Abstract
Background
Huachansu, a Chinese medicine that comes from dried toad venom from the skin glands of Bufo bufo gargarizans Cantor or B.melanotictus Schneider, has been used in the treatment of various cancers in China. We conducted a pilot study, using a phase I trial design, of Huachansu in patients with advanced cancer.
Patients and Methods
Huachansu was administered intravenously for 14 days followed by 7 days off (1 cycle). Without significant adverse events or progressive disease, treatment continued beyond 2 cycles. The dose of Huachansu (in mL/ m2) was escalated as follows with three patients per cohort: 10 (level 1), 20 (level 2), 40 (level 3), 60 (level 4), and 90 (level 5) mL/m2.
Results
Fifteen patients (hepatocellular cancer, N = 11; non-small cell lung cancer, N = 2; pancreatic cancer, N = 2) were enrolled in the trial and no dose limiting toxicities (DLT) were found. Eleven patients had no drug-related toxicity greater than grade I. Six (40%) had stable disease (median duration = 6.0 months; range 3.5 to 11.1 months). One of these patients (with hepatocellular cancer) had 20% regression (duration = 11 months) (dose level 1). Quality of life improved for patients with stable disease. Plasma bufalin concentration reached maximal levels at the end of the 2-hr infusion and was propotional to the amount of drug being administered (0.81-3.38, ng/mL).
Conclusions
No DLT was observed with the use of Huachansu at doses up to eight times higher than typically used in China. Six patients had prolonged stable disease or minor tumor shrinkage.
Keywords: pancreatic cancer, hepatocellular cancer, non-small cell lung cancer, phase I, traditional Chinese medicine
INTRODUCTION
Chansu, initially recorded in traditional Chinese medicine more than 1000 years ago, is the dried toad venom or the dried secretion from the skin glands of Bufo bufo gargarizans Cantor or B.melanotictus Schneider.1 It has been used as a local anesthetic, a cardiotonic agent, and a diuretic 1, 2 and is widely used to treat cancer at oncology clinics in China.1-3
Huachansu is a sterilized hot water extract of dried toad skin that has been prepared for injection. It is manufactured by Anhui Jinchan Biochemistry Company Ltd., in Huaibei, China (Chinese FDA (ISO9002)). The chemical components and pharmacologic activity of Huachansu have been investigated since the 1980s. Its two primary biologically active chemical components are indole alkaloids (bufotenine, bufotenidine, cinobufotenine, and serotonin) and steroidal cardiac glycosides (more than 28 have been identified, including bufalin, resibufogenin, cinobufagin, cinobufotalin, marinobufagin and bufotalin).3 Recent studies have demonstrated that bufalin, resibufogenin, and cinobufagin are the three major cardiac glycosides to which the antitumor activity of Huachansu can be attributed.4 A recent review article posits the emerging role of cardiac glycosides in the prevention and/or treatment of proliferative diseases such as cancer and suggests that Na, K-ATPase is a possible drug target for these group of compounds. Cardiac glycosides are involved in complex cell-signal transduction pathways and result in selective control of human tumor but not normal cellular proliferation; thus, cardiac glycosides may represent a promising form of targeted cancer chemotherapy.5
Chinese clinical trials conducted since the 1970s have shown repeatedly that Huachansu has anticancer activity. For example, total response (complete response [CR] plus partial response [PR]) rates of 10% and 16% were observed in patients with advanced hepatocellular carcinoma and lung cancer, respectively, with only mild adverse effects.6, 7 Furthermore, when used in combination with chemotherapy or radiotherapy, Huachansu synergistically enhanced the efficacy of the conventional therapies and reduced their toxicity.8
Although this seemingly well-tolerated traditional medicine is commonly used in China and is approved by the Chinese Food and Drug Adminstration, no formal trials of the agent have been done that examine dose-toxicity relationships. The doses generally used in China are 20 mL/m2 or 20-25 mL. We felt that this agent merited further exploration using a more formal, Western medicine-based approach, and that this pilot trial should also explore a broader range of doses. Therefore, we conducted a collaborative study between Fudan University Cancer Hospital and The University of Texas M. D. Anderson Cancer Center which is part of the International Center of Traditional Chinese Medicine for Cancer (funded in part by R21CA108084 and U19CA12153031 from the National Cancer Institute). Herein, we report the results of our pilot study, using a phase I clinical trial design, of Huachansu in patients with heptaocellular carcinoma, non-small cell lung cancer, or pancreatic cancer.
