Abstract
Background
Recent studies indicate an increased frequency of mutations in the gene for Gaucher disease, glucocerebrosidase (GBA), among patients with Parkinson disease. An international collaborative study was conducted to ascertain the frequency of GBA mutations in ethnically diverse patients with Parkinson disease.
Methods
Sixteen centers participated, including five from the Americas, six from Europe, two from Israel and three from Asia. Each received a standard DNA panel to compare genotyping results. Genotypes and phenotypic data from patients and controls were analyzed using multivariate logistic regression models and the Mantel Haenszel procedure to estimate odds ratios (ORs) across studies. The sample included 5691 patients (780 Ashkenazi Jews) and 4898 controls (387 Ashkenazi Jews).
Results
All 16 centers could detect GBA mutations, L444P and N370S, and the two were found in 15.3% of Ashkenazi patients with Parkinson disease (ORs = 4.95 for L444P and 5.62 for N370S), and in 3.2% of non-Ashkenazi patients (ORs = 9.68 for L444P and 3.30 for N370S). GBA was sequenced in 1642 non-Ashkenazi subjects, yielding a frequency of 6.9% for all mutations, demonstrate that limited mutation screens miss half the mutant alleles. The presence of any GBA mutation was associated with an OR of 5.43 across studies. Clinically, although phenotypes varied, subjects with a GBA mutation presented earlier, and were more likely to have affected relatives and atypical manifestations.
Conclusion
Data collected from sixteen centers demonstrate that there is a strong association between GBA mutations and Parkinson disease.
INTRODUCTION
Several lines of evidence suggest an association between parkinsonism and mutations in the gene encoding the lysosomal enzyme glucocerebrosidase (GBA), which is deficient in Gaucher disease. In this rare Mendelian disorder, lysosomal accumulation of glucocerebroside results in a broad spectrum of disease manifestations including hepatosplenomegaly, anemia, thrombocytopenia, bone disease and, at times, neurologic involvement.1, 2 Multiple independent studies have reported an increased frequency of GBA mutations in different cohorts with parkinsonism 3–21 However, different genome wide association studies have not identified this locus, and the picture of association has remained somewhat unclear, as many studies were not large enough to unequivocally label GBA mutations as risk alleles for typical Parkinson disease.
Recognition of the association between GBA mutations and parkinsonism began in the clinic, with the identification of rare patients with Gaucher disease who also developed parkinsonian symptoms. Clinical descriptions appeared as case reports, 22, 23 larger patient series24, 25 and prospective studies.26 Pedigree analyses revealed that relatives of patients with Gaucher disease, many of whom were obligate heterozygotes, had an increased incidence of Parkinson disease.27, 28
Almost 300 different GBA mutations have been identified in patients with Gaucher disease including missense, nonsense and frame shift mutations as well as insertions, deletions and complex alleles.29 Different expression studies have demonstrated that many of these mutations result in a significant loss of glucocerebrosidase activity 30.The GBA gene, located on 1q21–22, includes 11 exons, and has a highly similar pseudogene 16kb downstream. The 85 kb region surrounding GBA is particularly gene rich, encompassing seven genes and two pseudogenes.31 Recombination within and around the GBA locus is relatively frequent,32 complicating genotypic analyses.
The frequency and distribution of GBA mutations varies among different populations. Among Ashkenazi Jews, where the carrier frequency is between 1 in 12 and 1 in 16 1, 6, one common mutation, N370S, accounts for approximately 70% of mutant alleles.1 In other ethnicities, the carrier frequency is usually less than 1%, and the associated mutations are more diverse. While N370S is also common in European populations, and is exclusively associated with non-neuronopathic Gaucher disease, it has not been encountered among Asians.33 A second relatively frequent and pan-ethnic mutation, associated with neuronopathic Gaucher disease, is L444P, which can be a point mutation or part of a complex allele encompassing additional pseudogene sequence.32
This international collaborative study of GBA mutations in Parkinson disease was undertaken to better ascertain the frequency of GBA mutations by pooling individual data from sixteen centers, representing four continents, including 5691 cases and 4898 controls. Findings based on the data from 13 of the 16 centers (4185 of the cases and 3597 controls) have been previously published 5–16, 19. The goals were to establish the frequency of GBA mutations among different sites, to explore the range of GBA mutations encountered and to identify clinical features shared by GBA mutation carriers.
Methods
Patients and procedures
Researchers known to be genotyping Parkinson disease cohorts for GBA mutations were solicited for this collaboration. Sixteen centers participated and data were collected and analyzed at the National Human Genome Research Institute (NGHRI) Bethesda, Maryland (Table 1). The centers included subjects from North America (four groups), South America (one group), Asia (three groups), Israel (two groups) and Europe (six groups). Ethnicity was by self-report. Ashkenazi Jews provided the origin of grandparents. Informed consent was obtained under the supervision of each local ethics committee. All subjects fulfilled the UK Parkinson Disease Brain Bank Clinical Diagnostic Criteria for Parkinson disease.34
Table 1.
