sutures before the sutures are tied. Figure 1B used with permission from John Wiley and Sons.34
Details of the surgical procedure have been described.29 Postoperatively, animals were allowed unrestrained activity. After recovery from anesthesia, they were permitted to resume normal cage activity and nutrition ad libitum. The animals were weightbearing on the nonoperated leg by 2 hours after surgery, and they regained normal gait within 1 week. At the designated time points, the animals were euthanized. The knees were harvested, and mechanical testing was performed to measure the structural properties of the bone-ACL-bone complex.30,41 After mechanical testing, the knees were processed for histologic examination. In addition, 4 intact knees were identified to serve as intact ACL controls.
Collagen Platelet Composite Manufacture
The CPC was made in our laboratory as previously described.29 In brief, rat tail tendons were solubilized in an acidic enzyme solution to create an acid-soluble collagen slurry. The slurry was neutralized with sodium bicarbonate to a pH of 7.4, and the platelet solution was added. To make the platelet solution, blood was obtained autologously for each animal at the time of surgery, and centrifugation was used to remove the majority of the red blood cells and produce a platelet-rich plasma solution with an average platelet concentration of 1 279 000 ± 775 000 platelets/mm3. For platelets in the CPC, the average enhancement factor (relative to the systemic level) was 2.86 ± 1.69. The neutralized collagen and platelet-rich plasma were mixed in a 1:1 ratio to produce the CPC.
Magnetic Resonance Imaging
Just before sacrifice, the animals were anesthetized, and the size of the ACL repair mass was measured using volumetric sequences on MRI at 1.5 tesla (GE Medical Systems, Milwaukee, Wisconsin), with an 8-channel phased-array coil. Scanning was performed with the knees placed in maximum extension (between 30° and 45° of flexion). The ligament cross-sectional area and length were measured using a gapless PD sagittal series through the knee using a 3-dimensional FIESTA (fast image employing steady-state) acquisition protocol (repetition time = 4.9 msec, echo time = 1.6 msec, 0.8-mm slice thickness with 0-mm gap, field of view = 16, bandwidth = 62.5, and flip angle of 65°). The length and cross-sectional area measurements were used to assess the material properties of the graft.
Biomechanical Testing
The bone-ligament-bone ACL complexes were tested in uniaxial tension to determine the structural properties of the ACL or ACL repair.41 The tests were performed with the loading axis of the material-testing system aligned with the long axis of the ACL.48 Testing was performed with the knee flexed at 30° of flexion. Each specimen was tested to failure at 20 mm per minute.20,39 The yield load, displacement at yield, and linear stiffness were determined from the force-displacement curve for each specimen. The yield load represented the point along the force-displacement curve where the mechanical behavior of the ACL complex departed from linear behavior and, for the purposes of this analysis, was defined as the point where the linear stiffness declined by at least 2% from its maximum value. The displacement at yield was recorded at this same point, and linear stiffness was defined as the maximum slope of the force-displacement curve.
Histology
After mechanical testing, the knees were fixed in formalin, decalcified (DELTA-Cal, Delta Products Group, Aurora, Illinois), and cut in a sagittal plane through the ACL scar mass. The knee sections were then dehydrated, embedded in paraffin, and microtomed into 7-micron sections. These sections were placed onto custom glass slides (Corning 75 × 50 mm Plain Microscope Slides, Corning Incorporated, Corning, New York) and stored at 4°C, until staining with hematoxylin and eosin or α–smooth muscle actin antibodies. Hematoxylin and eosin was used to determine cell density and collagen formation, whereas α–smooth muscle actin immunohistochemistry was used to determine vascularity. One of the animals in the 6-week group had very little ACL tissue in both knees after mechanical testing, so both knees (suture repair and CPC) were excluded from the histologic analysis. The ACL was identified in each section, and 5 regions were marked for analysis: 1 at the femoral insertion site, 1 at the tibial insertion site, and 3 evenly spaced regions between the 2 insertion sites. For the hematoxylin and eosin slides, 3 photomicrographs were taken at each region: the first at magnification ×4 without polarized light, the next at magnification ×4 with polarized light, and the third at magnification ×40 without polarized light (Model BX51TF, Olympus Optical Co, Ltd, Tokyo, Japan). The cellular density was determined by counting all the cell nuclei in a 0.1-mm2 area at each region at magnification ×40. The collagen organization and fiber orientation were determined at each region using photomicrographs taken under polarized light. Photomicrographs were taken at corresponding regions on the slides treated with α–smooth muscle actin. On these slides, vascularity was determined by counting all the vessels in a 10-mm2 area at each region. All analyses were performed by an observer who was blinded to the treatment groups and time points of the photomicrographs.
