Abstract
Environmental factors, including social interaction, can alter the effects of drugs of abuse on behavior. The present study was conducted to examine the effects of social stimuli on oral phencyclidine (PCP) self-administration by rhesus monkeys. Ten adult rhesus monkeys (M. mulatta) were housed side by side in modular cages that could be configured to provide visual, auditory, and olfactory stimuli provided by another monkey located in the other side of the paired unit. During the first experiment, monkeys self-administered PCP (0.25 mg/ml) and water under concurrent fixed ratio (FR) 16 schedules of reinforcement with either a solid or a grid (social) partition separating each pair of monkeys. In the second experiment, a PCP concentration-response relationship was determined under concurrent progressive ratio (PR) schedules of reinforcement under the solid and grid partition conditions. Under the concurrent FR 16 schedules, PCP and water self-administration was significantly higher during exposure to a cage mate through a grid partition than when a solid partition separated the monkeys. The relative reinforcing strength of PCP, as measured by PR break points, was greater during the grid partition condition compared to the solid partition condition indicated by an upward shift in the concentration-response curve. To determine whether the social stimuli provided by another monkey led to activation of the hypothalamic-pituitary-adrenal (HPA) axis, which may have evoked the increase of PCP self-administration during the grid partition condition, a third experiment was conducted to examine cortisol levels under the two housing conditions. A modest, but nonsignificant increase in cortisol levels was found upon switching from the solid to the grid partition condition. The results suggest that social stimulation among monkeys in adjoining cages leads to enhanced reinforcing strength of PCP.
Keywords: Cortisol, Fixed ratio, Pair-housing, PCP, Progressive ratio, Rhesus monkey, Self-administration, Social stimuli
1. Introduction
Environmental factors, including social activity, can modulate the behavioral effects of abused substances and contribute to the perpetual cycle of drug use. Previous research using human subjects has shown that social behavior was altered following consumption of ethanol (Pliner and Cappell, 1974), amphetamine (Griffiths et al., 1977), opiates (Babor et al., 1976), and marijuana (Babor et al., 1974). The effects of drugs on social behavior in laboratory animals have also been documented. In nonhuman primates, a relationship has been demonstrated between social stimuli and the effects of psychoactive drugs on social behavior. Sassenrath and Chapman (1976) found that acute and chronic treatment with Δ9-THC produced changes in the dynamics of a social group of rhesus monkeys. PCP has been shown to alter social behavior in groups of nonhuman primates as well. Miller et al. (1973) found that PCP, as well as amphetamine and chlorpromazine, altered performance on a cooperative shock avoidance task when administered to one member of a pair of monkeys, and changed the social behavior of a well-established group of monkeys. Chronic, noncontingent PCP administration produced a dose-dependent increase in affiliative behaviors, such as grooming and playing, among group-housed capuchin monkeys (Linn et al., 1999). The role of social stimuli, however, has not been investigated as a factor mediating the reinforcing effects of PCP.
While drugs of abuse can alter social behavior, social stimuli can, in turn, alter behavioral and subject-rated measures of drug effects in humans. Jones (1971) found that when marijuana was smoked in isolation, subjects reported higher rates of sedation, relaxation, and dizziness, but higher levels of elation and lower levels of sedation when it was consumed in a group setting. Few studies have been conducted in human subjects to determine the influence of social context on the reinforcing effects of drugs. Kelly et al. (1994) reported that subjects smoked more marijuana when it was available during a period of social access. Several studies have directly examined the role of social stimuli on the reinforcing and behavioral effects of drugs in laboratory animals. Tomie and colleagues (2005; 2004) demonstrated that brief exposure to social stimuli provided by a conspecific rat was associated with faster acquisition of drinking an unsweetened ethanol solution and more rapid escalation of ethanol intake compared to groups not exposed to brief social stimuli, and greater levels of ethanol intake compared to water intake.
Antecedent variables related to social behavior, including rearing conditions and hierarchical rank, can also influence drug-taking behaviors in laboratory animals. Schenk and colleagues (1987) found that rats reared in isolation later acquired cocaine self-administration more rapidly than age-matched rats reared in groups. Others have found that morphine self-administration and physical dependence were greater and more severe, respectively, in rats raised in isolation compared to those raised in groups (Marks-Kaufman and Lewis, 1984). Higley et al. (1991) found that peer-raised monkeys consumed more alcohol, were more distressed, and had higher serum cortisol levels than monkeys that were raised by their natural mothers. Examination of ethanol consumption among monkeys in established dominance hierarchies have yielded mixed results. Peretti and Lewis (1969) found that an initially subordinate male monkey consumed the most ethanol in the group and increased his rank from the lowest to second highest after a nine-day period of ethanol self-administration. Conversely, Crowley et al. (1990) found that dominant monkeys consumed more alcohol than subordinate monkeys. Morgan et al. (2002) found that cynomolgus monkeys ranked as subordinate within a social group had significantly lower levels of the D2 subtype of dopamine receptors in the basal ganglia and self-administered significantly more cocaine at intermediate doses than their dominant counterparts, indicating that social status can affect neurobiological mechanisms that mediate the reinforcing effects of cocaine. Changes in environmental context can also affect drug self-administration. Isolating monkeys that were normally housed in social groups produced increases in alcohol self-administration (Higley et al., 1991; Kraemer and McKinney, 1985). Other findings, however, suggest that type of social context does not necessarily influence ethanol self-administration (Flory et al., 2006; Vivian et al., 1999).