MATERIALS AND METHODS
Patients
Patients with stage III or IV hepatocellular carcinoma, non-small cell lung cancer, or pancreatic cancer were considered eligible for enrollment in the trial, which was conducted at the Fudan University Cancer Hospital, in Shanghai, China. Inclusion criteria were as follows: ≥18 years of age; histologic or cytologic diagnosis of one of the three aforementioned cancers with measurable lesions; life expectancy of at least 3 months; performance status of ≥ 60 (Karnofsky performance status [KPS] scale); and adequate organ function. Patients also must not have received any previous chemotherapy or radiotherapy for at least 3 weeks and have recovered from any toxic effects of such therapy. Patients previously treated with Huachansu must have discontinued treatment at least 6 months earlier. Exclusion criteria were central nervous system involvement; need for concurrent radiotherapy or other chemotherapy; leukemia or myelodysplastic syndrome; pregnancy or lactation; symptomatic peripheral neuropathy; concurrent infection requiring intravenous antibiotics; and a history of allergy to toad skin products. Patients were prohibited from taking other medicines, herbs, supplements, tonics, etc. Written informed consent, indicating the patients awareness of the investigational nature of this study, was obtained from all patients.
Study Design
The study was collaboratively designed and conceived by faculty from the Fudan University Cancer Hospital and M. D. Anderson and the protocol was approved by both institutional review boards. Two nurses from Fudan University Cancer Hospital spent 3 months at M. D. Anderson undergoing research nurse training and two physicians underwent 2 months of faculty research training. During the course of the trial faculty and staff from M. D. Anderson visited Fudan University Cancer Hospital four times to review the trial and video conferences were conducted two times each month.
Patients were treated in cohorts of 3 per dose level at 5 dose levels. For each subsequent cohort of patients, dose escalation depended on the toxicity profile in the previous cohort. The typical dose of Huachansu used in China is 20-25 mL (approximatelty 15 mL/m2). The planned dose escalation schedule for Huachansu was as follows; level l, 10 mL/m2; level 2, 20 mL/m2; level 3, 40 mL/m2; level 4, 60 mL/m2; and level 5, 90 mL/m2. The treatment was repeated daily for 14 days followed by 7 days off (1 cycle). In the absence of treatment delays, treatment was continued beyond 2 cycles. After 2 cycles, most patients received other treatments in combination with Huachansu.
Anhui Jinchan Biochemistry Company Ltd. provided the drug for this trial from the same lot. There are a number of quality control strategies in place to improve and ensure product quality. The toad skins are acquired from designated source provinces (Anhui and Shandong) in China and the skin samples are only collected in the Fall. There is an established fingerprinting for the toad skin raw material, the semi-final extracts, and final product of Huachansu. In addition, to eliminate the variation in the extracts of the toad skins collected from different geographical regions, each final product lot is a mix, at a fixed ratio, of toad skin extracts prepared from each province source. Finally, analytical methods have been established to monitor the quality of extract and product. As a regular quality control measure, the concentrations of certain compounds in the extract are measured and HPLC fingerprinting is compared in the semi-final and final products. Based on these quality control methods Huachsnsu has good consistency and reproducibility.
The consistency of three separate lots of Huachansu were analyzed at M. D. Anderson and levels of bufalin (18.0 to 19.5 ng/mL) and resibufogenin (17.7 to 19.0 ng/mL) were remarkably close (less than 10% variation). In addition, molecular fingerprinting of three lots showed they were very similar. In each lot there were seven individual peaks with peak areas greater than 2% of total peak area. The seven peaks among the three lots had similar retention time and peak area (less than 20% variation). Even though there was good consisteinceny from lot to lot it was considered best for this trial to use Huachansu processed in a single lot.
Outcomes Measures
Toxicity in each cohort of 3 patients treated at a particular dose level was graded according to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE) (http://ctep.info.nih.gov). Response and progression were evaluated using the criteria proposed by the Response Evaluation Criteria in Solid Tumors (RECIST) Committee.9
Evaluation of quality of life was preformed using the M. D. Anderson Symptom Inventory (MDASI) pre-treament and then weekly thereafter. The MDASI consists of a core list of symptoms that are common to all cancer diagnoses and treatments. The MDASI was found to have good reliability and validity in a study of over 500 outpatients with cancer.10
Patient Evaluation
Pre-treatment evaluations included a complete history and physical examination; complete blood count (CBC); differential, platelet, and renal and hepatic function tests; cardiac perfusion scan; electrocardiogram; and radiologic imaging studies and tumor marker evaluation to document the extent of disease. Radiologic tests were done no more than 1 month prior to the start of treatment, and all other tests were done no more than 2 weeks prior to the start of therapy. Patients also completed the MDASI within 2 weeks of the start of treatment.