Demographic data from the different centers
| Center Name | Sample Size (Number of AJ) |
Male: Female Ratio (unknown) |
Mean Age of Collection |
Number of Mutations |
Percent Mutations |
Mutations Screened |
Clinical Data Provided* |
Percent Ashkenazi# |
|---|---|---|---|---|---|---|---|---|
| Brazil | N370S, L444P G377S |
Limited | 0 | |||||
| Patients | 65 | 42:23 | 54.1 | 4 | 6.2% | |||
| Controls | 264 | 169:95 | 54.4 | 0 | 0% | |||
| NYC, USA | Full sequencing |
Full | 58.3 | |||||
| Patients | 275 (177) | 171:104 | 65.6 | 34 | 12.4% | |||
| Controls | 140 (65) | 73:67 | 62.8 | 3 | 2.14% | |||
| France | N370S, L444P, D409H |
Full | 0 | |||||
| Patients | 297 | 185:112 | 57.8 | 12 | 4.0% | |||
| Controls | 251 | 142:109 | 57.8 | 1 | 0.39% | |||
| Haifa, IL | D409H, 84GG, V394L, IVS2+1, R496H |
Partial | 100.0 | |||||
| Patients | 162 (162) | 94:65 (3) | 68.5 | 40 | 24.7% | |||
| Controls | NP | NP | NP | NP | NP | |||
| Italy | L444P, N370S | Full | 0 | |||||
| Patients | 395 | 244:151 | 66.5 | 11 | 2.8% | |||
| Controls | 483 | 180:303 | 56.9 | 1 | 0.21% | |||
| Norway | L444P, N370S | Limited | 0 | |||||
| Patients | 311 | 186:123 (2) | NP | 7 | 2.3% | |||
| Controls | 473 | 267:206 | 64.1 | 8 | 1.69% | |||
| NHGRI, USA | Full sequencing |
Partial | 0.2 | |||||
| Patients | 539 | 275:166 (98) | 73.5 | 29 | 5.4% | |||
| Controls | 209 (1) | 100:109 | 67.6 | 6 | 2.87% | |||
| Portugal | Full sequencing |
Limited | 0 | |||||
| Patients | 231 | 110:121 | 65.3.0 | 15 | 6.5% | |||
| Controls | 482 | Unknown | 65.5 | 6 | 1.24% | |||
| Rostock, DE | Full sequencing |
Partial | 0 | |||||
| Patients | 298 | 190:108 | 64.3 | 18 | 6.0% | |||
| Controls | 212 | 105:107 | 74.5 | 5 | 2.4% | |||
| Singapore | L444P, N370S | Partial | 0 | |||||
| Patients | 329 | 170:158 (1) | 70.3 | 8 | 2.4% | |||
| Controls | 201 | 99:102 | 64.2 | 0 | 0% | |||
| Taiwan | L444, recNciI, R120W, some full sequencing |
Full | ||||||
| Patients | 559 | 304:255 | 69.1 | 22 | 3.9% | 0 | ||
| Controls | 377 | 198:179 | 60.5 | 4 | 1.06% | |||
| Tel Aviv, IL | 84GG, IVS2+1, N370S, V394L, D409H, L444P, R496H, RecTL |
Limited | 99.9 | |||||
| Patients | 420 (419) | 262:158 | 68.0 | 81 | 19.3% | |||
| Controls | 321 (321) | 159:162 | 65.3 | 13 | 4.05% | |||
| Japan | full sequencing | Limited | 0 | |||||
| Patients | 534 | 282:252 | 65.3 | 50 | 9.4% | |||
| Controls | 546 | 294:252 | 44.8 | 2 | 0.37% | |||
| Tubingen, DE | L444P, N370S | Partial | 0 | |||||
| Patients | 377 | 222:155 | 64.8 | 12 | 3.2% | |||
| Controls | 325 | 192:132 | 58.3 | 0 | 0% | |||
| Toronto, CA | N370S, K178T, L444P, 84GG, R329C, IVS2+1,recNcii I |
Partial | 1.1 | |||||
| Patients | 88 (2) | 51:37 | 55.0 | 5 | 5.7% | |||
| Controls | 96 | 27:69 | 69.6 | 1 | 1.0% | |||
| Seattle, USA | N370S, L444P, recNciiI |
Full | 1.5 | |||||
| Patients | 811 (20) | 607:204 | 66.9 | 24 | 2.9% | |||
| Controls | 518 | 193:324 | 65.2 | 2 | 0.39% | |||
Clinical data: Limited-age, gender, ethnicity, Partial-age, gender, ethnicity, family history and some information on symptoms, Complete- data provided includes age, gender, ethnicity, duration, family history, presenting symptoms, prominent clinical findings, cognitive changes, and scores on Hoehn and Yahr and/or Unified Parkinson Disease Rating scales
total percent Ashkenazi across all sites, 11.3%.
As the detection methods and number of mutations that could be identified varied greatly from center to center, 12 standard DNA samples from patients with Gaucher disease were genotyped by each center and the results were analyzed at the NHGRI. All participating groups could reliably detect mutations N370S and L444P, unless the mutant allele included large stretches of GBA pseudogene sequence. Four centers could identify 4–9 specific selected point mutations (Table 1). In addition, five participating centers sequenced all exons of GBA, and in a sixth, a subgroup was sequenced. Thus, results evaluating two mutations, 4–9 mutations, and the entire coding sequence were analyzed separately (see supplementary table 1). Two frequent GBA variants, E326K and T369M, were evaluated, but not included as mutant alleles.
The extent of clinical data collected from each site varied (Table 1). Some study centers provided only age, family history, sex, ethnicity and diagnosis, while others reported more complete data, including presenting symptoms, age at disease onset, specific clinical manifestations, response to medications and standardized Hoehn and Yahr and/or Unified Parkinson’s Disease Rating Scale scores. Only one proband was collected per family and subjects with diagnoses other than Parkinson disease were excluded.
Controls were screened for signs or symptoms of parkinsonism and centers attempted to match for age, sex and ethnicity. Controls with a family history of Parkinson disease were removed due to their increased risk for ultimately developing parkinsonism.
Study Design
The a priori aim in this study was to conduct a combined analysis of risk associated with GBA mutations from different centers including both published and unpublished data. Our analyses utilized patient level data from both published and unpublished studies. We assessed associations between Parkinson disease and GBA mutations not only in all available genotyped samples, but also in distinct subgroups of the total population (for detailed analyses of subgroups please refer to supplemental text).
Statistical Analyses
Descriptive statistics were calculated and stratified by study center. Available data on family history of Parkinson disease and seven clinical features of Parkinson disease (asymmetric onset, bradykinesia, cognitive changes, orthostatic hypotension, postural instability, rest tremor and rigidity) were analyzed. Clinical features were compared betweens cases carrying GBA mutations and cases without mutations, means were compared using two-tailed Student’s T-tests and frequency differences between patients with and without GBA mutations were assessed using chi-squared tests.
The primary aim was to estimate the odds ratios (ORs) associated with mutations in GBA across cohorts. To accomplish this we conducted fixed effects Mantel-Haenszel analyses using all available cases and controls from each study center. These models calculate crude ORs and confidence intervals from counts of mutations in cases and controls in each study, then combining the ORs from study specific 2×2 tables into a summary measure. Three separate Mantel-Haenszel analyses were performed, using any mutation, N370S and L444P as the independent variables. No multiple test correction was used in our analyses.
The heterogeneity of effects in the Mantel-Haenszel analyses were evaluated using Woolf’s test for heterogeneity 35 Possible analyses of interactions contributing to heterogeneity of ORs were limited by the data available for analysis, so several additional Mantel-Haenszel analyses were carried out. First, we excluded data from Japan and Norway, both together and independently, based on the assumption that general genetic homogeneity in these samples could be compounded by active recruitment of family members of cases, influencing the independence (or in the Norwegian cohort, the non-independence) of mutation frequency differences between cases and controls. An additional analysis performed omitting data from Tel Aviv, the center with the most robust OR with respect to the standard error of the estimate, confirmied that it was not inflating the combined OR.