Histomorphometry
For cellularity and vascularity, the number of cells or blood vessels was counted in the regions specified above. Between 39 and 572 cells and between 0 and 152 blood vessels were counted in each region. Nonnumerical characteristics—such as morphology, cell orientation, and crimp characteristics and orientation—were measured on a 3-point scale (0 to 2). For morphologic characteristics, scoring was based on activity and appearance. A score of 0 was given for spheroid cells, which were chondrocytic quiescent fibrocytes, had a round shape, were smallest of all cells seen, and had a nuclear cytoplasmic ratio of nearly 1. A score of 1 was given for fusiform cells, which were elongated and had an average nuclear cytoplasmic ratio of 0.4. A score of 2 was given to ovoid cells: activated ovoid fibroblasts had an average nuclear cytoplasmic ratio of 0.2 or less, a large amount of cytoplasm, and a round shape. For cell orientation, a score of 0 was given for cells that were randomly oriented, 1 for cells that were 50% oriented, and 2 for cells that were mostly or all oriented along the longitudinal axis of the healing ACL. For collagen density, a score of 0 was given for sparse collagen crimp, 1 was given when 50% of the ligament had collagen crimp, and 2 was given when most or the entire analyzed segment had visible collagen crimp.
Statistical Methods
Statistical comparisons were performed to compare the dependent variables between the suture and CPC groups. To compare cellularity, vascularity, and ligament cross-sectional area, t tests were used. Paired t tests were employed in 4- and 6-week samples when the same animal received both treatments. For knees obtained after 3 months of healing, unilateral surgeries were performed with each treatment as a separate group; therefore, statistical analysis at this time point used an unpaired t test. All categorical data (eg, presence of collagen) were compared using a chi-square test. For the mechanical testing studies, single-factor analysis of variance was used with the Bonferroni-Dunn post hoc test to make comparisons between groups. Finally, Pearson correlation coefficients were performed to evaluate the association between histologic and mechanical values. A P value of .05 was deemed statistically significant for all measures.
A power analysis was conducted to determine the number of samples required to have a reasonable chance of determining if observed changes were significant. Given the magnitude of the paradigm shift that would be required to change practice from ACL reconstruction to suture repair, we were interested in noting only large effects, because a small improvement in suture repair functionality would be less likely to affect clinical adoption. Therefore, we defined a clinically significant improvement as a 75% change over the suture values. On the basis of prior observed means and standard deviations of ACL repairs in the porcine model, we predicted that 4 animals in each group would provide a power of 85% and a confidence interval of 95% to detect a clinically significant improvement of 75%. All statistical analyses were performed using SAS StatView 3.0 (SPS Institute, Cary, North Carolina).
RESULTS
Biomechanical Testing
At 3 months of healing, the CPC-treated ligaments had significantly better functional measures than the ligaments treated with suture repair alone. The CPC ligaments had a 76% greater yield at load (P = .05), a 320% increase in linear stiffness (P = .015), and a 47% decrease in the displacement at yield (P = .05) (Table 2). Although higher means were noted for yield load and linear stiffness for the CPC groups at the 4- and 6-week time points, these differences were statistically significant at only the 3-month time point.
TABLE 2.
Average Mechanical and Histomorphometric Valuesa
| 4 Weeks |
6 Weeks |
3 Months |
|||||
|---|---|---|---|---|---|---|---|
| Characteristics | Intact | Suture | CPC | Suture | CPC | Suture | CPC |
| Yield load (N) | 713 ± 118 | 55.3 ± 52.9 | 79.8 ± 39.8 | 29.6 ± 18.9 | 46.9 ± 35.7 | 79.7 ± 44.9 | 126 ± 53.3b |
| Stiffness (N/mm) | 154 ± 20 | 17.6 ± 16.6 | 20.5 ± 10.1 | 8.1 ± 4.9 | 16.5 ± 11.5 | 12.5 ± 6.4 | 40.2 ± 21.8b |
| Yield displacement (mm) | 5.8 ± 1.4 | 8.3 ± 4.0 | 6.6 ± 1.6 | 6.9 ± 1.3 | 5.8 ± 1.7 | 5.5 ± 0.9 | 3.8 ± 1.5c |
| Cellularity (n per mm2) | 815 ± 615 | 2609 ± 991 | 2597 ± 1383 | 2235 ± 632 | 2315 ± 697 | 1352 ± 528 | 1814b ± 533 |
| Cell shape | 1, 1 | 2, 1.72 | 2, 1.84 | 1, 0.87 | 1, 0.87 | 0, 0.4 | 1, 0.96b |
| Cell orientation | 2, 2 | 1, 1.04 | 1, 1.08 | 1, 0.87 | 1, 1.27 | 1, 1.1 | 1, 0.72 |
| Vascularity (n per mm2) | 0.3 ± 0.2 | 5.2 ± 3.3 | 3.98 ± 2.0 | 6.55 ± 2.4 | 5.89 ± 3.3 | 3.7 ± 1.9 | 3.6 ± 1.7 |
| Collagen density | 2, 2 | 1, 1.44 | 1, 1.48 | 2, 1.46 | 1, 1.26 | 2, 1.55 | 2, 1.64 |
For yield load, stiffness, yield displacement, cellularity, and vascularity, a mean and standard deviation are given. For cell shape, cell orientation, and collagen density, a median and mean are given. CPC, collagen-platelet composite.