On-study assessments included history and physical examination after each of the first 2 cycles and weekly assessments of CBC, platelet count and differential, hepatic and renal function tests, EKG, and MDASI. Appropriate radiologic and tumor marker studies were repeated after the first 2 courses of therapy.
Determination of Plasma Bufalin Content
To delineate the role of bufadienolides in antitumor efficacy and the cardiac toxicity of Huachansu, we drew 5 mL of blood for testing at the following time points: pre-dose; mid-infusion; end of infusion; 24 hours after infusion; day 14 of cycle (pre-dose); and day 21 of cycle (before the next infusion cycle). To prepare the sample for the determination of the plasma bufalin content, we diluted 1.5 mL of plasma with 1.5 mL of PBS and then applied to a preconditioned Sep-Pak solid phase extraction cartridge (3 cc 100 mg, Waters Corp., Milford, MA). The column was then washed with 3 mL of water and bufalin was eluated with 3 mL of ethyl acetate, after which the effluent was collected and evaporated. The dried sample was then shipped to M. D. Anderson for analysis of bufalin content, where the extract was reconstituted in 100 μl methanol: 0.2% formic acid (1:1) prior to analysis by LC/MS/MS. The specific bufalin content was determined using a validated assay previously developed at M. D. Anderson.
An LC/MS/MS analytical method was developed for measuring levels of bufadienolides levels including bufalin, cinobufagin, resibufogenin, and cinobufotalin, in the Huachansu injection as well as in human plasma specimens. The limit of detection of this method was around 0.15 ng/mL. The levels of bufalin, cinobufagin, cinobufotalin, and resibufogenin in the Huachansu injectate used in this phase I clinical trial were 14.3±0.03, 3.35 ± 0.1, 21.5 ± 0.22, and 24.5 ± 2.18 ng/mL, respectively. However, since studies have shown that bufalin is the major bufodienolide with the most pronounced anticancer activity,11-13 we measured only the bufalin concentration in plasma by LC/MS/MS. Reverse-phase HPLC electrospray ionization MS was performed using a Micromass Quattro Ultima tandem mass spectrometer (Waters Corp) equipped with an Agilent 1100 HP binary pump high-pressure LC inlet. Bufalin was separated using a Luna C8 5μ (4.6 × 100 mm) LC column (Phenomenex, Torrence, CA). The mobile phase consisted of 0.2% formic acid (aqueous), pH 3.0, and methanol; the flow rate was 250 μl/min with a column temperature of 50°C. The sample injection volume was 25 μl. The mass spectrometer was operated in the electrospray positive-ion mode with a cone voltage of 75 V. Fragmentation of all compounds was performed using argon as the collision gas at a cell pressure of 2.1 × 10−3 torr. The collision energy was 12 V. Bufalin was detected using positive ionization and multiple-reaction monitoring of the transition ions 387 > 161 for bufalin.
RESULTS
Patient Characteristics
From January 2005 through July 2006, 15 patients (3 at each of the 5 dose levels) were enrolled in this study. All patients were evaluated for toxicity and response. Patient demographics and disease characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic | |
|---|---|
| General | |
| Male:female ratio | 11:4 |
| Median age, y (range) | 55 (29-70) |
| Evaluable for toxicity | 15 |
| Evaluable for response | 14 |
| Diagnosis | |
| Hepatocellular carcinoma | 11 |
| Pancreatic cancer | 2 |
| Non-small cell lung cancer | 2 |
| Disease stage | |
| III | 2 |
| IV | 13 |
| Prior therapy | |
| Yes | 12 |
| No | 3 |
| KPS | |
| 90 | 7 |
| 80 | 6 |
| 70 | 2 |
Dose Levels
Fifteen patients received 38 cycles of Huachansu (3 received 1 cycle; 10 received 2 cycles; 1 received 7 cycles; and 1 received 8 cycles).
Toxicities/Side Effects
Mild adverse events were observed at each dose level; all were grade I or II and no grade III or IV toxicities were observed. Eleven patients had no drug-related toxicity greater than grade I. Events known to be or suspected of being related to either Huachansu or to the disease state are shown in Table 2. There were no abnormalities in hepatic and renal function tests.
Table 2.