To follow up the results from the Mantel-Haenszel analyses, multivariate logistic regression models were used to examine the association between GBA mutations and Parkinson disease in subgroups stratified by level of sequencing coverage and Ashkenazi/ non-Ashkenazi status. These stratified models combined data for participants across studies, including all participants with complete data for covariates of self-reported ethnicity, age at sampling and gender. Identical parameters were used in logistic regression models testing the association with variants E326K and T369M. Chi-square tests of heterogeneity were used to compare effect size differences between Ashkenazi and non-Ashkenazi stratified models assessing risk attributable to all GBA mutations, and N370S and L444P separately across all sequencing depths. Detailed results and description of these regressions can be found in supplementary materials.
All data analyses were conducted using R 2.8.0 36 Source code for plotting of meta-analysis results is available in the package r.meta maintained by Thomas Lumley (available from http://cran.cnr.berkeley.edu/).
RESULTS
A total of 5691 genotyped patients with Parkinson disease were evaluated, including 780 Ashkenazi Jewish subjects and 4911 patients with no known Ashkenazi ancestry. The 4898 controls genotyped included 387 Ashkenazi Jewish individuals and 4511 with other ethnicities. Table 1 lists the frequency of mutations and demographics for each individual study. Full sequencing was performed on 1859 patient and 1674 control samples