CPC group is significantly higher than the suture group (P < .05).
CPC group is significantly lower than the suture group (P < .05).
Gross Observations
The intact porcine ACL was grossly similar to the human ACL. The ligament was fan shaped with a broad insertion site on the tibia and a smaller insertion site on the medial aspect of the lateral femoral condyle. There was a thin synovial covering on all surfaces of the intact ligament, which was often contiguous with the anterior ligamentum mucosum and the posterior synovium covering the PCL and posterior capsule. At 4 weeks after transection, both groups had a scar mass in the region of the ACL that was irregular and covered with thick synovial tissue. Ligaments in the CPC group had a much thicker scar mass than that of the suture group (Figure 2).
Figure 2.
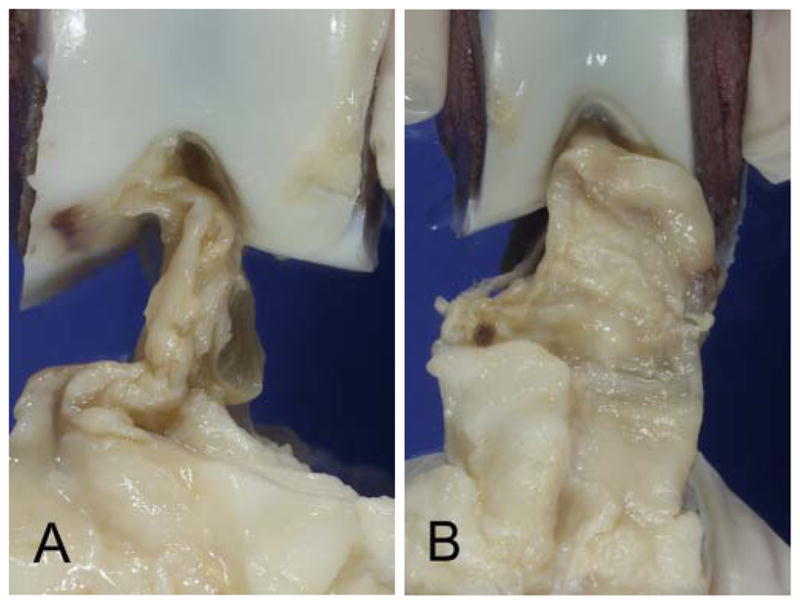
Scar mass between treatment groups at 4 weeks after surgery: A, suture repair–only group; B, collagen platelet composite group. Ligaments treated with collagen platelet composites had larger scar masses at 4 weeks.
At 6 weeks, there was no significant change in the gross appearance of the ACL scars from the 4-week time point. By 3 months after surgery, the fibers of the scar mass had become more parallel in both groups, with a thinner synovium covering the scar; the scar mass also decreased in size. The CPC-treated ligament retained a larger overall scar mass than that of the untreated ligament (Figure 3). In no case was hypertrophic repair tissue noted to be impinging in the notch on gross evaluation of the knees at the 3-month time point. In many cases, the CPC-treated ligaments grossly resembled the intact ACL.
Figure 3.
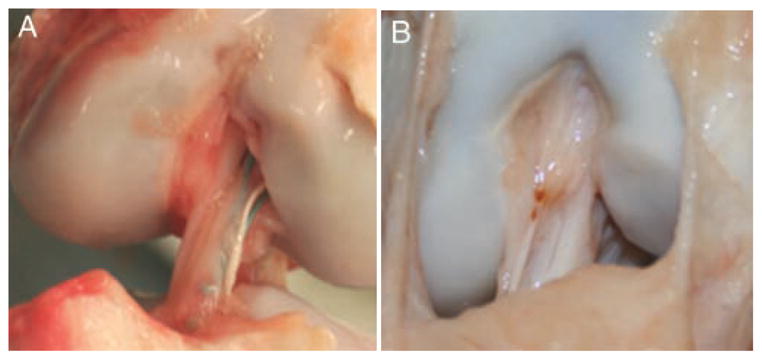
Scar mass between treatment groups at 3 months after surgery: A, suture repair–only group; B, collagen platelet composite group. Ligaments treated with collagen platelet composites have a larger and more organized scar mass at 3 months, with a gross appearance closer to that of an intact porcine ACL. Both photographs are taken with the knee in 90° of flexion.
Scar Mass Size
On MRI scans, the minimum and maximum cross-sectional areas of the scar masses for both groups were determined at 6 weeks and 3 months of healing. At 6 weeks, there was no significant difference in the minimum cross-sectional area of the ligaments of the suture and CPC groups (20.7 ± 8.0 and 21.6 ± 3.7, respectively; P > .05) and no significant difference in the maximum cross-sectional area (38.5 ± 19.4 and 50.2 ± 20.6, respectively; P > .05). At 3 months, there was no significant difference in the minimum cross-sectional area of the ligaments of the suture and CPC groups (39.3 ± 23.8 and 29.8 ±13.2, respectively; P > .05) and no significant difference in the maximum cross-sectional area (71.3 ± 40.2 and 57.2 ± 37.7, respectively; P > .05).