Toxicity profile
| I | II | III | IV | |
|---|---|---|---|---|
| Leukopenia | 1 | 0 | 0 | 0 |
| Thrombocytopenia | 1 | 0 | 0 | 0 |
| Loss of appetite | 1 | 0 | 0 | 0 |
| Constipation | 1 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 |
| Rash | 1 | 1 | 0 | 0 |
| Myalgia | 1 | 0 | 0 | 0 |
| Dizziness | 1 | 0 | 0 | 0 |
| Oral ulcer | 0 | 1 | 0 | 0 |
| Dyspnea | 0 | 1 | 0 | 0 |
| Premature ventricular contraction | 1 | 0 | 0 | 0 |
| Hypertension | 0 | 1 | 0 | 0 |
Toxicities by body system are described below:
Hematologic toxicities
Two types of hematologic toxicity were associated with Huachansu use. One patient developed grade I thrombocytopenia on day 6 after receiving the level 2 dose, but this resolved after 27 days. Another patient developed grade I leukopenia on day 14 after receiving the level 4 dose, but this resolved after 7 days.
Gastrointestinal toxicities
One patient given the level 1 dose of Huachansu had loss of appetite starting on day 10, but the patient recovered his appetite after 30 days. One patient who received the level 2 dose had grade I constipation on the first day, but this condition resolved after 7 days. One patient given the level 5 dose developed grade I diarrhea 2 days after receiving the medication, and the toxicity did not resolve until the completion of the study.
Mucocutaneous toxicities
Two types of mucocutaneous toxicity were observed: dental ulcers and rashes. A grade II dental ulcer occurred in 1 patient a day after receipt of a level 1 dose, but this condition resolved by 3 days later. One patient who received a level 5 dose had a grade II rash on day 21 that lasted 20 days; another patient given a level 2 dose had a grade I rash during maintenance therapy with Huachansu that lasted 1 week.
Cardiovascular toxicites
One patient who received the level 1 dose had grade I premature ventricular contraction on day 20 that resolved on the same day. The same patient also had grade II hypertension 16 hours after treatment, which resolved by 4 hours later. There were no other abnormities observed on the serial EKGs for other patients.
Other toxic effects
Grade I myalgia was noted in 1 patient treated with the level 1 dose that occurred 50 days after medication was started (maintenance therapy), and this condition persisted for the duration of treatment; grade II dyspnea occurred on day 4 in 1 patient given a level 3 dose; the dyspnea resolved within 30 minutes. Finally, persistent grade I dizziness occurred in 1 patient 5 days after receiving a level 5 dose.
Maximum Tolerated Dose
There were no grade III/IV adverse events. As a result, no dose-limiting toxicities (DLT) were established. In addition, no cardiac toxicities were observed.
Response
One patient with hepatocelluar cancer dropped out of the study after one cycle of treatment as they had bone metastasis that needed radiation and they redused to provide a new CT scan (patient number 9, Table 3). Therapy was classified as having failed in this patient (Table 3). Six (40%) had stable disease and 9 (60%) had progressive disease. One patient with hepatocellular carcinoma treated with the level 1 dose had a 20% reduction in tumor mass (RECIST)9 and stable disease in response to Huachansu alone that lasted for 11.1 months (Table 3, Figure 1). AFP levels dropped from 138 to 70 ug/L after four cycles of treatment and then started to climb again after 8 cycles (107 ug/L). One patient with hepatocellular carcinoma treated with the level 2 dose who also had metastases to the lung had stable disease in response to Huachansu alone that lasted 8.0 months . Four other patients had stable disease for 6, 3.5, 8, and 5.5 months, respectively (see Table 3). AFP levels were examined in all patients.
Table 3.
