Abstract
The α7 subunit of the nicotinic acetylcholine receptor (NAchRα7) is one of the principal brain receptors for nicotine and is thought to be a mediator of nicotine’s pro-cognitive effects. While nicotine is known to interact with the stress axis, little is known about the effect of stress or corticosteroids on the expression in the hippocampus, a brain region important to both cognition and stress reactivity. We examined the effects of chronic (21 day) restraint stress (CRS) and adrenalectomy with hormone replacement with the selective mineralocorticoid receptor (MR) agonist aldosterone, the selective glucocorticoid receptor (GR) agonist RU28,362 or corticosterone for 7 days, on the hippocampal expression of NAchRα7 mRNA and protein, as measured by 125I α-Bungarotoxin autoradiography. We found that CRS increase the levels of NAchRα7 mRNA in the CA1, CA3 and Dentate gyrus while levels of the protein were lowered by the same treatment. Corticosteroid replacement showed a GR specific increase in NAchRα7 mRNA, consistent with a corticosteroid mediated effect of CRS. While the mechanism behind these observations is as yet unclear, they may be neuroprotective against the damaging effects of CRS or an example of adaptation to the allostatic load produced by CRS.
1. Introduction
Nicotine is one of the most commonly used stimulants known to man, and it has a profound and well publicized negative impact upon public health. The interaction of nicotine with the stress axis is well known, and nicotine use shares a high co-morbidity with a number of psychiatric disorders (1, 2). In addition to its pathogenic role, nicotine has also been shown to have pro-cognitive effects in humans and animals (3, 4). Studies of the effects of adrenalectomy and sub-chronic corticosterone treatment have shown that both treatments alter nicotine tolerance and binding of the NAchRα7 selective ligand α-Bungarotoxin in the hippocampus and other brain regions (5–10). In addition to containing nicotinic receptors, the hippocampus is rich in both MR and GR receptors and is a nexus for many of the effects of stress on the brain, as well as being a center for spatial cognition and declarative memory formation. Chronic stress has been shown to have a number of effects on hippocampal structure and function, including dendritic remodeling and impairment of spatial and declarative memory in both man and animals (11, 12).
The present study examined the impact of chronic restraint stress and pharmacologic manipulation of corticosteroid levels, after removal of the source of endogenous corticosteroids by adrenalectomy, upon the expression of the α7 subunit of the nicotinic acetylcholine receptor in the hippocampus. Adrenalectomy allows the manipulation of corticosteroid levels without the confound of background levels of endogenous corticosteroids, while replacement in the drinking water produces a diurnal rhythm of corticosteroid levels approximating those occurring in the intact animal.
2. Results
Chronic restraint stress
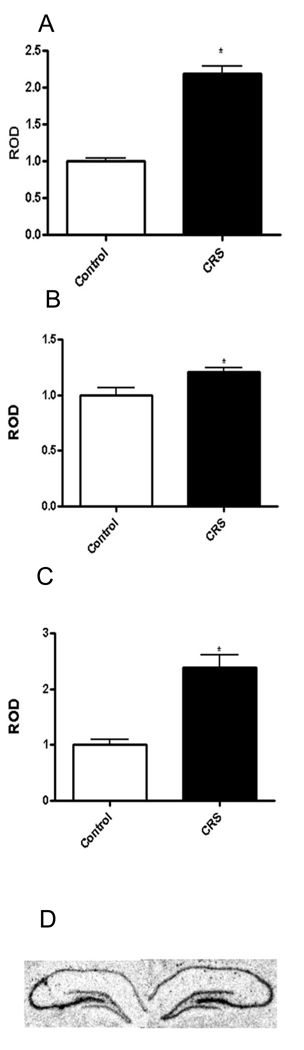
As figure 1 shows, NAchR α7 mRNA was significantly elevated by CRS in CA1 (138±1.0%), CA3 (21 ±2.8%), and DG (118±4.6%) (n=8, p<0.0001, 0.02 and 0.0001 respectively).