Histomorphometry
Intact porcine ACLs (n = 4) had an average cellularity of 815 ± 615 cells per square millimeter (see Table 2). These cells were uniformly fusiform and aligned along the longitudinal axis of the ligament; they were also evenly spaced and nestled between fascicles of collagen. Vascularity averaged 0.3 ± 0.2 vessels per square millimeter without significant variation along the ligament (P > .05; see Appendix, available in the online version of this article at http://ajs.sagepub.com/supplemental/). The intact ligament was characterized by a uniform pattern of collagen crimp with a consistent wavelength, width, and longitudinal orientation.
At 4 weeks after transection and repair of the ligament, the cell density in both repair groups increased more than 300% (P < .001). For cell shape, both groups changed to the larger ovoid shape of the activated fibroblast. The average vascularity in the ligament increased approximately 15-fold in both treatment groups (P < .001). At 6 weeks, the cell number decreased, and there was a significant change in cell shape to a fusiform shape (P < .001). Between 4 and 6 weeks, a 50% increase in vascularity was found in the CPC group (P = .03), however no significant difference was found in vascularity between the two groups (P > .05). At 3 months, the CPC group had 25.5% higher cellularity (P = .015). Between 6 weeks and 3 months, the suture group had shifted to a more spheroid cell shape, whereas the CPC group maintained a fusiform cell shape. The CPC group had a significantly higher proportion of fusiform cells at the 3-month time point (P < .001) (Figure 4). Vascularity decreased by 40% at 3 months in both groups with no significant difference between groups. Although multiple changes occurred in the repaired ligament, at all time points there was a significant difference in cellularity, cell shape, vascularity, and collagen organization between intact ligament and repaired ligament (P < .05).
Figure 4.
Changes in suture and CPC groups over time. Blood vessels are noted as the ringlike structures staining positive for α-SMA (arrow points to example). Notice the change in cellularity from ovoid cells in both groups at 4 weeks to spheroid cells in the suture group and fusiform cells in the CPC group at 3 months; note also the increase in blood vessels in both groups at 6 weeks. The arrows in 64× H&E polarized indicate bundles of collagen, which are more consistent in the CPC-treated ligaments at 3 months. The size bar for 160× H&E is 10 microns; for 20× α-SMA, 100 microns; for 64× H&E polarized, 100 microns. CPC, collagen-platelet composite; H&E, hematoxylin and eosin; α-SMA, α–smooth muscle actin, where red is a positive stain.
Pearson Correlations
No significant relationships were found, at any time point in either test group, between cellularity and vascularity and between cellularity and mechanical values (P > .05). Vascularity, however, did show some interesting relationships. A Pearson correlation coefficient of −.90 (P = .038) was found between vascularity and stiffness at 4 weeks in the suture group, with repairs of higher vascularity having lower stiffness (Figure 5). In the suture group, Pearson correlation coefficients were found between vascularity and displacement at yield at 4 weeks and 3 months, .931 (P = .022) and .963 (P = .037), respectively. In the CPC group at 4 weeks, an almost-significant relationship was found between vascularity and displacement at yield, .858 (P = .063).
Figure 5.
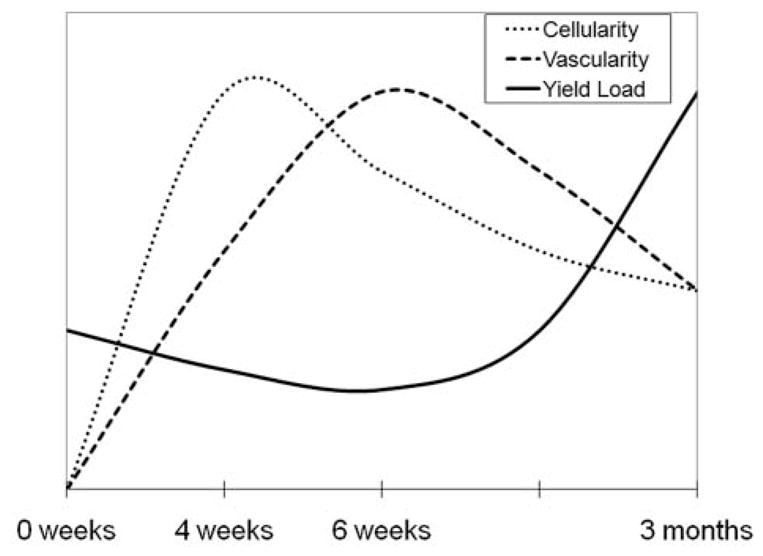
The relationship among mechanical properties, cellularity, and vascularity over the 3-month healing period. Cellularity of the wound site peaks at 4 weeks; vascular density peaks at 6 weeks. Yield load of the healing ligament does not start to significantly increase until after the cellularity and vascularity peaks, at 3 months.