Response to Huachansu
| No. | Diagnosis | Disease Stage | Prior Therapy | Cycles received | Dose (mL/m2) | KPS Base-line | KPS After 1 Cycle | KPS After 2 Cycles | Response | Duration of Response (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HCC | IV | Yes | 2 | 10 | 90 | 80 | 70 | PD | |
| 2 | NSCLC | IV | No | 2 | 10 | 80 | 70 | 70 | PD | |
| 3 | HCC | IV | No | 8 | 10 | 90 | 90 | 90 | SD | 11.1 |
| 4 | HCC | IV | Yes | 7 | 20 | 90 | 90 | 90 | SD | 8.0 |
| 5 | PC | IV | Yes | 2 | 20 | 70 | 70 | 70 | PD | |
| 6 | HCC | III | Yes | 2 | 20 | 90 | 90 | 90 | SD | 6.0 |
| 7 | NSCLC | IV | Yes | 2 | 40 | 70 | 70 | 70 | PD | |
| 8 | HCC | IV | Yes | 1 | 40 | 80 | 80 | \ | PD | |
| 9 | HCC | IV | Yes | 1 | 40 | 80 | \ | \ | PD | |
| 10 | HCC | IV | Yes | 2 | 60 | 80 | 80 | 80 | SD | 3.5 |
| 11 | HCC | IV | Yes | 2 | 60 | 90 | 90 | 90 | PD | |
| 12 | HCC | IV | Yes | 2 | 60 | 90 | 90 | 90 | SD | 8.0 |
| 13 | HCC | IV | Yes | 2 | 90 | 80 | 80 | 80 | SD | 5.5 |
| 14 | PC | IV | Yes | 1 | 90 | 80 | 70 | \ | PD | |
| 15 | HCC | III | No | 2 | 90 | 90 | 90 | 90 | PD |
HCC --- hepatocellular carcinoma
NSCLC --- non-small cell lung cancer
PC --- pancreatic cancer
SD --- stable disease
PD --- progressive disease
Figure 1.
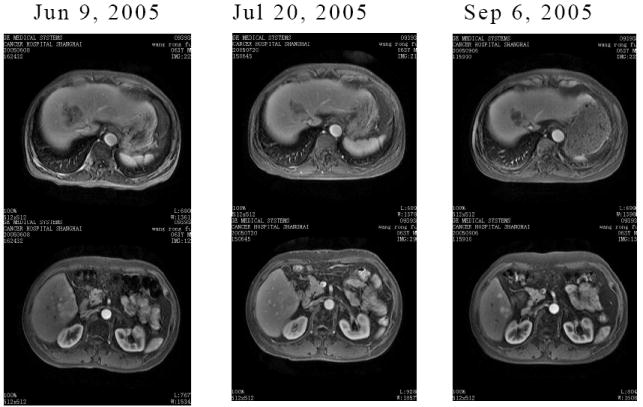
CT scans of the abdomen for patient 3 with hepatocellular carcinoma showing a 20% reduction in tumor mass from baseline (left) to the second cycle of treatment (right).
Plasma Bufalin Determination
Table 4 shows a clear dose-dependent increase in bufalin levels (bufalin levels in the plasma from patients given level 1 and 2 doses were too low for detection). Although no formal kinetics analyses were performed because of our limited data, it was clearly shown that maximum levels of bufalin were reached 2 hours after the infusion. Accumulation of bufalin within patients (at least in plasma) over the dosing period was not observed, as evidenced by the absence of detectable bufalin in samples collected 24 hours after the drug infusion. Similarly, the plasma bufalin concentration in all the samples collected on day 14 was not higher than that at 2 hours after the first day’s infusion, again suggesting a lack of accumulation of the drug in plasma.
Table 4.
Determination of plasma bufalin levels by LC/MS/MS
| Plama Bufalin Level (ng/mL) | |||
|---|---|---|---|
| Time point | Dose level 3 | Dose level 4 | Dose level 5 |
| Predose | 0 | 0 | 0 |
| 1 hr | 0.17 ± 0.07 | 0.55± 0.34 | 2.02 ± 0.68 |
| 2 hr | 0.81 ± 0.14 | 1.46 ± 0.46 | 3.38 ± 0.69 |
| 24 hr | 0 | 0.26 ± 0.16 | 0 |
| 14 days | 0.23 ± 0.10 | 0.92 ± 0.59 | 1.67 ± 1.31 |
| 21 days | 0 | 0.16 ± 0.06 | 0 |
Values are presented as the mean of three experimental values from the same treatment group.