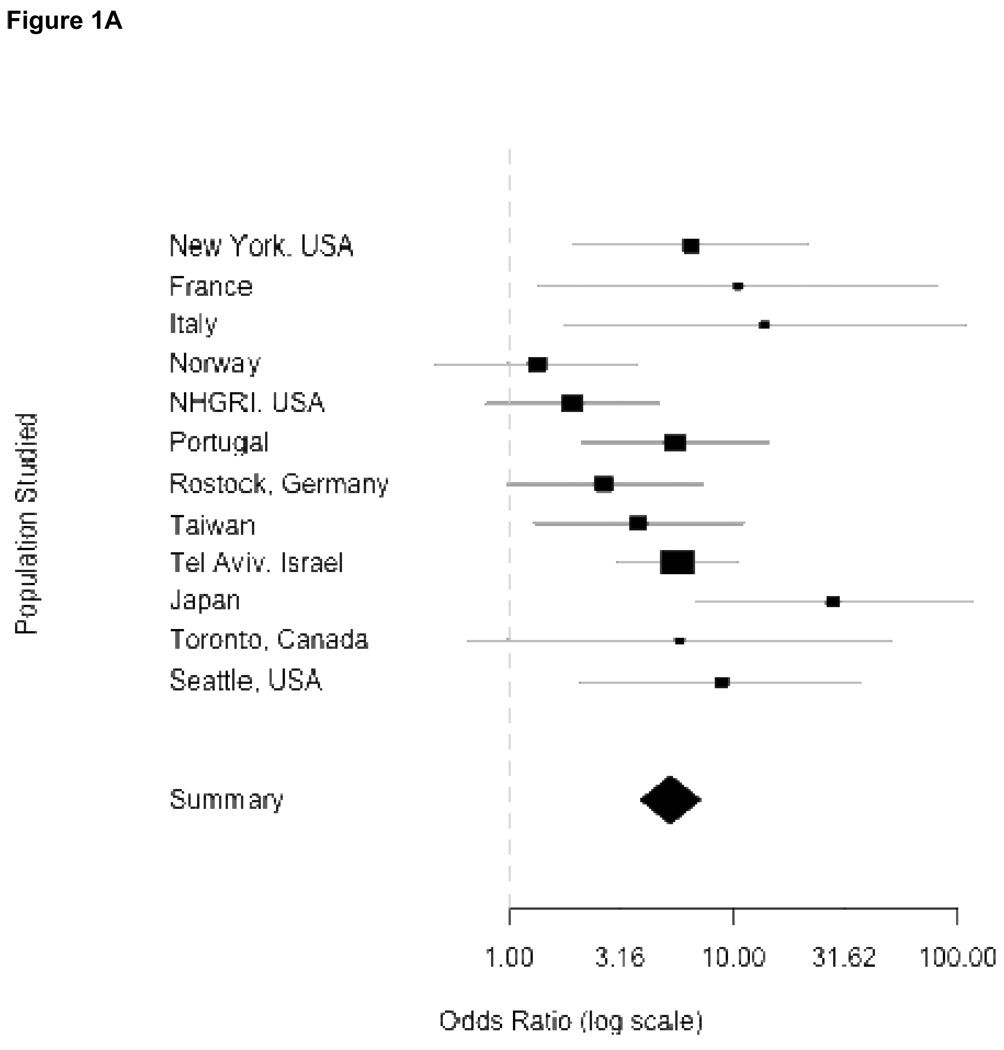
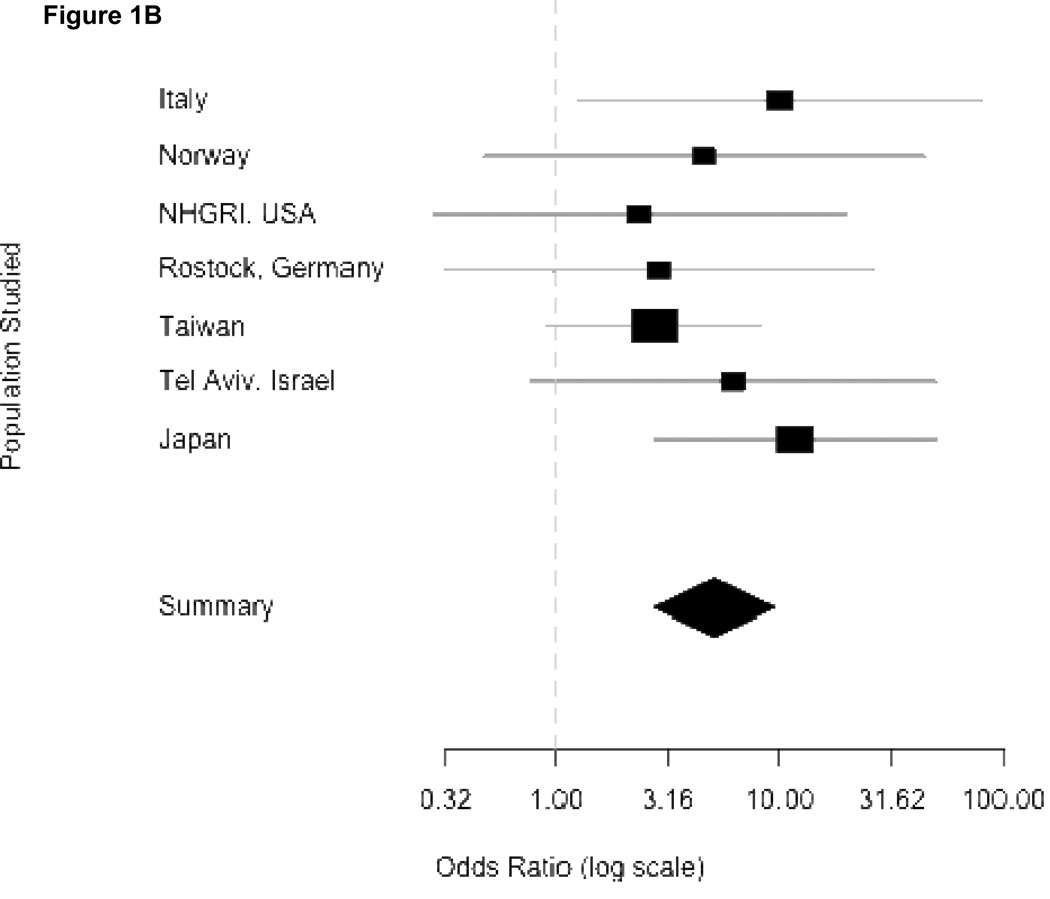
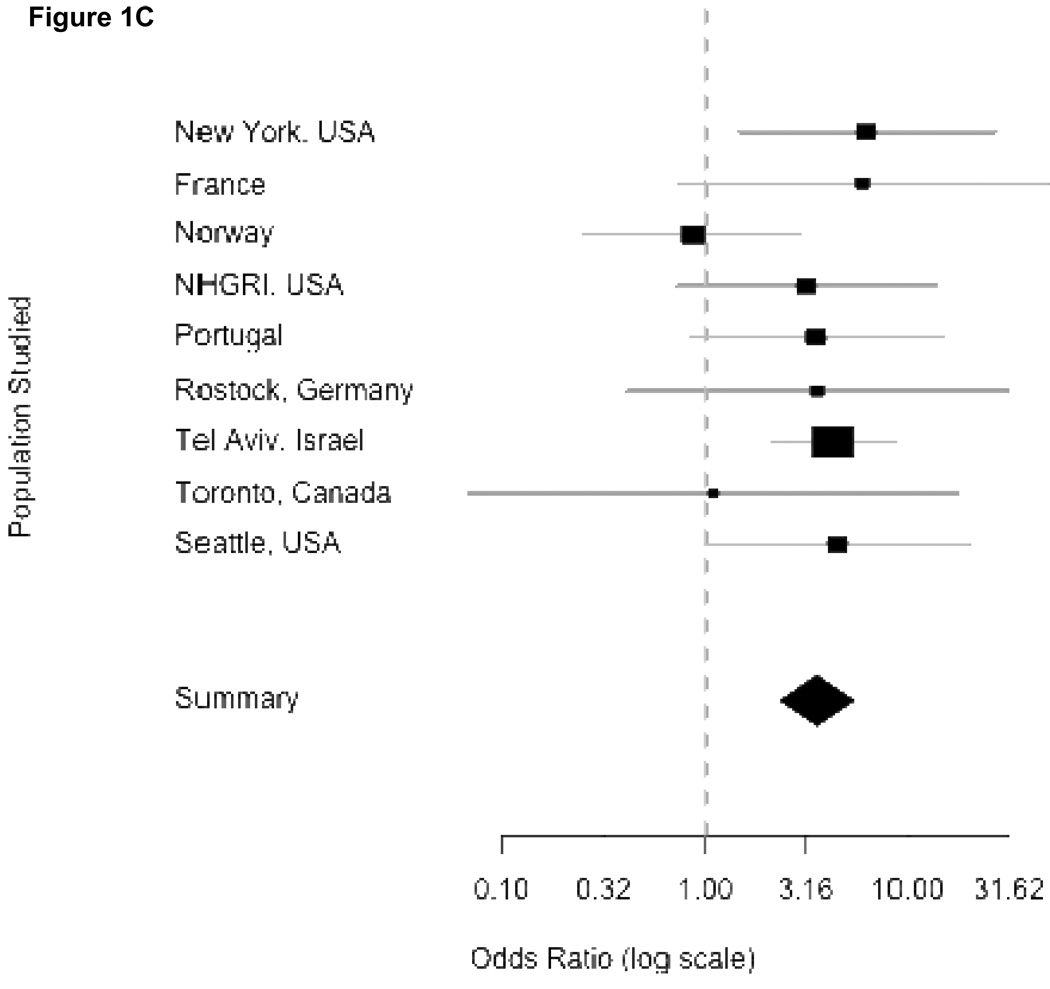
In Figure 1, the odds ratios, standard errors and confidence limits from the Mantel-Haenszel procedure for each independent study are shown graphically, where the precision of the effect estimate is reflected in the size of the squares, then combining odds across study sites. Panel 1A summarizes the results using any mutation as an independent variable. Each center had an over-representation of mutations among patients compared to controls, although confidence intervals varied considerably. Eight centers had an OR greater than 5. Because one center did not provide individual controls (Haifa, Israel) and three centers did not find mutations among their controls (Brazil, Singapore and Tubingen, Germany), they do not appear in the forest plots. The overall combined OR denoted by the diamond symbol demonstrates how greatly the confidence interval is reduced when the individual studies are combined. Panels 1B and 1C show the individual ORs for GBA mutations L444P and N370S respectively. While the results are overwhelmingly positive for each mutation, they were higher for L444P.