DISCUSSION
This report is the first to show progressive functional healing of the ACL with improved yield load, displacement at yield, and linear stiffness properties over time. The changes in structural properties were accompanied by significant changes in the ligament tissue biology. In addition, the placement of a CPC in the wound site resulted in repairs with improved structural properties, greater cellularity, and a more fusiform cell shape. The use of a CPC has been found to stimulate ACL graft healing after ACL reconstruction.10 The present study is different in that CPC is stimulating healing of the ACL rather than replacing it.
This study is the first to show functional healing with primary ACL repair at 3 months in any animal model. Studies examining primary repair methods in humans have found this treatment gives results no better than those of nonoperative treatment, with most patients experiencing pain and instability.31,40 Regarding other ligaments, previous studies have documented the healing of the medial collateral ligament (MCL) as occurring in 3 phases: hemostasis and inflammation, cellular proliferation and matrix deposition, and matrix deposition and long-term remodeling.12 On the basis of the results reported here, it appears that after suture repair, the phases of healing in the ACL follow a similar course, with the phases being (1) inflammation, (2) cellular proliferation, (3) vascular proliferation, (4) vascular pruning, and (5) collagen remodeling. Although these phases were present in both groups (suture and CPC), they resulted in a more functional result (improved yield load and stiffness of the repairs) when a bioactive scaffold supplemented the sutures.
The first phase noted here was a cellular proliferation phase, which occurred between 4 and 6 weeks after injury. This phase was characterized by repair tissue with a cellular density more than 3 times that of the intact ACL, and it activated ovoid fibroblasts throughout the wound site. Some new vessels were noted during this phase, but no significant organization of collagen into bundles could be identified. Woo et al,49 studying changes in the MCL with a combined ACL and MCL injury, found that MCL cellularity is at its maximum at 6 weeks after transection (although the 4-week time point was not examined). Frank et al,12 studying dissection of the rabbit MCL, observed a decrease in cell number from 3 to 6 weeks, similar to what this study found. Thus, the cellular proliferation phase noted in the healing ACL is consistent with that observed in other models of soft tissue repair.
The vascular proliferation phase of ACL repair was noted at the 6-week time point, and it was characterized by increasing vascularity of the wound site (more than 20-fold greater than that in the intact ligament) and by decreasing cellular density. During this phase, the increased vessel number in the wound site was predominantly composed of small blood vessels (ie, capillaries), whereas the regions of ligament near the bone had fewer larger vessels (arterioles and venules). As a comparison, the MCL healing study by Frank et al12 found an increase in vascularization up until 3 weeks. Arnoczky et al4 examined the revascularization of a patellar tendon graft used to replace the canine ACL and so observed an increase in vascularization up until 20 weeks. Rougaff and Shelbourne37 conducted biopsies of human patellar tendon autografts used for ACL repair. They found that capillary invasion into the patellar tendon started at 3 weeks and increased in prevalence up to 8 weeks after surgery. Thus, the increase in vascularity at 6 weeks, as observed in the healing ACL, is consistent with the healing of other ligaments and so may be faster than the revascularization of ACL grafts in animal models. Note that platelet-rich plasma has been associated with increases in myofibroblast formation and vascularization, although this study did not note a significant difference produced by CPCs.5,6,22
Vascular pruning was noted between 6 weeks and 3 months as the large number of disorganized capillaries in the wound site changed to smaller numbers of arterioles, with an increasing degree of organization of the vessels into paths that were parallel to the newly deposited collagen bundles. In a study looking at the vascular invasion of a canine patellar tendon graft, vascular response subsided by 26 weeks.4 In human ACL rupture, the vascularity decreased from the 16- to 20-week time point to the 52- to 104-week time point.27 Thus, the observation of a decrease in vascularity as healing proceeds is consistent with prior reports, although this phase occurred earlier here than what was reported in ACL grafts and nonhealing ACL tissue.
At 3 months, collagen deposition and remodeling were noted in the healing ACLs. During this phase, many collagen bundles were aligned in the ACL, but they were not as yet uniformly aligned with the longitudinal axis as were those in the intact ligament. Other studies have shown that collagen fiber orientation is the best predictor of strength.8,11,26,33 Therefore, with time, additional alignment of the collagen could result in increased yield loads and stiffness of the healing ligament tissue. This remodeling phase was also characterized by a decrease in hypercellularity and hypervascularity, as seen in the earlier time points; however, cell and vessel density remained higher than that in the intact ligaments, thereby suggesting that healing is not yet complete for these parameters. Frank et al12 found that collagen remodeling took place from 6 to 14 weeks but that little changed between 14 and 40 weeks. Woo et al,50 in a rabbit MCL study, stated that collagen remodeling begins several weeks after injury and may continue for months or even years. In this model of healing, collagen remodeling takes place between 6 weeks and 3 months, and it likely continues beyond the final time point noted in this study.