Figure 1.
A) shows relative levels of NAchRα7 mRNA in the CA1 of control rats and those subjected to CRS. B) shows mRNA levels in the CA3 and C) shows levels in the dentate gyrus. D) shows representative autoradiograms of the hippocampus of control (left) and CRS (right) treated rats. *-p<0.05, n=8.
Chronic steroid treatment
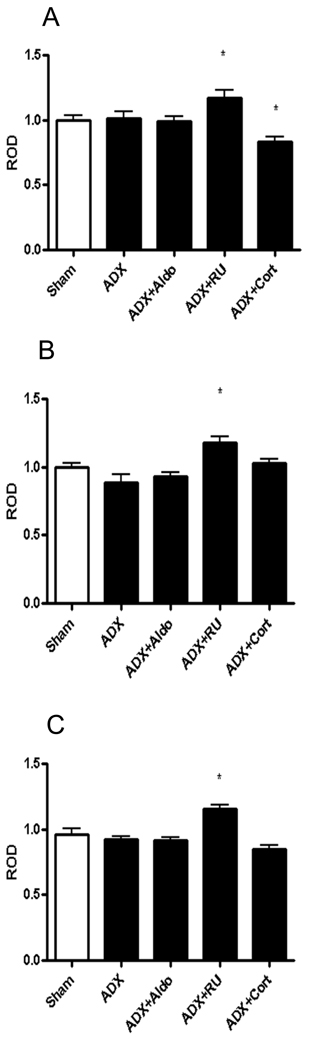
As can be seen in figure 2, The α7 NAchR subunit showed a main effect of treatment in CA1, CA3 and DG (n=7, F=3.512, 6.462, and 6.087 respectively; p<.0001, 0.007, 0.002 respectively). Adrenalectomized (ADX) animals co-treated with RU28,362 showed significant elevations in expression in CA1 (17±4.8%), CA3 (18±3.7%) and DG (15±2.3%) when compared to sham (n=7, p<0.05). RU28,362 co-treatment also produced significantly higher levels of NAchR α7 mRNA than ADX with aldosterone (n=7, p<0.05) and ADX with corticosterone (n=7, p<0.05) co-treatment in all three of the aforementioned regions. ADX + RU28,362 also induced mRNA expression significantly higher than ADX plus vehicle treatment in both the CA3 and DG (n=7, p<0.05).
Figure 2.
A) shows relative levels of NAchRα7 mRNA in the CA1 of rats given either sham surgery, adrenalectomy (ADX), ADX plus aldosterone replacement, ADX plus RU28,362 replacement, or ADX plus corticosterone replacement. B) shows mRNA levels in the CA3 and C) shows levels in the dentate gyrus. *-p<0.05, n=8.
125I α-Bungarotoxin autoradiography
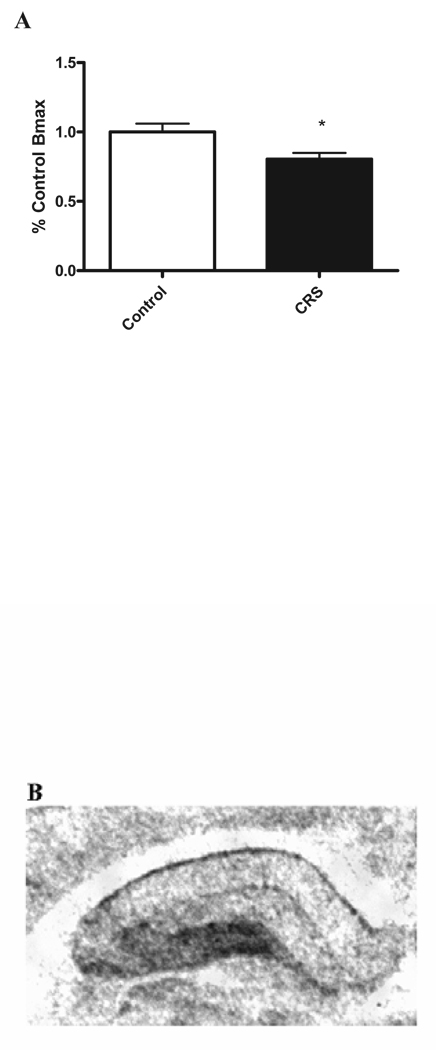
In contrast to the effect of adrenal steroids and stress on NAchRα7 mRNA levels, 125I α-Bungarotoxin autoradiography showed a 20%±5.6% reduction in levels of NAchRα7 binding in the dentate gyrus after chronic restraint of (n=8, p<0.025, see figure 3.) and a trend towards a reduction in binding after adrenalectomy and replacement with RU28,362 (data not shown). No effect of any treatment was observed upon 125I α-Bungarotoxin binding in the CA1 or CA3.