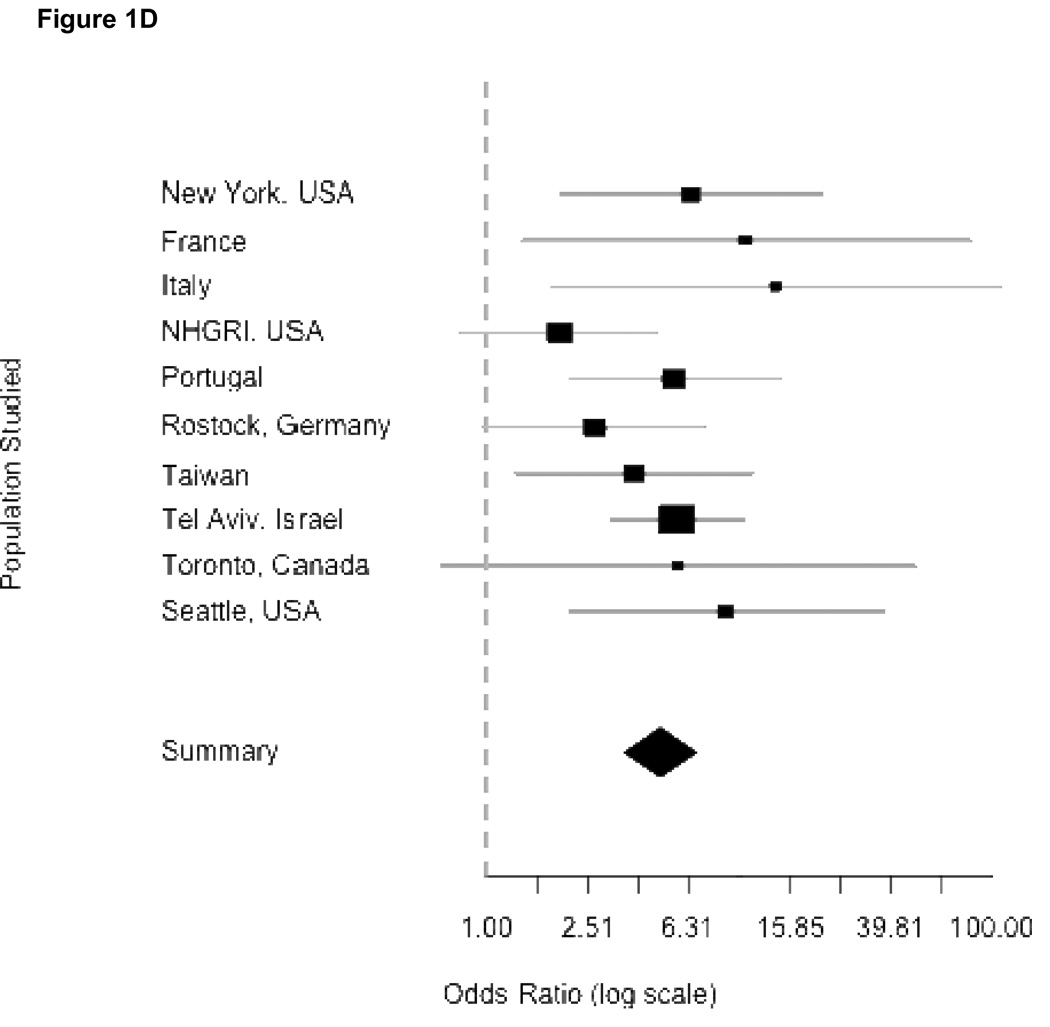
Figure 1.
Odds ratios from meta-analyses. Panels correspond to combined risk estimates associated with possessing (A) any GBA mutation, (B) mutation L444P (C) mutation N370S and (D) any GBA mutation excluding Japan and Norway study centers. Horizontal grey lines indicate 95% confidence intervals of estimates. Point estimates per study population are indicated by squares where height is inversely proportional to the standard error of the estimates. Diamonds represent the summary odds ratio whose width indicates the 95% confidence intervals.
Combined ORs for Mantel-Haenszel analyses were homogenous for L444P and N370S (Woolf’s test for heterogeneity, p-values of 0.347 and 0.499 respectively). This allows for confidence in reporting the Mantel-Haenszel combined ORs attributable to N370S (OR = 3.96, 95% CI 2.6–6.02) and L444P (OR = 6.73, 95% CI 4.5–15.42). However, the Mantel-Haenszel OR in the model for risk attributable to all GBA mutations was significantly heterogeneous (Woolf’s test for heterogeneity, p-value = 0.0207). After excluding Tokyo and Norway, the OR was slightly attenuated (OR = 5.43, 95% CI 3.89–7.57), but heterogeneity was no longer significant (Woolf’s test, p-value = 0.414), and further excluding Tel Aviv resulted in a homogenous (Woolf’s test, p-value = 0.3386) OR of 5.3, (95% CI 3.59–7.94). Independent exclusions of data from either Norway or Japan showed homogenous ORs (Japan excluded: OR = 4.91, 95% CI 3.6–6.7, Woolf’s test p-value = 0.1447; Norway excluded: OR = 6.35, 95% CI 4.6–8.75, Woolf’s test p-value = 0.0991) (Panel 1D). Thus, we can confidently report a Mantel-Haenszel OR of 5.43 associated with GBA mutations even when these centers are excluded.
Overall, when screening solely for N370S and L444P, one of these two mutations were found in 15.3% of Ashkenazi Jewish patients versus 3.4% of controls, and in 3.2% of non-Ashkenazi patients versus 0.6% of controls. As expected, N370S was particularly prevalent among Ashkenazi Jews (OR 5.62, 95%CL 3.04–10.39) and was not seen among any of the Asian patients or controls (supplementary Table 1).