Quality of Life Outcomes
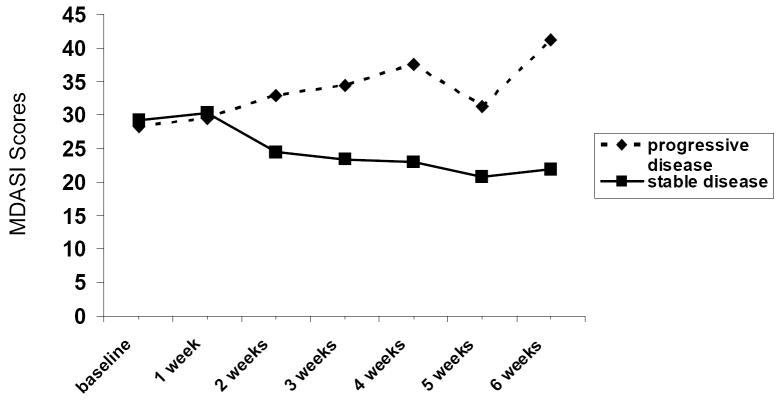
There were no significant changes over time in patients’ overall cancer-related symptoms as assessed by the MDASI during the first cycle of treatment (baseline: 28.7 ± 26.1; week 1: 29.8 ± 30.8; week 2: 29.5 ± 32.3; week 3: 30.0 ± 33.5; week 4: 30.3 ± 32.3; paired t-tests compared to baseline) and no association between symptoms and treatment dose (data not shown). Although there was a large variance in cancer-related symptoms among patients and among patients by the dose levels they received starting at baseline, this was attributed to the initial extent of disease and remained relatively stable within the groups of patients by dose during the first cycle of treatment. Not surprisingly, patients with stable disease showed a reduction in their MDASI scores over time and patients with progressive disease showed an increase in their MDASI scores over time, although the differences between the two groups did not reach statistical significance, likely due to the small sample size (PROC MIXED procedure in SAS V9.1.3) (see Figure 2).
Figure 2.
Severity of cancer-related symptoms across two cycles of treatment for groups of patients with stable disease and progressive disease.
DISCUSSION
In this trial there were no DLTs associated with the administration of Huachansu, though we gave doses considerably higher than the conventional doses used in China (conventional doses = 20-25 mL; our highest dose = 162 mL). The grade I and II side effects that may have been related to Huachansu observed in this study were hematologic, gastrointestinal, mucocutaneous, and cardiovascular in nature (see Table 2). These side effects did not appear dose related, and it was unclear for many of them whether they were genuinely related to drug or rather to the underlying disease. Eleven patients (73%) had no toxicities greater than grade I. Importantly, significant cardiac toxicity was not observed despite the administration of high doses of this cardiac glycoside-containing compound. There were also no statistically significant changes in cancer-related symptoms associated with the administration of Huachansu. The dose was not escalated beyond level 5 (i.e., 90 mL/m2, for a total of 162 mL), even though no DLT’s were detected at this high dose, as this was already approximately eight times the dose generally used in China (20-25 ml) and because of the observation of stable disease at the low doses that are comparable to the dose commonly used.14
Although there were no complete or partial responses in this study, some patients had stable disease (6 of 15 patients with a median duration of 6 months [range, 3.5 to 11.1 months]). Of particular note, 1 patient with hepatocellular carcinoma had a 20% reduction in tumor mass that lasted for more than 11 months.
Several studies have suggested the potential therapeutical value of cardiac glycosides such as digoxin, oleandrin, and bufalin in the treatment of various cancers.15-21 Bufadienolides (cardiac glycosides derived from Chansu), including bufalin, cinobufagin, and epoxybufanolides, have been found to inhibit tumor cell proliferation and induce apoptosis in human leukemia HL-60 and U937 cells, prostate cancer PC3 and DU145 cells, and human epidemoid carcinoma KB cells.12, 13, 21, 22 These glycosides induce differentiation and apoptosis in a broad range of human leukemia cell lines through alteration of expression of c-myc and bcl-2. Additionally, it was found that bufalin inhibited proliferation of human leukemia U927 cells by the activation of mitogen-activated protein kinase (MAPK) via a signaling pathway that included Ras, Raf-1, and MAPK kinase-1.23 Furthermore, research suggests that bufalin induces cell cycle changes in human leukemia cells by reducting levels of topoisomerase II.13, 22
Studies conducted in China have suggested that Huachansu can inhibit tumor cell growth and improve immunologic function.24 Indeed, in vitro experiments showed that Huachansu suppressed the growth of various human cancer cell lines 25, 26 and inhibited the proliferation of MGC-80-3 and SMMC-7721 cells by causing S phase cell cycle arrest and inhibiting Bcl-2 expression.27 In addition, we previously found that the proliferation of human melanoma BRO cells was inhibited by Huachansu via G2/M cell cycle arrest (unpublished observations, P. Yang). This anticancer activity might be associated with activation of the MAPK pathway, as evidenced by increased pERK in BRO cells (unpublished observations, P. Yang), suggesting that cardiac glycosides at least partially contribute to the anticancer activity of Huachansu.11 The same product that was used in the in vitro work was used in this trial. Furthermore, Huachansu has previously been found to markedly inhibit the biosynthesis of DNA and RNA in H22 AH, ascites hepatoma cells 28 and was also effective in reducing tumor volume and increasing survival in mice bearing Lewis lung tumor cells.29 Therefore, Huachansu appears to have important antineoplastic properties that warrant further clinical testing.