Rhesus monkeys are inherently social animals living in groups with highly developed social structures. Consequently, social stimuli can serve as robust reinforcers in individually housed nonhuman primates (see Anderson, 1998 for a review). Butler (1954) found that rhesus monkeys will perform an operant task at a greater rate to gain visual access to another monkey than to see food, a toy train, or an empty chamber. These results were later confirmed in another study in which monkeys were trained to lever press by reinforcement with visual observation of another monkey, compared to an empty room (Butler, 1958). Squirrel monkeys can discriminate between auditory stimuli in an operant “go/no-go” procedure when behavior is reinforced by contact with another monkey (Hupfer and Maurus, 1975). More recently, studies have shown that male monkeys will relinquish liquid deliveries in a choice procedure when the alternative reinforcer is viewing pictures of the perinea of female monkeys and the faces of high-ranking monkeys (Deaner et al., 2005). Thus, there is evidence suggesting that social stimuli, as well as viewing images of other monkeys can serve as reinforcing stimuli.
The finding that concurrent availability of nondrug alternative reinforcers reduces drug self-administration has been well-established in human and nonhuman subjects. In humans, heroin (Comer et al., 1998) and cocaine (Hart et al., 2000) self-administration decreased when a monetary alternative was concurrently available. In rhesus monkeys, concurrent access to saccharin solutions reduced self-administration of orally delivered PCP (Carroll, 1985; Cosgrove and Carroll, 2003) and smoked cocaine (Comer et al., 1994).
The present study was conducted to examine the effects of exposing monkeys to social stimuli on PCP self-administration. For the first experiment, the reinforcing effects of PCP (0.25 mg/ml) and water were examined under concurrent FR 16 schedules under three housing conditions: 1) when the monkeys were housed in their original single cages, 2) in new modular cages with a solid partition separating the pair, and 3) when the solid partition was replaced with a grid partition allowing access to social stimuli. A second experiment was conducted to examine the reinforcing strength of several concentrations of PCP (0.125, 0.25, 0.5, and 1.0 mg/ml) and water using a concurrent PR schedule during the grid and solid partition conditions. Given that social stimuli can serve as positive reinforcing stimuli in individually housed monkeys, we hypothesized that noncontingent exposure to a same-sex cage mate would reduce PCP self-administration in a manner consistent with providing nondrug alternative reinforcers. Additionally, because social stimuli can lead to activation of the hypothalamic-pituitary-adrenal (HPA) axis (Gust et al., 1993), we sought to determine whether exposure to a monkey in an adjoining cage served as a stressful stimulus. Thus, in a third experiment, cortisol levels obtained from saliva samples were analyzed to examine the possible involvement of stress in mediating changes in PCP self-administration as a result of the social stimuli.
2.Methods
2.1. Subjects
Ten adult rhesus monkeys (Macaca mulatta; eight males and two females) served as subjects. All monkeys had previous experience self-administering PCP concentrations ranging from 0.0625 to 1.0 mg/ml under both FR and PR schedules. Immediately prior to this study, monkeys were maintained on concurrent FR 16 schedules of reinforcement for PCP (0.25 mg/ml) and water deliveries. The monkeys were maintained at 85% of their free-feeding body weights (7.2-12.5 kg for males and 7.0-8.1 kg for females) by adjusting their daily food allotment (Harlan Teklad monkey chow, Bartonville, IL). Monkeys were given fresh fruit, vegetables, or trail mix daily to supplement their diet. Following the daily experimental sessions, movies were played for enrichment. Other enrichment objects including a hanging wooden log and one loose toy were provided to the monkeys under all conditions. Experiments were conducted in two humidity- and temperature-controlled vivarium rooms under a 12-h light/dark cycle (lights on at 0600h) that contained 10 or 12 monkeys each. All procedures and protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee under protocol number 0410A6475. Laboratory facilities were accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). Laboratory practices were carried out in accordance with the Principles of Laboratory Animal Care (National Research Council, 2003).
2.2. Apparatus
At the beginning of the study, monkeys were individually housed in two-tiered stand-alone units with top and bottom cages measuring 76 cm in height × 83 cm in width × 100 cm in depth (Lab Products, Maywood, NJ). Following a period of self-administration in which PCP (0.25 mg/ml) and water were concurrently available under FR 16 schedules in the original cages, monkeys were transferred to new, modular two-tiered pair-housing cages arranged in a side-by-side configuration to allow interaction between two pairs (one pair on the top row, and one pair on the bottom row). The pair-housing units were stainless steel custom-made cages (84 cm in height × 86 cm in width × 79 cm in depth; Suburban Surgical, Chicago, IL) consisting of solid back and side walls, a barred front door, grid floors and a primate perch. The interior side walls could be replaced with a grid partition that had 2.5 cm × 2.5 cm open links between each set of cross bars; the solid exterior-facing side walls were modified to accommodate intelligence panels. The cages could be configured to allow 1) full unrestrained contact, 2) limited physical contact through the grid partition while still allowing visual, auditory, and olfactory exchange in an adjoining cage, or 3) no exposure with solid partitions in place. The solid interior wall or the grid partitions were the configurations that were compared in this study.
Each intelligence panel contained 2 brass spouts (1.2 cm in diameter) that extended 2.7 cm into the cage through circular apertures in the wall about 45 cm above the cage floor and were mounted equidistantly from the center and sides of the cage. There were 3 stimulus lamps placed equidistantly on the panel. Two green-colored stimulus lamps were located above the spouts. One stimulus lamp flashed (10 Hz) to indicate drug availability while the other remained continuously lit to indicate water availability. A red center stimulus lamp remained extinguished and was not used in this study. Each spout was mounted on clear Plexiglas with 2 green and 2 white stimulus lamps embedded within it. The small green and white stimulus lamps were illuminated upon lip contact for PCP or water, respectively. When the appropriate number of lip contact responses had been made, a solenoid valve opened allowing 0.6 ml of liquid to flow from the 2000-ml reservoirs suspended above and mounted to the outside of the panel attached to the cage. Scheduling and recording of events were accomplished using Med-PC software (Med-PC® for Windows) and associated interfaces (Med Associates, St Albans, VT) located in an adjacent room.