Figure 3.
A) shows relative levels of 125I α-Bungarotoxin binding in the dentate gyrus in control and CRS treated rats. B) shows a representative autoradiogram of the rat hippocampus. *-p<0.05, n=8.
Pyknotic Cell Counts
adrenalectomy produced a 46% (n=8, p< 0.05) increase in the number of pyknotic cells in the dentate gyrus relative to sham adrenalectomized animals, whereas other treatments showed no significant effect on pyknotic cell number and in no treatment was the number of pyknotic cells higher than 5% (data not shown).
3. Discussion
We have shown that chronic stress produces substantial increases in NAchRα7 mRNA while decreasing binding to the receptor in the hippocampal formation, changes which suggest that the NAchRα7 may be important to hippocampal adaptations to stress or allostatic overload (defined as an attempted adaptation to an environmental challenge which has the capacity to produce pathophysiology (13)). This appears to be the case with CRS and NAchRα7, as mRNA is up-regulated while protein levels are reduced, suggesting increased turnover and, perhaps “wear” on the system, although alternative explanations are not excluded by the data presented here. For example, stress may alter trafficking or post-translational modification of NAchRα7 protein, or, mRNA may be upregulated indirectly due to the level of stimulation of the receptors during stress, though the RU28,362 data argue against the latter interpretation.
That adrenalectomy and treatment with the selective GR agonist RU28,362 recapitulates some of this effect seems to indicate that corticosterone is at least partially responsible for the effects we observed with chronic restraint stress. Corticosterone is the principal endogenous ligand for both the GR and the mineralocorticoid receptor (MR) in rodents, and has a higher affinity for the latter. That a selective activation of the GR has a similar effect to CRS suggests that balanced activation of MR and GR in the hippocampus maintains homeostatic levels of NAchRα7 whereas excess GR activation produces up-regulation of the sub-unit mRNA. It is also possible that the differing effects of RU28,362 and corticosterone are due to differing activity at non-genomic GR sites (14). Other groups have observed a corticosterone mediated reduction in α-bungarotoxin binding in the hippocampus using corticosterone pellets (10) in mice. That we did not may be explicable due to species differences or by the fact that our animals received corticosterone in their drinking water and therefore showed a behaviorally induced circadian rhythm in corticosterone levels, which is likely to produce different effects than chronic steady state levels of the steroid. Similarly, though it has been reported that ADX results in an increase in α-bungarotoxin binding in the hippocampus of mice (6) this effect was not present in all mouse strains (15), and our results suggest it is absent in the Sprague-Dawley rat.
That CRS produces such a substantial increase in NAchRα7 mRNA, while reducing labeling with the NAchRα7 selective ligand 125I α-bungarotoxin, implies a higher turnover of receptors. This may be a result of failed adaptation to the allostatic overload produced by CRS. Since NAchRα7 activation is associated with improved cognition (4), the reduction in NAchRα7 levels may contribute to the cognitive deficits observed after chronic stress (11), though further studies will be required to assess this hypothesis. The NAchRα7 gene, CHRNA7, contains a glucocorticoid response element (GRE) (16) and we found that selective agonism of the GR with RU28,362 produced the expected increase in NAchRα7 mRNA. That said, the difference in the magnitude of the stress and RU28,362 effects admits of mechanisms in addition to direct activation of GR. It is quite possible that some of the effect is due to increases in glutamate signaling, which increases in the hippocampus during stress (17). The CHRNA7 gene contains multiple SP1 elements (16) and SP1 family transcription factors are highly regulated by glutamatergic signalling in neurons (18).