We were not able to conduct formal pharmacokinetic analyses in this trial in largely because Huachansu is not a conventional drug. Rather, it is a complex extract containing multiple chemical components. There are few biomarkers or specific chemical constituents that could be used to assess pharmacokinetic parameters, and our preclinical research suggests that the antitumor activity of Huachansu can be attributed to multiple components of the extract and not a single active principal ingredient. This is one of the challenges of researching natural products. Despite these challenges, it should be noted that the product has a uniform manufacturing process and three different lots tested had virtually identical proportion of bufalin and fingerprints. We were able to measure, in a limited fashion, plasma concentrations of bufalin, a known cardiac glycoside that has been reported to have anticancer activity against multiple cancer cell lines.12, 13, 21, 22 Bufalin was not detected in the plasma of patients who received level 1 and 2 doses probably because of the relatively low level of bufalin in the Huachansu injection and a minimum the detection level of 0.15 ng/mL using our assay. Regardless, we were at least able to quantify the bufalin levels in the patients who received dose levels 3-5. There was a dose-dependent increase in bufalin levels, with maximum levels of bufalin reached 2 hours after the infusion and no suggestion of accumulation of the drug in plasma, which may be due to the drug’s short half-life. Given that the patient with stable disease and 20% tumor shrinkage was treated with the level 1 dose, there was no clear correlation between the plasma bufalin content and antitumor effect of Huachansu. However, the small number of patients precluded making an association between dose and response. A phase II clinical trial of Huachansu is currently ongoing at the Fudan University Cancer Hospital for patients with advanced pancreatic cancer. This is part of the International Center of Traditional Chinese Medicine for Cancer funded by the NCI. Patients are randomized and treated with gemcitabine and Huachansu (20 mL/m2) or gemcitabine and placebo. The 20mL/m2 dose of Huachansu in that trial was chosen based on historical use and on our finding of the best response at the lower doses in the current trial. We conclude the Huachansu is well tolerated, even at doses eight times those normally administered in China, and that, as a single agent, it can result in disease stabilization in a subset of patients.
Acknowledgments
Support was provided in part by National Cancer Institute (NCI) grants CA108084 and CA12153031. Huachansu provided by Anhui Jinchan Biochemistry Company Ltd. We thank Dr. Joseph Chiang and Jennifer McQuade for all their support with language, culture, and politics. The Office of Scientific Publications, The University of Texas M. D. Anderson Cancer Center, for their helpful editorial comments on this article
References
- 1.Huang KC. The pharmacology of Chinese Herbs. CRC Press; 1993. Anesthetic and muscle-relaxing herbs: Chan Su; pp. 114–115. [Google Scholar]
- 2.Yan ZH, Weng WJ, Gao XM, Pang JZ, Sheng LS, Geng SS, et al. Chan Su. Chinese Materia Medica. 1979 [Google Scholar]
- 3.Su YH, Nu X. Evaluation of pharmacodynamic effect of pharmaceutical agents of Chan Su. J Beijing Univ of TCM. 2001;24:51–54. [Google Scholar]
- 4.Su YH, Huang XQ, Zhang DZ, Zhang YN, Xie JM, Linh CQ. HPLC separation and determination of bufadienolide in cinobufacini injection. Chinese Traditional Patent Med. 2003;25:24–27. [Google Scholar]
- 5.Newman RA, Yang P, Pawlus AD, Block KI. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8(1):36–49. doi: 10.1124/mi.8.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Hang L. Clinical effect of HuaChanSu injection in combination with chemotherapy in advanced lung cancer. Henan Journal of Oncology. 2002;15 [Google Scholar]
- 7.Zhongjie S, Chengen P, Guojun W. Clinical observation on HuaChanSu in treating hepatocellular carcinoma after transarterial chemoembolization (TACE) Cancer Prevention and Treatment. 2002;29:67–69. [Google Scholar]