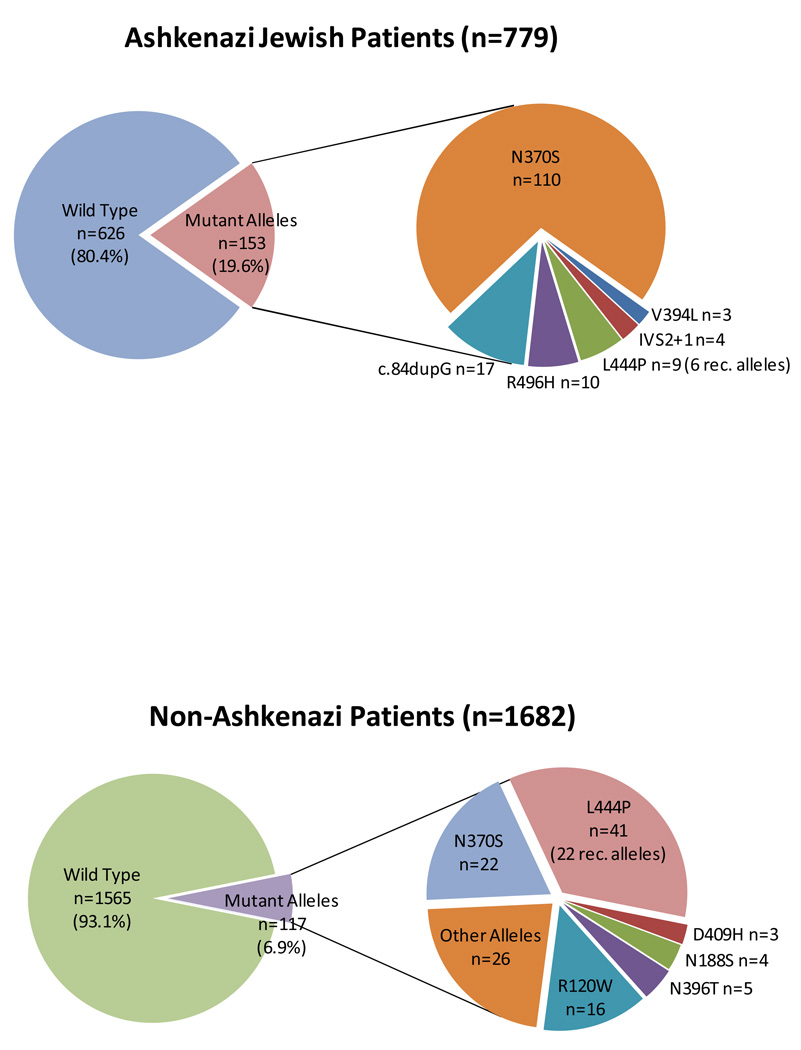
All Ashkenazi Jewish subjects were screened for the presence or absence of 6–8 different GBA mutations (Table 1). Including this larger number of screened mutations increased the OR for any mutation to 6.48 (95%CL 3.78–11.09). The distribution of specific GBA mutations among Ashkenazi Jewish patients with Parkinson disease, where ~20% of patients carried a GBA mutation, is shown in Figure 2A, demonstrating that 20% of the identified mutant alleles were not L444P or N370S. Among non-Ashkenazi Jewish subjects the entire GBA coding region was sequenced in 1682 patients and 1609 controls. In patients where full sequencing was performed, the OR for any GBA mutation was 6.51 (95%CL 3.62–11.7). Figure 2B illustrates the distribution of mutations identified among non- Ashkenazi patients. The mutations identified indicate that as many as 46% of mutant alleles could be missed when focusing solely on N370S and L444P. Moreover, 22 of 43 subjects with L444P carried other pseudogene sequence and hence had recombinant alleles.
Figure 2.
The distribution of mutations identified. (A) Ashkenazi Jewish patients with Parkinson disease. 19.6% carried a GBA mutation. Insert demonstrates the distribution of the different mutations identified. (B) Non-Ashkenazi patients with Parkinson disease with full sequencing. 6.9% carried a GBA mutation. The insert shows that among the 117 mutations identified, 54% were not N370S or L444P.
Full sequencing data identified two common GBA variants, E326K and T369M which are not pathogenic in subjects with Gaucher disease37. Neither demonstrated a significant association with Parkinson disease.
Seventeen patients (15 Ashkenazi) carried two GBA mutations. Genotype N370S/N370S was observed in fourteen, N370S/ R496M in two and N370S/V394L in one.
Age at onset was found to be significantly lower among subjects with GBA mutations (p-value <0.001), with a mean age of 54.9 in subjects with GBA mutations as compared to 58.8 in subjects without. The mean length of disease duration from diagnosis to evaluation was 7.8 years both groups.
Information about family history of parkinsonism was available on 4401 of the patients studied. 17.8% of participants without GBA mutations reported a first or second degree relative with Parkinson disease, as compared to 24.0% of subjects with a GBA mutation (p-value =0.0057).
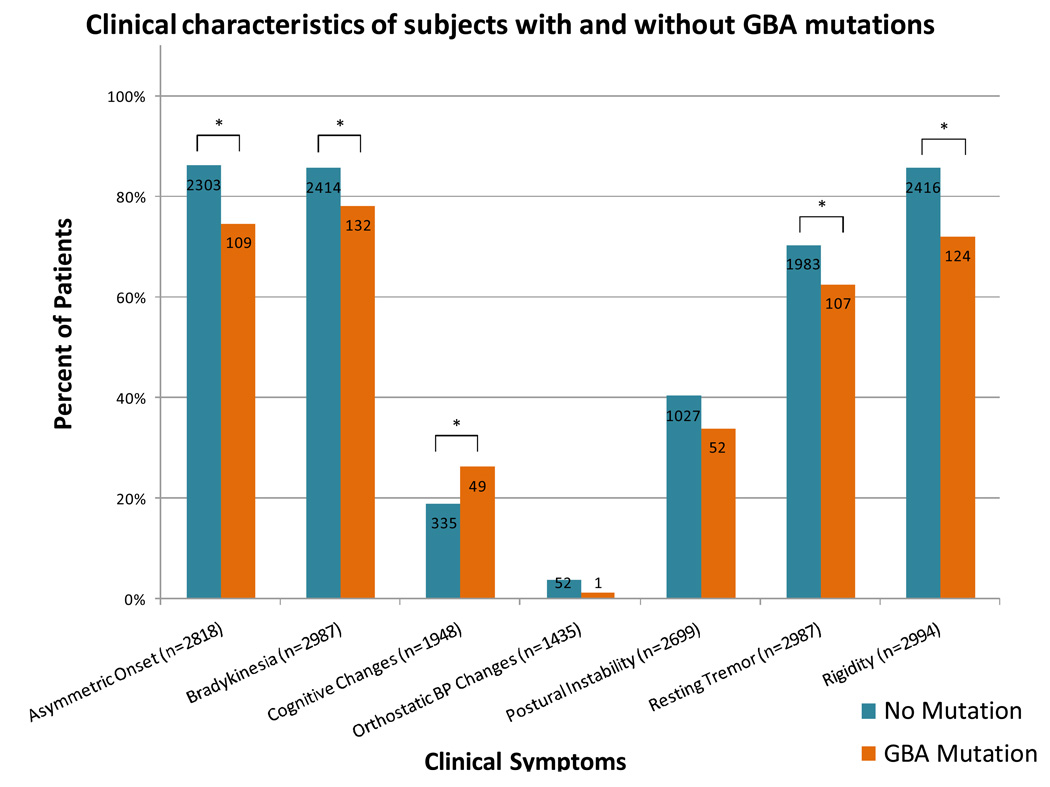
The frequency of seven clinical findings associated with Parkinson disease in subjects with and without GBA mutations is shown in Figure 3. Generally, the symptom profile for the two groups was similar, although mutations were associated with a significantly lower frequency of asymmetric onset (p-value<0.0001), bradykinesia (p-value=0.0001), resting tremor (p-value=0.0298), and rigidity (p-value<0.0001). There were no significant differences between orthostatic blood pressure changes (p-value=0.2591) or postural instability (p-value=0.1194) among subjects with and without GBA mutations. The presence or absence of cognitive changes was recorded for 1948 patients, reported as present in 26.3% of patients with mutations and 19.0% without (p-value= 0.0071).