2.3. Self-administration procedure
All monkeys were initially trained to self-administer PCP (0.25 mg/ml) and water according to concurrent FR 16 schedules of reinforcement during 3-h daily sessions. Experimental sessions were conducted 7 days per week from 1000 to 1300. Completion of FRs for lip contacts on the two spouts were independent of each other, such that responding toward the FR on one spout was unaffected by responses on the other spout. There was no scheduled timeout following completion of the FR contingency and subsequent liquid delivery. Water and PCP were available on alternating sides each day to control for side preferences.
2.3.2. Progressive ratio schedule
Following the demonstration of stable responding, defined as no steadily increasing or decreasing trends in PCP deliveries over a 5-day period, the relative reinforcing strength of PCP was examined. Concurrent PR schedules of reinforcement were used whereby monkeys self-administered PCP and water as in previous studies (e.g., Carroll et al., 2005). Successive liquid deliveries were contingent upon a systematically increasing progression of ratio values 8, 16, 32, 64, 128, 178, 256, 356, 512, 712, 1024, 1424, 2048, 2848, and 4096. Upon completion of each fixed ratio, 20 individual deliveries (0.6 ml each) were available upon lip contacts with the spout (FR 1). There was a 30-s limited hold to allow for consumption of the 20 deliveries. There was no timeout after each liquid delivery, but the schedule terminated for the day once a 30-min period without completion of a ratio occurred. The break point was defined as the last completed ratio requirement. The session could last up to 3 h, although break points were typically reached within 1.5 h. In the present study, no monkeys exceeded a break point of 1424 responses. The break point was not likely constrained by the 30-min limited hold period, as previous work from this laboratory has demonstrated that monkeys were able to complete a ratio of 2848 within this period (Rodefer and Carroll, 1999). After the break point had been reached, the end of the session was signaled by extinguishing the lights over the spouts and deactivation of the lip contact input and associated stimulus lamps mounted behind the spout. Self-administration under the PR schedule was examined first under the solid partition condition and subsequently under the grid partition condition.
Different concentrations of PCP were substituted when the criterion for stability of 5 consecutive days with no steadily increasing or decreasing trend in break point was met. PCP concentrations of 0.125, 0.25, 0.5, and 1.0 mg/ml were tested in a counterbalanced order across monkeys, and the break point was allowed to stabilize at each concentration for at least 5 days. Water was concurrently available under the same, but independent, PR schedule.
2.4. Salivary cortisol collection
In a third experiment, monkeys self-administered PCP and water under concurrent FR 16 schedules during the solid and grid partition conditions. Saliva samples were collected at 0900-0930 hours (prior to commencement of the self-administration sessions) on the last day of the solid wall condition (baseline), then subsequently on the first and the 14th days of the grid partition condition. Salivary cortisol was collected using the method modified from that developed by Roma (2006) for obtaining saliva samples in infant rhesus macaques. Salivary cortisol content is highly correlated with serum cortisol levels (Salimetrics, 2006), and provides a low-stress, minimally invasive alternative to sedation and venipuncture for serum collection (Boyce et al., 1995). Flavored, braided absorbent cotton dental rolls (15.2 cm in length, 0.63 cm in diameter; Richmond Dental, Charlotte, NC) were placed in a clamp apparatus which clipped to the front of each monkeys' cage. The cotton rolls were prepared by soaking them in a solution that consisted of 71.12% tap water, 28.45% granulated sugar, 0.28% red food coloring (McCormick and Co., Hunt Valley, MD) and 0.14% flavoring oil concentrate (Tropical Punch, LorAnn Oils, Lansing, MI). The cotton rolls were saturated with this solution and then allowed to dry completely at room temperature. The cotton rolls were refrigerated until ready for use. On the days of sample collection, the dried cotton rolls were placed in the clamp apparatus secured to the front of each monkey's cage. The clamp apparatus was modified from that described by Roma (2006). Briefly, it consisted of a U-shaped cable bolt mounted onto a 23-cm Plexiglas platform. Two eye hooks were attached into at either end of the platform and double-sided clips attached the apparatus to the bars on the front of the cage. Monkeys were allowed to chew on the cotton rolls for up to 10 min or longer if necessary to obtain at least 0.6 ml of saliva. Following the 10-min sample collection period, the clamp apparati were removed from the cages, the cotton rolls containing saliva were removed and placed in a Salivette tube (Sarstedt, Newton, NC) and centrifuged for 10 min at 3000 rpm. The saliva was extracted from the Salivette tube by pipette and frozen until it was taken for analysis by the Fairview-University Diagnostics Laboratory at the University of Minnesota. Saliva samples were analyzed in duplicate for their cortisol content using enzyme immunoassay (EIA; Saliametrics, State College, PA). The inter- and intra-assay coefficients of variance were 5.3% and 3.5%, respectively.
2.5. Drugs
Phencyclidine HCl was obtained from the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC). The PCP solutions were mixed in tap water 24 h prior to each session, and they were stored at room temperature. The PCP concentrations (0.125, 0.25, 0.5, and 1.0 mg/ml) refer to the weight of the HCl salt.