Given that NAchRα7 is expressed in most inhibitory interneurons in the hippocampus (19), its levels would have an impact on GABA tone in the region. The NAchRα7 is also directly neuroprotective of hippocampal neurons (20–22); these two observations demonstrate that intact NAchRα7 function may be important to the ability of the hippocampus to resist excitotoxic insult and the damaging effects of stress.
Our results are of broader interest given the effects of nicotine and the NAchRα7 on learning and memory, dementia (4, 23) and schizophrenia (24) as well as the role of stress and hypothalamic-pituitary axis activity in nicotine addiction and relapse (3, 25). The emerging neuroprotective role of NAchRα7 (26) also adds important context to our observations.
4. Experimental Procedures
Animals
Male Sprague-Dawley rats were obtained from Charles River (Kingston, NY) at 70 days of age. Animals were housed 2–3 per cage (same age cage mates) in clear polycarbonate cages with wood chip bedding. All animals were maintained on a 12 h light-dark schedule (lights on at 0800 h) and the temperature was kept at 21±2°C. All animals had ad libitum access to food and water. All procedures were carried out in accordance with the guidelines established by the NIH Guide for the Care and Use of Laboratory Animals.
Chronic restraint stress
To assess the effect of CRS on NAchRα7 levels we subjected rats to a 6 hour a day restraint for 3 weeks. Animals were left undisturbed after arrival for one week after delivery. Animals were restrained in wire mesh restrainers, secured at the head and tail ends with clips. Chronic stress was administered for 6 hours daily for 21 days. These animals were sacrificed 18 hours after the last stress to ensure that neither circulating corticosteroids (18 hours later being close to the circadian nadir in corticosterone secretion) nor the acute effects of stress contaminated the results. Animals were returned to their home cages immediately after termination of the stressor, unless immediately sacrificed. Brains were removed and flash frozen on dry ice then stored at −80°C until processing. All animals were killed between 1300 and 1700 h.
Steroid treatments
In order to determine the contribution of adrenal steroids to the regulation of NAchRα7 mRNA in the hippocampal formation we adrenalectomized rats and treated them with several corticosteroid receptor agonists. These treatments followed those administered in (27, 28) with some modification. Animals were anesthetized using ketamine and xylazine and the adrenal glands removed, save for one group which received a sham surgery. During the same surgery, osmotic mini-pumps (Alzet, Cupertino, CA) were implanted between the scapula. These pumps delivered either vehicle, the mineralocorticoid receptor agonist aldosterone at 10mg/hour or the glucocorticoid receptor agonist RU28,362 at 10µg/hour. Animals who underwent ADX received 0.9% saline in their drinking water and one group received 400µg/ml corticosterone in addition to the saline. Seven days after the completion of the surgeries the animals were sacrificed and their brains frozen as described above.
In Situ Hybridization
Brain sections were cut at 20 µm on a cryostat and placed on Fisher Biotech ProbeOn Plus slides (Fisher, Pittsburgh, PA). In situ hybridization began with a tailing reaction to radioactively label the oligonucleotide probes with 35S. The probe sequences are those described by Ryan and Loiacono (29) ( α7 accession number: L31619, mRNA sequence 701–746, 1031–1076, 1161–1206) . Processing of the slides followed methods as previously described (30) with some modification as described in (31). Anatomical locations were determined with the assistance of the atlas of Paxinos and Watson (32). Optical density was determined using MCID 5.0 (Imaging Research, St. Catharine’s, OT, Canada).