Figure 3.
Clinical manifestations of Parkinson disease reported in subjects without (teal) and with (orange) GBA mutations. The number of subjects with data recorded for each symptom is indicated in each respective bar. * = p-value<0.05; significant difference between clinical symptom of patients with and without a GBA mutation.
DISCUSSION
The results of this analysis overwhelmingly support the association between GBA mutations and Parkinson disease. The combined study demonstrates that this finding is not exclusive to a specific ethnicity, nor associated with any specific GBA mutation. While mutations in GBA are likely to be a susceptibility factor rather than a Mendelian cause of Parkinson disease, the high frequency of mutations among ethnically diverse heterogeneous cohorts of subjects with Parkinson disease render mutations in this gene among the most common and universally found risk factors for Parkinson disease identified to date.
The major limitation of this study was the unavoidable differences in data ascertainment among the different sites. Moreover, some sites were more successful in matching cases and controls with regard to age and gender. To ensure that the analysis was not driven by a small subset of centers, we evaluated the variance in ORs across centers. Excluding the center with the most precise estimate (Tel Aviv), and the centers with the most extreme ORs (Norway and Japan) only resulted in a slight attenuation of the combined OR, which in all analyses remained 5.4 or higher.
This study confirms the need to sequence all exons to accurately ascertain the frequency of GBA mutations in both patients and controls. The combined ORs may be underestimated because so many of the centers performed limited mutation analyses. Our data demonstrate that among non-Ashkenazi cases as many as 46% of mutant alleles can be missed when screening for only two mutations. Furthermore, analysis of specific mutations may produce a serious bias. While in sequenced samples the frequency of GBA mutations was 6.9% among 1642 patients (OR 6.51), only 36% of the samples included in our entire analysis were fully sequenced. Adequate sample size and accurate genotyping in controls are imperative to avoid underestimating rare variants.
Despite the difficulty in determining the phenotypic profile associated with GBA mutations from this study, which intentionally only included subjects that met diagnostic criteria for Parkinson disease, some trends are apparent. Subjects carrying mutations presented on average 4 years earlier, were more likely to have a family history of Parkinson disease, and had less bradykinesia and rest tremor and more cognitive changes reported, a trend supporting other reports.3, 5, 6, 17, 19, 24, 25. However because of the exclusion criteria used in this analysis, our findings do not accurately reflect the full spectrum of parkinsonian symptoms associated with GBA mutations. An increased frequency of GBA mutations has also been described in cohorts with Lewy body disorders 38–40 although not in multiple system atrophy 41, and further studies are warranted.
Now that GBA is a well-validated risk factor for Parkinson disease, the ultimate challenge is to establish the mechanisms contributing to this association .Both a gain of function mechanism due to enhanced protein aggregation or lysosmal dysfunction 42 or a loss of function related to fluctuations in levels of ceramide 43 have been postulated. Further research is in progress to elucidate the pathophysiology of both disorders,, facilitate more accurate genetic counseling and develop new therapeutic strategies.
Supplementary Material
ACKNOWLEGEMENTS
Supported in part by the Intramural Research Program of the National Human Genome Research and the National Institute on Aging (NIA project number Z01AG000957-05) at the National Institutes of Health, Department of Health and Human Services; the National Parkinson Foundation, Miami, Florida; the Merit Review Award from the Department of Veterans Affairs, Seattle, Washington;; the German National Genome Network (NGFNplus 01GS08134; German Ministry for Education and Research); the French Parkinson’s Disease Genetics Study Group; the Duke-NUS Graduate Medical School, the National Medical Research Council, Biomedical Research Council, Singapore Millennium Foundation; the SingHealth Research and Staff from Singapore General Hospital, National Neuroscience Institute; the Tel Aviv Sourasky Medical Center Grant of Excellence and Wolfson and Kahn Foundations; the Portuguese FCT grant; the NS36630, Parkinson’s Disease Foundation, UL1 RR024156; the NSC-95-2314-B-182A-061 from the National Science Council, Executive Yuan, Taiwan; and a Grant-in-Aid for Scientific Research on Priority Areas, Applied Genomics, and Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors acknowledge Dora Yearout, B.S. for technical assistance, John W. Roberts, M.D. for subject recruitment at the University of Washington, Seattle, Washington, Jae Choi, B.S. from NHGRI for preparation of the figures and Neil Oden from Emmes Corporation, Rockville, Maryland for statistical assistance.
Footnotes
DISCLOSURE
Dr. Barg reports receiving research support from Johnson and Johnson, lecture fees from Novartis, Teva, GlaxoSmithKline, UCB Pharma and Boehniger and consulting fees from Novartis and Teva. Dr. Gasser reports receiving research support from Novartis, the German Research Ministry (National Genome Network, NGFN) European Commission (NEURON), consulting fees from Cephalon Pharma and Merck Serono- lecture fees from Boehringer- Ingelheim, Merck Serono, Novartis and Schwarz Pharma, and has a patent for the detection of LRRK2 mutations. Dr. Perera receives grant support from FAPESP, CNPq and Genzyme of Brazil. Dr. Rozenberg received grant support form Genzyme of Brazil. Dr. Wu and Dr. Lee-Chen received grant support from the National Science Council, Expectative Yuan, Taiwan. Dr. Fahn reports receiving grant support from the Parkinson’s Disease Foundation, the Smart Family Foundation , lecture fees from Boehringer- Ingelheim and consulting fees from Anteres Pharma, Boehringer- Ingelheim, Elsai, EMD-Serono, Genzyme, IMPAX Pharma, Intec Pharm, Kyowa, Novartis, Teva, Valeant, Vernalis, and serves on the Merz Pharm Safety Monitoring Board. Dr. Ottman reports consulting fees from Ortho-McNeil and Janssen Scientific Affairs, LLC., and equity in Trigeminal Solutions, Inc. All other authors had nothing relevant to disclose.