2.6. Data analysis
Numbers of PCP and water deliveries obtained under concurrent FR 16 schedules of reinforcement during various cage conditions (i.e., new cage with a solid partition, new cage with a grid partition, and subsequent solid partition) were analyzed using separate one-way (cage condition × PCP or water) repeated measures analyses of variance (ANOVAs). A two-tailed paired t-test was used to determine whether statistically significant differences in PCP self-administration occurred when monkeys were moved from their original cages to the new cage with a solid partition. Numbers of PCP and water deliveries obtained under the PR schedule were analyzed using separate two-way (PCP concentration × grid or solid partition) repeated measures ANOVAs. Break points for PCP deliveries were analyzed similarly; however, since break point data likely violate the homogeneity of variance assumption (Richardson and Roberts, 1996), break points (final ratios completed) were log transformed and subsequently analyzed using a two-way repeated measures ANOVA. Newman-Keuls post-hoc tests were performed when overall significant effects were detected (P < 0.05). Salivary cortisol levels were analyzed using a one-way repeated measures ANOVA comparing cortisol levels on the last day in which the solid partition was in place, and the first and 14th days that the grid partition was in place. A Spearman nonparametric correlation was used to examine the relationship between cortisol levels and numbers of PCP deliveries.
3. Results
3.1. Overt behavior
During the grid partition (social) conditions, monkeys interacted with each other through various non-vocal submissive gestures (e.g., lip-smacking, grimacing), aggressive gestures (e.g., lunging at each other), and displayed non-interactive behaviors such as vocalizations (e.g., barking) and pacing. There were no formal quantifications of overt behaviors in the present study, and no attempts were made to determine dominance among the pairs.
3.2. Self-administration of PCP and water under concurrent FR 16 schedules
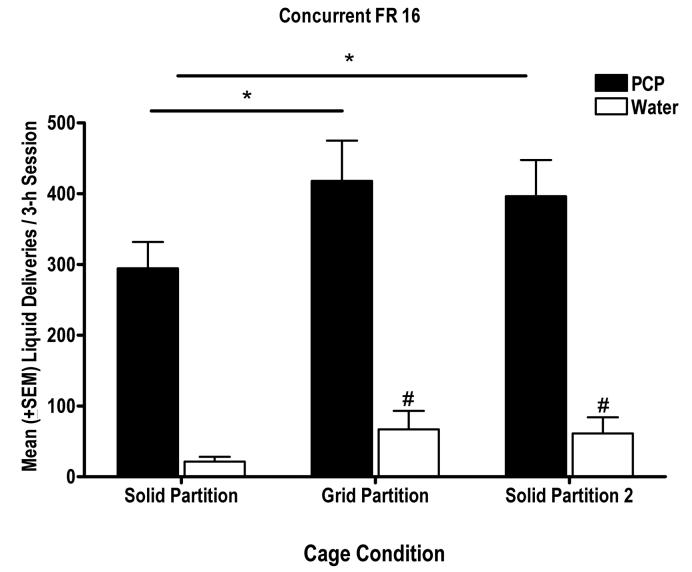
Figure 1 shows the mean (+SEM) numbers of PCP and water deliveries obtained during 3-h experimental sessions when the monkeys were in the new cages with the solid partitions in place (solid partition), when the grid partitions replaced the solid partitions (grid partition), and when they were returned to the solid partition condition (solid partition 2). The results from a paired two-tailed t-test comparison indicated that there was no significant change in PCP deliveries when monkeys were transferred from their original cages to the new cages with the solid partitions in place (t(9) = 1.11; results not shown). However, PCP self-administration was significantly altered by changing of partition conditions [F(2, 29) = 4.22, P < 0.05]. Upon exposure to their cage mates through the grid partition, monkeys self-administered significantly more PCP than when the solid partitions separated them (P < 0.05). When the solid partitions were restored following exposure to the cage mate, the number of PCP deliveries did not significantly decrease, but remained significantly greater compared to the previous solid partition condition (P < 0.05). A separate one-way repeated measures ANOVA indicated that water deliveries also differed as a function of partition condition [F(2, 29) = 4.58; P < 0.05). Specifically, the number of water deliveries increased when the grid partition was in place (P < 0.05) and remained significantly greater during the subsequent solid partition condition when compared to the initial solid partition condition (P < 0.05).
Figure 1.
Mean (+ SEM) numbers of PCP (filled bars) and water (unfilled bars) deliveries obtained under various cage conditions for the group of monkeys under a concurrent FR 16 schedule of reinforcement. Each bar represents mean (+ SEM) liquid deliveries obtained during 5 consecutive days when monkeys were housed in the new cage with solid partition in place (solid partition), the new cage with the grid partition in place (grid partition), and when the solid partition was restored (solid partition 2). *P < 0.05 compared to PCP deliveries obtained under the solid partition condition; #P < 0.05 compared to water deliveries obtained under the solid partition condition.
3.3. Progressive ratio performance and concentration-response determination
As shown in Figure 2, break point values were significantly higher when monkeys were exposed to their cage mate under the grid partition condition [F(1, 79) = 15.13; P < 0.01]. Post-hoc tests indicate significant differences in break points at 0.125 (P < 0.01), 0.25 (P < 0.01) and at 0.5 mg/ml (P < 0.05). The effect on break point values was also concentration-dependent within the grid and solid partition conditions [F(3, 79) = 5.27; P < 0.01]. Under the grid partition condition, break points reached at 0.5 mg/ml were significantly greater (P < 0.05) than break points reached when 0.125 mg/ml was the available solution. Under the solid partition condition, significant differences in break points were found between 0.125 and 0.5 mg/ml (P < 0.01) and 0.125 and 1.0 mg/ml (P < 0.05), as well as between 0.25 mg/ml and 0.5 mg/ml (P < 0.05).
Figure 2.