It is important to note the decrease in structural properties at the 6-week time point, when revascularization is occurring. In a study on the patellar tendon of the rabbit, Tohyama and Yasuda43 noted a 42% decrease in mechanical strength at the time of maximal revascularization, or 6 weeks. In sheep ACL grafts, the highest vascularity was seen between 6 and 12 weeks, which was noted as the nadir in strength for the grafts.47 Thus, the revascularization period in several soft tissues apparently occurs between 6 and 12 weeks, and it is a phase accompanied by a decrease in strength (Figure 5). This factor is an important one to take into account when designing postoperative rehabilitation protocols so that undue stress is not placed on the healing tissue during this phase.
The addition of a CPC to the repair site of the ACL induced histologic changes and improved the functional performance of the healing tissue. At the 3-month time point, the addition of CPC resulted in an overall increase in ligament cellularity, and more of the cells had a fusiform cell shape. The increase in cellularity is likely to have resulted in the presence of a greater number of cells that might be capable of producing and organizing collagen. In addition, the fusiform shape of the fibroblast has been associated with increased collagen secretion and crimp formation.1,7,46 One or both of these findings could in turn explain the increase in yield load seen in the CPC ligaments at the 3-month time point.
One of the 6-week animals showed very little scar formation in both the suture knee and the CPC-treated knee. This paucity of ACL tissue may have been due to a failure in the neutralization or setting of the hydrogel or to an early forceful kicking motion in the animal postoperatively; however, that it occurred in both knees suggests that this animal may have had some defect in its intrinsic wound-healing capability.
This study agrees with the findings in previous research on CPC-enhanced primary ACL repair in that the structural properties of CPC-treated ligaments increase.29 The current study further advances the use of CPC to treat ACL injuries in a number of ways. This study examined the healing knees up to 3 months postoperatively; it examined the histologic appearance of the ligament; and it demonstrated an increase in vascularity matched by a loss of strength that had not been previously noted.
These studies compared the use of suture repair alone with suture repair supplemented with CPC. The observation that CPC improved the biomechanical function of the repairs at 15 weeks is interesting. Prior studies have demonstrated that the use of platelet-rich plasma alone28 or the use of a collagen scaffold alone (B.C.F. et al, unpublished data) is ineffective at improving the biomechanical properties of an ACL repair. This finding suggests that the combination of a provisional scaffolding (collagen in this experiment) and a source of stimulatory cytokines (as found in platelets) may be necessary to achieve enhanced functional healing and that the use of one component or the other alone may not produce the same result.
The principal weakness of this study is the limited time points studied. Selecting 4 weeks as the minimum time point means that the acute inflammatory phase of healing was not evaluated in this work. Similarly, by not having a time point greater than 3 months, remodeling was not yet complete. Whereas studying ligaments 6 months after surgery would provide further knowledge on augmented healing, the current funding for this area of research unfortunately limits the length of study. Although additional studies at earlier and later time points are needed to completely define ACL healing, this study provides data about the cellular proliferation, vascular proliferation, vascular pruning, and early remodeling phases in the functionally healing ACL. In addition, this study was performed in an immature large-animal model. Working with immature animals allows us to focus on the population that stands to suffer the longest period of disability owing to premature osteoarthritis after an ACL tear. The applicability of these results to adults is not known, because the effects of skeletal maturity on ligament healing have yet to be defined. In addition, whereas large-animal models have clear advantages over smaller models, particularly when vascularization is of interest, these models are clearly different from the human condition; the animals are quadruped and there is no ability to control the postoperative rehabilitation. Also, although the anatomy and morphology of the pig knee are similar to those of the human,51 there may be subtle differences that are not yet appreciated. However, these models provide a place to study ACL healing with traditional methods that require destruction of the healing tissue (biomechanical testing and histology). Animal models also required that histologic analysis be performed on tissues that were biomechanically tested. Biomechanical testing would not alter the main features of cellularity and vascularity, and although crimp could be affected, there is no a reason to believe that it would have disproportionately affected one group. This experimentation clearly cannot be performed in a clinical trial; thus, it provides key insights into the ACL healing process that may contribute to our understanding of how to encourage and enhance ACL healing in the future. In addition, with the relatively large interanimal variability often encountered in these large-animal models, the use of a bilateral model greatly amplifies the power of the study to determine significant differences between treatment groups. In the groups where each animal had one experimental knee and an associated internal control, the power of the study is significantly increased to detect treatment effects. However, this approach limits the number of treatments to one experimental side and one control side. The choice of what might be the optimal contralateral control is often a difficult one. We had recently completed a study demonstrating the use of a collagen scaffold alone (ie, without concentrated platelets), which did not result in a significant improvement in the biomechanical properties of the simpler suture repair (B.C.F. et al, unpublished data); as such, we elected in the current study to continue with the consistent use of a suture-only control. Finally, at least some of the kinematic abnormalities that occur in the ACL-deficient and ACL-reconstructed knee are inherently rotational.3,42,45 However, testing methods to measure these types of abnormalities in the porcine knee are not yet standardized and were thus not included in this study.