REFERENCES
- 1.Beutler EGG. Gaucher Disease. In: Scriver CBA, Sly W, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. pp. 3635–3668. [Google Scholar]
- 2.Sidransky E. Gaucher disease: complexity in a "simple" disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 5.Clark LN, Ross BM, Wang Y, et al. Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease. Neurology. 2007;69:1270–1277. doi: 10.1212/01.wnl.0000276989.17578.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 7.Sato C, Morgan A, Lang AE, et al. Analysis of the glucocerebrosidase gene in Parkinson's disease. Mov Disord. 2005;20:367–370. doi: 10.1002/mds.20319. [DOI] [PubMed] [Google Scholar]
- 8.Eblan MJ, Nguyen J, Ziegler SG, et al. Glucocerebrosidase mutations are also found in subjects with early-onset parkinsonism from Venezuela. Mov Disord. 2006;21:282–283. doi: 10.1002/mds.20766. [DOI] [PubMed] [Google Scholar]
- 9.Spitz M, Rozenberg R, Pereira Lda V, Reis Barbosa E. Association between Parkinson's disease and glucocerebrosidase mutations in Brazil. Parkinsonism Relat Disord. 2008;14:58–62. doi: 10.1016/j.parkreldis.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Bras J, Paisan-Ruiz C, Guerreiro R, et al. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mata IF, Samii A, Schneer SH, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol. 2008;65:379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toft M, Pielsticker L, Ross OA, Aasly JO, Farrer MJ. Glucocerebrosidase gene mutations and Parkinson disease in the Norwegian population. Neurology. 2006;66:415–417. doi: 10.1212/01.wnl.0000196492.80676.7c. [DOI] [PubMed] [Google Scholar]
- 13.De Marco EV, Annesi G, Tarantino P, et al. Glucocerebrosidase gene mutations are associated with Parkinson's disease in southern Italy. Mov Disord. 2008;23:460–463. doi: 10.1002/mds.21892. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler SG, Eblan MJ, Gutti U, et al. Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Molecular genetics and metabolism. 2007;91:195–200. doi: 10.1016/j.ymgme.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu YR, Chen CM, Chao CY, et al. Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol Neurosurg Psychiatry. 2007;78:977–979. doi: 10.1136/jnnp.2006.105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan EK, Tong J, Fook-Chong S, et al. Glucocerebrosidase mutations and risk of Parkinson disease in Chinese patients. Arch Neurol. 2007;64:1056–1058. doi: 10.1001/archneur.64.7.1056. [DOI] [PubMed] [Google Scholar]
- 17.Nichols WC, Pankratz N, Marek DK, et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2008 doi: 10.1212/01.wnl.0000327823.81237.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009 doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsui J, Mizuta I, Toyoda A, et al. Mutations for Gaucher disease confer high susceptibility to Parkinson disease. Arch Neurol. 2009;66:571–576. doi: 10.1001/archneurol.2009.72. [DOI] [PubMed] [Google Scholar]
- 20.Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fidani L. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci Lett. 2009;452:87–89. doi: 10.1016/j.neulet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 21.The PDGene Database. [Accessed June 24, 2009];Alzheimer Research Forum. 2009 http://www.pdgene.org/.
- 22.Machaczka M, Rucinska M, Skotnicki AB, Jurczak W. Parkinson's syndrome preceding clinical manifestation of Gaucher's disease. Am J Hematol. 1999;61:216–217. doi: 10.1002/(sici)1096-8652(199907)61:3<216::aid-ajh12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Tayebi N, Callahan M, Madike V, et al. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab. 2001;73:313–321. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- 24.Neudorfer O, Giladi N, Elstein D, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996;89:691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 25.Tayebi N, Walker J, Stubblefield B, et al. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab. 2003;79:104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 26.Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65:1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E. Parkinsonism among Gaucher disease carriers. J Med Genet. 2004;41:937–940. doi: 10.1136/jmg.2004.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halperin A, Elstein D, Zimran A. Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis. 2006;36:426–428. doi: 10.1016/j.bcmd.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum Mutat. 2008;29:567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 30.Montfort M, Chabas A, Vilageliu L, Grinberg D. Functional analysis of 13 GBA mutant alleles identified in Gaucher disease patients: Pathogenic changes and "modifier" polymorphisms. Hum Mutat. 2004;23:567–575. doi: 10.1002/humu.20043. [DOI] [PubMed] [Google Scholar]
- 31.Winfield SL, Tayebi N, Martin BM, Ginns EI, Sidransky E. Identification of three additional genes contiguous to the glucocerebrosidase locus on chromosome 1q21: implications for Gaucher disease. Genome Res. 1997;7:1020–1026. doi: 10.1101/gr.7.10.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tayebi N, Stubblefield BK, Park JK, et al. Reciprocal and nonreciprocal recombination at the glucocerebrosidase gene region: implications for complexity in Gaucher disease. Am J Hum Genet. 2003;72:519–534. doi: 10.1086/367850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan L, Hsu CM, Tsai CH, Lee CC, Hwu WL, Tsai FJ. Mutation analysis of Gaucher disease patients in Taiwan: high prevalence of the RecNciI and L444P mutations. Blood Cells Mol Dis. 2006;36:422–425. doi: 10.1016/j.bcmd.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 36.R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2005 (Accessed at http://www.R-project.org.)
- 37.Park JK, Tayebi N, Stubblefield BK, et al. The E326K mutation and Gaucher disease: mutation or polymorphism? Clin Genet. 2002;61:32–34. doi: 10.1034/j.1399-0004.2002.610106.x. [DOI] [PubMed] [Google Scholar]
- 38.Goker-Alpan O, Giasson BI, Eblan MJ, et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 39.Clark LN, Kartsaklis LA, Wolf Gilbert R, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66:578–583. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrer MJ, Williams LN, Algom AA, et al. Glucosidase-beta variations and Lewy body disorders. Parkinsonism Relat Disord. 2008 doi: 10.1016/j.parkreldis.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segarane B, Li A, Paudel R, et al. Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology. 2009;72:1185–1186. doi: 10.1212/01.wnl.0000345356.40399.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Depaolo J, Goker-Alpan O, Samaddar T, Lopez G, Sidransky E. The association between mutations in the lysosomal protein glucocerebrosidase and parkinsonism. Mov Disord. 2009 doi: 10.1002/mds.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: Potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275:5767–5773. doi: 10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.