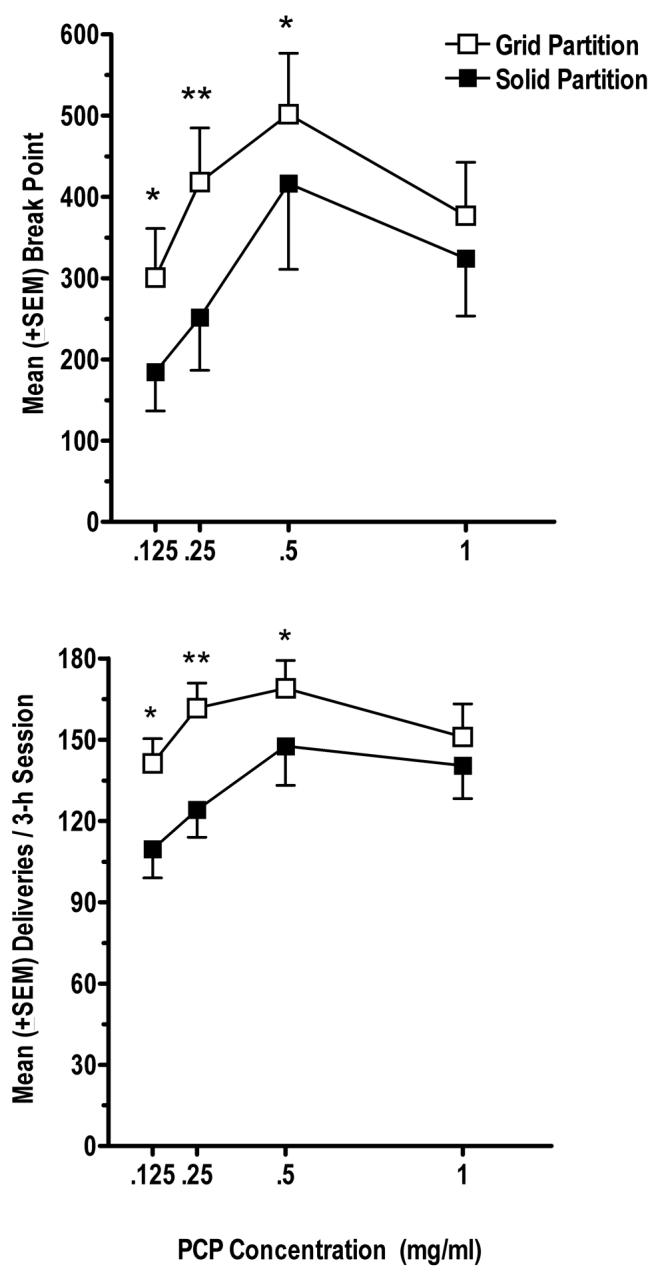
Upper panel. Mean (± SEM) PR break points (final ratio completed) obtained in the group of monkeys during the grid partition (open symbols) and solid partition (filled symbols) conditions as a function of PCP concentration. *P < 0.05, **P < 0.01 compared to the solid partition condition. Lower panel. Mean (± SEM) numbers of PCP deliveries obtained under a concurrent PR schedule of reinforcement as a function of PCP concentration. Other details as in upper panel.
Similarly, monkeys self-administered significantly greater numbers of PCP deliveries when they were exposed to social stimuli compared to the solid partition condition [F(1, 79) = 19.87; P < 0.01]. Post-hoc tests revealed significant differences in PCP deliveries at 0.125 (P < 0.01), 0.25 (P < 0.01), and 0.5 mg/ml PCP (P < 0.05) during the grid partition condition compared to the solid partition condition. The effect was also concentration-dependent within each partition condition [F(3, 79) = 5.75; P < 0.01]. Under the grid partition condition, significantly greater numbers of PCP deliveries were obtained at 0.5 mg/ml than at 0.125 mg/ml (P < 0.05). Under the solid partition condition, significantly greater numbers of PCP deliveries were obtained at 0.5 mg/ml (P < 0.01) and at 1.0 mg/ml (P < 0.05) compared to 0.125 mg/ml. A separate two-way ANOVA conducted on water deliveries failed to reveal a statistically significant difference between partition conditions or between concurrent PCP concentrations.
3.4. Salivary cortisol
Salivary cortisol was collected in 8 of 10 monkeys. Reliable samples could not be collected from 2 monkeys because the dental rolls were destroyed before samples could be obtained. Cortisol levels were modestly, but not significantly [F(2, 23) = 1.92, P = 0.18], elevated during the grid partition conditions (Table 1). Further, on the days that cortisol was collected, no significant differences were found in the number of PCP deliveries under the solid and grid partition conditions [F(2, 23) = 2.48, P > 0.05]. No significant correlation was found between the change in numbers of PCP deliveries and the change in cortisol levels from the solid to grid partition conditions (Spearman r = −.0004).
Table 1.
Mean (±SEM) cortisol levels and PCP deliveries as a function of cage partition configuration.
| Cage Partition Configuration |
Mean (±SEM) Cortisol (μg/dl) |
Mean (±SEM) PCP Deliveries |
|---|---|---|
| Solid Partition | 0.54 (0.11) | 139.6 (19.11) |
| Grid Partition Day 1 | 0.50 (0.07) | 122.5 (12.21) |
| Grid Partition Day 14 | 0.68 (0.16) | 160.0 (15.12) |
4. Discussion
The findings of the present study indicate that PCP self-administration is sensitive to changes in social context. Under both concurrent FR 16 and concurrent PR schedules, PCP self-administration increased when monkeys were exposed to social stimuli in adjoining cages through a grid partition, compared to when they were individually housed (solid partition). Initially, monkeys were tested under concurrent FR 16 schedules for 0.25 mg/ml PCP and water deliveries. The numbers of PCP deliveries were comparable when the monkeys were transferred from their original cages to new pair-housing cages under solid partition conditions, indicating a transition between cages was not sufficient to alter self-administration. However, both PCP and water deliveries increased significantly when the grid partition replaced the solid partition exposing the monkeys to their cage mates. Upon reexamination of self-administration under solid partition conditions, PCP and water deliveries remained elevated instead of returning to levels obtained prior to exposure to another monkey in an adjoining cage. The concomitant increase in PCP and water deliveries during exposure to social stimuli may reflect a general increase in consummatory behavior. When subsequently tested under a PR schedule, in which subsequent deliveries were obtained under increasingly demanding conditions, a significant difference between housing conditions was observed, but water deliveries remained unaffected by the change in housing condition. Under the PR schedule, PCP self-administration was concentration-dependent during both the grid and solid partition conditions. At low and intermediate concentrations, the numbers of PCP deliveries obtained and break points reached were significantly greater during the grid partition conditions. These suggest that PCP's reinforcing strength was modulated by the presence of social stimuli. The effect of social stimuli on water deliveries obtained under the FR, but not PR schedule, may be attributable to the schedule of reinforcement under which the monkeys were maintained.