In summary, this study for the first time documents healing of the ACL over a 3-month time course, with significant improvements in ACL function noted with the addition of a CPC. The histologic changes that occurred with the use of CPC—namely, greater cell density in the wound site and a more fusiform cell shape—help to characterize enhanced ACL repair. Although additional studies at earlier and later time points are clearly needed to completely define the entire process of ACL healing, this model of enhanced suture repair provides important insights that support the possibility of treating ACL injuries with enhanced primary repair methods.
Supplementary Material
Acknowledgments
Funding for this project was received from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, grant No. AR052772 (M.M.M.). We acknowledge the contributions of Eduardo Abreu and Matthew Palmer for the production of the collagen-platelet composites; David Zurakowski for assistance with the statistical analyses; Mark Kelly and Arthur Nedder for the veterinary care; David Spenciner, David Paller, and Ryan Rich for the biomechanical testing; and Lena Liu for the histologic sample preparations.
Footnotes
One or more authors has declared a potential conflict of interest: Dr Murray is a paid consultant, founder, and stockholder in Connective Orthopaedics. Dr Fleming is a paid consultant for Connective Orthopaedics.
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav.
References
- 1.Aggeler J, Frisch SM, Werb Z. Changes in cell shape correlate with collagenase gene expression in rabbit synovial fibroblasts. J Cell Biol. 1984;98:1662–1671. doi: 10.1083/jcb.98.5.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen RB, Gormsen J. Fibrin dissolution in synovial fluid. Acta Rheumatol Scand. 1970;16:319–333. [PubMed] [Google Scholar]
- 3.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 4.Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon: an evaluation of graft revascularization in the dog. J Bone Joint Surg Am. 1982;64(2):217–224. [PubMed] [Google Scholar]
- 5.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67(1):30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Cáceres M, Hidalgo R, Sanz A, Martinez J, Riera P, Smith PC. Effect of platelet-rich plasma on cell adhesion, cell migration, and myofibroblastic differentiation in human gingival fibroblasts. J Periodontol. 2008;79(4):714–720. doi: 10.1902/jop.2008.070395. [DOI] [PubMed] [Google Scholar]
- 7.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 8.Doillon CJ, Dunn MG, Bender E, Silver FH. Collagen fiber formation in repair tissue: development of strength and toughness. Coll Relat Res. 1985;5(6):481–492. doi: 10.1016/s0174-173x(85)80002-9. [DOI] [PubMed] [Google Scholar]
- 9.Feagin JA, Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 10.Fleming BC, Spindler KP, Palmer MP, et al. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank C, McDonald D, Wilson J, Eyre D, Shrive N. Rabbit medial collateral ligament scar weakness is associated with decreased collagen pyridinoline crosslink density. J Orthop Res. 1995;13(2):157–165. doi: 10.1002/jor.1100130203. [DOI] [PubMed] [Google Scholar]
- 12.Frank C, Woo SLY, Amiel D, Harwood F, Gomez M, Akeson W. Medial collateral ligament healing a multidisciplinary assessment in rabbits. Am J Sports Med. 1983;11(6):379–389. doi: 10.1177/036354658301100602. [DOI] [PubMed] [Google Scholar]
- 13.Fu FH, Bennett CH, Lattermann C, Ma CB. Current trends in anterior cruciate ligament reconstruction, part 1: biology and biomechanics of reconstruction. Am J Sports Med. 1999;27:821–830. doi: 10.1177/03635465990270062501. [DOI] [PubMed] [Google Scholar]
- 14.Gillquist J, Messner K. Anterior cruciate ligament reconstruction and the long-term incidence of gonarthrosis. Sports Med. 1999;27:143–156. doi: 10.2165/00007256-199927030-00001. [DOI] [PubMed] [Google Scholar]
- 15.Gulotta LV, Rodeo SA. Biology of autograft and allograft healing in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):509–524. doi: 10.1016/j.csm.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Harrold AJ. The defect of blood coagulation in joints. J Clin Pathol. 1961;14:305–308. doi: 10.1136/jcp.14.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heijne A, Axelsson K, Werner S, Biguet G. Rehabilitation and recovery after anterior cruciate ligament reconstruction: patients’ experiences. Scand J Med Sci Sports. 2008;18(3):325–335. doi: 10.1111/j.1600-0838.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 18.Järvelä T, Paakkala T, Kannus P, Järvinen M. The incidence of patellofemoral osteoarthritis and associated findings 7 years after anterior cruciate ligament reconstruction with a bone-patellar tendon-bone autograft. Am J Sports Med. 2001;29:18–24. doi: 10.1177/03635465010290010701. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures: a long-term follow-up study. Am J Sports Med. 1990;18:354–358. doi: 10.1177/036354659001800404. [DOI] [PubMed] [Google Scholar]
- 20.Katsuragi R, Yasuda K, Tsujino J, Keira M, Kaneda K. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28:47–56. doi: 10.1177/03635465000280012001. [DOI] [PubMed] [Google Scholar]