- 8.Suxia H, Qing Z, Dongxiu L. Clinical effect of HuaChanSu combined with radiotherapy in cancer treatment. Shanxi Journal of Medicine. 2002;31 [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer: The M. D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transduction function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 12.Kamano Y, Nogawa T, Yamashita A, Hayashi M, Inoue M. Isolation and structure of a 20,21-epoxybufanolides series from Chansu. J Na Prod. 2002;65:1001–05. doi: 10.1021/np0200360. [DOI] [PubMed] [Google Scholar]
- 13.Masuda Y, Kawazoe N, Nakajo S, Yoshida T, Kuroiwa Y, Nakaya K. Bufalin induces apoptosis and influences the expression of apoptosis-related genes in human leukemia cells. Leuk Res. 1995;19:549–56. doi: 10.1016/0145-2126(95)00031-i. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wang Q, Lu w, Zhang x, Zheng J, Wang Q. Chemotherapeutic effect of Huachansu on human lung cancer- 106 case report. Chinese Journal of Clinical Oncology and Rehabilitation. 2001;8(4):90–91. [Google Scholar]
- 15.Haux J, Solheim O, Isaksen T, Angelsen A. Digitoxin, non-toxic concentrations, inhibits proliferation and induces cell death in prostate cancer cell lines. Z Onkol. 2000;32:14–20. [Google Scholar]
- 16.Huang YT, Chuedh SC, Teng CM, Guh JH. Investigation of ouabain-induced anticancer effect in human androgen-independent prostate cancer PC-3 cells. Biochemical Pharmacology. 2004;67:727–33. doi: 10.1016/j.bcp.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Jing Y, Ohizumi H, Kawazoe N, Hashimoto S, Masuda Y, Nakajo S, et al. Selective inhibitory effect of bufalin on growth of human tumor cells in vitro: association with the induction of apoptosis in leukemia HL-60 cells. Jpn J cancer Res. 1994;85:645–51. doi: 10.1111/j.1349-7006.1994.tb02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson S, Lindholm P, Gullbo J, Larsson R, Bohlin L, Claeson P. Cytotoxicity of digitoxin and related cardiac glycosides in human tumor cells. Anticancer Drugs. 2001;12:475–83. doi: 10.1097/00001813-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Kawazoe N, Watabe M, Masuda Y, Nakajo S, Nakaya K. Tiam1 is involved in the regulation of bufalin-induced apoptosis in human leukemia cells. Oncogene. 1999;18:2413–21. doi: 10.1038/sj.onc.1202555. [DOI] [PubMed] [Google Scholar]
- 20.Mckonkey DJ, Lin Y, Nutt LK, Ozel HZ, Newman RA. Cardiac glycosides stimulate Ca2+increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Research. 2000;60:3807–12. [PubMed] [Google Scholar]
- 21.Yeh JY, Huang WJSFK, Wang PS. Effects of bufalin and cinobufalin on the proliferation of androgen dependent and independent prostate cancer cells. Prostate. 2003;54:112–24. doi: 10.1002/pros.10172. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto S, Jing Y, Kawazoe N. Bufalin reduces the level of topoisomerase II in human leukemia cells and affects the cytotoxicity of anticancer drugs. Leuk Res. 1997;21:875–83. doi: 10.1016/s0145-2126(97)00061-1. [DOI] [PubMed] [Google Scholar]
- 23.Xie ZJ, Cai T. Na+-K+-ATPase mediated signal transudation: from protein interaction to cellular function. Mol Interv. 2003;3:157–68. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 24.Chen GH. Advances in quality determination, pharmacological studies and clinical application of toad venom. Chinese Traditional and Herbal Drugs. 2001;32:184–86. [Google Scholar]
- 25.Cuo XD, Cuei YA, Wang JH, Qin S, Chen HY. The effect of cinobufacini on human gastric neoplasm cell line MGC-80-3. Chinese Clin Oncol. 2003;8:33–37. [Google Scholar]
- 26.Zhang ZY, Zhang KH, Wang ZW, Zhu JQ, Zhu ZH, Huang DQ. Cytotoxicity of HuaChanSu on three digestive tract tumor cells in vitro. Chinese Med Pharma Clin. 1999;15:28–29. [Google Scholar]
- 27.Cuo XD, Cuei YA, Qin S, Chen HY, Wang JH. Effect of cinobufacini on tumor cell cycle and expression of bcl-2 protein. Modern J Integrated Traditional Chinese and Western Med. 2003;12:567–68. [Google Scholar]
- 28.Guan J, Zhao XH, Jiang BS, Chen ZW, Qin QY. Preliminary study on the antitumor mechanism of cinobufacini injection. J Penfu Med College. 1993;18:78–81. [Google Scholar]
- 29.Lin Y, Fu J, Chen XW, Lin DQ. Experimental study on anticancer action of cinobufacini injection for pulmonary carcinoma. J Guangzhou Univ Traditional Chinese Med. 2003;20:69–71. [Google Scholar]