Based on previous findings with consummatory rewards (Carroll et al., 2001), we hypothesized that, because social stimuli provided by another monkey may serve as an alternative reinforcer, PCP self-administration would decrease when the monkeys were exposed to cage mates in an adjoining cage. However, the opposite effect was observed. Previous studies have demonstrated that concurrent access to nondrug consummatory substances reduce PCP and cocaine self-administration by monkeys (Carroll, 1985; Comer et al., 1994), presumably because rewarding substances compete with, or substitute for one another. In the case of consummatory substances, the drug and nondrug reinforcers may function as substitutes in behavioral economic terms (Carroll and Bickel, 1998), as intake of one substance decreases due to increased price, intake of the other increases. In the present study, the introduction of social stimuli increased PCP intake. This may have been due to the stimuli functioning as complements (i.e., when one increases, the other increases; Carroll and Bickel, 1998; Carroll et al., 2001). Complements (e.g., alcohol and cigarettes) tend to facilitate drug intake. Thus, in this study, social stimuli may be viewed as factors that increase drug self-administration.
An important consideration, however, is that the social stimuli provided by the cage mate was an involuntary situation, as the monkeys had no control over the opening or closing of the solid partition. This forced, or passive, condition may not have operated in the same way as when nondrug incentives are response contingent. The results of the present study are consistent with previous studies in rats orally self-administering ethanol and receiving involuntary access to social stimuli. Tomie et al. (2005; 2004) found that rats exposed to a conspecific rat during an experimental session in which ethanol was available demonstrated faster acquisition, more rapid escalation of ethanol consumption, and greater ethanol intake (g/kg) than rats not exposed to a conspecific rat, and consumed greater volumes of ethanol than a group of rats that only received water.
Another possible determinant causing an increase in PCP self-administration in the presence of social stimuli was a reaction to novelty in environmental conditions. Several studies have demonstrated the existence of a relationship between reactivity to novel stimuli and drug self-administration (Cain et al., 2005; Larson and Carroll, 2005; Nadal et al., 2002). The monkeys used in the present study had long histories of being individually housed; thus, the exposure to social stimulus of another monkey in close proximity was novel, possibly stimulating an increase in self-administration. Another potential explanation for the present results is that having the grid partition enabled the monkeys to watch other monkeys drink, and this may have stimulated both monkeys of the pair. It was noted in a previous study of monkeys smoking cocaine base that one monkey typically initiated his smoking trials earlier in the session and this, in turn, prompted the other monkeys to begin smoking (Carroll et al., 1990).
The findings of the present study are inconsistent with previous studies demonstrating greater self-administration of drugs by monkeys that are housed individually compared to those housed socially. Kraemer and McKinney (1985) demonstrated that monkeys that were normally group-housed consumed more alcohol when they were isolated from their cage mates, suggesting that a change in social situations was sufficient to alter self-administration. Hudson and Singer (1979) demonstrated that polydipsia could be generated by visual presentation of other monkeys, indicating that the effect of the social stimuli may not be leading to increased drug-seeking behavior, but rather a change in schedule-induced behavior. The results from the present study also support a possible effect of adjunctive behavior under simple reinforcement schedules, as both water and PCP consumption were affected by changing of partition condition under the concurrent FR 16 schedules of reinforcement, but not under the concurrent PR schedules. These results suggest that a separation of effects could be observed under more complicated schedules. Further research using different schedules of reinforcement on the effects of social stimuli on drug-maintained behaviors is warranted.
In contrast to serving as reinforcing stimuli, social stimuli can also induce stress responses, and stress, such as that which is evoked in a social defeat situation can enhance the reinforcing effects of cocaine in rats (Haney et al., 1995; Miczek et al., 2004; Miczek and Mutschler, 1996). Exposure to other types of stress, including exogenous administration of glucocorticoids, can potentiate cocaine self-administration (Deroche et al., 1997; Goeders, 2002; Piazza and Le Moal, 1998). Thus, one possible cause for increases in PCP self-administration was that the social stimuli generated a stress response. To determine whether a heightened stress response may have mediated an increase in PCP self-administration under the social condition (grid partition condition), salivary cortisol levels were compared under the solid and grid partition conditions. A slight, but nonsignificant, increase in salivary cortisol was observed 14 days after the grid partition was in place. It should be noted that no difference in PCP deliveries was observed as a function of partition condition on the days that cortisol was measured. This finding is in contrast to those results obtained in the first two experiments, indicating that PCP self-administration may have reached ceiling levels after prolonged exposure to the social stimulus condition. A dissipation in differences following an extended period of cocaine self-administration among a socially housed monkeys was also noted by Czoty et al. (2004).
While some studies conducted in nonhuman primates have shown that changes in social context are related to increases in stress responses (Higley et al., 1991; Ziegler et al., 1995), others have shown that serum cortisol does not necessarily increase in the monkey during exposure to social stimuli. Previous studies have shown that pair-housing in rhesus monkeys failed to result in increased serum cortisol levels (Reinhardt et al., 1991). In further support of the present findings, Tomie et al. (2005) demonstrated that corticosterone levels did not differ among groups of rats exposed to social stimuli during ethanol drinking sessions compared to those not exposed to the social stimuli. A conclusion regarding stress and the reinforcing effects of PCP during exposure to social stimuli in the present study remains tentative, as cortisol was not collected during the initial stages when robust differences in PCP self-administration were observed.