- 21.Korgsgaard M. The cruciate ligaments—a challenge for the scientific mind. Scand J Med Sci Sports. 2004;14(5):273–274. doi: 10.1111/j.1600-0838.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Reddy MS, Geurs N, et al. Efficacy of platelet-rich plasma on wound healing in rabbits. J Periodont. 2008;79(4):691–699. doi: 10.1902/jop.2008.070449. [DOI] [PubMed] [Google Scholar]
- 23.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 24.Lohmander LS, Östenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 25.Maletius W, Gillquist J. Long-term results of anterior cruciate ligament reconstruction with a Dacron prosthesis: the frequency of osteoarthritis after seven to eleven years. Am J Sports Med. 1997;25(3):288–293. doi: 10.1177/036354659702500303. [DOI] [PubMed] [Google Scholar]
- 26.Martin RB, Ishida J. The relative effect of collagen fiber orientation, porosity, density, and mineralization on bone strength. J Biomech. 1989;22(5):419–426. doi: 10.1016/0021-9290(89)90202-9. [DOI] [PubMed] [Google Scholar]
- 27.Murray MM, Martin SD, Martin TL, Spector M. Histologic changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82(10):1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27(5):639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 30.Murray MM, Spindler KP, Devin C. Use of a collagen platelet rich scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24(4):820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 31.Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament: a randomized study with short-term follow-up and observations. Clin Orthop Rel Res. 1985;198:87–93. [PubMed] [Google Scholar]
- 32.O’Donoghue DH, Rockwood CA, Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48:503–519. [PubMed] [Google Scholar]
- 33.Oxlund H, Barckman M, Ortoft G, Andreassen TT. Reduced concentration of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17(4):S365–S371. doi: 10.1016/8756-3282(95)00328-b. [DOI] [PubMed] [Google Scholar]
- 34.Palmer MP, Abreu EL, Mastrangelo A, et al. Injection temperature significantly affects in vitro and in vivo performance of collagen-platelet scaffolds. J Orthop Res. 2009;27(7):964–971. doi: 10.1002/jor.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlovich R, Jr, Goldberg SH, Bach BR., Jr Adolescent ACL injury: treatment considerations. J Knee Surg. 2004;17(2):79–93. doi: 10.1055/s-0030-1248203. [DOI] [PubMed] [Google Scholar]
- 36.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17(2):195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 37.Rougraff BT, Shelbourne KD. Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):9–14. doi: 10.1007/s001670050113. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz AL, Kelly M, Nutton RW. Arthroscopic ACL reconstruction: a 5–9 year follow-up. Knee. 2002;9(3):197–200. doi: 10.1016/s0968-0160(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 39.Sakai T, Yasuda K, Tohyama H, et al. Effects of combined administration of transforming growth factor-beta1 and epidermal growth factor on properties of the in situ frozen anterior cruciate ligament in rabbits. J Orthop Res. 2002;20:1345–1351. doi: 10.1016/S0736-0266(02)00065-7. [DOI] [PubMed] [Google Scholar]
- 40.Sandberg R, Balkfors B, Nilsson B, Westlin N. Operative versus non-operative treatment of recent injuries to the knee: a prospective randomized study. J Bone Joint Surg Am. 1987;69(8):1120–1126. [PubMed] [Google Scholar]
- 41.Spindler KP, Murray MM, Devin C, Nanney LB, Davidson JM. The central ACL defect as a model for failure of intra-articular healing. J Orthop Res. 2006;24(3):401–406. doi: 10.1002/jor.20074. [DOI] [PubMed] [Google Scholar]
- 42.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 43.Tohyama H, Yasuda K. Extrinsic cell infiltration and revascularization accelerate mechanical deterioration of the patellar tendon after fibroblast necrosis. J Biomech Eng. 2000;122(6):594–599. doi: 10.1115/1.1319659. [DOI] [PubMed] [Google Scholar]
- 44.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waite JC, Beard DJ, Dodd CA, Murray DW, Gill HS. In vivo kinematics of the ACL-deficient limb during running and cutting. Knee Surg Sports Traumatol Arthrosc. 2005;13(5):377–384. doi: 10.1007/s00167-004-0569-6. [DOI] [PubMed] [Google Scholar]
- 46.Watson PA. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991;5(7):2013–2019. doi: 10.1096/fasebj.5.7.1707019. [DOI] [PubMed] [Google Scholar]
- 47.Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. Am J Sports Med. 2001;29:751–761. doi: 10.1177/03635465010290061401. [DOI] [PubMed] [Google Scholar]
- 48.Woo SLY, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament complex: the effects of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 49.Woo SLY, Niyibizi C, Matyas J, Kavalkovich K, Weaver-Green C, Fox RJ. Medial collateral ligament healing: combined medial collateral and anterior cruciate ligament injuries studied in rabbits. Acta Orthop Scand. 1997;68(2):142–148. doi: 10.3109/17453679709003997. [DOI] [PubMed] [Google Scholar]
- 50.Woo SLY, Vogrin TM, Abramowitch SD. Healing and repair of ligament injuries in the knee. J Am Acad Orthop Surg. 2000;8(6):364–372. doi: 10.5435/00124635-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Eng. 1998;26(3):345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