125I α-Bungarotoxin autoradiography
We followed the procedure of (33) and (34) with some modification. 20 µm thaw mounted sections were brought to room temperature in a desiccator and pre-incubated in 1%BSA and 50mM Tris pH 7.4 for 30 minutes, followed by a one hour RT incubation with 5nM 125I α-Bungarotoxin (Perkin Elmer, Waltham, MA, USA) with or with out 1mM (−) nicotine to determine non-specific binding. Sections were then washed 4 times for 5 minutes in ice cold 50mM Tris, pH7.4, dried and placed on Kodak BioMax MR film for 3 days.
Pyknotic Cell Counts
Numbers of pyknotic cells were assessed following the method of Frye and McCormick (35). Sections were serial to those used for autoradiography and in situ. Slides containing these sections were processed to reveal Nissl substance beginning with a brief fixation in 4% paraformaldehyde in 0.1M PB for 15 minutes after which they were washed in distilled water three times for 2 minutes per wash. Sections were then dipped in 0.1% Cresyl Violet for 2 minutes and then dehydrated in ascending concentrations of ethanol prior to clearing in xylenes for 4 minutes. After drying the slides were coverslipped with permount. Pyknotic cells in the granule cell layer and subgranule zone of the dentate gyrus were identified in a 100× visual field as those having a small volume, membrane blebbing, and dark condensed nucleus and chromatin.
Statistics
Optical density measurements were analyzed by a one way ANOVA for the chronic steroid study and by Student’s t-test for the chronic stress study. Significant main effects and interactions in ANOVA were further analyzed using Tukey’s test, respectively. Differences are considered significant at p<0.05. All data are presented as mean ±SEM.
Acknowledgements
This work was supported by NIH MH 15125, MH41256 and MH065749. RGH was supported by the Gary R. Helman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13(9):1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 2.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 3.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 4.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184(3–4):523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 5.Robinson SF, Grun EU, Pauly JR, Collins AC. Changes in sensitivity to nicotine and brain nicotinic receptors following chronic nicotine and corticosterone treatments in mice. Pharmacol Biochem Behav. 1996;54(3):587–593. doi: 10.1016/0091-3057(95)02281-3. [DOI] [PubMed] [Google Scholar]
- 6.Grun EU, Pauly JR, Bullock AE, Collins AC. Corticosterone reversibly alters brain alpha-bungarotoxin binding and nicotine sensitivity. Pharmacol Biochem Behav. 1995;52(3):629–635. doi: 10.1016/0091-3057(95)00157-r. [DOI] [PubMed] [Google Scholar]
- 7.Grun EA, Pauly JR, Collins AC. Adrenalectomy reverses chronic injection-induced tolerance to nicotine. Psychopharmacology (Berl) 1992;109(3):299–304. doi: 10.1007/BF02245877. [DOI] [PubMed] [Google Scholar]
- 8.Pauly JR, Grun EU, Collins AC. Chronic corticosterone administration modulates nicotine sensitivity and brain nicotinic receptor binding in C3H mice. Psychopharmacology (Berl) 1990;101(3):310–316. doi: 10.1007/BF02244047. [DOI] [PubMed] [Google Scholar]
- 9.Caggiula AR, et al. The role of corticosteroids in nicotine's physiological and behavioral effects. Psychoneuroendocrinology. 1998;23(2):143–159. doi: 10.1016/s0306-4530(97)00078-4. [DOI] [PubMed] [Google Scholar]
- 10.Pauly JR, Collins AC. An autoradiographic analysis of alterations in nicotinic cholinergic receptors following 1 week of corticosterone supplementation. Neuroendocrinology. 1993;57(2):262–271. doi: 10.1159/000126368. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 13.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS. Steroid hormone actions on the brain: when is the genome involved? Horm Behav. 1994;28(4):396–405. doi: 10.1006/hbeh.1994.1036. [DOI] [PubMed] [Google Scholar]
- 15.Stitzel JA, Farnham DA, Collins AC. Chronic corticosterone treatment elicits dose-dependent changes in mouse brain alpha-bungarotoxin binding. Neuroscience. 1996;72(3):791–799. doi: 10.1016/0306-4522(95)00584-6. [DOI] [PubMed] [Google Scholar]