The role of HPA axis activity in facilitating drug self-administration may also depend on the substance of abuse under investigation (Moffett and Goeders, 2005; but see Sarnyai et al., 2001 for a review). Cocaine increases, while the mu-opioid agonist, fentanyl, decreases cortisol and adrenocorticotropic hormone in rhesus monkeys (Broadbear et al., 2004). Review of the literature suggests that the effect of NMDA antagonists on cortisol levels is equivocal. Broadbear et al. (2004) found that self-administered ketamine reduced plasma cortisol levels in rhesus monkeys. However, others have found that cortisol levels initially decrease, but later increase with sustained PCP-induced anesthesia in rhesus monkeys (Setchell et al., 1975). Others have shown that PCP and ketamine increase serum cortisol in rhesus monkeys (Elvidge et al., 1976), or fail to alter cortisol levels in baboons (Bentson et al., 2003). The activation or suppression of cortisol release may also depend on whether the drug is being self-administered or given noncontingently by the experimenter. Thus, it is possible that, due to their extensive histories of PCP self-administration, the monkeys had an altered HPA tone that consequently altered PCP's reinforcing effects.
In the present study, exposure to social stimuli provided by another monkey in an adjoining cage increased the reinforcing strength of PCP in rhesus monkeys. This finding has translational relevance, as social stimuli can alter the effects of drugs and drug taking patterns in humans. While the possibility remains that the increased PCP self-administration was mediated by the novelty of social context or stress resulting from it, it is important to acknowledge that social stimuli facilitate increased drug self-administration in rhesus monkeys.
Acknowledgements
This research was supported by National Institute on Drug Abuse grants R01 DA002486-27 and K05 DA015267-06 (MEC) and National Institute on Drug Abuse training grant T32 DA07097-24 (JLN). The authors wish to thank Peter G. Roma for providing expert advice on salivary cortisol collection and analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson J. Social stimuli and social rewards in primate learning and cognition. Behav Proc. 1998;42:159–175. doi: 10.1016/s0376-6357(97)00074-0. [DOI] [PubMed] [Google Scholar]
- Babor TF, Meyer RE, Mirin SM, McNamee HB, Davies M. Behavioral and social effects of heroin self-administration and withdrawal. Arch Gen Psychiatry. 1976;33:363–367. doi: 10.1001/archpsyc.1976.01770030067010. [DOI] [PubMed] [Google Scholar]
- Babor TF, Rossi AM, Sagotsky G, Meyer RE. Group behavior: Patterns of smoking. In: Mendelson JH, Rossi AM, Meyer RE, editors. The Use of Marijuana: A psychological and physiological inquiry. Plenum Press; New York: 1974. pp. 47–59. [Google Scholar]
- Bentson KL, Capitanio JP, Mendoza SP. Cortisol responses to immobilization with Telazol or ketamine in baboons (Papio cynocephalus/anubis) and rhesus macaques (Macaca mulatta) J Med Primatol. 2003;32:148–160. doi: 10.1034/j.1600-0684.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Champoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery-reared rhesus monkeys: reactivity to peer interactions and altered circadian activity. Dev Psychobiol. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Self-administration of fentanyl, cocaine and ketamine: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology (Berl) 2004;176:398–406. doi: 10.1007/s00213-004-1891-x. [DOI] [PubMed] [Google Scholar]
- Butler RA. The differential effect of visual and auditory incentives on the performance of monkeys. American Journal of Psychology. 1958;71:591–593. [PubMed] [Google Scholar]
- Butler RA. Incentive conditions which influence visual exploration. J Exp Psy. 1954;48:19–23. doi: 10.1037/h0063578. [DOI] [PubMed] [Google Scholar]
- Cain ME, Saucier DA, Bardo MT. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Carroll ME. Concurrent phencyclidine and saccharin access: presentation of an alternative reinforcer reduces drug intake. J Exp Anal Behav. 1985;43:131–144. doi: 10.1901/jeab.1985.43-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology (Berl) 2005;180:414–426. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Bickel WK. Behavioral-environmental determinants of the reinforcing functions of cocaine. In: Higgins ST, Katz JL, editors. Cocaine Abuse: Behavior, Pharmacology and Clinical Applications. Plenum, Inc.; New York: 1998. pp. 81–105. [Google Scholar]
- Carroll ME, Bickel WK, Higgins ST. Nondrug Incentives to Treat Drug Abuse: Laboratory and Clinical Developments. In: Overmier JB, editor. Animal Research and Human Health. American Psychological Association; Washington D.C.: 2001. pp. 139–154. [Google Scholar]
- Carroll ME, Krattiger KL, Gieske D, Sadoff D. Cocaine-base smoking in rhesus monkeys: reinforcing and physiological effects. Psychopharmacology (Berl) 1990;102:443–450. doi: 10.1007/BF02247123. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hunt VR, Carroll ME. Effects of concurrent saccharin availability and buprenorphine pretreatment on demand for smoked cocaine base in rhesus monkeys. Psychopharmacology (Berl) 1994;115:15–23. doi: 10.1007/BF02244746. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME. Effects of a non-drug reinforcer, saccharin, on oral self-administration of phencyclidine in male and female rhesus monkeys. Psychopharmacology (Berl) 2003;170:9–16. doi: 10.1007/s00213-003-1487-x. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Williams EA, Jones RH. Initiating ethanol drinking in a simian social group in a naturalistic setting. Alcohol Clin Exp Res. 1990;14:444–455. doi: 10.1111/j.1530-0277.1990.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther. 1997;281:1401–1407. [PubMed] [Google Scholar]