- 16.Leonard S, et al. Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59(12):1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 17.Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61(5):1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- 18.Mao XR, Moerman-Herzog AM, Chen Y, Barger SW. Unique aspects of transcriptional regulation in neurons--nuances in NFkappaB and Sp1-related factors. J Neuroinflammation. 2009;6:16. doi: 10.1186/1742-2094-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son JH, Winzer-Serhan UH. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs in rat hippocampal GABAergic interneurons. J Comp Neurol. 2008;511(2):286–299. doi: 10.1002/cne.21828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dajas-Bailador FA, Lima PA, Wonnacott S. The alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism. Neuropharmacology. 2000;39(13):2799–2807. doi: 10.1016/s0028-3908(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 21.Egea J, et al. Neuroprotection afforded by nicotine against oxygen and glucose deprivation in hippocampal slices is lost in alpha7 nicotinic receptor knockout mice. Neuroscience. 2007;145(3):866–872. doi: 10.1016/j.neuroscience.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Shin EJ, et al. Repeated intracerebroventricular infusion of nicotine prevents kainate-induced neurotoxicity by activating the alpha7 nicotinic acetylcholine receptor. Epilepsy Res. 2007;73(3):292–298. doi: 10.1016/j.eplepsyres.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Cincotta SL, Yorek MS, Moschak TM, Lewis SR, Rodefer JS. Selective nicotinic acetylcholine receptor agonists: potential therapies for neuropsychiatric disorders with cognitive dysfunction. Curr Opin Investig Drugs. 2008;9(1):47–56. [PubMed] [Google Scholar]
- 24.Deutsch SI, et al. Therapeutic implications of a selective alpha7 nicotinic receptor abnormality in schizophrenia. Isr J Psychiatry Relat Sci. 2005;42(1):33–44. [PubMed] [Google Scholar]
- 25.Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59(3):236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Mudo G, Belluardo N, Fuxe K. Nicotinic receptor agonists as neuroprotective/neurotrophic drugs. Progress in molecular mechanisms. J Neural Transm. 2007;114(1):135–147. doi: 10.1007/s00702-006-0561-z. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe Y, Weiland NG, McEwen BS. Effects of adrenal steroid manipulations and repeated restraint stress on dynorphin mRNA levels and excitatory amino acid receptor binding in hippocampus. Brain Res. 1995;680(1–2):217–225. doi: 10.1016/0006-8993(95)00235-i. [DOI] [PubMed] [Google Scholar]
- 28.Hunter RG, et al. Regulation of kainate receptor subunit mRNA by stress and corticosteroids in the rat hippocampus. PLoS One. 2009;4(1):e4328. doi: 10.1371/journal.pone.0004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan RE, Loiacono RE. Nicotinic receptor subunit mRNA in the thalamus of the rat: relevance to schizophrenia? Neuroreport. 2000;11(17):3693–3698. doi: 10.1097/00001756-200011270-00021. [DOI] [PubMed] [Google Scholar]
- 30.Hunter RG, et al. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Hunter RG, et al. Regulation of CART mRNA by stress and corticosteroids in the hippocampus and amygdala. Brain Res. 2007;1152:234–240. doi: 10.1016/j.brainres.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. New York: Academic Press; 1986. [Google Scholar]
- 33.Segal M, Dudai Y, Amsterdam A. Distribution of an alphabungarotoxin- binding cholinergic nicotinic receptor in rat brain. Brain Res. 1978;148(1):105–119. doi: 10.1016/0006-8993(78)90381-5. [DOI] [PubMed] [Google Scholar]
- 34.Orr-Urtreger A, et al. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17(23):9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frye CA, McCormick CM. Androgens are neuroprotective in the dentate gyrus of adrenalectomized female rats. Stress. 2000;3(3):185–194. doi: 10.3109/10253890009001122. [DOI] [PubMed] [Google Scholar]