- Elvidge H, Challis JR, Robinson JS, Roper C, Thorburn GD. Influence of handling and sedation on plasma cortisol in rhesus monkeys (Macaca mulatta) J Endocrinol. 1976;70:325–326. doi: 10.1677/joe.0.0700325. [DOI] [PubMed] [Google Scholar]
- Flory GS, Chen SA, Woltz LA, Magleby S, Higley JD. A computerized apparatus designed to automatically dispense, measure, and record alcohol consumption by individual members of a rhesus macaque social group: trait-like drinking across social- and single-cage conditions. Methods. 2006;38:178–184. doi: 10.1016/j.ymeth.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Stitzer ML, Corker K, Bigelow G, Liebson I. Drug produced changes in human social behavior: Facilitation by d-amphetamine. Pharmacol Biochem Behav. 1977;71:365–372. doi: 10.1016/0091-3057(77)90233-7. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK, Wilson ME. Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta) Horm Behav. 1993;27:318–331. doi: 10.1006/hbeh.1993.1024. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R, Singer G. Polydipsia in the Monkey Generated by Visual Display Schedules. Physiol Behav. 1979;22:379–381. doi: 10.1016/0031-9384(79)90101-x. [DOI] [PubMed] [Google Scholar]
- Hupfer K, Maurus M. Operant Conditioning of the Squirrel Monkey with Social Reinforcement. Naturwissenschaften. 1975;62:42–43. doi: 10.1007/BF00594053. [DOI] [PubMed] [Google Scholar]
- Jones RT. Marijuana-induced high: Influence of expectation, setting, and previous drug experience. Pharmacology Reviews. 1971;23:359–369. [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Mayr MT, Fischman MW. Effects of delta 9-tetrahydrocannabinol and social context on marijuana self-administration by humans. Pharmacol Biochem Behav. 1994;49:763–768. doi: 10.1016/0091-3057(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Kraemer GW, McKinney WT. Social separation increases alcohol consumption in rhesus monkeys. Psychopharmacology (Berl) 1985;86:182–189. doi: 10.1007/BF00431706. [DOI] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Wheel running as a predictor of cocaine self-administration and reinstatement in female rats. Pharmacol Biochem Behav. 2005;82:590–600. doi: 10.1016/j.pbb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Linn GS, O'Keeffe RT, Schroeder CE, Lifshitz K, Javitt DC. Behavioral effects of chronic phencyclidine in monkeys. Neuroreport. 1999;10:2789–2793. doi: 10.1097/00001756-199909090-00017. [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R, Lewis MJ. Early housing experience modifies morphine self-administration and physical dependence in adult rats. Addict Behav. 1984;9:235–243. doi: 10.1016/0306-4603(84)90015-7. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Covington HE, 3rd, Nikulina EM, Jr., Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 1996;128:256–264. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miller RE, Levine JM, Mirsky IA. Effects of psychoactive drugs on nonverbal communication and group social behavior of monkeys. J Pers Soc Psychol. 1973;28:396–405. doi: 10.1037/h0035028. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. Neither non-contingent electric footshock nor administered corticosterone facilitate the acquisition of methamphetamine self-administration. Pharmacol Biochem Behav. 2005;80:333–339. doi: 10.1016/j.pbb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of mammals in neuroscience and behavioral research. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Peretti PO, Lewis BR. Effects of Alcoholic Consumption on the Activity Patterns of Individual Rhesus Monkeys and Their Behavior in A Social Group. Primates. 1969;10:181–188. [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pliner P, Cappell H. Modification of affective consequences of alcohol. J Ab Psychol. 1974;83:418–425. doi: 10.1037/h0036884. [DOI] [PubMed] [Google Scholar]
- Reinhardt V, Cowley D, Eisele S. Serum cortisol concentrations of single-housed and isosexually pair-housed rhesus macaques. J Exp An Sci. 1991;34:73–76. [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Carroll ME. Concurrent progressive-ratio schedules to compare reinforcing effectiveness of different phencyclidine (PCP) concentrations in rhesus monkeys. Psychopharmacology (Berl) 1999;144:163–174. doi: 10.1007/s002130050990. [DOI] [PubMed] [Google Scholar]
- Roma PG. The SPIT method for simultaneous and unobtrusive collection of salivary cortisol from individually housed infant monkeys. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. Springer-Verlag; New York: 2006. pp. 429–460. [Google Scholar]
- Salimetrics 2006 http://www.salimetrics.com/pdf/ER%20Cort%20Research%20Kit%20Insert.pdf.
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Sassenrath EN, Chapman LF. Primate social behavior as a method of analysis of drug action: studies with THC in monkeys. Fed Proc. 1976;35:2238–2244. [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Shackleton CH, Himsworth RL. Studies on plasma corticosteroids in the rhesus monkey (Macaca mulatta) J Endocrinol. 1975;67:241–250. doi: 10.1677/joe.0.0670241. [DOI] [PubMed] [Google Scholar]
- Tomie A, Gittleman J, Dranoff E, Pohorecky LA. Social interaction opportunity and intermittent presentations of ethanol sipper tube induce ethanol drinking in rats. Alcohol. 2005;35:43–55. doi: 10.1016/j.alcohol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Tomie A, Uveges JM, Burger KM, Patterson-Buckendahl P, Pohorecky LA. Effects of ethanol sipper and social opportunity on ethanol drinking in rats. Alcohol Alcohol. 2004;39:197–202. doi: 10.1093/alcalc/agh055. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Higley JD, Linnoila M, Woods JH. Oral ethanol self-administration in rhesus monkeys: behavioral and neurochemical correlates. Alcohol Clin Exp Res. 1999;23:1352–1361. [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Horm Behav. 1995;29:407–424. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]